Does Regulated Deficit Irrigation Affect Pear Fruit Texture by Modifying the Stone Cells?
Abstract
:1. Introduction
2. Results
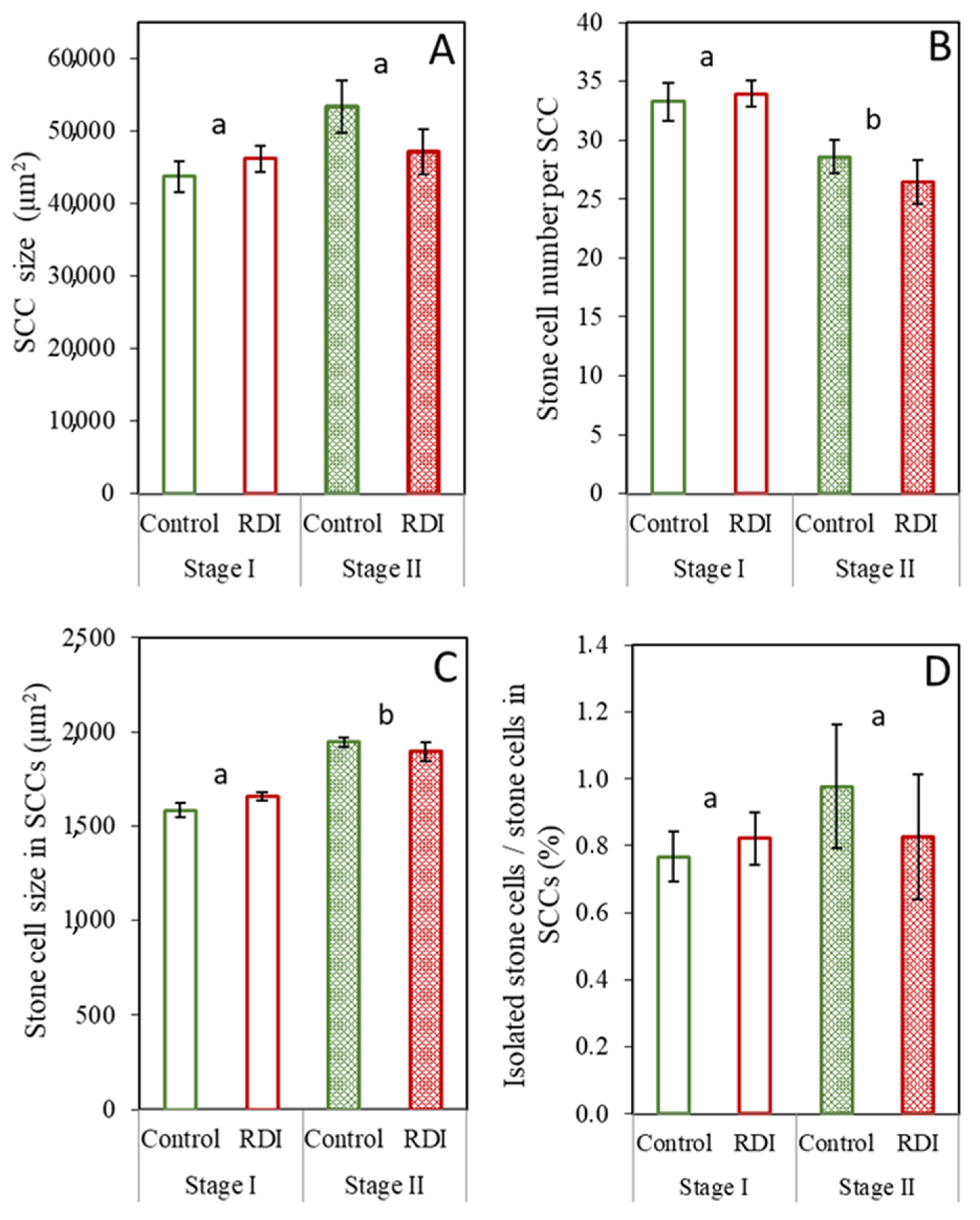
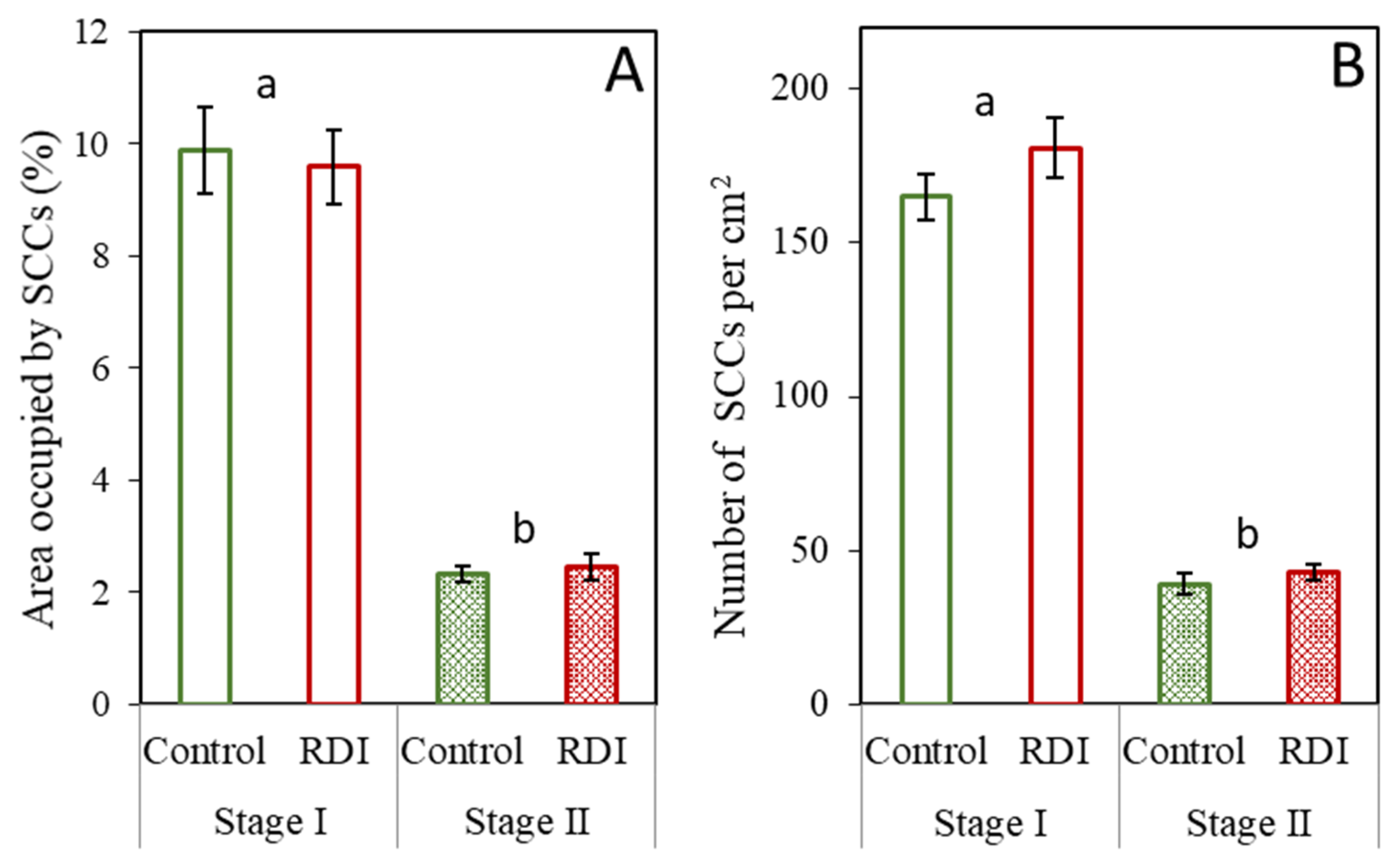
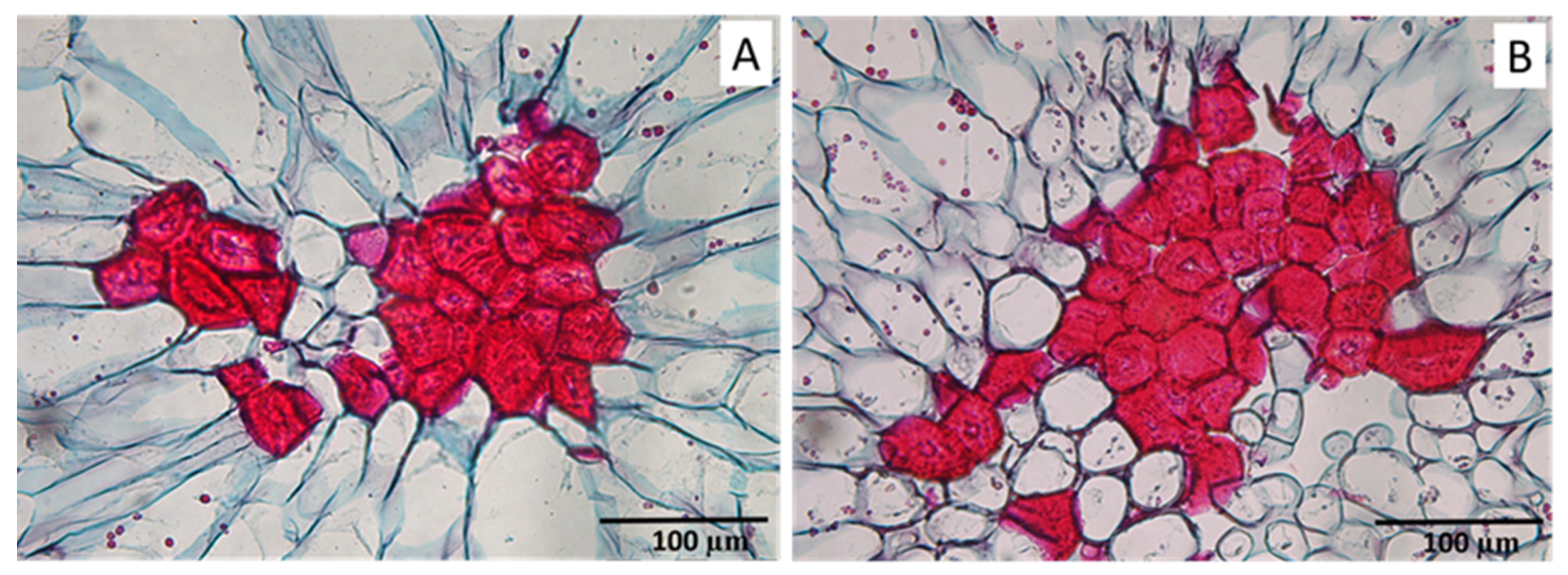
2.1. Stone Cells under RDI and Control Irrigation
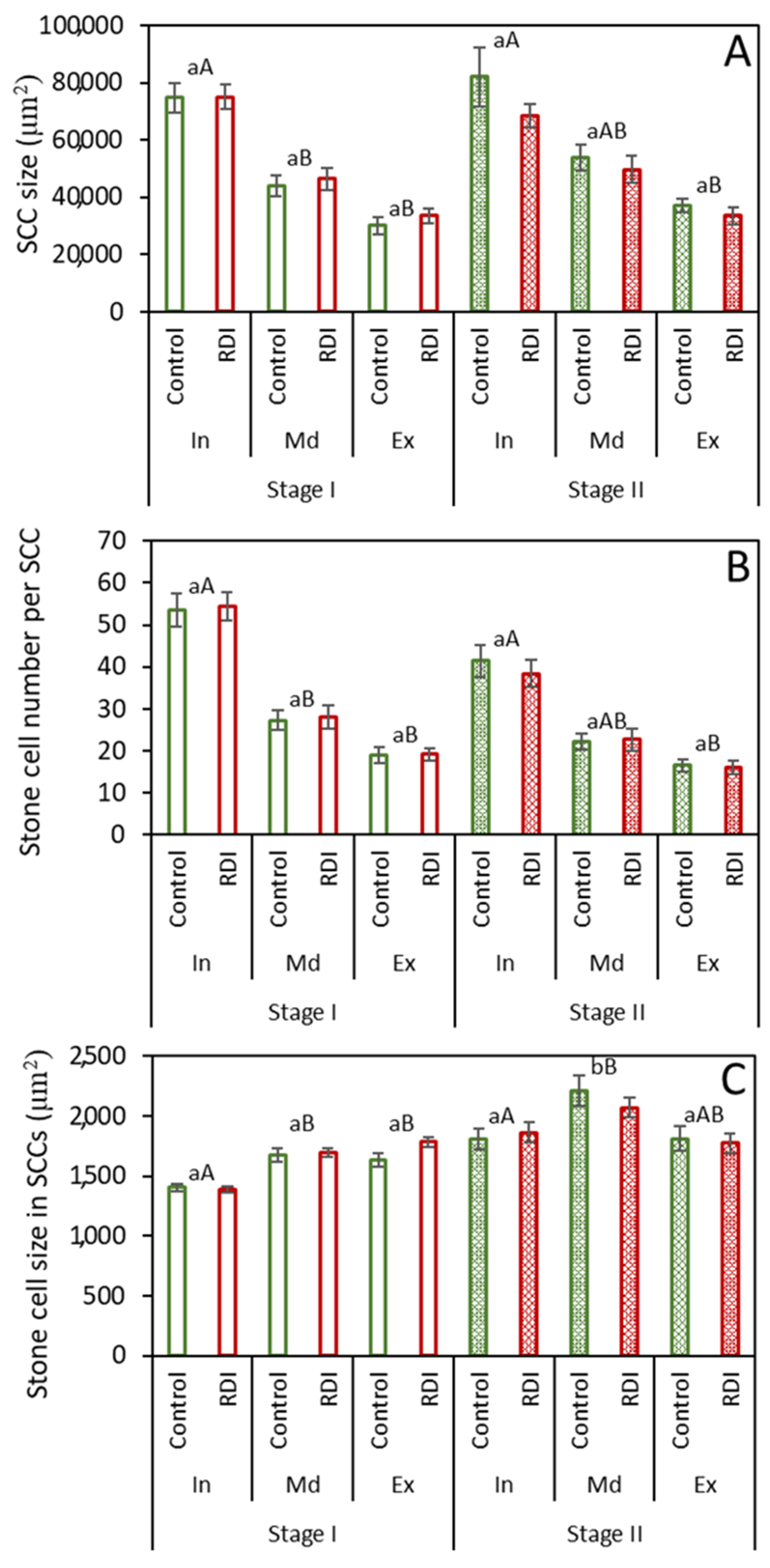
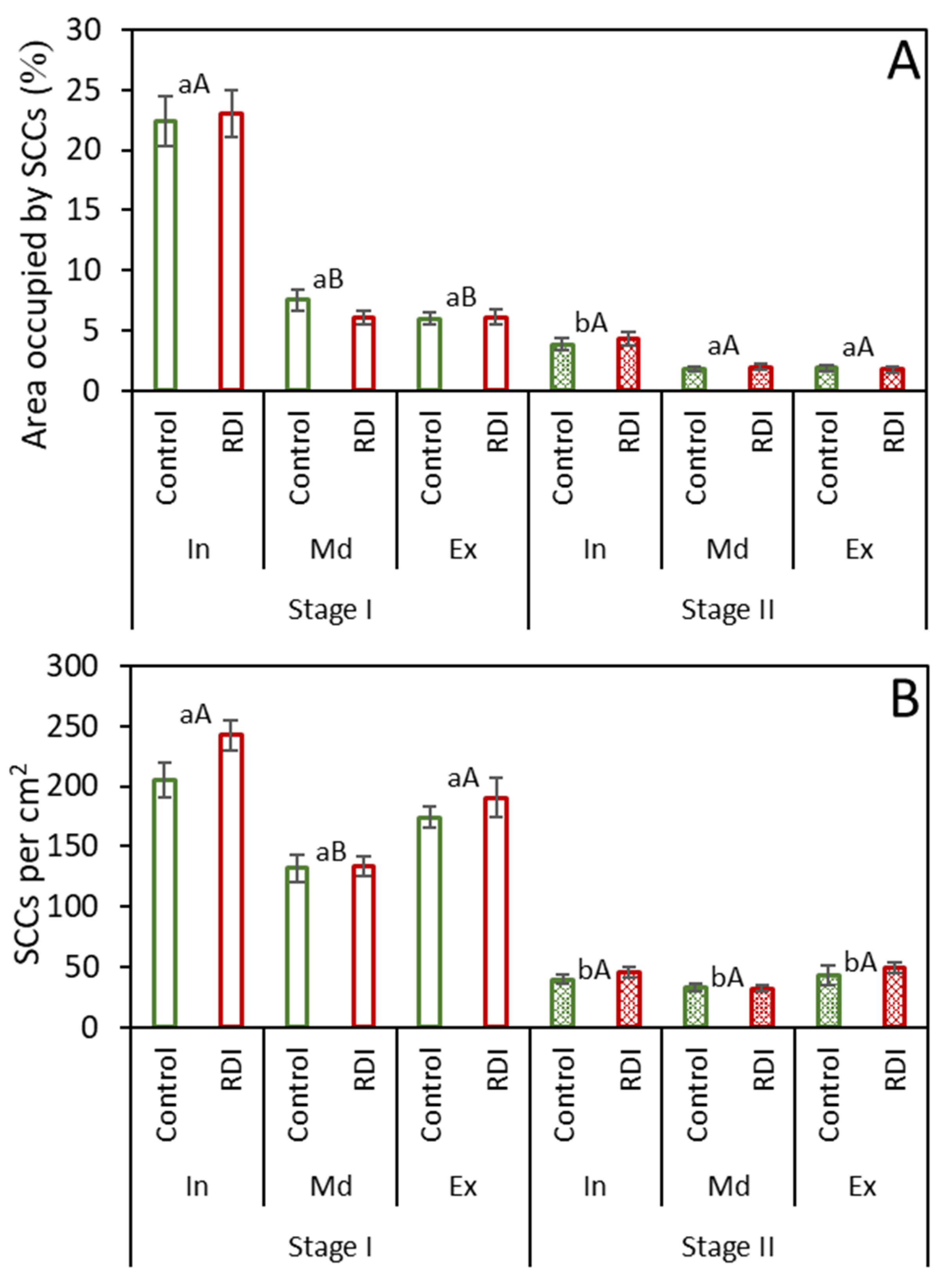
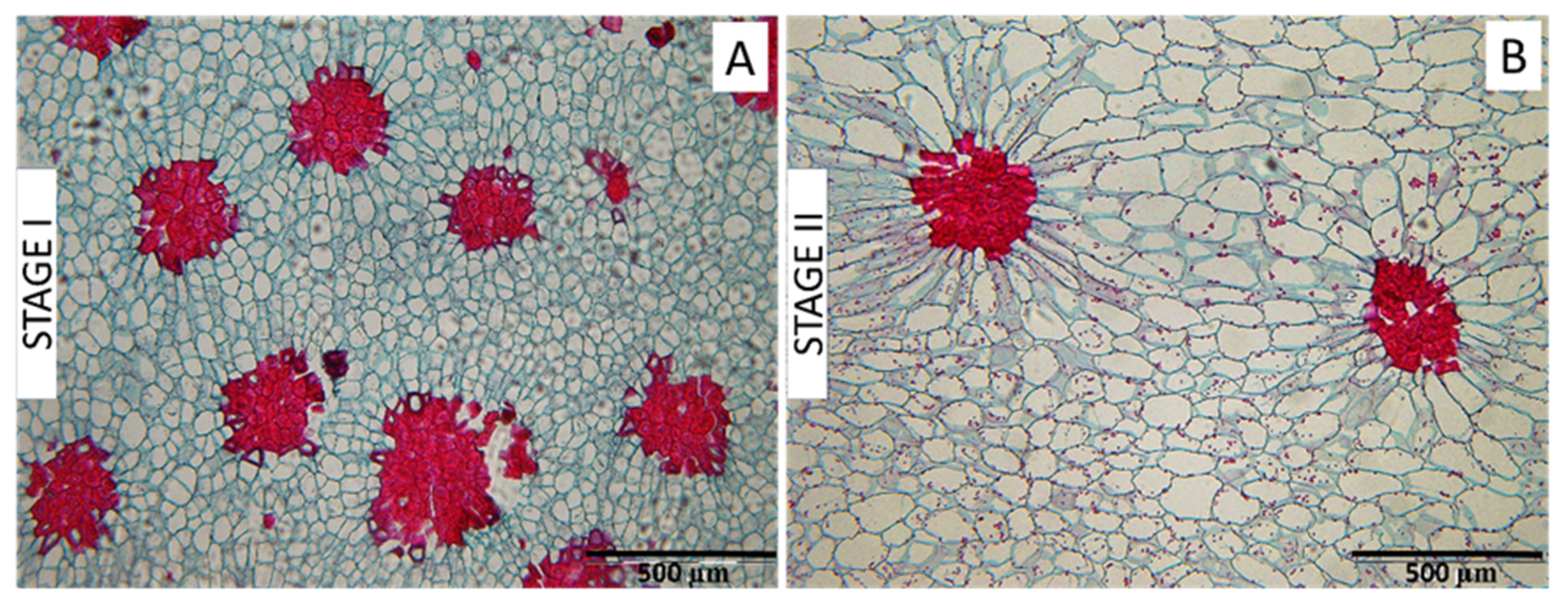
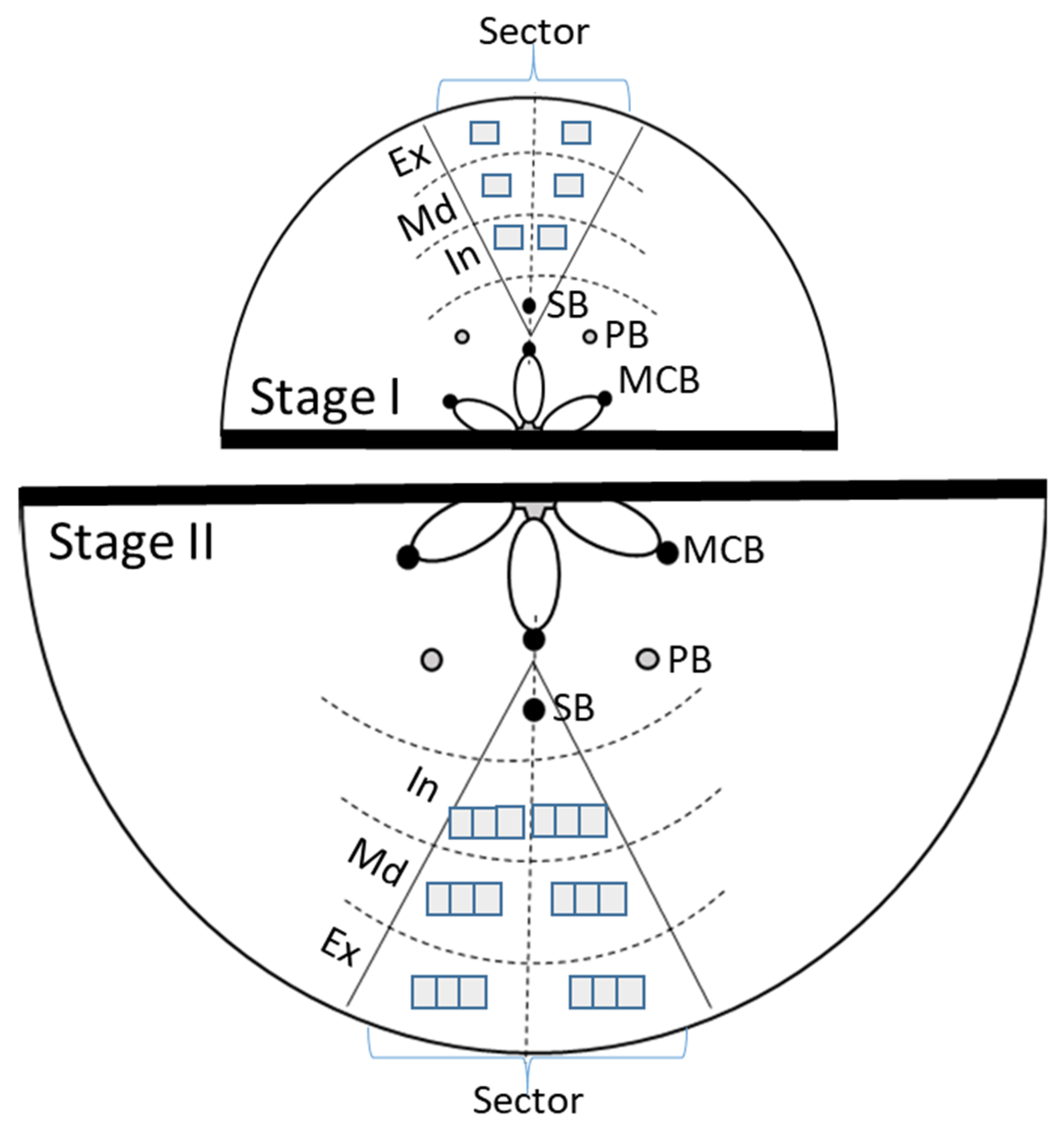
2.2. Stone Cell Dimensions and Presence in Different Fruit Zones
2.3. Cellular Differentiation in the Fruit Flesh
3. Discussion
3.1. Effect of RDI Strategies on Pear Fruit Stone Cells
3.2. Stone Cell and Cluster Development
3.3. Relationship between Parenchyma Development and SCCs
3.4. Microscope Image Analysis Techniques for Advancing SCC Studies
4. Materials and Methods
4.1. Growth Conditions and Irrigation Treatments
4.2. Fruit Tissue Sampling and Preparation
4.3. Histological Assessment
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katerji, N.; Mastrorilli, M.; Rana, G. Water Use Efficiency of Crops Cultivated in the Mediterranean Region: Review and Analysis. Eur. J. Agron. 2008, 28, 493–507. [Google Scholar] [CrossRef]
- Chalmers, D.; Burge, G.; Jerie, P.H.; Mitchell, P.D. The Mechanism of Regulation of ’Bartlett’ pear Fruit and Vegetative Growth by Irrigation Withholding and Regulated Deficit Irrigation. J. Am. Soc. Hortic. Sci. 1986, 111, 904–907. [Google Scholar] [CrossRef]
- Galindo, A.; Collado-González, J.; Griñán, I.; Corell, M.; Centeno, A.; Martín-Palomo, M.J.; Girón, I.F.; Rodríguez, P.; Cruz, Z.N.; Memmi, H.; et al. Deficit Irrigation and Emerging Fruit Crops as a Strategy to Save Water in Mediterranean Semiarid Agrosystems. Agric. Water Manag. 2018, 202, 311–324. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Van Den Ende, B.; Jerie, P.H.; Chalmers, D.J. Responses of ’Bartlett’ pear to Withholding Irrigation, Regulated Deficit Irrigation, and Tree Spacing. J. Am. Soc. Hortic. Sci. 1989, 114, 15–19. [Google Scholar] [CrossRef]
- Vélez-Sánchez, J.E.; Balaguera-López, H.E.; Alvarez-Herrera, J.G. Effect of Regulated Deficit Irrigation (RDI) on the Production and Quality of Pear Triunfo de Viena Variety under Tropical Conditions. Sci. Hortic. 2021, 278, 109880. [Google Scholar] [CrossRef]
- Caspari, H.W.; Behboudian, M.H.; Chalmers, D.J. Water Use, Growth, and Fruit Yield of ‘Hosui’ Asian Pears under Deficit Irrigation. J. Am. Soc. Hortic. Sci. 1994, 119, 383–388. [Google Scholar] [CrossRef]
- Behboudian, M.H.; Lawes, G.S.; Griffiths, K.M. The Influence of Water Deficit on Water Relations, Photosynthesis and Fruit Growth in Asian Pear (Pyrus Serotina Rehd.). Sci. Hortic. 1994, 60, 89–99. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Wang, W.; Ma, Y.; Huang, X. Yield and Growth of Mature Pear Trees under Water Deficit during Slow Fruit Growth Stages in Sparse Planting Orchard. Sci. Hortic. 2013, 164, 189–195. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Zhao, F.; Cheng, X.; Zhao, P.; Liu, S. Response of Pear Trees’ (Pyrus Bretschneideri ‘Sinkiangensis’) Fine Roots to a Soil Water Regime of Regulated Deficit Irrigation. Agronomy 2021, 11, 2316. [Google Scholar] [CrossRef]
- Bain, J.M. Some Morphological, Anatomical, and Physiological Changes in the Pear Fruit (Pyrus communis Var. Williams Bon Chrétien) during Development and Following Harvest. Aust. J. Bot. 1961, 9, 99–123. [Google Scholar] [CrossRef]
- Marsal, J.; Rapoport, H.F.; Manrique, T.; Girona, J. Pear Fruit Growth under Regulated Deficit Irrigation in Container-Grown Trees. Sci. Hortic. 2000, 85, 243–259. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Li, S.H.; Schneider, B.; Gershenzon, J. Microchemical Analysis of Laser-Microdissected Stone Cells of Norway Spruce by Cryogenic Nuclear Magnetic Resonance Spectroscopy. Planta 2007, 225, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Nii, N.; Kawahara, T.; Nakao, Y. The Development of Stone Cells in Japanese Pear Fruit. J. Hortic. Sci. Biotechnol. 2008, 83, 148–153. [Google Scholar] [CrossRef]
- Cai, Y.; Li, G.; Nie, J.; Lin, Y.; Nie, F.; Zhang, J.; Xu, Y. Study of the Structure and Biosynthetic Pathway of Lignin in Stone Cells of Pear. Sci. Hortic. 2010, 125, 374–379. [Google Scholar] [CrossRef]
- Cheng, X.; Cai, Y.; Zhang, J. Stone Cell Development in Pear. In The Pear Genome. Compendium of Plant Genomes; Korban, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 201–225. [Google Scholar] [CrossRef]
- Li, N.; Ma, Y.; Song, Y.; Tian, C.; Zhang, L.; Li, L. Anatomical Studies of Stone Cells in Fruits of Four Different Pear Cultivars. Int. J. Agric. Biol. 2017, 19, 610–614. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.H.; Kim, W.S.; Han, T.H.; Park, Y.S.; Gemma, H. Effect of Soil Water Stress on the Development of Stone Cells in Pear (Pyrus pyrifolia Cv. ’Niitaka’) Flesh. Sci. Hortic. 2006, 110, 247–253. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, S.H. Distribution of Stone Cell in Asian, Chinese, and European Pear Fruit and Its Morphological Changes. J. Appl. Bot. Food Qual. 2013, 86, 185–189. [Google Scholar] [CrossRef]
- Yan, C.; Yin, M.; Zhang, N.; Jin, Q.; Fang, Z.; Lin, Y.; Cai, Y. Stone Cell Distribution and Lignin Structure in Various Pear Varieties. Sci. Hortic. 2014, 174, 142–150. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Liu, C.; Liu, Y.; Lu, X.; Du, G.; Lyu, D. Biochemical Characterization and Expression Analysis of Lignification in Two Pear (Pyrus ussuriensis Maxim.) Varieties with Contrasting Stone Cell Content. Protoplasma 2020, 257, 261–274. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, J.-H.; Kim, W.-S.; Park, Y.-S.; Gemma, H. Effects of Calcium Chloride Spray on Peroxidase Activity and Stone Cell Development in Pear Fruit (Pyrus pyrifolia ’Niitaka’). J. Jpn. Soc. Hortic. Sci. 2007, 76, 191–196. [Google Scholar] [CrossRef]
- Sterling, C. Sclereid Development and the Texture of Barlett Pears. J. Food Sci. 1954, 19, 433–443. [Google Scholar] [CrossRef]
- Pandey, V.P.; Singh, S.; Dwivedi, U. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Torrecillas, A.; Corell, M.; Galindo, A.; Pérez-López, D.; Memmi, H.; Rodríguez, P.; Cruz, Z.N.; Centeno, A.; Intrigliolo, D.S.; Moriana, A. Agronomical Effects of Deficit Irrigation in Apricot, Peach, and Plum Trees. Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops; Academic Press: Cambridge, MA, USA, 2018; pp. 87–109. [Google Scholar] [CrossRef]
- Esau, K. Anatomy of Seed Plants; John Wiley & Sons: New York, NY, USA, 1898. [Google Scholar]
- Tao, S.; Khanizadeh, S.; Zhang, H.; Zhang, S. Anatomy, Ultrastructure and Lignin Distribution of Stone Cells in Two Pyrus Species. Plant Sci. 2009, 176, 413–419. [Google Scholar] [CrossRef]
- Xue, C.; Yao, J.L.; Qin, M.F.; Zhang, M.Y.; Allan, A.C.; Wang, D.F.; Wu, J. PbrmiR397a Regulates Lignification during Stone Cell Development in Pear Fruit. Plant Biotechnol. J. 2019, 17, 103–117. [Google Scholar] [CrossRef]
- Rallo, P.; Rapoport, H.F. Early Growth and Development of the Olive Fruit Mesocarp. J. Hortic. Sci. Biotechnol. 2001, 76, 408–412. [Google Scholar] [CrossRef]
- Jin, Q.; Yan, C.; Qiu, J.; Zhang, N.; Lin, Y.; Cai, Y. Structural Characterization and Deposition of Stone Cell Lignin in Dangshan Su Pear. Sci. Hortic. 2013, 155, 123–130. [Google Scholar] [CrossRef]
- Gong, X.; Xie, Z.; Qi, K.; Zhao, L.; Yuan, Y.; Xu, J.; Rui, W.; Shiratake, K.; Bao, J.; Khanizadeh, S.; et al. PbMC1a/1b Regulates Lignification during Stone Cell Development in Pear (Pyrus bretschneideri) Fruit. Hortic. Res. 2020, 7, 59. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, H.; Liu, C.; Li, X.; Liu, Y.; Lyu, D.; Du, G. Production of Reactive Oxygen Species by PuRBOHF Is Critical for Stone Cell Development in Pear Fruit. Hortic. Res. 2021, 8, 249. [Google Scholar] [CrossRef]
- Zhao, S. New Discoveries of Stone Cell Differentiation in Fruitlets of “Yali” Pears (Pyrus bretschneideri Rehd.). J. Food Agric. Environ. 2005, 11, 937–942. [Google Scholar]
- Gucci, R.; Lodolini, E.M.; Rapoport, H.F. Water Deficit-Induced Changes in Mesocarp Cellular Processes and the Relationship between Mesocarp and Endocarp during Olive Fruit Development. Tree Physiol. 2009, 29, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company: London, UK, 1940. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 2000; Volume 198, ISBN 0195089561. [Google Scholar]

| Size (µm2) | ||||||
|---|---|---|---|---|---|---|
| Zone | Parenchyma Cells (General) | Parenchyma Cells around Cluster | ||||
| Stage I | Control | In | 2147 ± 67.51 | aA | 1853 ± 89.44 | aB |
| Md | 3034 ± 109.95 | aA | 2763 ± 116.29 | aB | ||
| Ex | 3154 ± 42.84 | aA | 2772 ± 62.40 | aB | ||
| RDI | In | 1879 ± 49.62 | aA | 1787 ± 66.86 | aA | |
| Md | 2956 ± 99.95 | aA | 2691 ± 126.30 | aB | ||
| Ex | 2889 ± 108.04 | aA | 2558 ± 125.96 | aB | ||
| Stage II | Control | In | 9472 ± 438.13 | bA | 7985 ± 411.44 | bB |
| Md | 13,435 ± 528.35 | bA | 12,560 ± 349.52 | bB | ||
| Ex | 13,139 ± 489.40 | bA | 11,724 ± 494.09 | bB | ||
| RDI | In | 8706 ± 178.79 | bA | 7276 ± 225.49 | bA | |
| Md | 13,400 ± 428.39 | bA | 11,143 ± 483.39 | bB | ||
| Ex | 11,327 ± 570.13 | bA | 9658 ± 373.16 | bB | ||
| Circularity (1–0) | |||
|---|---|---|---|
| Parenchyma Cells (General) | Parenchyma Cells around Cluster | ||
| Stage I | Control | 0.67 ± 0.01 aA | 0.67 ± 0.01 aA |
| RDI | 0.62 ± 0.01 aA | 0.68 ± 0.01 aA | |
| Stage II | Control | 0.58 ± 0.01 bA | 0.42 ± 0.01 bB |
| RDI | 0.55 ± 0.01 bA | 0.45 ± 0.01 bB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peco, J.D.; Rapoport, H.F.; Centeno, A.; Pérez-López, D. Does Regulated Deficit Irrigation Affect Pear Fruit Texture by Modifying the Stone Cells? Plants 2023, 12, 4024. https://doi.org/10.3390/plants12234024
Peco JD, Rapoport HF, Centeno A, Pérez-López D. Does Regulated Deficit Irrigation Affect Pear Fruit Texture by Modifying the Stone Cells? Plants. 2023; 12(23):4024. https://doi.org/10.3390/plants12234024
Chicago/Turabian StylePeco, Jesús D., Hava F. Rapoport, Ana Centeno, and David Pérez-López. 2023. "Does Regulated Deficit Irrigation Affect Pear Fruit Texture by Modifying the Stone Cells?" Plants 12, no. 23: 4024. https://doi.org/10.3390/plants12234024
APA StylePeco, J. D., Rapoport, H. F., Centeno, A., & Pérez-López, D. (2023). Does Regulated Deficit Irrigation Affect Pear Fruit Texture by Modifying the Stone Cells? Plants, 12(23), 4024. https://doi.org/10.3390/plants12234024







