Decrease in Soil Functionalities and Herbs’ Diversity, but Not That of Arbuscular Mycorrhizal Fungi, Linked to Short Fire Interval in Semi-Arid Oak Forest Ecosystem, West Iran
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Fire History
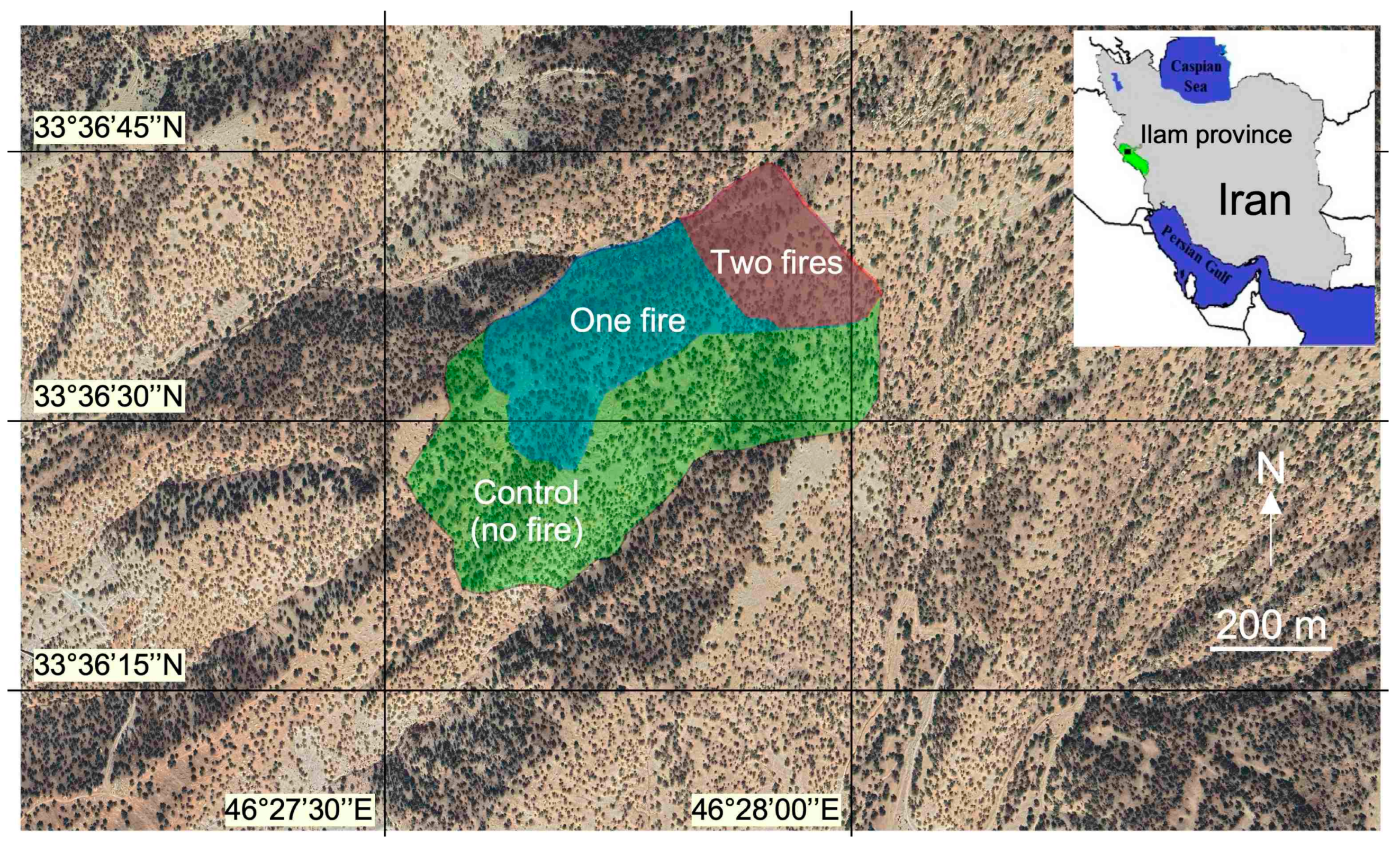
2.2. Data Collection for Physics and Chemistry of Soil
2.3. Diversity of Vascular Plants and Arbuscular Mycorrhizal Fungi
2.4. Statistical Analysis
3. Results
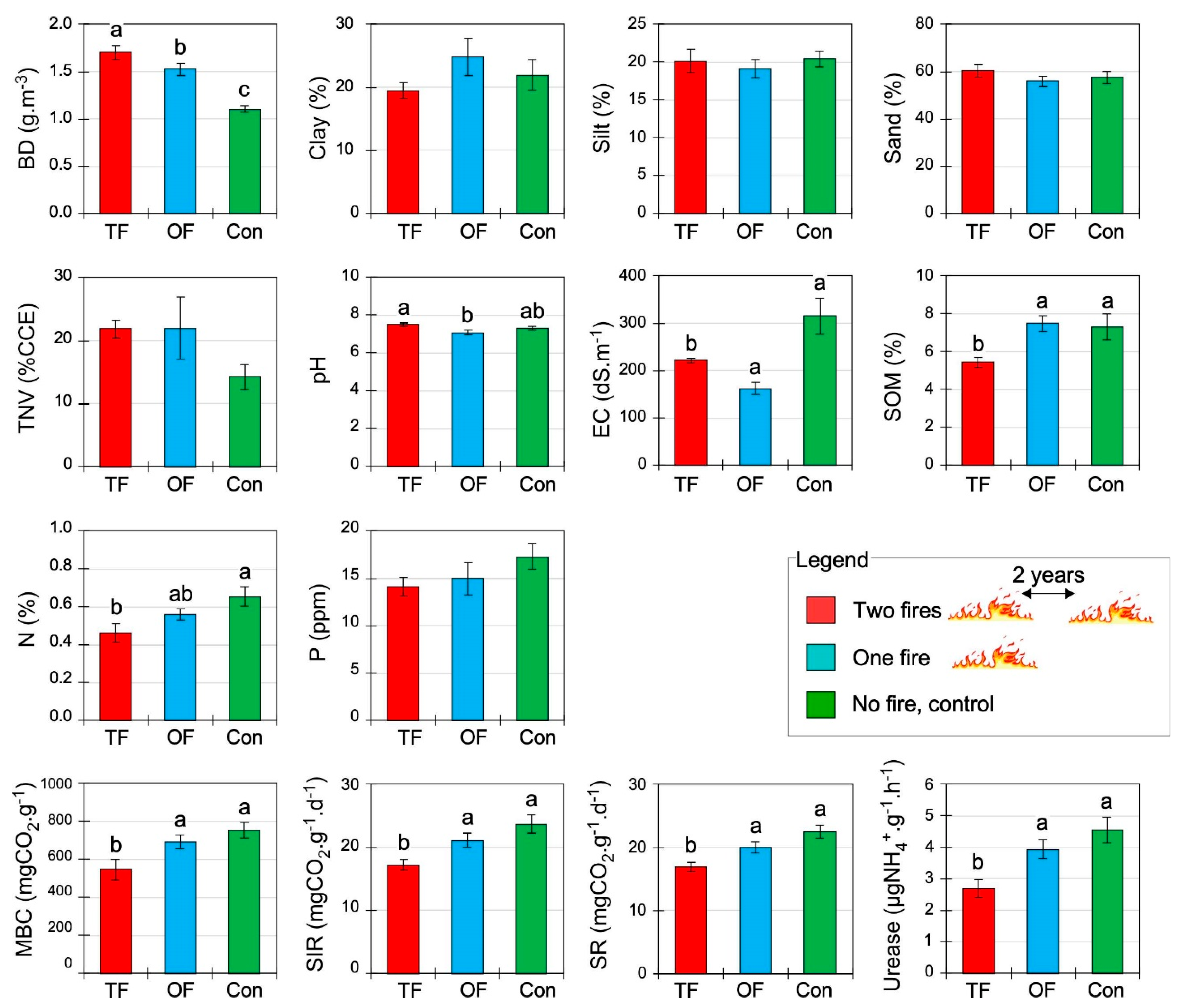
3.1. Effect of Fire Treatment on Soil Properties
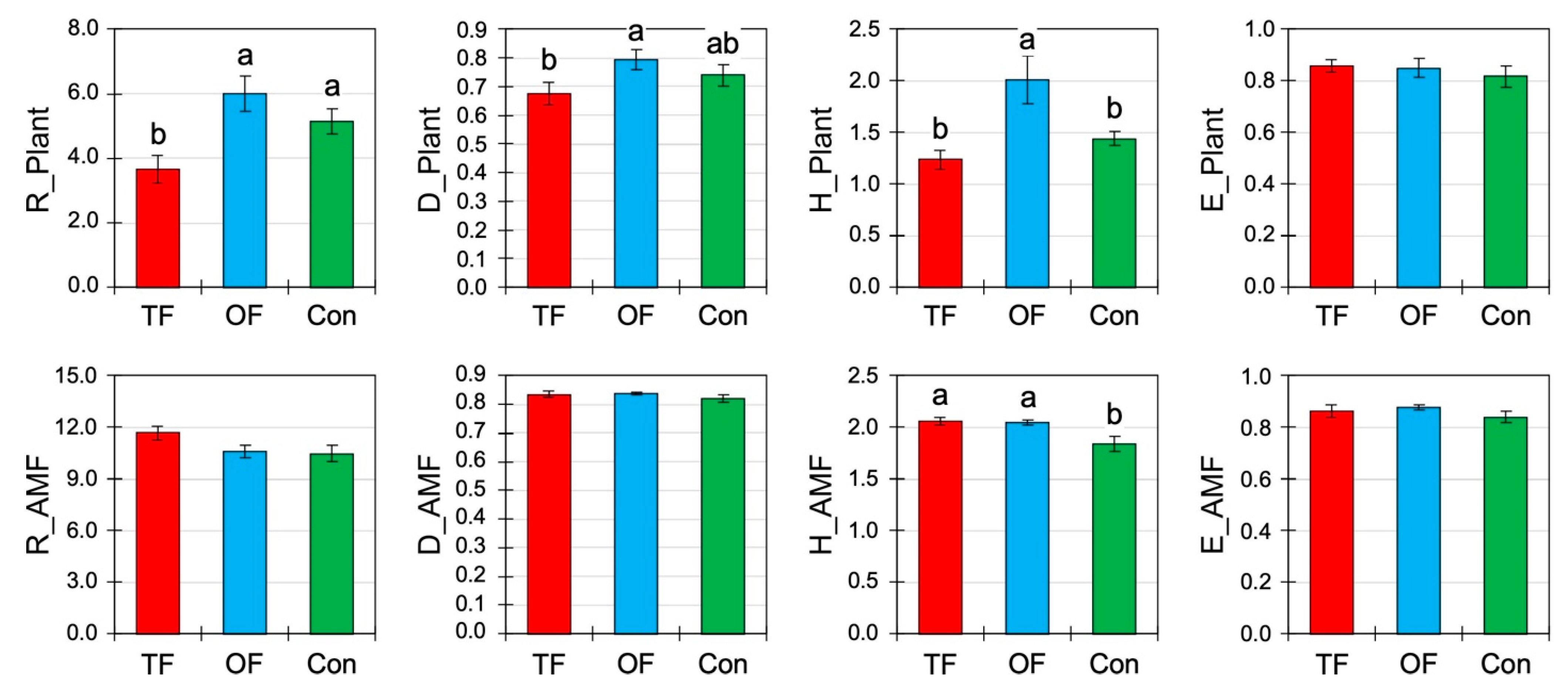
3.2. Effects of Fire Treatment on Community Diversity
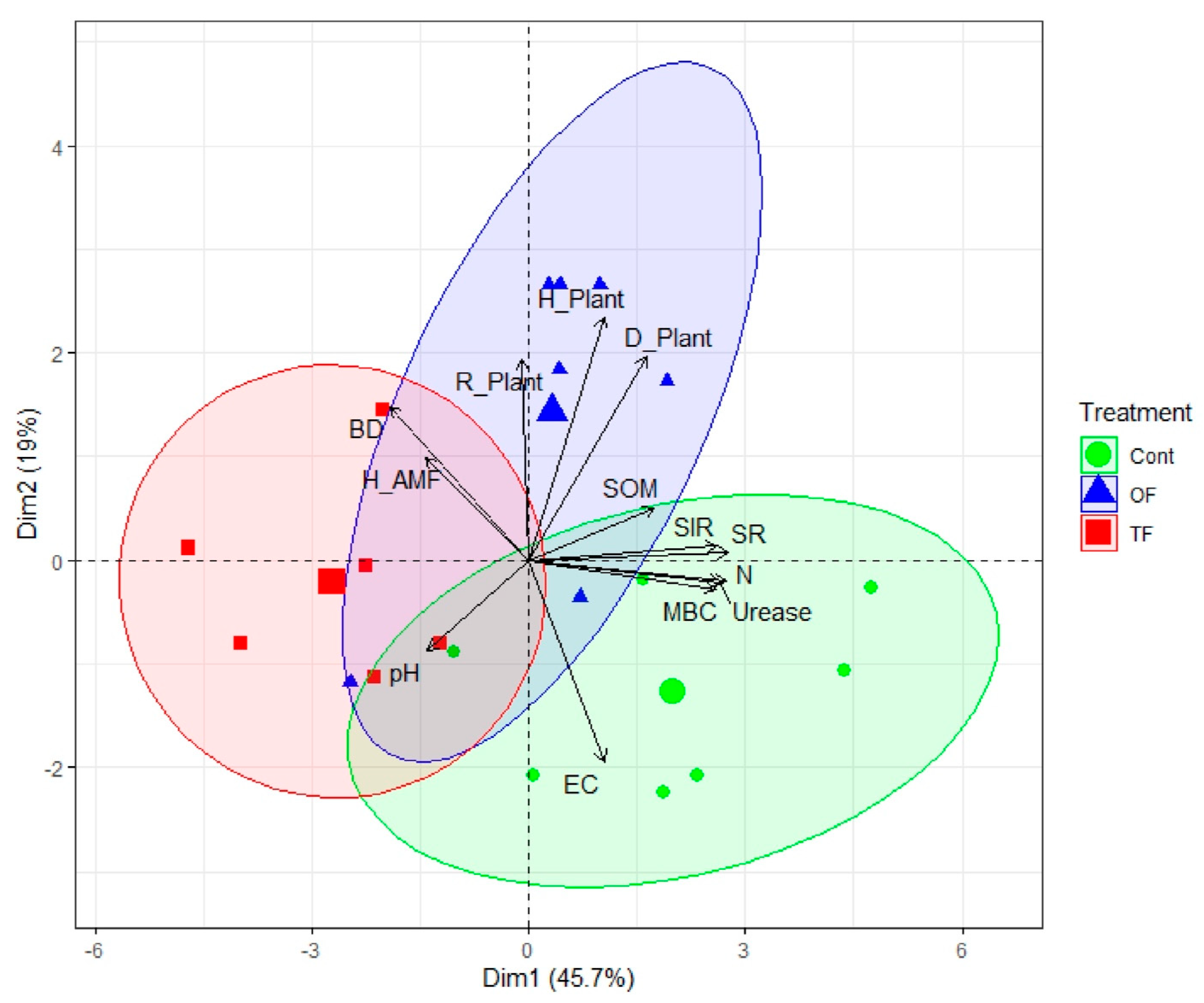
3.3. Soil Properties and Biodiversity under Different Fire Treatments
3.4. Direct and Indirect Effects of Fire on Biological Diversity and Soil Propperties
4. Discussion
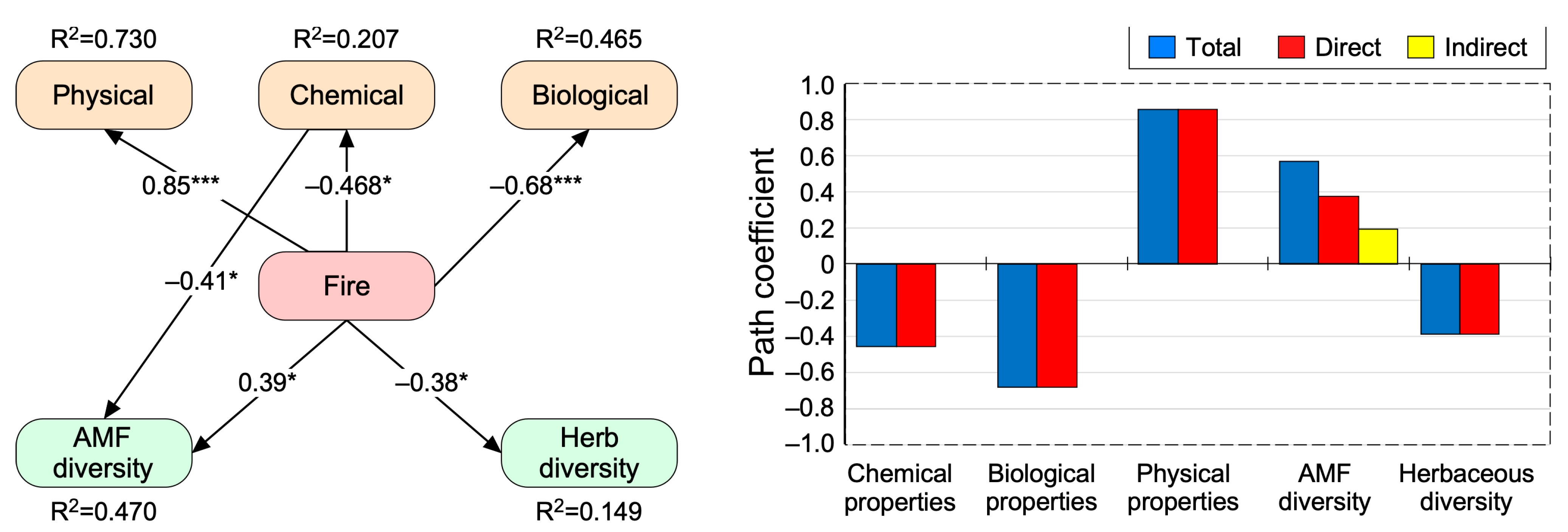
4.1. Short Fire Intervals Increased Bulk Density and Decreased Electrical Conductivity
4.2. Depleted Biochemical Soil Properties
4.3. Biodiversity Changes in Herbs and Arbuscular Mycorrhizal Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kodandapani, N.; Cochrane, M.A.; Sukumar, R. Conservation threat of increasing fire frequencies in the Western Ghats, India. Conserv. Biol. 2004, 18, 1553–1561. [Google Scholar] [CrossRef]
- Aleman, J.C.; Blarquez, O.; Gourlet-Fleury, S.; Bremond, L.; Favier, C. Tree cover in Central Africa: Determinants and sensitivity under contrasted scenarios of global change. Sci. Rep. 2017, 7, 41393. [Google Scholar] [CrossRef] [PubMed]
- Carcaillet, C.; Desponts, M.; Robin, V.; Bergeron, Y. Long-Term Steady-State Dry Boreal Forest in the Face of Disturbance. Ecosystems 2020, 23, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Cogoni, D.; Calderisi, G.; Fenu, G. Short-Term Effects and Vegetation Response after a Megafire in a Mediterranean Area. Land 2022, 11, 2328. [Google Scholar] [CrossRef]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Owen, S.M.; Patterson, A.M.; Gehring, C.A.; Sieg, C.H.; Baggett, L.S.; Fulé, P.Z. Large, high-severity burn patches limit fungal recovery 13 years after wildfire in a ponderosa pine forest. Soil Biol. Biochem. 2019, 139, 107616. [Google Scholar] [CrossRef]
- Fournier, T.; Fèvre, J.; Carcaillet, F.; Carcaillet, C. For a few years more: Reductions in plant diversity 70 years after the last fire in Mediterranean forests. Plant Ecol. 2020, 221, 559–576. [Google Scholar] [CrossRef]
- Ray, T.; Malasiya, D.; Rajpoot, R.; Verma, S.; Dar, J.A.; Dayanandan, A.; Raha, D.; Lone, P.; Pandey, P.; Khare, P.K.; et al. Impact of Forest Fire Frequency on Tree Diversity and Species Regeneration in Tropical Dry Deciduous Forest of Panna Tiger Reserve, Madhya Pradesh, India. J. Sustain. For. 2020, 40, 831–845. [Google Scholar] [CrossRef]
- Bond, W.J.; van Wilgen, B.W. Fire and Plants; Chapman & Hall: London, UK, 1996; 263p. [Google Scholar]
- Peterson, D.W.; Reich, P.B. Fire frequency and tree canopy structure influence plant species diversity in a forest grassland ecotone. Plant Ecol. 2008, 194, 5–16. [Google Scholar] [CrossRef]
- Heydari, M.; Rostamy, A.; Najafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in Zagros oak (Quercus brantii Lindl.) forests in Iran. J. For. Res. 2016, 28, 95–104. [Google Scholar] [CrossRef]
- Chick, M.P.; Nitschke, C.R.; Cohn, J.S.; Penman, T.D.; York, A. Factors influencing above-ground and soil seed bank vegetation diversity at different scales in a quasi-Mediterranean ecosystem. J. Veg. Sci. 2018, 29, 684–694. [Google Scholar] [CrossRef]
- Keeley, J.E. Seed germination and life history syndromes in the California chaparral. Bot. Rev. 1991, 57, 81–116. [Google Scholar] [CrossRef]
- Pingree, M.R.A.; DeLuca, T.H. Function of Wildfire-Deposited Pyrogenic Carbon in Terrestrial Ecosystems. Front. Environ. Sci. 2017, 5, 53. [Google Scholar] [CrossRef]
- Wardle, D.A.; Zackrisson, O.; Hörnberg, G.; Gallet, C. The Influence of Island Area on Ecosystem Properties. Science 1997, 277, 1296–1299. [Google Scholar] [CrossRef]
- Lombao, A.; Barreiro, A.; Fontúrbel, M.T.; Martína, A.; Carballas, T.; Díaz-Raviña, M. Effect of repeated soil heating at different temperatures on microbial activity in two burned soils. Sci. Total Environ. 2021, 799, 149440. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef]
- Dove, N.C.; Hart, S.C. Fire Reduces Fungal Species Richness and In Situ Mycorrhizal Colonization: A Meta-Analysis. Fire Ecol. 2017, 13, 37–65. [Google Scholar] [CrossRef]
- Taudière, A.; Richard, F.; Carcaillet, C. Review on fire effects on ectomycorrhizal symbiosis, an unachieved work for a scalding topic. For. Ecol. Manag. 2017, 391, 446–457. [Google Scholar] [CrossRef]
- Barreiro, A.; Martín, A.; Carballas, T.; Díaz-Raviña, M. Long-term response of soil microbial communities to fire and fire-fighting chemicals. Biol. Fertil. Soils 2016, 52, 963–975. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.D.C.; Seoane, S.; Gil-Sotres, F. Intra-annual variation in biochemical properties and the biochemical equilibrium of different grassland soils under contrasting management and climate. Biol. Fertil. Soils 2011, 47, 633–645. [Google Scholar] [CrossRef]
- Wardle, D.A.; Walker, L.R.; Bardgett, R.D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 2004, 305, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Salgado, M.M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological soil quality indicators: A review. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; WorldScientific: Badajos, Spain, 2010; pp. 319–332. [Google Scholar]
- Rutigliano, F.; D’Ascoli, R.; De Santo, A.V. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol. Biochem. 2004, 36, 1719–1729. [Google Scholar] [CrossRef]
- Atlas, R.M. Diversity of microbial communities. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1984; Volume 7, pp. 1–47. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; Lombao, A.; Barreiro, A.; Martín, A.; Carballas, T. Medium-term impact of post-fire emergency rehabilitation techniques on a shrubland ecosystem in Galicia (NW Spain). J. Soil Sci. 2018, 8, 322–346. [Google Scholar] [CrossRef]
- Smith, M.D.; van Wilgen, B.W.; Burns, C.E.; Govender, N.; Potgieter, A.L.F.; Andelman, S.; Biggs, H.C.; Botha, J.; Trollope, W.S.W. Long-term effects of fire frequency and season on herbaceous vegetation in savannas of the Kruger National Park, South Africa. J. Plant Ecol. 2012, 6, 71–83. [Google Scholar] [CrossRef]
- Simpson, K.J.; Ripley, B.S.; Christin, P.; Belcher, C.M.; Lehmann, C.E.R.; Thomas, G.H.; Osborne, C.P. Determinants of flammability in savanna grass species. J. Ecol. 2015, 104, 138–148. [Google Scholar] [CrossRef]
- Willis, K.J.; Braun, M.; Sümegi, P.; Toth, A. Does soil change cause vegetation change or vice versa? A temporal perspective from Hungary. Ecology 1997, 78, 740–750. [Google Scholar] [CrossRef]
- Magomani, M.I.; Van Tol, J.J. The impact of fire frequency on selected soil physical properties in a semi-arid savannah Thornveld. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2018, 69, 43–51. [Google Scholar] [CrossRef]
- DeBano, L.F.; Neary, D.G.; Folliot, P.F. Chapter 2: Effects on soil physical properties. In Wildland Fire in Ecosystems: Fire Effects on Soil and Water; Neary, D.G., Ryan, K.C., DeBano, L.F., Eds.; General Technical Report RMRS-GTR-42; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2005; Volume 4, pp. 29–52. 250p. [Google Scholar]
- Almendros, G.; González-Vila, F.J.; Martin, F. Fire-induced transformation of soil organic matter from an oak forest: An experimental approach to the effects of fire on humic substances. Soil Sci. 1990, 149, 158–168. [Google Scholar] [CrossRef]
- Mirzaei, J. Impacts of two spatially and temporally isolated anthropogenic fire eventson soils of oak-dominated Zagros forests of Iran. Turk. J. Agric. For. 2016, 40, 109–119. [Google Scholar] [CrossRef]
- Moritz, M.A.; Parisien, M.-A.; Batllori, E.; Krawchuk, M.A.; Van Dorn, J.; Ganz, D.J.; Hayhoe, K. Climate change and disruptions to global fire activity. Ecosphere 2012, 3, 49. [Google Scholar] [CrossRef]
- Moradizadeh, H.; Heydari, M.; Omidipour, R.; Mezbani, A.; Prévosto, B. Ecological effects of fire severity and time since fire on the diversity partitioning, composition and niche apportionment models of post-fire understory vegetation in semi-arid oak forests of Western Iran. Ecol. Eng. 2019, 143, 105694. [Google Scholar] [CrossRef]
- Roshan, S.A.; Heydari, M.; Wait, A.; Uddin, S.M.; Lucas-Borja, M.E.; Keeley, J.E. Divergent successional trajectories of soil seed bank and post-fire vegetation in a semiarid oak forest: Implications for post-fire ecological restoration. Ecol. Eng. 2022, 182, 106736. [Google Scholar] [CrossRef]
- Enright, N.J.; Fontaine, J.B.; Westcott, V.C.; Lade, J.C.; Miller, B.P. Fire interval effects on persistence of resprouter species in Mediterranean-type shrublands. Plant Ecol. 2011, 212, 2071–2083. [Google Scholar] [CrossRef]
- Mahood, A.L.; Balch, J.K. Repeated fires reduce plant diversity in low-elevation Wyoming big sagebrush ecosystems (1984–2014). Ecosphere 2019, 10, e02591. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Allen, S.; Mackenzie, B.D.E.; Yates, C.J.; Gosper, C.R.; Keith, D.A.; Merow, C.; White, M.D.; Wenk, E.; Maitner, B.S.; et al. High fire frequency and the impact of the 2019–2020 megafires on Australian plant diversity. Divers. Distrib. 2021, 27, 1166–1179. [Google Scholar] [CrossRef]
- Debyle Norbert, V. Fire, logging, and debris disposal effects on soil and water in northern coniferous forests. In International Union of Forest Research Organizations, Div. 1, Proceedings of the XVI IUFRO World Congress, Oslo, Norway, 1976; U.S. Government Printing Office: Washington, DC, USA, 1976; pp. 201–212. [Google Scholar]
- Ulery, A.L.; Graham, R.C.; Amrhein, C. Wood-ash composition and soil pH following intense burning. Soil Sci. 1993, 57, 135–140. [Google Scholar] [CrossRef]
- Pourreza, M.; Hosseini, S.M.; Sinegani, A.A.S.; Matinizadeh, M.; Dick, W.A. Soil microbial activity in response to fire severity in Zagros oak (Quercus brantii Lindl.) forests, Iran, after one year. Geoderma 2014, 213, 95–102. [Google Scholar] [CrossRef]
- Heydari, M.; Omidipour, R.; Abedi, M.; Baskin, C. Effects of fire disturbance on alpha and beta diversity and on beta diversity components of soil seed banks and aboveground vegetation. Plant Ecol. Evol. 2017, 150, 247–256. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part I. Physical and Mineralogical Properties; Klute, A., Ed.; Agronomy Series; ASA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Kalra, Y.P.; Maynard, D.G. Methods Manual for Forest Soil and Plant Analysis; Information Report; NOR-X-319; Northern Forestry Center, Northwest Region, Forestry Canada: Edmonton, AB, Canada, 1991; 125p. [Google Scholar]
- Allison, L.E.; Moodie, C.D. Carbonate. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; Black, C.A., Ed.; Agronomy Series; ASA: Madison, WI, USA, 1965; pp. 1379–1398. [Google Scholar]
- Bremner, J.; Mulvaney, M. Nitrogen total. In Methods of Soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, R.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular: Washington, DC, USA, 1954; Volume 939, 19p.
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; 608p. [Google Scholar]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Gerdemann, J.; Nicolson, T. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez, Y. Manual for the Identification of VA Mycorrhizal Fungi; Synergistic Publications: Gainesville, FL, USA, 1989; 286p. [Google Scholar]
- Mozaffarian, V. Flora of Iram; Farhang Moaser Publication: Tehran, Iran, 2008; 700p. [Google Scholar]
- Hubbert, K.; Preisler, H.; Wohlgemuth, P.; Graham, R.; Narog, M. Prescribed burning effects on soil physical properties and soil water repellency in a steep chaparral watershed, southern California, USA. Geoderma 2006, 130, 284–298. [Google Scholar] [CrossRef]
- Granged, A.J.; Jordán, A.; Zavala, L.M.; Muñoz-Rojas, M.; Mataix-Solera, J. Short-term effects of experimental fire for a soil under eucalyptus forest (SE Australia). Geoderma 2011, 167–168, 125–134. [Google Scholar] [CrossRef]
- Brye, R. Soil physiochemical changes following 12 years of annual burning in a humid–subtropical tallgrass prairie: A hypothesis. Acta Oecol. 2006, 30, 407–413. [Google Scholar] [CrossRef]
- Chief, K.; Young, M.H.; Shafer, D.S. Changes in Soil Structure and Hydraulic Properties in a Wooded-Shrubland Ecosystem following a Prescribed Fire. Soil Sci. Soc. Am. J. 2012, 76, 1965–1977. [Google Scholar] [CrossRef]
- Allen, E.B.; Steers, R.J.; Dickens, S.J. Impacts of Fire and Invasive Species on Desert Soil Ecology. Rangel. Ecol. Manag. 2011, 64, 450–462. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Erickson, T.E.; Martini, D.; Dixon, K.W.; Merritt, D.J. Soil physicochemical and microbiological indicators of short, medium and long term post-fire recovery in semi-arid ecosystems. Ecol. Indic. 2016, 63, 14–22. [Google Scholar] [CrossRef]
- Rodríguez, J.; González-Pérez, J.A.; Turmero, A.; Hernández, M.; Ball, A.S.; González-Vila, F.J.; Arias, M.E. Wildfire effects on the microbial activity and diversity in a Mediterranean forest soil. Catena 2017, 158, 82–88. [Google Scholar] [CrossRef]
- Girona-García, A.; Ortiz-Perpiñá, O.; Badía-Villas, D.; Martí-Dalmau, C. Effects of prescribed burning on soil organic C, aggregate stability and water repellency in a subalpine shrubland: Variations among sieve fractions and depths. Catena 2018, 166, 68–77. [Google Scholar] [CrossRef]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Effect of heating on some physical and chemical parameters related to soil aggregation and erodibility. Soil Sci. 1988, 146, 255–261. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C. Effect of 25 annual cattle manure applications on soluble and exchangeable cations in soil. Soil Sci. 2002, 167, 126–134. [Google Scholar] [CrossRef]
- Wardle, D.A.; Hörnberg, G.; Zackrisson, O.; Kalela-Brundin, M.; Coomes, D.A. Long-Term Effects of Wildfire on Ecosystem Properties Across an Island Area Gradient. Science 2003, 300, 972–975. [Google Scholar] [CrossRef]
- Fekete, I.; Kotroczó, Z.; Varga, C.; Hargitai, R.; Townsend, K.; Csányi, G.; Várbiró, G. Variability of Organic Matter Inputs Affects Soil Moisture and Soil Biological Parameters in a European Detritus Manipulation Experiment. Ecosystems 2012, 15, 5792–5803. [Google Scholar] [CrossRef]
- Nichols, L.; Shinneman, D.J.; McIlroy, S.K.; de Graaff, M.-A. Fire frequency impacts soil properties and processes in sagebrush steppe ecosystems of the Columbia Basin. Appl. Soil Ecol. 2021, 165, 103967. [Google Scholar] [CrossRef]
- Neff, J.; Harden, J.W.; Gleixner, G. Fire effects on soil organic matter content, composition, and nutrients in boreal interior Alaska. Can. J. For. Res. 2005, 35, 2178–2187. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2017, 553, 194–198. [Google Scholar] [CrossRef]
- Driscoll, K.; Arocena, J.; Massicotte, H. Post-fire soil nitrogen content and vegetation composition in Sub-Boreal spruce forests of British Columbia’s central interior, Canada. For. Ecol. Manag. 1999, 121, 227–237. [Google Scholar] [CrossRef]
- Srikanthasamy, T.; Barot, S.; Koffi, F.K.; Tambosco, K.; Marcangeli, Y.; Carmignac, D.; N’Dri, A.B.; Gervaix, J.; Le Roux, X.; Lata, J. Short-term impact of fire on the total soil microbial and nitrifier communities in a wet savanna. Ecol. Evol. 2021, 11, 9958–9969. [Google Scholar] [CrossRef]
- Sinha, P.; Hobbs, P.V.; Yokelson, R.; Bertschi, I.T.; Blake, D.R.; Simpson, I.J.; Gao, S.; Kirchstetter, T.W.; Novakov, T. Emissions of trace gases and particles from savanna fires in southern Africa. J. Geophys. Res. Atmos. 2003, 108, 8487. [Google Scholar] [CrossRef]
- Heydari, M.; Faramarzi, M.; Pothier, D. Post-fire recovery of herbaceous species composition and diversity, and soil quality indicators one year after wildfire in a semi-arid oak woodland. Ecol. Eng. 2016, 94, 688–697. [Google Scholar] [CrossRef]
- Arroyo-Vargas, P.; Fuentes-Ramírez, A.; Muys, B.; Pauchard, A. Impacts of fre severity and cattle grazing on early plant dynamics in old-growth Araucaria-Nothofagus forests. For. Ecosyst. 2019, 6, 44. [Google Scholar] [CrossRef]
- Caon, L.; Vallejo, V.R.; Ritsema, C.J.; Geissen, V. Effects of wildfire on soil nutrients in Mediterranean ecosystems. Earth-Sci. Rev. 2014, 139, 47–58. [Google Scholar] [CrossRef]
- Huston, M.A. Disturbance, productivity, and species diversity: Empiricism vs. logic in ecological theory. Ecology 2014, 95, 2382–2396. [Google Scholar] [CrossRef]
- Burkle, L.A.; Myers, J.A.; Belote, R.T. Wildfire disturbance and productivity as drivers of plant species diversity across spatial scales. Ecosphere 2015, 6, 202. [Google Scholar] [CrossRef]
- Abedi, M.; Omidipour, R.; Hosseini, S.V.; Bahalkeh, K.; Gross, N. Fire disturbance effects on plant taxonomic and functional β-diversity mediated by topographic exposure. Ecol. Evol. 2022, 12, e8552. [Google Scholar] [CrossRef]
- Long, R.L.; Stevens, J.C.; Griffiths, E.M.; Adamek, M.; Gorecki, M.J.; Powles, S.B.; Merritt, D.J. Seeds of Brassicaceae weeds have an inherent or inducible response to the germination stimulant karrikinolide. Ann. Bot. 2011, 108, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Schwilk, D.; Zavala, N. Germination response of grassland species to plant-derived smoke. J. Arid. Environ. 2012, 79, 111–115. [Google Scholar] [CrossRef]
- Jefferson, L.V.; Pennacchio, M.; Havens, K.; Forsberg, B.; Sollenberger, D.; Ault, J. Ex situ germination responses of Mid-western USA prairie species to plant-derived Smoke. Am. Midl. Nat. 2008, 159, 251–256. [Google Scholar] [CrossRef]
- Naghipour, A.A.; Bashari, H.; Khajeddin, S.J.; Tahmasebi, P.; Iravani, M. Effects of smoke, ash and heat shock on seed germi-nation of seven species from Central Zagros rangelands in the semi-arid region of Iran. Afr. J. Range Forage Sci. 2016, 33, 67–71. [Google Scholar] [CrossRef]
- Barlow, J.; Peres, C. Fire-mediated dieback and compositional cascade in an Amazonian forest. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1787–1794. [Google Scholar] [CrossRef]
- Malkisnon, D.; Wittenberg, L.; Beeri, O.; Barzilai, R. Effects of Repeated Fires on the Structure, Composition, and Dynamics of Mediterranean Maquis: Short- and Long-Term Perspectives. Ecosystems 2011, 14, 478–488. [Google Scholar] [CrossRef]
- Massad, T.J.; Balch, J.K.; Davidson, E.A.; Brando, P.M.; Mews, C.L.; Porto, P.; Quintino, R.M.; Vieira, S.A.; Junior, B.H.M.; Trumbore, S.E. Interactions between repeated fire, nutrients, and insect herbivores affect the recovery of diversity in the southern Amazon. Oecologia 2012, 172, 219–229. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Huston, M. A General Hypothesis of Species Diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Kondoh, M. Unifying the relationships of species richness to productivity and disturbance. Proc. R. Soc. B Boil. Sci. 2001, 268, 269–271. [Google Scholar] [CrossRef]
- Taudière, A.; Bellanger, J.-M.; Carcaillet, C.; Hugot, L.; Kjellberg, F.; Lecanda, A.; Lesne, A.; Moreau, P.-A.; Scharmann, K.; Leidel, S.; et al. Diversity of foliar endophytic ascomycetes in the endemic Corsican pine forests. Fungal Ecol. 2018, 36, 128–140. [Google Scholar] [CrossRef]
- Sun, H.; Santalahti, M.; Pumpanen, J.; Köster, K.; Berninger, F.; Raffaello, T.; Jumpponen, A.; Asiegbu, F.O.; Heinonsalo, J. Fungal Community Shifts in Structure and Function across a Boreal Forest Fire Chronosequence. Appl. Environ. Microbiol. 2015, 81, 7869–7880. [Google Scholar] [CrossRef]
- Su, W.-Q.; Tang, C.; Lin, J.; Yu, M.; Dai, Z.; Luo, Y.; Li, Y.; Xu, J. Recovery patterns of soil bacterial and fungal communities in Chinese boreal forests along a fire chronosequence. Sci. Total. Environ. 2021, 805, 150372. [Google Scholar] [CrossRef]
| Parameter | Code | Method Name and References |
|---|---|---|
| Bulk density (g.cm−3) | BD | common core method, mass and volume [46] |
| Granulometry (%) | sand, silt, clay | hydrometry [47] |
| Soil pH | pH | Standard, pH-H2O [48] |
| Lime content as the Total Neutralizing Value (Lime), as a percentage of Calcium Carbonate Equivalent (%CCE) | TNV | titration with NaOH [49] |
| Electrical conductivity (dS.m−1) | EC | aqueous soil extract [48] |
| Total nitrogen (%) | N | Kjeldahl method [50] |
| Available phosphorus (mg.kg−1, ppm) | P | spectrophotometer [51] |
| Soil organic matter (%) | SOM | chromic acid titration [52] |
| Microbial biomass carbon of soil (mgCO2.g−1) | MBC | [53] |
| Soil basal respiration (mgCO2.g−1.d−1) | SR | [54] |
| Substrate-induced respiration (mgCO2.g−1.d−1) | SIR | [55] |
| Soil urease enzymatic activity based on soil dry weight (dwt) and per hour (µgNH4+.g−1soil dwt.h−1) | Urease | colorimetry [56] |
| Index Name | Formula | Range of Values | Properties |
|---|---|---|---|
| Menhinick | R > 0 | Richness controlling for the effect of observation size (rarefied richness) | |
| Gini-Simpson | 0 ≤ D < 1 | Diversity highlighting abundant taxa | |
| Shannon | 0 < H′ ≤ ln(S) | Diversity highlighting low-abundance taxa | |
| Pielou’s evenness | 0 < E ≤ 1 | Determines the equality of the community in individuals’ number per taxon, where H′max = ln(S) |
| Soil Physics | Soil Chemistry | Soil Biology | ||||||
|---|---|---|---|---|---|---|---|---|
| Property | F | p | Property | F | p | Property | F | p |
| BD | 29.134 | <0.001 | TNV | 1.818 | 0.192 | MBC | 6.029 | 0.010 |
| Sand | 0.779 | 0.475 | pH | 4.009 | 0.037 | SR | 9.294 | 0.002 |
| Silt | 0.292 | 0.751 | EC | 10.013 | 0.001 | SIR | 7.586 | 0.004 |
| Clay | 1.240 | 0.314 | N | 4.791 | 0.022 | Urease | 7.444 | 0.005 |
| P | 1.378 | 0.279 | SOM | 4.710 | 0.024 | |||
| Plant community diversity | AMF community diversity | |||||||
| Index | F | p | Index | F | p | |||
| Richness | 6.26 | 0.009 | Richness | 2.234 | 0.138 | |||
| Simpson | 6.846 | 0.007 | Simpson | 0.892 | 0.428 | |||
| Shannon | 6.846 | 0.007 | Shannon | 5.984 | 0.011 | |||
| Evenness | 0.357 | 0.705 | Evenness | 0.942 | 0.409 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzaei, J.; Heydari, M.; Omidipour, R.; Jafarian, N.; Carcaillet, C. Decrease in Soil Functionalities and Herbs’ Diversity, but Not That of Arbuscular Mycorrhizal Fungi, Linked to Short Fire Interval in Semi-Arid Oak Forest Ecosystem, West Iran. Plants 2023, 12, 1112. https://doi.org/10.3390/plants12051112
Mirzaei J, Heydari M, Omidipour R, Jafarian N, Carcaillet C. Decrease in Soil Functionalities and Herbs’ Diversity, but Not That of Arbuscular Mycorrhizal Fungi, Linked to Short Fire Interval in Semi-Arid Oak Forest Ecosystem, West Iran. Plants. 2023; 12(5):1112. https://doi.org/10.3390/plants12051112
Chicago/Turabian StyleMirzaei, Javad, Mehdi Heydari, Reza Omidipour, Nahid Jafarian, and Christopher Carcaillet. 2023. "Decrease in Soil Functionalities and Herbs’ Diversity, but Not That of Arbuscular Mycorrhizal Fungi, Linked to Short Fire Interval in Semi-Arid Oak Forest Ecosystem, West Iran" Plants 12, no. 5: 1112. https://doi.org/10.3390/plants12051112
APA StyleMirzaei, J., Heydari, M., Omidipour, R., Jafarian, N., & Carcaillet, C. (2023). Decrease in Soil Functionalities and Herbs’ Diversity, but Not That of Arbuscular Mycorrhizal Fungi, Linked to Short Fire Interval in Semi-Arid Oak Forest Ecosystem, West Iran. Plants, 12(5), 1112. https://doi.org/10.3390/plants12051112







