Abstract
Melatonin (MT) is considered a new plant hormone having a universal distribution from prokaryotic bacteria to higher plants. It has been characterized as an antistress molecule playing a positive role in the acclimation of plants to stress conditions, but its impact on plants under non-stressed conditions is not well understood. In the current research, we evaluated the impact of MT application (10 and 100 μM) on photosystem II (PSII) function, reactive oxygen species (ROS) generation, and chlorophyll content on mint (Mentha spicata L.) plants in order to elucidate the molecular mechanism of MT action on the photosynthetic electron transport process that under non-stressed conditions is still unclear. Seventy-two hours after the foliar spray of mint plants with 100 μM MT, the improved chlorophyll content imported a higher amount of light energy capture, which caused a 6% increase in the quantum yield of PSII photochemistry (ΦPSII) and electron transport rate (ETR). Nevertheless, the spray with 100 μM MT reduced the efficiency of the oxygen-evolving complex (OEC), causing donor-side photoinhibition, with a simultaneous slight increase in ROS. Even so, the application of 100 μM MT decreased the excess excitation energy at PSII implying superior PSII efficiency. The decreased excitation pressure at PSII, after 100 μM MT foliar spray, suggests that MT induced stomatal closure through ROS production. The response of ΦPSII to MT spray corresponds to a J-shaped hormetic curve, with ΦPSII enhancement by 100 μM MT. It is suggested that the hormetic stimulation of PSII functionality was triggered by the non-photochemical quenching (NPQ) mechanism that stimulated ROS production, which enhanced the photosynthetic function. It is concluded that MT molecules can be used under both stress and non-stressed conditions as photosynthetic biostimulants for enhancing crop yields.
1. Introduction
Photosynthesis is a fundamental process to plant growth and development, but the plant’s capability to achieve high photosynthetic activity simply depends on the environmental conditions [1]. Enhancing photosynthetic efficiency and improving crop performance stand as crucial and highly significant research challenges [2,3,4]. Improving the quantum yield of photosystem II (PSII) stands as a pathway toward achieving increased efficiency and productivity in photosynthesis [5].
Photosystem II (PSII) uses solar energy to provide electrons by oxidizing water. At PSII in the oxygen-evolving complex (OEC), the oxidation of H2O results in oxygen (O2), protons (H+), and electrons (e−) [6]. The e− are transferred to NADP+, and coupled with this transfer, the proton gradient that is established drives the synthesis of ATP [6,7]. The activity of PSII is regularly censored by chlorophyll a fluorescence measurements [8,9,10,11]. Chlorophyll a fluorescence analysis is used extensively for acquiring information regarding the amount of absorbed light energy used for photochemistry (ΦPSII), the amount of regulated non-photochemical energy loss in PSII (ΦNPQ), and the amount of nonregulated energy loss in PSII (ΦNO) [12,13,14]. The sum of ΦPSII + ΦNPQ + ΦNO is equal to 1 [12].
During the conversion of the light energy to chemical energy, reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide anion radical (O2•−), and singlet-excited oxygen (1O2), are constantly produced [7,15,16,17]. However, they are scavenged by different antioxidant mechanisms [15,16,17,18,19,20]. When ROS production is not well adjusted by the antioxidant mechanisms, photooxidative stress develops [21].
Melatonin (MT) is an indole molecule (N-acetyl-5-methoxytryptamine) naturally appearing in roots, leaves, fruits, and seeds [22,23], which was first discovered in the animal kingdom [24]. Melatonin in plants, which is called also phytomelatonin [25], was detected in 1995 by various research groups [22,26,27,28]. The MT molecule plays crucial roles in an extensive variety of physiological processes, e.g., germination, root and shoot growth, photosynthesis, stomatal closure, osmoregulation, secondary metabolism, leaf senescence, circadian cycle regulation, flowering, and fruit setting, and in the protection against biotic and abiotic factors [29,30,31,32,33]. The identification in the model plant Arabidopsis thaliana of the first plant melatonin receptor, named PHYTOMELATONIN RECEPTOR 1 (AtPMTR1) [34], unlocked the door to be considered a new plant hormone [29]. Melatonin has been shown to have a universal distribution from prokaryotic bacteria to higher plants, being a phylogenetically conserved molecule [35]. Melatonin activates or deactivates certain metabolic pathways, not merely by regulating gene and protein expression but also through post-translational modifications of proteins [36]. It has been characterized as an antistress molecule playing a positive role in a number of environmental stresses, e.g., in low and high temperatures, salinity, drought, toxic chemicals, UV radiation, fungal diseases, and plant–pathogen interactions [37,38]. Melatonin is related to plant hormones, e.g., abscisic acid (ABA), cytokinins (CTK), gibberellins (GAs), ethylene (ETH), indole acetic acid (IAA), jasmonic acid (JA), brassinosteroids (BR), salicylic acid (SA), and strigolactone (SL) [39,40]. Plants have been found to possess much higher MT levels compared to animals, possibly as a compensatory response to their lack of mobility, to withstand harmful environmental conditions [40]. High MT concentrations have been measured in widespread beverages like tea, coffee, beer, and wine, and also in popular crops like wheat, rice, corn, oats, and barley [40].
Exogenous application of MT can penetrate the plasma membranes increasing the endogenous MT levels [23,41]. Endogenous MT is produced from tryptophan as an intermediate product of the shikimate pathway in the chloroplasts [42]. Melatonin under diverse stress conditions has a fundamental function in preserving the chlorophyll molecules and the photosynthetic function [43]. Additionally, MT interacts with other molecules like ROS, nitric oxide (NO), and Ca2+ to regulate the redox network [44,45]. Melatonin and ROS signaling have been shown to be interrelated coordinately [30]. Melatonin-induced plant stress tolerance is linked with up-regulation of stress-induced transcription factors [46]. Melatonin performs a key role in protein quality control in plants and thus functions as a pleiotropic molecule under both non-stress and stress conditions [46].
Melatonin (MT) has been extensively reported to contribute to the acclimation of plants to stress conditions [47]. The positive regulation of MT on photosynthetic efficiency and redox homeostasis under stress conditions has been frequently confirmed [48,49]. Under saline-alkali stress conditions, exogenous MT increased the efficiency of light energy capture and electron transport and improved soybean photosynthesis [50]. In rice plants under salt stress conditions, exogenous MT enhanced photosynthetic function by improving antioxidant capacity, increasing the xanthophyll pool size, and enhancing photosynthetic enzyme activities [47]. Furthermore, exogenous MT application increased strawberry fruit yield and quality under salinity stress [42]. During chilling stress, exogenous MT enhanced violaxanthin de-epoxidase activity accelerating the photoprotective heat dissipation of excitation energy, i.e., the non-photochemical quenching (NPQ), mitigating photoinhibition [51]. Ιn grafted Carya cathayensis plants under drought stress, MT regulated metabolic processes, including photosynthesis, antioxidant system, and gene expression [52]. Recently, Karumannil et al. [33] reviewed the molecular mechanisms of MT impact on photosynthetic function in different environmental conditions. However, the molecular mechanisms of the possible interaction between MT and photosynthetic function under non-stressed conditions have seldom been studied [53].
In the current study, we evaluated the consequences of exogenous MT application on the PSII function of Mentha spicata plants, under non-stressed conditions. We also evaluated the impact of MT application on ROS generation, and chlorophyll content, in order to elucidate the molecular mechanism of MT action on photosynthetic electron transport that under non-stressed conditions is still unclear.
2. Results
2.1. Melatonin Impact on Chlorophyll Content
The chlorophyll content of mint plants, 72 h after the spray with 10 μM melatonin (MT) did not differ from those that were sprayed with distilled water (dH2O) (Figure 1). However, an 18% increase (p < 0.05) in chlorophyll content was observed in plants that were sprayed with 100 μM MT compared to control plants (Figure 1).
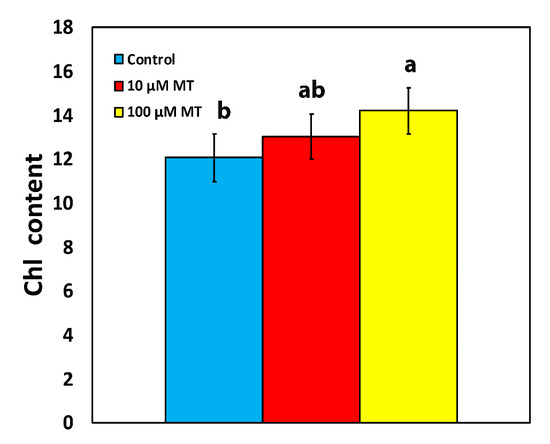
Figure 1.
Changes in the chlorophyll content of Mentha spicata leaves 72 h after the spray with 10 and 100 μM MT, in comparison to control leaves (sprayed with distilled water). Different lowercase letters symbolize statistical differences (p < 0.05). The error bars in columns symbolize SD.
2.2. Changes in the Efficiency of the Oxygen Evolving Complex and the Maximum Efficiency of PSII Photochemistry by Melatonin
A malfunction of the oxygen-evolving complex (OEC) was observed in mint plants, 72 h after the spray with MT, showing a decreased efficiency of 2.5% (p < 0.05) at 10 μM MT and of 6% (p < 0.05) at 100 μM MT, compared to control values (Figure 2a). An analogous pattern was observed in the maximum efficiency of PSII photochemistry (Fv/Fm), with a decreased efficiency of 0.5% (p < 0.05) at 10 μM MT and of 1% (p < 0.05) at 100 μM MT, compared to plants sprayed with dH2O (Figure 2b).
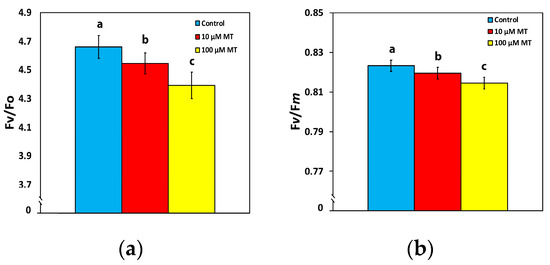
Figure 2.
Changes in the efficiency of the oxygen-evolving complex (OEC) (Fv/Fo) (a), and the maximum efficiency of PSII photochemistry (Fv/Fm) (b), 72 h after the spray of Mentha spicata leaves with 10 and 100 μM MT, in comparison to control leaves (sprayed with distilled water). Different lowercase letters symbolize statistical differences (p < 0.05). The error bars in columns symbolize SD.
2.3. Partitioning of the Absorbed Light Energy after Foliar Application of Melatonin
To estimate the partitioning of the captured light energy at PSII, we assessed the effective quantum yield of PSII photochemistry (ΦPSII), the quantum yield of regulated non-photochemical energy loss in PSII (ΦNPQ), and the quantum yield of non-regulated energy loss in PSII (ΦNO), with their sum (ΦPSII + ΦNPQ + ΦNO) to be equal to 1 [12].
The ΦPSII of mint plants 72 h after the spray with 10 μM MT did not differ from those that were sprayed with dH2O (Figure 3a) at the growth light intensity (GL 200 μmol photons m−2 s−1) and at high light intensity (HL, intensity 1000 μmol photons m−2 s−1). In contrast, in mint plants, 72 h after the spray with 100 μM MT, ΦPSII increased (p < 0.05) by 6% at the GL intensity, but there was no difference at the HL intensity compared to plants that were sprayed with dH2O (Figure 3a).
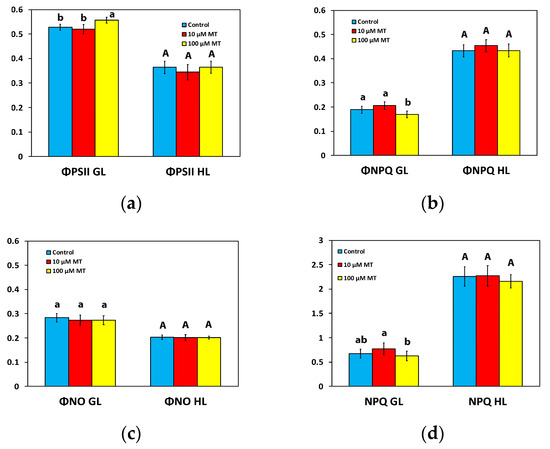
Figure 3.
Changes in the allocation of the absorbed light energy; the effective quantum yield of PSII photochemistry (ΦPSII) (a), the quantum yield of regulated non-photochemical energy loss in PSII (ΦNPQ) (b), the quantum yield of non-regulated energy dissipated in PSII (ΦNO) (c); and the photoprotective heat dissipation of excitation energy, i.e., the non-photochemical quenching (NPQ) (d); assessed all at the growth light intensity (GL, 200 μmol photons m−2 s−1), and at a high light intensity (HL, 1000 μmol photons m−2 s−1), 72 h after the spray of Mentha spicata leaves with 10 and 100 μM MT, compared to control leaves. Different lowercase or uppercase letters symbolize statistical differences (p < 0.05). The error bars in columns symbolize SD.
ΦNPQ, at both the GL intensity and the HL intensity, of mint plants sprayed with 10 μM MT did not differ from those that were sprayed with dH2O (Figure 3b). However, in mint plants, 72 h after the spray with 100 μM MT, ΦNPQ decreased (p < 0.05) by 10% at the GL intensity, but it did not differ from those that were sprayed with dH2O at the HL intensity (Figure 3b).
MT treatment had no impact on the quantum yield of non-regulated energy loss in PSII (ΦNO) at both the GL intensity and the HL intensity (Figure 3c).
2.4. Changes in Non-Photochemical Quenching by Melatonin Spray
The non-photochemical quenching (NPQ) of mint plants 72 h after the spray with 10 μM MT did not differ from those that were sprayed with dH2O at both the GL and the HL intensity (Figure 3d). In contrast, in mint plants, 72 h after the spray with 100 μM MT, NPQ decreased (p < 0.05) by 7% at the GL intensity, but there was no difference at the HL intensity compared to plants that were sprayed with dH2O (Figure 3d).
2.5. Melatonin Impact on PSII Reaction Centers and Their Efficiency
Photochemical quenching (qp) that represents the fraction of open PSII reaction centers, or in other words the redox state of quinone A (QA), did not differ at both the GL intensity and the HL intensity, in mint plants sprayed with 10 μM MT compared to those that were sprayed with dH2O (Figure 4a). However, in mint plants, 72 h after the spray with 100 μM MT, qp increased (p < 0.05) by 6% at the GL intensity, but there was no difference at the HL intensity compared to plants that were sprayed with dH2O (Figure 4a). The efficiency of open reaction centers (Fv′/Fm′) in mint plants sprayed with 10 μM MT decreased at the GL intensity compared to those that were sprayed with dH2O but remained the same to controls at the HL intensity (Figure 4b). In contrast, in mint plants sprayed with 100 μM MT, Fv′/Fm′ remained the same as controls at the GL intensity (Figure 4b) but decreased at the HL intensity compared to plants that were sprayed with dH2O (Figure 4b).
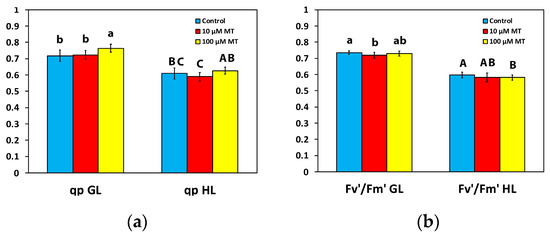
Figure 4.
Changes in the fraction of open PSII reaction centers (qp), a measure of the redox state of quinone A (QA) (a), and the efficiency of excitation energy capture by the open PSII reaction centers (Fv′/Fm′) (b); assessed all at the growth light intensity (GL, 200 μmol photons m−2 s−1), and at a high light intensity (HL, 1000 μmol photons m−2 s−1), 72 h after the spray of Mentha spicata leaves with 10 and 100 μM MT, in comparison to control leaves (sprayed with distilled water). Different lowercase or uppercase letters symbolize statistical differences (p < 0.05). The error bars in columns symbolize SD.
2.6. Changes in the Electron Transport Rate and the Excess Excitation Energy by Melatonin Spray
The electron transport rate (ETR) of mint plants 72 h after the spray with 10 μM MT did not differ from those that were sprayed with dH2O at both the GL intensity and the HL intensity (Figure 5a). In contrast, in mint plants, 72 h after the spray with 100 μM MT, ETR increased (p < 0.05) by 6% at the GL intensity, but there was no difference at the HL intensity compared to plants that were sprayed with dH2O (Figure 5a).
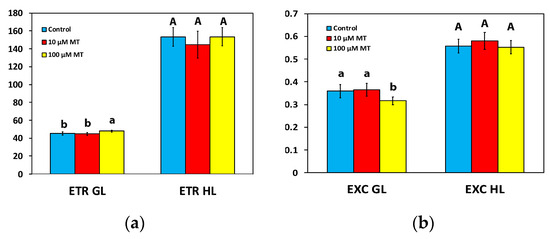
Figure 5.
Changes in the electron transport rate (ETR) (a), and the relative excess excitation energy at PSII (EXC) (b); assessed all at the growth light intensity (GL, 200 μmol photons m−2 s−1) and a high light intensity (HL, 1000 μmol photons m−2 s−1), 72 h after the spray of Mentha spicata leaves with 10 and 100 μM MT, in comparison to control leaves (sprayed with distilled water). Different lowercase or uppercase letters symbolize statistical differences (p < 0.05). The error bars in columns symbolize SD.
The excess excitation energy at PSII (EXC) in mint plants, 72 h after the spray with 100 μM MT, decreased (p < 0.05) by 12% at the GL intensity, but there was no difference at the HL intensity compared to plants that were sprayed with dH2O (Figure 5b). In mint plants sprayed with 10 μM MT, EXC did not differ from those sprayed with dH2O at both GL and HL intensity (Figure 5b).
2.7. Melatonin Impact on PSII Excitation Pressure
The excitation pressure at PSII, based on the “lake” model for the photosynthetic unit (1-qL) in mint plants, 72 h after the spray with 100 μM MT, decreased (p < 0.05) by 11% and 4%, at the GL and the HL intensity, respectively, compared to plants that were sprayed with dH2O (Figure 6). In mint plants sprayed with 10 μM MT, excitation pressure did not differ from those sprayed with dH2O at both GL and HL intensity (Figure 6).
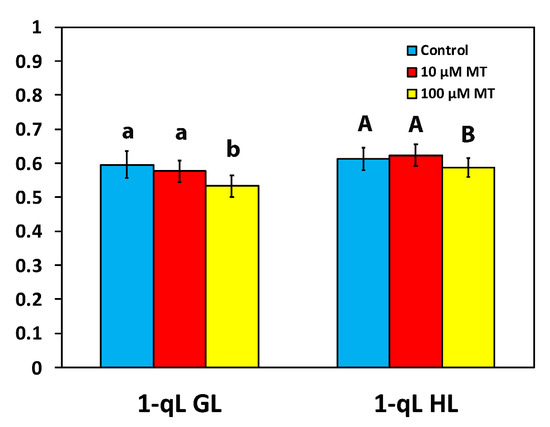
Figure 6.
Changes in the excitation pressure at PSII (based on the “lake” model for the photosynthetic unit), assessed at the growth light intensity (GL, 200 μmol photons m−2 s−1), and at a high light intensity (HL, 1000 μmol photons m−2 s−1), 72 h after the spray of Mentha spicata leaves with 10 and 100 μM MT, in comparison to control leaves (sprayed with distilled water). Different lowercase or uppercase letters symbolize statistical differences (p < 0.05). The error bars in columns symbolize SD.
2.8. Melatonin Impact on Reactive Oxygen Species Generation
Low MT foliar spray concentration (10 μM) did not seem to induce any reactive oxygen species (ROS) accumulation (Figure 7b), compared to plants that were sprayed with dH2O (Figure 7a). However, foliar spray with 100 μM MT induced a slight increase in ROS generation, especially on the leaf’s midvein (arrows, Figure 7c).
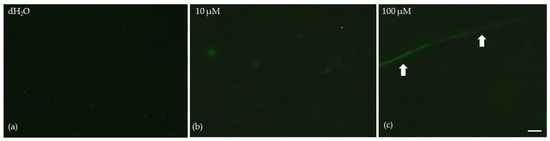
Figure 7.
Reactive oxygen species (ROS) production 72 h after the spray of Mentha spicata leaves with distilled water (dH2O) (a), with 10 μM MT (b), and 100 μM MT (c). The slight light green color denotes a slight ROS generation, arrows point to the midvein. Scale bar: 200 μm.
2.9. Melatonin-Induced Hormetic Responses of Photosystem II
There was a decline in the effective quantum yield of PSII photochemistry (ΦPSII) in mint plants, 72 h after the spray with 10 μM MT at both the GL and HL intensity (Figure 8a). This effect changed after the spray with 100 μM MT, with ΦPSII increasing above the control level at both GL and HL intensity (Figure 8a). This pattern of hormesis corresponds to a J-shaped hormetic response curve (Figure 8a).
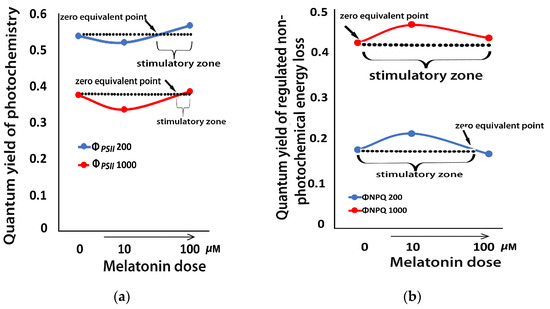
Figure 8.
A J-shaped hormetic response curve of ΦPSII (a), and an inverted J-shaped hormetic response curve of ΦNPQ (b), 72 h after the spray of Mentha spicata leaves with distilled water (control 0 μM MT) or with 10 and 100 μM MT, assessed either at the growth light intensity (200 μmol photons m−2 s−1), or at a high light intensity (1000 μmol photons m−2 s−1).
In contrast to ΦPSII, the photoprotective quantum yield of regulated non-photochemical energy loss in PSII (ΦNPQ), 72 h after the spray with 10 μM MT at both the GL and HL intensity, increased, while it decreased with 100 μM MT (Figure 8b), showing an inverted J-shaped hormetic response pattern (Figure 8b).
3. Discussion
Chlorophyll molecules serve as the principal pigments for absorbing light energy and transferring it to the reaction centers (RCs). Melatonin, which, in plants, is synthesized in mitochondria and chloroplasts through two paths that both are based on tryptophan [33], has revealed exceptional protective effects on chlorophyll molecules [53], controlling both the degradation and synthesis of chlorophyll molecules and protecting photosynthetic proteins [53]. A higher chlorophyll content, as we observed after the spray with 100 μM MT (Figure 1), can lead to the formation of larger light-harvesting complexes (LHCs), resulting in an increased capture of light energy and consequently enhancing ΦPSII and ETR [54,55,56,57,58], as it was detected (Figure 3a and Figure 5a). The observed improvement in photosynthetic function, at the GL following the spray with 100 μM MT, can be attributed to the enhanced light absorption. However, MT spray resulted in the malfunction of the OEC (Figure 2a) that caused donor-side photoinhibition [55,59,60,61], reflected in the reduced Fv/Fm (Figure 2b). When the OEC fails to efficiently reduce the chlorophyll molecule at the PSII RC, it results in damaging oxidations in PSII [59]. Consequently, donor-side photoinhibition is often associated with the production of ROS [55,62,63,64]. The minor increase in ROS generation that we observed (Figure 7c), as a result of donor-side photoinhibition (Figure 2b), can be attributed to a malfunction of the OEC (Figure 2a).
The non-photochemical quenching (NPQ) mechanism, by dissipating surplus light energy, serves as a protective measure for the photosynthetic apparatus against the detrimental impacts of ROS [7,56,65]. While a minimal level of ROS is necessary for maintaining life, a slight increase in ROS levels triggers molecular tolerance mechanisms, which are generally considered beneficial. Nevertheless, elevated levels of ROS are recognized as detrimental to plants [7,66,67,68,69,70,71]. NPQ functions as a photoprotective mechanism that inhibits the formation of ROS [72,73,74,75,76]. The reduction of excitation energy dissipation as heat through NPQ by 7%, 72 h after the spray with 100 μM MT (Figure 3d), can explain the slight increase in ROS generation (Figure 7c). However, this slight increase in ROS production can be considered as favorable for triggering defense stress responses [66,77,78]. The surplus light energy dissipated as heat by NPQ reduces the efficiency of PSII photochemistry (down-regulation of PSII) [20,21,74]. The increased excitation energy dissipation as heat through NPQ, 72 h after the spray with 10 μM MT compared to the spray with 100 μM MT (Figure 3d), decreased ΦPSII (Figure 3a). An increased NPQ, as was observed in mint plants sprayed with 10 μM MT, compared to plants sprayed with 100 μM MT (Figure 3d), decreases the ETR (Figure 5a), preventing the ROS formation (see Figure 7b), which occurs during photoinhibition (Figure 2b) [79].
The increased ETR of mint plants at the GL, following the spray with 100 μM MT, (Figure 5a), could be due to a decreased NPQ (Figure 3d) [79,80]. The observed donor-side photoinhibition, reflected by the reduced Fv/Fm (Figure 2b), decreased NPQ (Figure 3d), enhancing the ETR (Figure 5a) [63,81]. The increased effective quantum yield of PSII photochemistry (ΦPSII), 72 h after the spray with 100 μM MT at the GL intensity (Figure 3a), resulted in increased values of ETR (Figure 5a). Simultaneously, there was a reduction in excess excitation energy at PSII (Figure 5b), indicating enhanced efficiency of PSII. Enhancing photosynthesis is a critical challenge faced by plant scientists, especially in light of the ever-increasing global demand for food [2,82,83]. The ultimate goal of improving photosynthetic efficiency can be accomplished by optimizing the allocation of absorbed light energy [84,85].
As a result of the increased ΦPSII with 100 μM MT at the GL intensity (Figure 3a), the controlled non-photochemical energy loss in PSII (ΦNPQ) decreased by 10% (p < 0.05) (Figure 3b), while the unregulated energy loss in PSII (ΦNO) remained unchanged (Figure 3c). An increased ΦPSII can be attributed either to an increased efficiency of RCs (Fv′/Fm′) or/and to an increased number of open RCs (qp) [86]. The increased ΦPSII, with 100 μM MT at the GL intensity (Figure 3a), was rather due to the increased fraction of open PSII RCs (qp) (Figure 4a) than due to increased efficiency of the RCs (Fv′/Fm′) (Figure 4b). In Chara australis application of 10 μM MT to the artificial pond water, increased ΦPSII by 34% was attributed to an increased fraction of open PSII RCs, rather than increased efficiency of each RC [87]. More open RCs reflect higher photosynthetic efficiency [87].
The excitation pressure at PSII, based on the “lake” model for the photosynthetic unit (1 − qL) [12], in mint plants sprayed with 100 μM MT, decreased at both the GL and the HL intensity (Figure 6), which corresponds to diminished stomatal opening [88]. It seems that 100 μM MT could have induced the stomatal closure of mint plants through ROS production [34]. MT-induced stomatal closure is possibly regulated by H2O2 production and Ca2+ influx [34]. Fluctuations in the parameter 1 − qL reflect alterations in the redox state of QA [12], which act as a signal to the stomatal guard cells [89]. Consistent with this hypothesis, the parameter 1 − qL was linearly correlated to the stomatal conductance in tobacco plants [90]. It seems that stomatal movement is not controlled by the Calvin–Benson cycle but instead by the redox state (QA) [91]. As stomatal closure is a recognized process used by plants to restrict the penetration of pathogens, also known as stomatal immunity [92], MT is now acquiring consideration for its ability to prevent pathogen invasion and induce responses to biotic stress in plants [34,93,94,95].
Hormesis can commonly be exploited as an assessable measure of biological plasticity through adaptive responses under disruption of homeostasis [70,96,97,98]. These adaptive responses, which can be triggered by exposing plants to a low level of a factor that causes disruption of homeostasis, can result in protecting plants through the stimulation of cellular defence mechanisms [66,96,97]. Elucidating the molecular mechanisms that trigger hormesis in plants aims to accomplish higher crop productivity [55,97]. Higher crop productivity can be achieved by more efficient utilization of the absorbed light energy [5,99,100].
Hormetic–biphasic dose–response relationships were commonly observed in plants [55,96,101,102]. Melatonin has been shown to induce biphasic dose–response relationships in a series of studies including plants and animals [102]. In mint plants, MT induced a biphasic dose–response of ΦPSII with a J-shaped hormetic response curve to be enhanced by 100 μM MT (Figure 8a). Hormetic stimulation of PSII functionality can be triggered by NPQ, which can stimulate ROS production [55,96,103]. The process of NPQ dissipates in a harmless way the excess excitation energy (EXC) and decreases ETR to avoid ROS creation, thus NPQ can control a range of the level of ROS [96,103,104,105]. The slight increase in ROS level, 72 h after the spray with 100 μM MT (Figure 3d), is suggested to trigger the molecular mechanisms that are considered favorable for enhancing photosynthetic function [98,103]. ROS are considered as signaling hormetic molecules, which result in a biphasic dose–response effect on physiological end-points, such as photosynthesis [104,105]. ROS signaling can be favorable and essential for acclimation, regulating different pathways [106,107]. ROS play essential roles in the acclimation process of plants to environmental stress conditions as signal transduction molecules. Hormesis relies highly on the choice of dose range, duration of exposure, and experimental design [55,70,96,103,108,109,110,111,112,113,114]. Consequently, PSII hormetic responses can be observed only in appropriate planned studies [55,96].
Under non-stressed conditions, exogenous MT application in Chara australis increased the number of open RCs of PSII, thus improving ΦPSII [87], as we also observed in Mentha spicata plants. In contrast to our results, in which 100 μM MT reduced Fv/Fm due to donor-side photoinhibition, Yang et al. [115] suggested that the application of MT might alleviate PSII inhibition and partially display a direct antioxidant effect. They concluded that the application of 200 μM MT in the tea plant (Camellia sinensis (L.) Kuntze) stimulated photosynthesis and the expression of genes related to chlorophyll metabolism in a dose-dependent manner [115]. A dose-dependent increase in chlorophyll content was also noticed in our experiments (Figure 1), and enriched chlorophyll content by MT priming under high-temperature stress was observed in the tall fescue [116]. In agreement with our results, MT priming under high-temperature stress increased ΦPSII by increasing the fraction of RCs and decreased NPQ and the excessive excitation energy [116]. Exogenously applied MT in different crops improved not only crop yield but also quality by active regulation of several traits of plant development and growth, under either stressed or non-stressed conditions [31,53,117,118,119,120,121].
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
Mint (Mentha spicata L.) plants were obtained from a plant nursery and transferred to a growth chamber with 16 h light and 8 h dark cycles, 210 ± 10 μmol photons m−2 s−1 light intensity, 21 ± 1/18 ± 1 °C day/night temperature, and relative humidity 55 ± 5/60 ± 5% day/night.
Melatonin (N-acetyl-5-methoxytryptamine) (MT) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in ethanol (20 mg mL−1), before being further diluted with ultra-pure water [42,122]. Mint plants were foliar-sprayed until full wetting (15 mL plant−1), with 10 μM MT, 100 μM MT, or distilled water (dH2O) (control). Control plants were sprayed with dH2O with an equal amount of ethanol to that in MT-sprayed plants. To prevent MT from dropping into the soil, the surface of the soil was shielded by an aluminum foil that was detached after the spray. Since MT may be photo-responsive, the plants were sprayed during the dark cycle [123].
Leaf samples from M. spicata were taken 72 h after the spray from 4 to 5 plants with 3 independent biological replicates (n = 12–15) for the following measurements.
4.2. Chlorophyll Content
Relative chlorophyll content was measured in Mentha spicata leaves 72 h after the foliar spray with distilled water (control), 10 μM MT, and 100 μM MT, using a portable Chlorophyll Content Meter (Model Cl-01, Hansatech Instruments Ltd., Norfolk, UK). Values were expressed in relative units [63,124].
4.3. Chlorophyll Fluorescence Measurements
Chlorophyll a fluorescence was measured in Mentha spicata plants using a chlorophyll fluorometer imaging-PAM M-Series (Heinz Walz GmbH, Effeltrich, Germany), as described in detail previously [125]. Fluorescence was excited by blue LED in dark-adapted leaves with saturating pulses (SPs) of 6000 μmol photons m−2 s−1. Measurements on M. spicata leaves were conducted 72 h after the foliar spray with distilled water (control), 10 μM MT, and 100 μM MT. The actinic light (AL) used was 200 μmol photons m−2 s−1 corresponding to the growth light (GL) or 1000 μmol photons m−2 s−1 corresponding to a high light (HL) intensity. The chlorophyll fluorescence parameters, described in Table S1, were estimated using Win V2.41a software (Heinz Walz GmbH, Effeltrich, Germany). For each treatment, 12–15 leaves of the same developmental age were measured.
4.4. Reactive Oxygen Species Detection
In vivo imaging of ROS in mint leaves was performed 72 h after the foliar spray with distilled water (control), 10 μM MT, and 100 μM MT as described previously [126]. Thirty min after incubation of the leaves in the dark with 25 μM 2′, 7′-dichlorofluorescein diacetate (DCF-DA, Sigma Aldrich, Chemie GmbH, Schnelldorf, Germany), they were observed with a Zeiss AxioImager Z2 epi-fluorescence microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) that was equipped with an AxioCam MRc5 digital camera (Carl Zeiss MicroImaging GmbH, Göttingen, Germany).
4.5. Statistical Analysis
Data are presented as mean values ± SD and were tested for normality using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. The population of variances was not equal, so significant differences between the three treatments were determined using Welch ANOVA followed by a post hoc analysis with the Games–Howell test. All analyses were performed using SPSS version 28.0 (IBM, Chicago, IL, USA) for Windows. Values were considered significantly different at p < 0.05.
5. Conclusions
We observed a hormetic response of ΦPSII, which was probably triggered by NPQ that stimulated ROS production at 100 μM MT. The application of 100 μM MT in mint plants increased the chlorophyll content, possibly resulting in increased LHCs and increased light energy capture that enhanced ETR. In addition, 100 μM MT decreased the excess excitation energy at PSII and the excitation pressure at PSII, indicating an improved PSII efficiency. Improving photosynthetic function is of great importance for improving plant productivity and grain yield. Therefore, MT can potentially be used as a photosynthetic biostimulant that can be applied to plants exogenously to enhance crop yields while reducing the use of chemical fertilizers, also under non-stressed conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12234025/s1, Table S1: Definitions of the chlorophyll fluorescence parameters used in the experiments.
Author Contributions
Conceptualization, M.M.; methodology, M.M., I.S., I.-D.S.A., J.M. and F.M.; software, B.Ş., S.İ. and J.M.; validation, M.M.; formal analysis, M.M., I.S., B.Ş., S.İ. and J.M.; investigation, I.S., B.Ş. and S.İ.; resources, M.M. and F.M.; data curation, M.M., I.S., I.-D.S.A., B.Ş., S.İ., J.M. and F.M.; writing—original draft preparation, M.M. and J.M.; writing—review and editing, M.M., I.S., I.-D.S.A., B.Ş., S.İ., J.M. and F.M.; visualization, M.M., I.S. and J.M.; supervision, M.M.; project administration, M.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509531. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Ann. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R. When there is too much light. Plant Physiol. 2001, 125, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Wolosiuk, R.A.; Malkin, R. Photosynthesis. In Biochemistry & Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 508–566. [Google Scholar]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Moustakas, M.; Guidi, L.; Calatayud, A. Editorial: Chlorophyll fluorescence analysis in biotic and abiotic stress, volume II. Front. Plant Sci. 2022, 13, 1066865. [Google Scholar] [CrossRef]
- McAusland, L.; Atkinson, J.A.; Lawson, T.; Murchie, E.H. High throughput procedure utilising chlorophyll fluorescence imaging to phenotype dynamic photosynthesis and photoprotection in leaves under controlled gaseous conditions. Plant Methods 2019, 15, 109. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection of biotic and abiotic stress on plants by chlorophyll fluorescence imaging analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S. Editorial: Reactive oxygen species in chloroplasts and chloroplast antioxidants under abiotic stress. Front. Plant Sci. 2023, 14, 1208247. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Photoprotective mechanism of the non-target organism Arabidopsis thaliana to paraquat exposure. Pest. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Chen, Q.; Arnao, M.B. Phytomelatonin: An emerging new hormone in plants. J. Exp. Bot. 2022, 73, 5773–5778. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; Van Onckelen, H. Melatonin in higher plant determined by radioimmunoassay and liquid chromatography-mass spectrometry. Biol. Rhythm Res. 1995, 26, 406–409. [Google Scholar]
- Van Tassel, D.; Roberts, N.; Oenill, S.; O’Neill, S.D. Melatonin from higher plants: Isolation and identification of N-acetyl 5-methoxytryptamine. Plant Physiol. 1995, 108S, 101. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X.; et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J. Pineal Res. 2020, 68, e12640. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Q.; Ahammed, G.J.; Zhou, J. Functions and prospects of melatonin in plant growth, yield, and quality. J. Exp. Bot. 2022, 73, 5928–5946. [Google Scholar] [CrossRef]
- Khan, D.; Cai, N.; Zhu, W.; Li, L.; Guan, M.; Pu, X.; Chen, Q. The role of phytomelatonin receptor 1-mediated signaling in plant growth and stress response. Front. Plant Sci. 2023, 14, 1142753. [Google Scholar] [CrossRef] [PubMed]
- Karumannil, S.; Khan, T.A.; Kappachery, S.; Gururani, M.A. Impact of exogenous melatonin application on photosynthetic machinery under abiotic stress conditions. Plants 2023, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Rodríguez-Ruiz, M.; Muñoz-Vargas, M.A.; González-Gordo, S.; Reiter, R.J.; Palma, J.M. Interactions of melatonin, reactive oxygen species, and nitric oxide during fruit ripening: An update and prospective view. J. Exp. Bot. 2022, 73, 5947–5960. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernãndez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernãndez-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Sanie Khatam, A.; Rastegar, S.; Aboutalebi Jahromi, A.; Hassanzadeh Khankahdani, H.; Akbar Bagherian, S.A. Biochemical and physiological mechanism induced by melatonin in Mexican lime (Citrus aurantifolia Swingle) plants: Cold and freezing stress. Acta Physiol. Plant. 2023, 45, 98. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Abadía, J.; Marjani, M. Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.S.; Ahmed, S.; Ikram, A.U.; Hannan, F.; Yasin, M.U.; Wang, J.; Zhao, B.; Islam, F.; Chen, J. Phytomelatonin: A key regulator of redox and phytohormones signaling against biotic/abiotic stresses. Redox Biol. 2023, 64, 102805. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hwang, O.J.; Back, K. Phytomelatonin as a signaling molecule for protein quality control via chaperone, autophagy, and ubiquitin–proteasome systems in plants. J. Exp. Bot. 2022, 73, 5863–5873. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Wei, H.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Bakyani, M.R.F.; Alinia, M.; Kazemeini, S.A.; Abadía, J.; Dadkhodaie, A. Foliar application of melatonin improves the salt tolerance, ion and redox homeostasis and seed oil fatty acid profile in Camelina sativa. Plants 2022, 11, 3113. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Wang, G.; Du, Y.; Zhang, Z.; Yu, G.; Ren, C.; Zhang, Y.; Du, J. Exogenous melatonin enhances soybean (Glycine max (L.) Merr.) seedling tolerance to saline-alkali stress by regulating antioxidant response and DNA damage repair. Physiol. Plant. 2022, 174, e13731. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Liu, B.; Zhang, S. Exogenous melatonin mitigates photoinhibition by accelerating non-photochemical quenching in tomato seedlings exposed to moderate light during chilling. Front. Plant Sci. 2017, 8, 244. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.F.; Xu, D.B.; Tao, S.C.; Chong, S.L.; Yan, D.L.; Li, Z.; Yuan, H.W.; Zheng, B.S. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, Y.; Qin, X.; Ding, C.; Chen, Y.; Tang, Z.; Huang, Y.; Reiter, R.J.; Yuan, S.; Yuan, M. New insights into the role of melatonin in photosynthesis. J. Exp. Bot. 2022, 73, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Zhu, X.; Melis, A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Adamakis, I.-D.S.; Moustaka, J.; Apostolova, E. A hormetic spatiotemporal photosystem II response mechanism of salvia to excess zinc exposure. Int. J. Mol. Sci. 2022, 23, 11232. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Light harvesting control in plants. FEBS Lett. 2018, 592, 3030–3039. [Google Scholar] [CrossRef]
- Nelson, N.; Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef]
- Anderson, J.M.; Park, Y.I.; Chow, W.S. Unifying model for the photoinactivation of photosystem II in vivo: A hypothesis. Photosynth. Res. 1998, 56, 1–13. [Google Scholar] [CrossRef]
- Sarvikas, P.; Hakala, M.; Pätsikkä, E.; Tyystjärvi, T.; Tyystjärvi, E. Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 391–400. [Google Scholar] [CrossRef]
- Széles, E.; Kuntam, S.; Vidal-Meireles, A.; Nagy, V.; Nagy, K.; Ábrahám, Á.; Kovács, L.; Tóth, S.Z. Single-cell microfluidics in combination with chlorophyll a fluorescence measurements to assess the lifetime of the Chlamydomonas PSBO protein. Photosynthetica 2023, 61, 13–20. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Moustakas, M.; Dendrinou-Samara, C. Impact of coated zinc oxide nanoparticles on photosystem II of tomato plants. Materials 2023, 16, 5846. [Google Scholar] [CrossRef] [PubMed]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.-D.S.; Mourdikoudis, S.; Dendrinou-Samara, C.; Moustakas, M. Modification of tomato photosystem II photochemistry with engineered zinc oxide nanorods. Plants 2023, 12, 3502. [Google Scholar] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Ouzounidou, G.; Moustakas, M. Hormesis responses of photosystem II in Arabidopsis thaliana under water deficit stress. Int. J. Mol. Sci. 2023, 24, 9573. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Mittler, R. A systemic whole-plant change in redox levels accompanies the rapid systemic response to wounding. Plant Physiol. 2021, 186, 4–8. [Google Scholar] [CrossRef]
- Roach, T.; Na, C.S.; Stöggl, W.; Krieger-Liszkay, A. The non-photochemical quenching protein LHCSR3 prevents oxygen-dependent photoinhibition in Chlamydomonas reinhardtii. J. Exp. Bot. 2020, 71, 2650–2660. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Gunell, S.; Lempiäinen, T.; Rintamäki, E.; Aro, E.M.; Tikkanen, M. Enhanced function of non-photoinhibited photosystem II complexes upon PSII photoinhibition. Biochim. Biophys. Acta (BBA)-Bioenerg. 2023, 1864, 148978. [Google Scholar] [CrossRef]
- Paul, M.J. Improving photosynthetic metabolism for crop yields: What is going to work? Front. Plant Sci. 2021, 12, 743862. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nosberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Struik, P.C. Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: From leaf biochemistry to canopy physiology and crop ecology. J. Exp. Bot. 2015, 66, 6535–6549. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Lazár, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal. Behav. 2013, 8, e23279. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Busch, F.A. Opinion: The red-light response of stomatal movement is sensed by the redox state of the photosynthetic electron transport chain. Photosynth. Res. 2014, 119, 131–140. [Google Scholar] [CrossRef]
- Głowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, A.P.; Leonelli, L.; Leakey, A.D.B.; Ort, D.R.; Niyogi, K.K.; Long, S.P. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 868. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Long, S.P. Predicting light-induced stomatal movements based on the redox state of plastoquinone: Theory and validation. Photosynth. Res. 2019, 141, 83–97. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts in plants. Biomolecules 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, Z.; Ma, W.; Zhang, S.; Hou, S.; Wei, J.; Dong, S.; Yu, X.; Song, Y.; Gao, W.; et al. Melatonin functions in priming of stomatal immunity in Panax notoginseng and Arabidopsis thaliana. Plant Physiol. 2021, 187, 2837–2851. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.E.; Campos, M.L. Waking up for defense! Melatonin as a regulator of stomatal immunity in plants. Plant Physiol. 2022, 188, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R.D. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Sperdouli, I.; Hanć, A.; Dobrikova, A.; Apostolova, E.; Moustakas, M. Rapid hormetic responses of photosystem II photochemistry of clary sage to cadmium exposure. Int. J. Mol. Sci. 2021, 22, 41. [Google Scholar] [CrossRef]
- Li, Z.; Xing, F.; Xing, D. Characterization of target site of aluminum phytotoxicity in photosynthetic electron transport by fluorescence techniques in tobacco leaves. Plant Cell Physiol. 2012, 53, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Agathokleous, E. Accumulator plants and hormesis. Environ. Pollut. 2021, 274, 116526. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. New insights into the role of melatonin in plants and animals. Chem. Biol. Interact. 2019, 299, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.L.; Sperdouli, I.; Pyrri, I.; Adamakis, I.D.S.; Moustakas, M. Hormetic responses of photosystem II in tomato to Botrytis cinerea. Plants 2021, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.C.; Ozgur, R.; Uzilday, B. Reactive oxygen species: Connecting eustress, hormesis, and allostasis in plants. Plant Stress 2023, 8, 100164. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Müller-Moulé, P.; Gilmore, A.M.; Niyogi, K.K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Agathokleous, E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2021, 32, 889–898. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental hormesis: From cell to ecosystem. Curr. Opin. Environ. Sci. Health 2022, 29, 100378. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Editorial overview: Hormesis and dose-response. Curr. Opin. Toxicol. 2022, 30, 100343. [Google Scholar] [CrossRef]
- Yang, N.; Han, M.H.; Teng, R.M.; Yang, Y.Z.; Wang, Y.H.; Xiong, A.S.; Zhuang, J. Exogenous melatonin enhances photosynthetic capacity and related gene expression in a dose-dependent manner in the tea plant (Camellia sinensis (L.) Kuntze). Int. J. Mol. Sci. 2022, 23, 6694. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xing, M.; Hu, T.; Ji, M.; Li, X.; Amombo, E.; Shao, A.; Xu, X.; Fu, J. Photosystem II photochemical adjustment of tall fescue against heat stress after melatonin priming. J. Plant Physiol. 2022, 275, 153758. [Google Scholar] [CrossRef] [PubMed]
- Kayaa, A.; Doganla, Z.B. Melatonin improves the multiple stress tolerance in pepper (Capsicum annuum). Sci. Hortic. 2019, 256, 108509. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Nazir, F.; Saleem, M. Melatonin in business with abiotic stresses in plants. Physiol. Mol. Biol. Plants 2020, 26, 1931–1944. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Zia-Ur-Rehman, M.; Hussain, S.M.; Qayyum, M.F.; Rizwan, M.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Ali, S. Combined effects of zinc oxide nanoparticles and melatonin on wheat growth, chlorophyll contents, cadmium (Cd) and zinc uptake under Cd stress. Sci. Total Environ. 2023, 864, 161061. [Google Scholar] [CrossRef]
- Muhammad, H.M.D.; Naz, S.; Lal, M.K.; Tiwari, R.K.; Ahmad, R.; Nawaz, M.A.; Das, R.; Altaf, M.A. Melatonin in business with abiotic stresses in vegetable crops. Sci. Hortic. 2024, 324, 112594. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Gholami, R.; Abdelrahman, M.; Tran, L.P. Exogenous melatonin mitigates salinity-induced damage in olive seedlings by modulating ion homeostasis, antioxidant defense, and phytohormone balance. Physiol. Plant. 2021, 173, 1682–1694. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Borek, M.; Bączek-Kwinta, R.; Rapacz, M. Photosynthetic activity of variegated leaves of Coleus × hybridus hort. cultivars characterised by chlorophyll fluorescence techniques. Photosynthetica 2016, 54, 331–339. [Google Scholar] [CrossRef]
- Moustaka, J.; Panteris, E.; Adamakis, I.D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).