Functional Groups Response to Water Deficit in Mediterranean Ecosystems
Abstract
1. Introduction
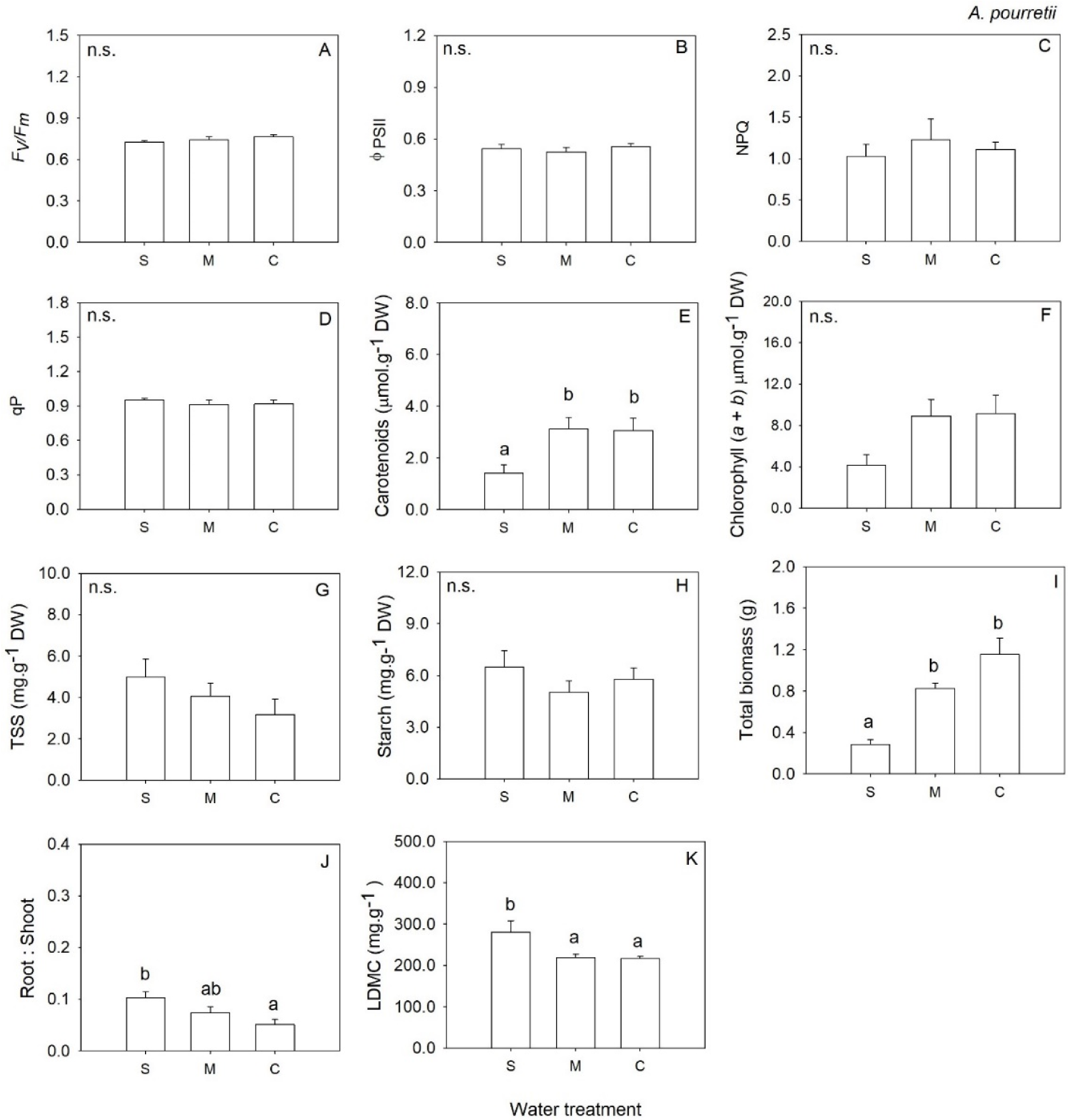
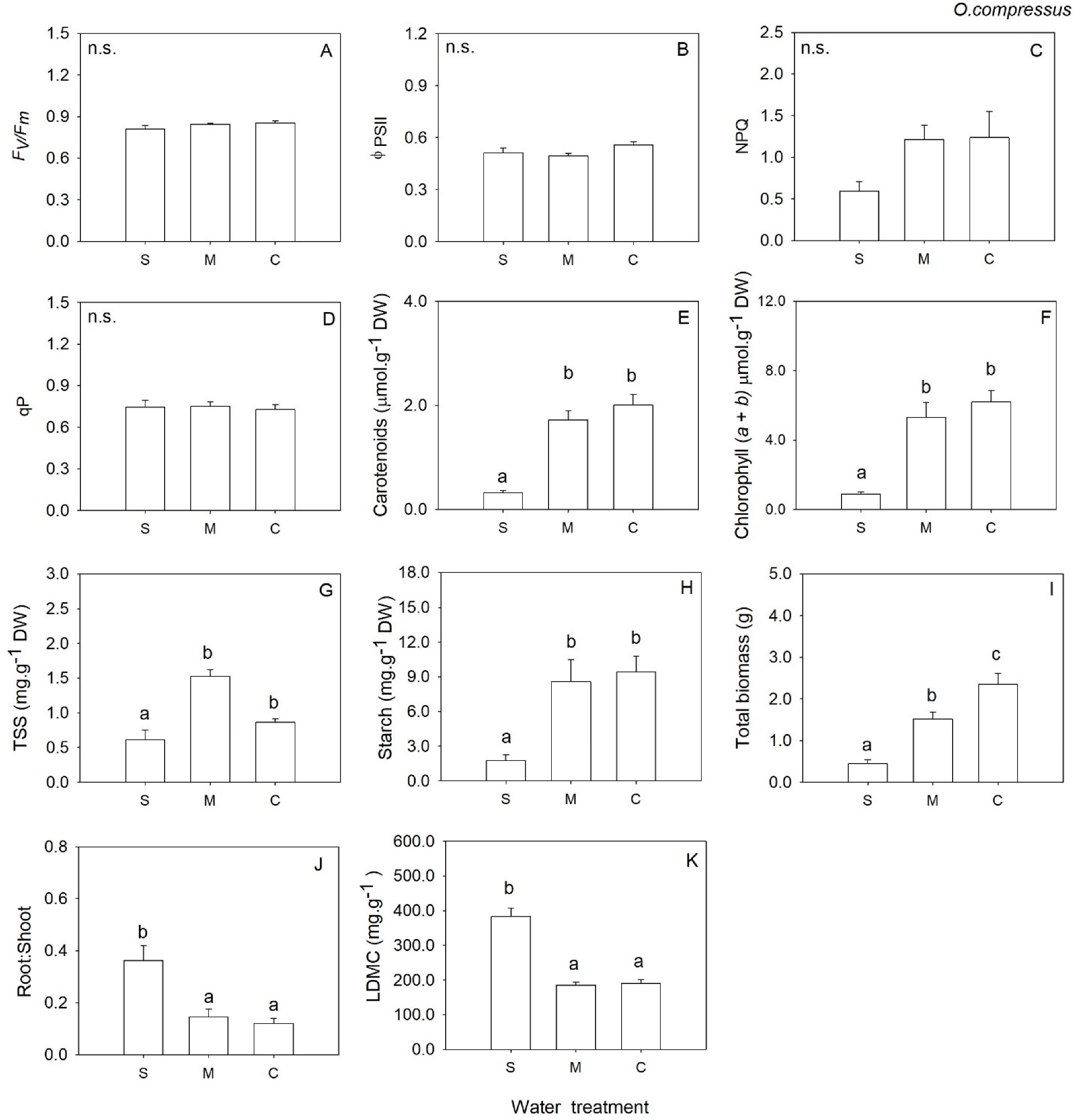
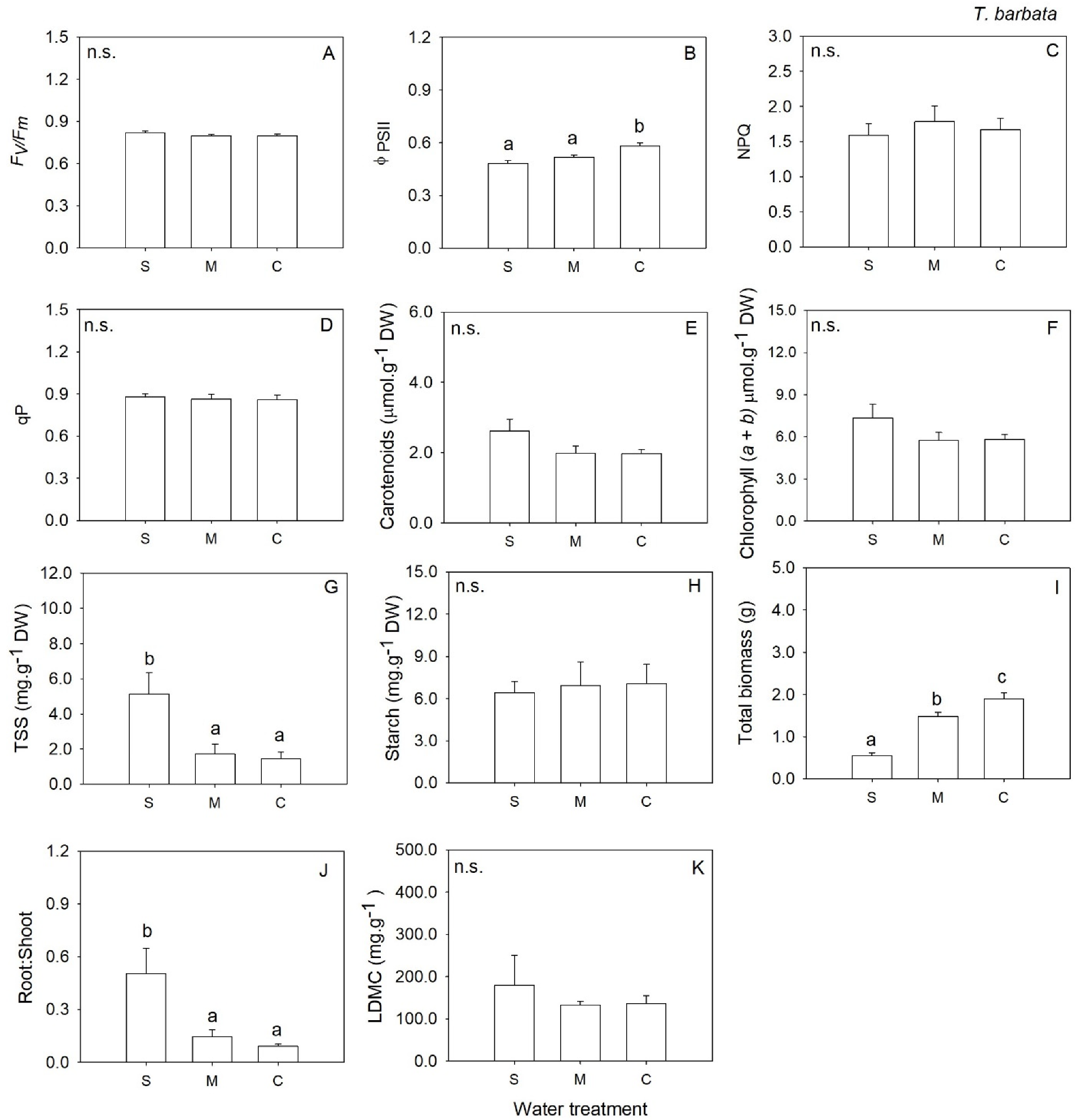
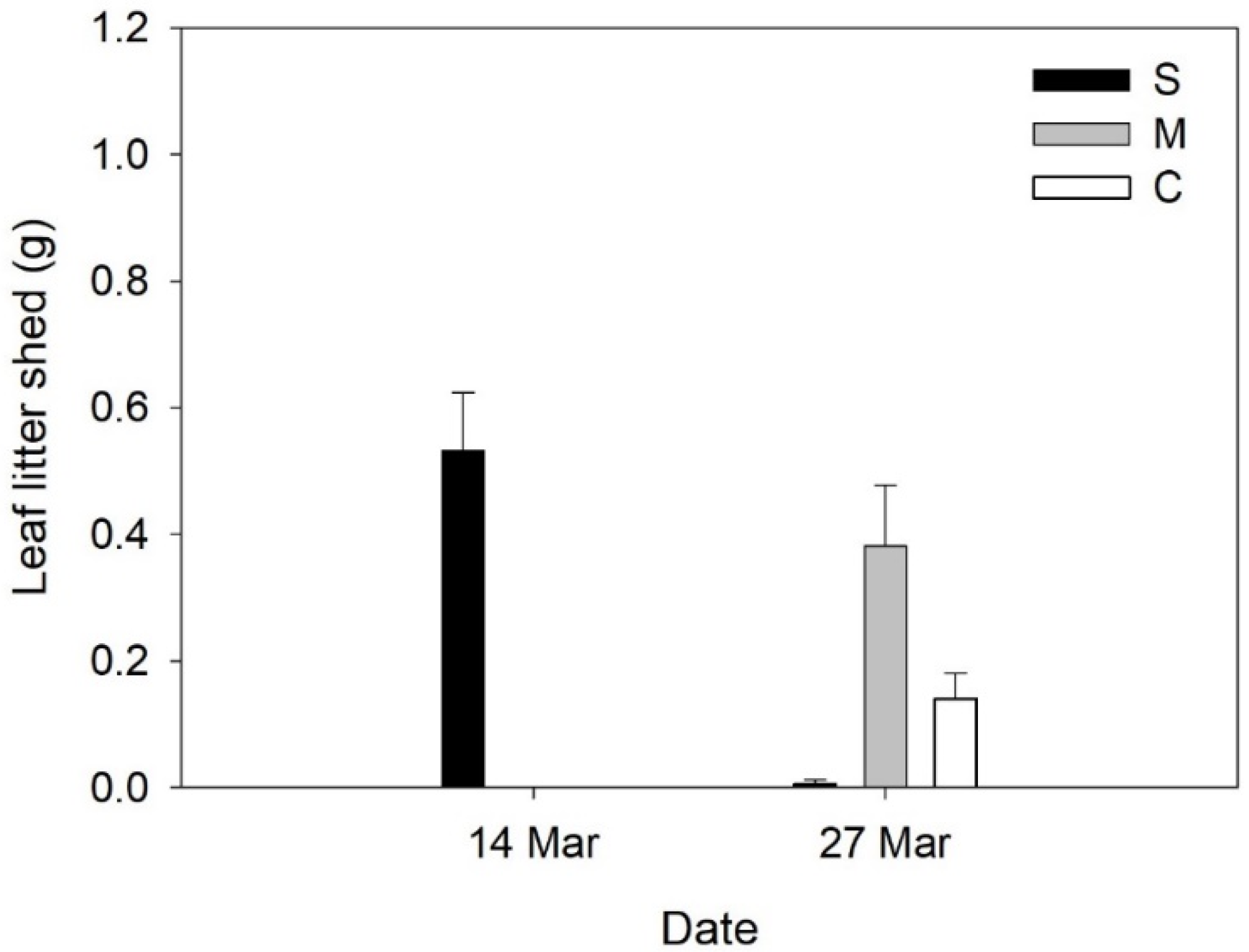
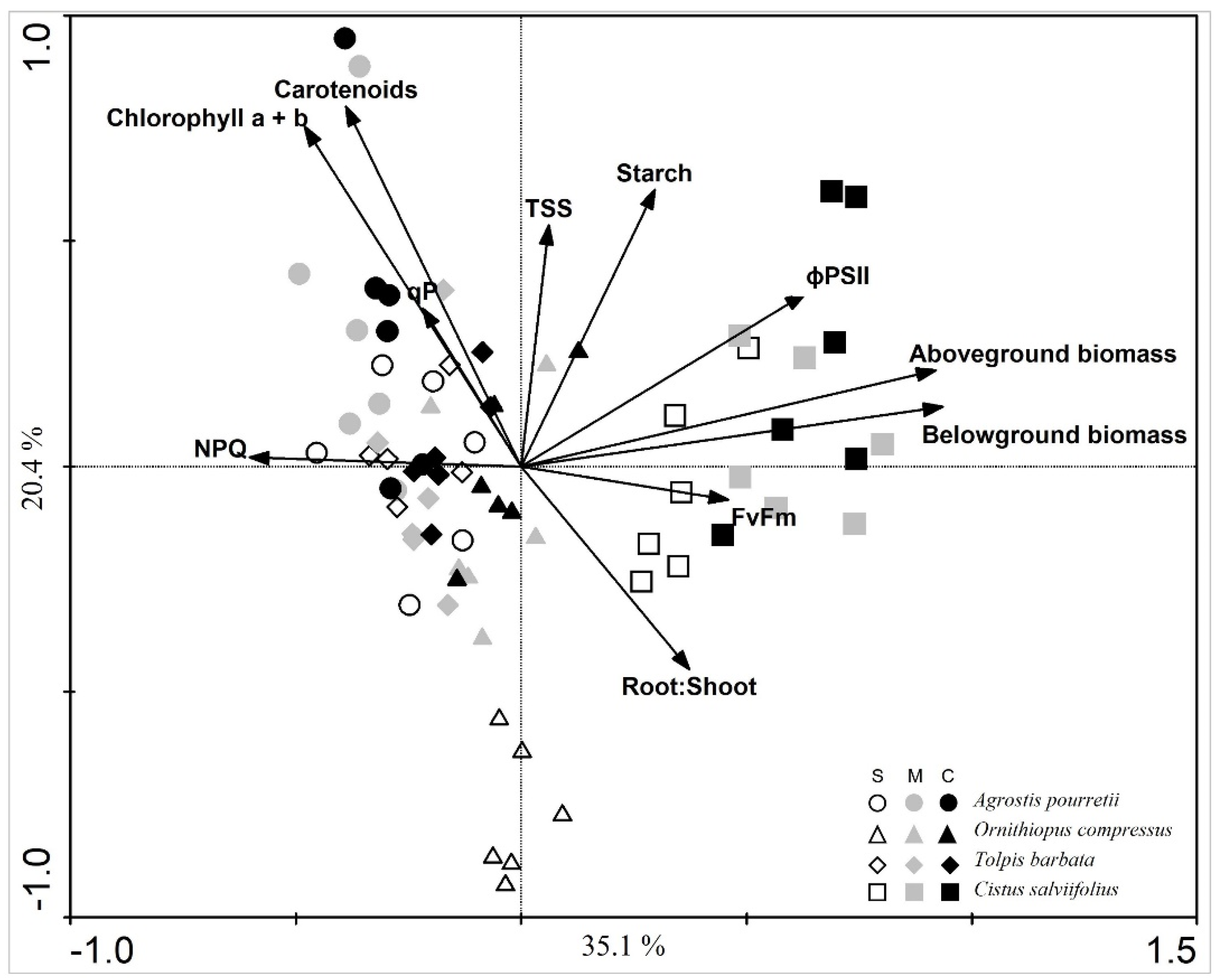
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Set Up
4.2. Water Deficit Treatment
4.3. Sampling and Measurements
4.3.1. Plant Water Content, Chlorophyll a Fluorescence and Pigments Content
4.3.2. Plant Carbohydrates Contents
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- IPCC. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Castro, H.; Barrico, L.; Rodríguez-Echeverría, S.; Freitas, H. Trends in Plant and Soil Microbial Diversity Associated with Mediterranean Extensive Cereal-Fallow Rotation Agro-Ecosystems. Agric. Ecosyst. Environ. 2016, 217, 33–40. [Google Scholar] [CrossRef]
- García-Palacios, P.; Shaw, E.A.; Wall, D.H.; Hättenschwiler, S. Temporal Dynamics of Biotic and Abiotic Drivers of Litter Decomposition. Ecol. Lett. 2016, 217, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant Secondary Metabolites: A Key Driver of Litter Decomposition and Soil Nutrient Cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Castro, H.; Castro, P. Mediterranean Marginal Lands in Face of Climate Change: Biodiversity and Ecosystem Services. In Climate Change Management; Castro, P., Azul, A., Leal Filho, W., Azeiteiro, U., Eds.; Springer: Cham, Switzerland, 2009; pp. 175–187. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field. Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- de Dato, G.D.; Micali, M.; Abou Jaoudé, R.; Liberati, D.; De Angelis, P. Earlier Summer Drought Affects Leaf Functioning of the Mediterranean Species Cistus monspeliensis L. Environ. Exp. Bot. 2013, 93, 13–19. [Google Scholar] [CrossRef]
- Castro, H.; Freitas, H. Aboveground Biomass and Productivity in the Montado: From Herbaceous to Shrub Dominated Communities. J. Arid Environ. 2009, 73, 506–511. [Google Scholar] [CrossRef]
- Turner, N.C. Adaptation to Water Deficits: A Changing Perspective. Aust. J. Plant Physiol. 1986, 13, 175–190. [Google Scholar] [CrossRef]
- Dodd, I.C.; Ryan, A.C. Whole-Plant Physiological Responses to Water-Deficit Stress. In eLS; Wiley: Hoboken, NJ, USA, 2016; pp. 1–9. ISBN 9780470015902. [Google Scholar]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding Plant Responses to Drought—From Genes to the Whole Plant. Funct. Plant Biol. 2003, 30, 239. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-Oxidative Stress Markers as a Measure of Abiotic Stress-Induced Leaf Senescence: Advantages and Limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W.; López-Pozo, M.; Polutchko, S.K. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Acebron, K.; Matsubara, S.; Jedmowski, C.; Emin, D.; Muller, O.; Rascher, U. Diurnal Dynamics of Nonphotochemical Quenching in Arabidopsis Npq Mutants Assessed by Solar-Induced Fluorescence and Reflectance Measurements in the Field. New Phytol. 2021, 229, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll Fluorescence and Oxidative Stress Endpoints to Discriminate Olive Cultivars Tolerance to Drought and Heat Episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Peñuelas, J. Drought-Induced Oxidative Stress in Strawberry Tree (Arbutus unedo L.) Growing in Mediterranean Field Conditions. Plant Sci. 2004, 166, 1105–1110. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in Carotenoids, Tocopherols and Diterpenes during Drought and Recovery, and the Biological Significance of Chlorophyll Loss in Rosmarinus officinalis Plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Balaguer, L.; Pugnaire, F.I.; Armas, C.; Valladares, F.; Manrique, E. Ecophysiological Signi Cance of Chlorophyll Loss and Reduced Photochemical Ef Ciency under Extreme Aridity In. Plant Soil 2002, 240, 343–352. [Google Scholar] [CrossRef]
- Jones, M.; Osmond, C.; Turner, N. Accumulation of Solutes in Leaves of Sorghum and Sunflower in Response to Water Deficits. Funct. Plant Biol. 1980, 7, 193. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, H.; Fujita, D.; Basra, S. Review Article Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Magaña Ugarte, R.; Escudero, A.; Gavilán, R.G. Assessing the Role of Selected Osmolytes in Mediterranean High-Mountain Specialists. Front. Ecol. Evol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- González-Orenga, S.; Al Hassan, M.; Llinares, J.V.; Lisón, P.; López-Gresa, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Qualitative and Quantitative Differences in Osmolytes Accumulation and Antioxidant Activities in Response to Water Deficit in Four Mediterranean Limonium Species. Plants 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Santos, C.; Serodio, J.; Silva, A.M.S.; Dias, M.C. Physiological Performance of Drought-Stressed Olive Plants When Exposed to a Combined Heat–UV-B Shock and after Stress Relief. Funct. Plant Biol. 2018, 45, 1233. [Google Scholar] [CrossRef]
- Wang, J.Y.; Turner, N.C.; Liu, Y.X.; Siddique, K.H.M.; Xiong, Y.C. Effects of Drought Stress on Morphological, Physiological and Biochemical Characteristics of Wheat Species Differing in Ploidy Level. Funct. Plant Biol. 2017, 44, 219. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.M.; Tronina, Ł.; García-Plazaola, J.I.; Esteban, R.; Pereira, J.S.; Manuela Chaves, M. Resilience of a Semi-Deciduous Shrub, Cistus Salvifolius, to Severe Summer Drought and Heat Stress. Funct. Plant Biol. 2015, 42, 219–228. [Google Scholar] [CrossRef]
- Puglielli, G.; Catoni, R.; Spoletini, A.; Varone, L.; Gratani, L. Short-Term Physiological Plasticity: Trade-off between Drought and Recovery Responses in Three Mediterranean Cistus Species. Ecol. Evol. 2017, 7, 10880–10889. [Google Scholar] [CrossRef]
- Catoni, R.; Gratani, L.; Varone, L. Physiological, Morphological and Anatomical Trait Variations between Winter and Summer Leaves of Cistus Species. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 442–449. [Google Scholar] [CrossRef]
- Jongen, M.; Hellmann, C.; Unger, S. Species-Specific Adaptations Explain Resilience of Herbaceous Understorey to Increased Precipitation Variability in a Mediterranean Oak Woodland. Ecol. Evol. 2015, 5, 4246–4262. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J.; Silva, S.; Santos, C. Photosynthetic Parameters of Ulmus minor Plantlets Affected by Irradiance during Acclimatization. Biol. Plant. 2013, 57, 33–40. [Google Scholar] [CrossRef]
- Jongen, M.; Unger, S.; Fangueiro, D.; Cerasoli, S.; Silva, J.M.N.; Pereira, J.S. Resilience of Montado Understorey to Experimental Precipitation Variability Fails under Severe Natural Drought. Agric. Ecosyst. Environ. 2013, 178, 18–30. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Dovrat, G.; Meron, E.; Shachak, M.; Golodets, C.; Osem, Y. Plant Size Is Related to Biomass Partitioning and Stress Resistance in Water-Limited Annual Plant Communities. J. Arid Environ. 2019, 165, 1–9. [Google Scholar] [CrossRef]
- de Oliveira, J.M.P.F.; Santos, C.; Araújo, M.; Oliveira, M.M.; Dias, M.C. High-Salinity Activates Photoprotective Mechanisms in Quercus Suber via Accumulation of Carbohydrates and Involvement of Non-Enzymatic and Enzymatic Antioxidant Pathways. New For. 2022, 53, 285–300. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Xu, L.K. Sensitivity of with of Roots versus Leaves to Water Stress: Biophysical Analysis and Relation to Water. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root Development in Horticultural Plants Grown under Abiotic Stress Conditions—A Review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Garnier, E.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; Dolezal, J.; Eriksson, O.; Fortunel, C.; Freitas, H.; Golodets, C.; et al. Assessing the Effects of Land-Use Change on Plant Traits, Communities and Ecosystem Functioning in Grasslands: A Standardized Methodology and Lessons from an Application to 11 European Sites. Ann. Bot. 2007, 99, 967–985. [Google Scholar] [CrossRef]
- van Kooten, O.; Snel, J.F.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Osaki, M.; Shinano, T.; Tadano, T. Redistribution of Carbon and Nitrogen Compounds from the Shoot to the Harvesting Organs during Maturation in Field Crops. Soil Sci. Plant Nutr. 1991, 37, 117–128. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The Meta-Analysis of Response Ratios in Experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]


| Effect | Water Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | A. pourretti | O. compressus | T. barbata | C. salviifolius | ||||||||
| Response Variables | df | F | p | df | F | p | df | F | p | df | F | p |
| Physiological | ||||||||||||
| Fv/Fm | 2 | 1.20 | 0.327 | 2 | 2.10 | 0.157 | 2 | 0.91 | 0.425 | 2 | 0.23 | 0.800 |
| ΦPSII | 2 | 0.44 | 0.653 | 2 | 0.55 | 0.589 | 2 | 9.31 | 0.003 | 2 | 1.28 | 0.306 |
| NPQ | 2 | 0.31 | 0.735 | 2 | 2.79 | 0.094 | 2 | 0.11 | 0.899 | 2 | 0.56 | 0.584 |
| qP | 2 | 0.41 | 0.671 | 2 | 0.09 | 0.919 | 2 | 0.15 | 0.859 | 2 | 0.61 | 0.559 |
| Chl (a + b) | 2 | 3.50 | 0.057 | 2 | 20.02 | <0.001 | 2 | 0.97 | 0.404 | 2 | 1.43 | 0.275 |
| Carotenoids | 2 | 5.30 | 0.018 | 2 | 34.75 | <0.001 | 2 | 1.46 | 0.265 | 2 | 1.24 | 0.321 |
| Total Soluble Sugars | 2 | 1.44 | 0.270 | 2 | 19.39 | <0.001 | 2 | 8.29 | 0.004 | 2 | 0.79 | 0.471 |
| Starch | 2 | 0.93 | 0.417 | 2 | 19.25 | <0.001 | 2 | 0.04 | 0.961 | 2 | 1.16 | 0.339 |
| Morphological | ||||||||||||
| LDMC | 2 | 7.07 | 0.009 | 2 | 48.09 | <0.001 | 2 | 0.67 | 0.528 | 2 | 15.66 | <0.001 |
| Total biomass | 2 | 30.01 | <0.001 | 2 | 25.65 | <0.001 | 2 | 36.01 | <0.001 | 2 | 62.99 | <0.001 |
| Root: Shoot | 2 | 4.95 | 0.022 | 2 | 12.62 | 0.001 | 2 | 9.12 | 0.003 | 2 | 0.27 | 0.764 |
| Belowground biomass | 2 | 2.34 | 0.131 | 2 | 6.02 | 0.012 | 2 | 0.13 | 0.877 | 2 | 6.69 | 0.008 |
| Aboveground biomass | 2 | 32.72 | <0.001 | 2 | 39.1 | <0.001 | 2 | 38.01 | <0.001 | 2 | 58.08 | <0.001 |
| Species | Water Treatment | |||
|---|---|---|---|---|
| Severe Stress | Moderate Stress | Control | ANOVA Results | |
| A. pourretti | 6.17 ± 0.54 (a) | 22.50 ± 2.69 (b) | 28.33 ± 2.29 (b) | F = 30.97, p < 0.001 |
| O. compressus | 0.00 ± 0.00 (a) | 15.17 ± 1.92 (b) | 17.67 ± 2.62 (b) | F = 26.02, p < 0.001 |
| T. barbata | 10.80 ± 3.6 (a) | 42.67 ± 4.5 (b) | 47.67 ± 4.17 (b) | F = 21.41, p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, H.; Dias, M.C.; Sousa, J.P.; Freitas, H. Functional Groups Response to Water Deficit in Mediterranean Ecosystems. Plants 2023, 12, 1471. https://doi.org/10.3390/plants12071471
Castro H, Dias MC, Sousa JP, Freitas H. Functional Groups Response to Water Deficit in Mediterranean Ecosystems. Plants. 2023; 12(7):1471. https://doi.org/10.3390/plants12071471
Chicago/Turabian StyleCastro, Helena, Maria Celeste Dias, José Paulo Sousa, and Helena Freitas. 2023. "Functional Groups Response to Water Deficit in Mediterranean Ecosystems" Plants 12, no. 7: 1471. https://doi.org/10.3390/plants12071471
APA StyleCastro, H., Dias, M. C., Sousa, J. P., & Freitas, H. (2023). Functional Groups Response to Water Deficit in Mediterranean Ecosystems. Plants, 12(7), 1471. https://doi.org/10.3390/plants12071471








