Exploring the Bioactive Compounds in Some Apple Vinegar Samples and Their Biological Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties and Total Phenolic and Flavonoid Content
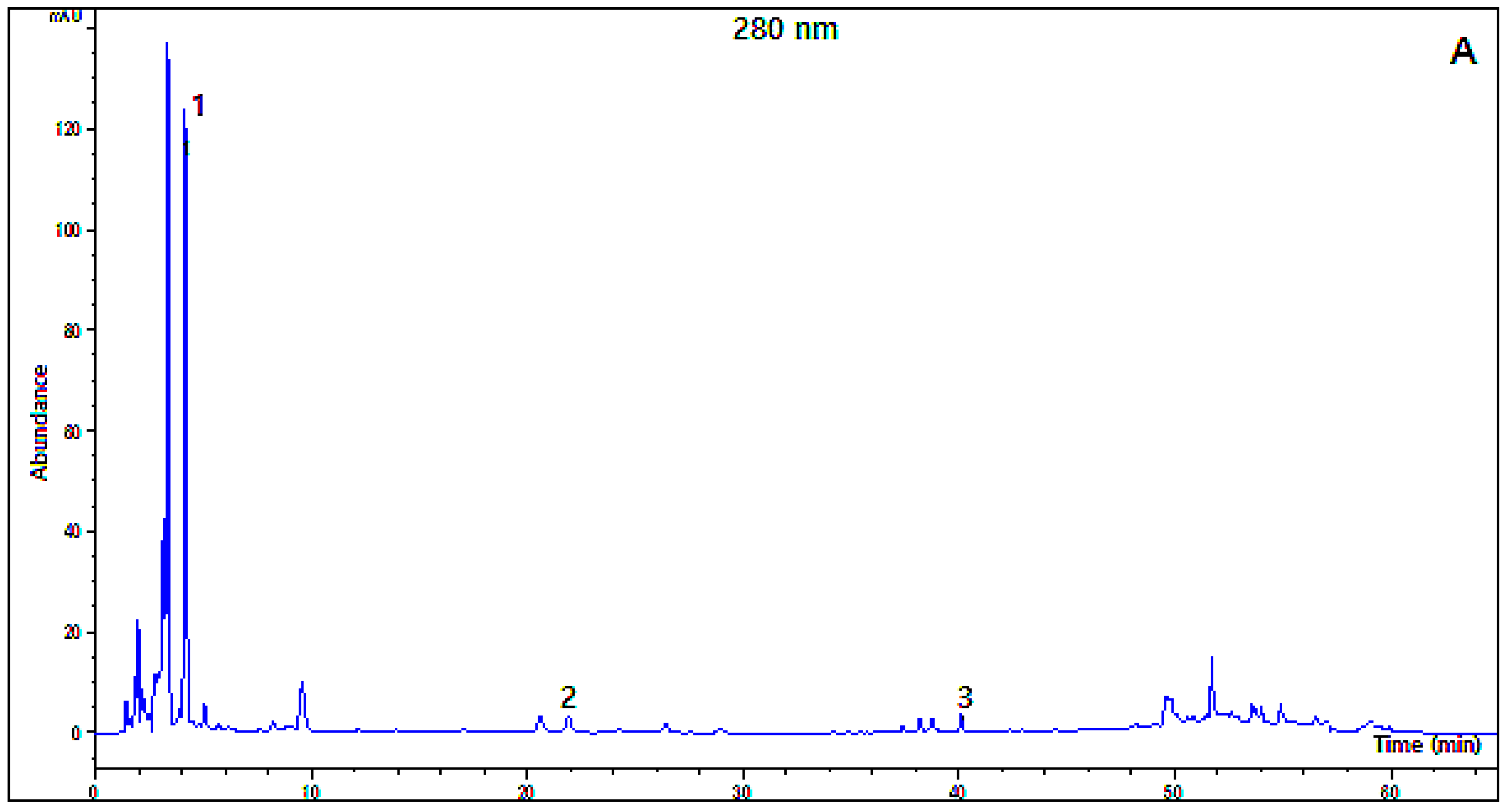
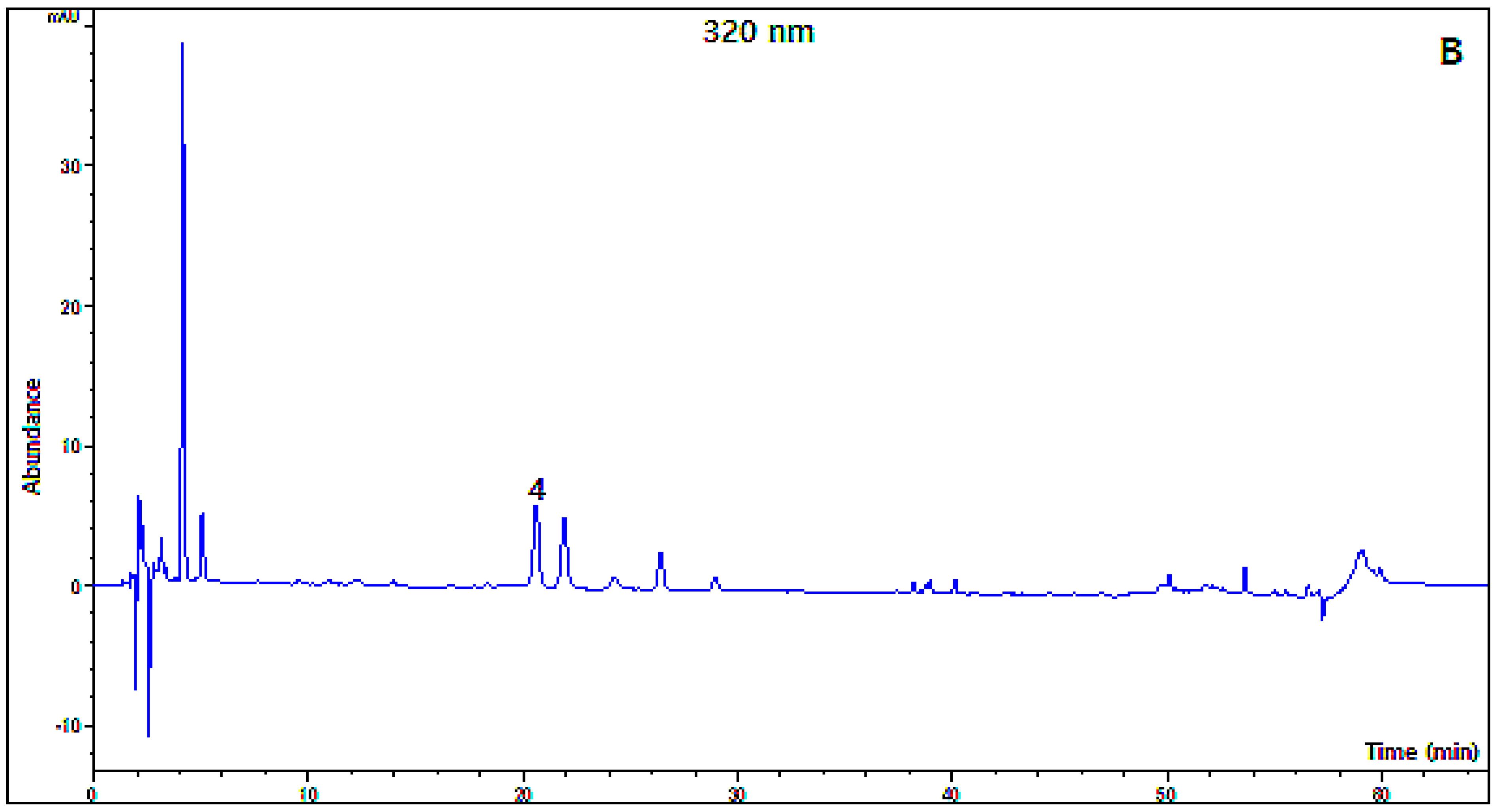
2.2. Organic Acid and Polyphenol Composition
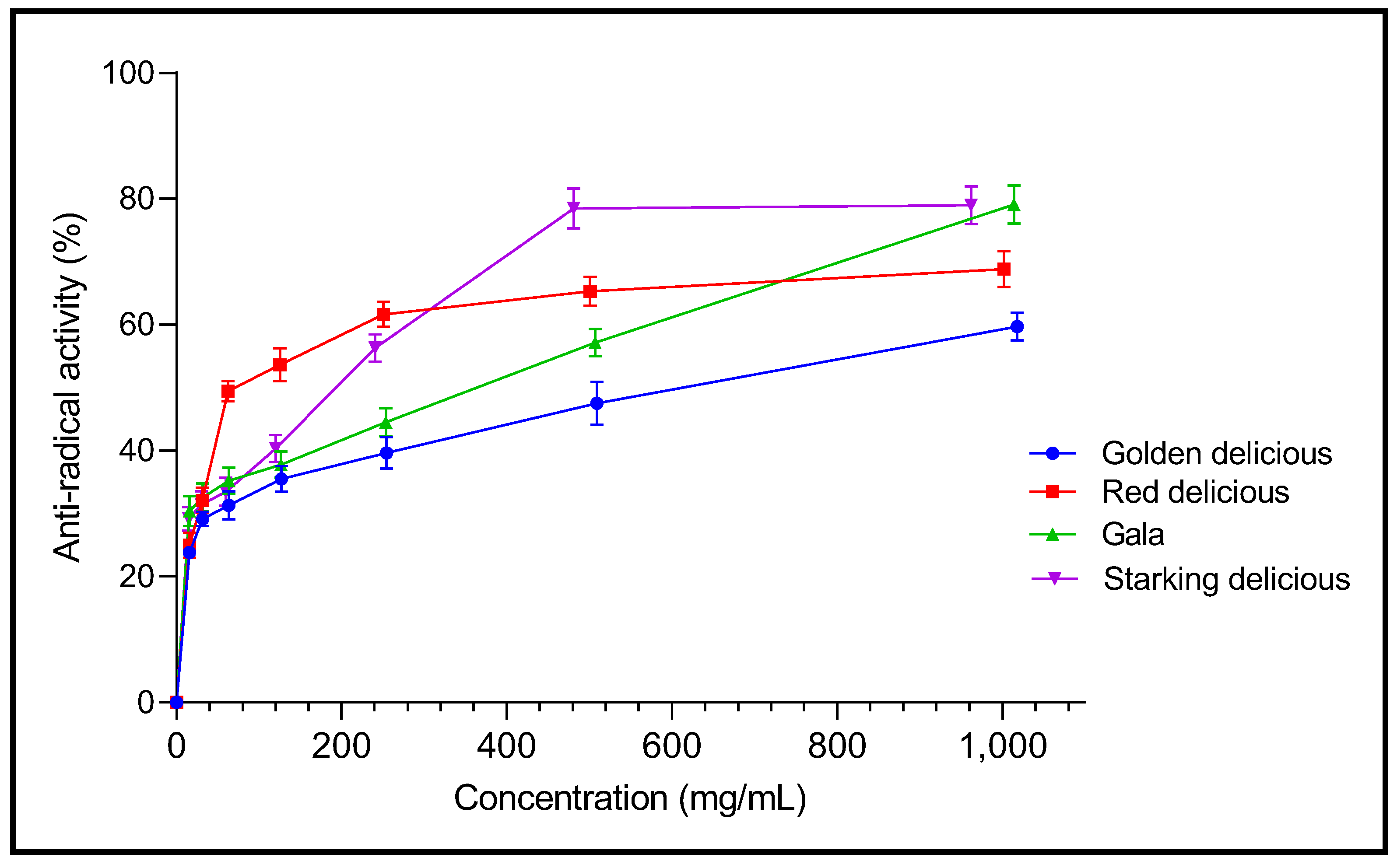
2.3. Antioxidant Capacity
2.4. Antimicrobial Activity
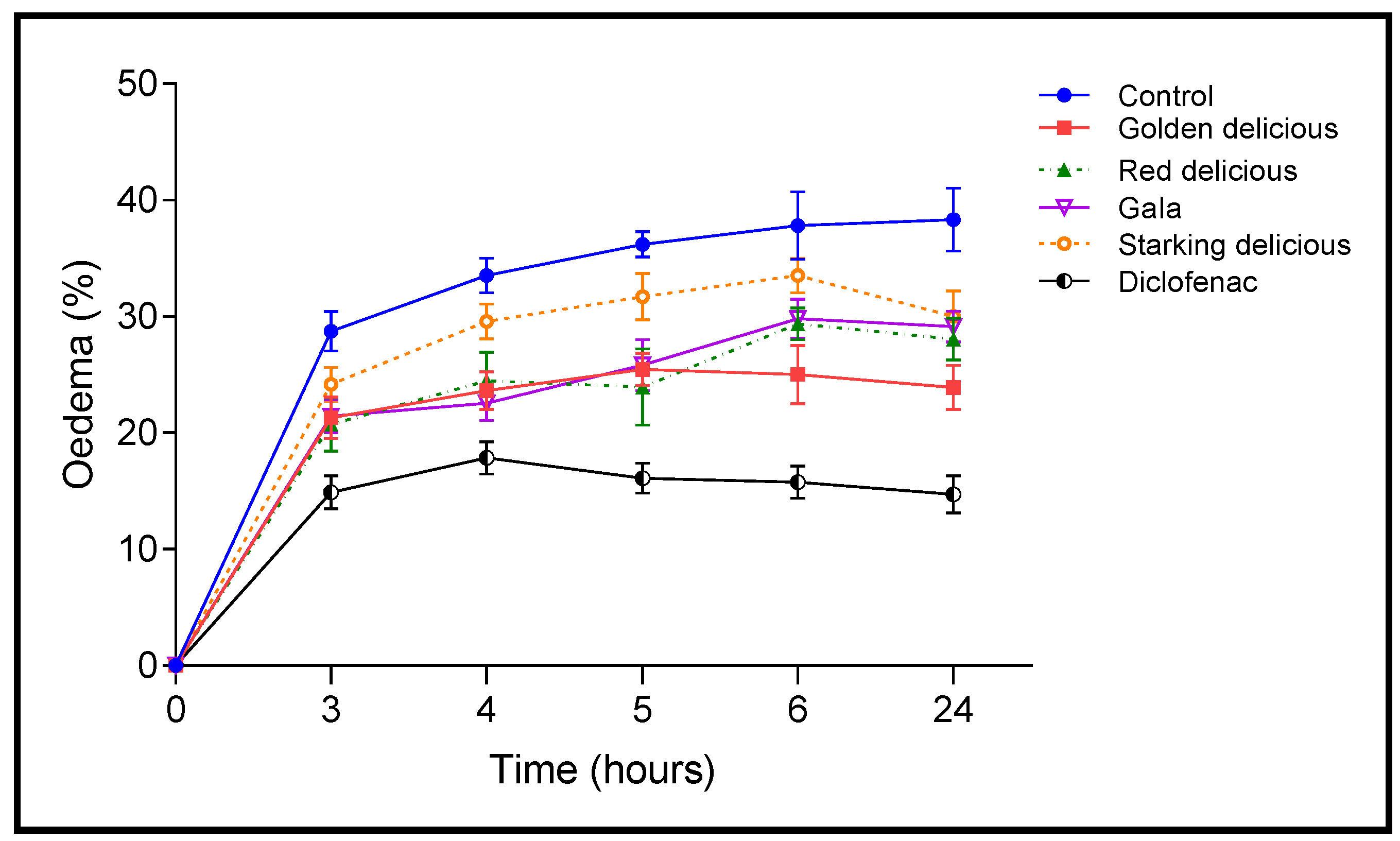
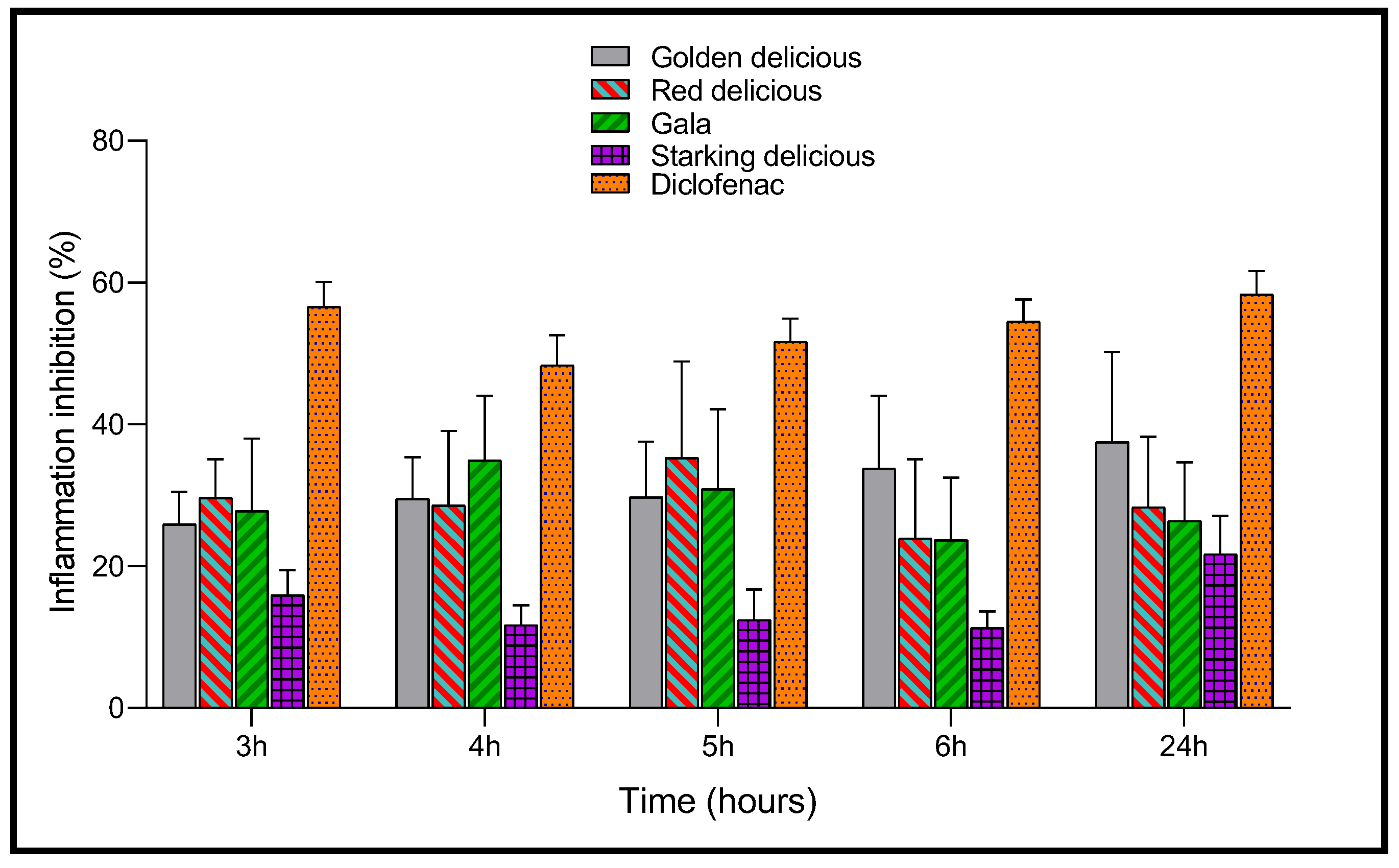
2.5. Anti-Inflammatory Activity
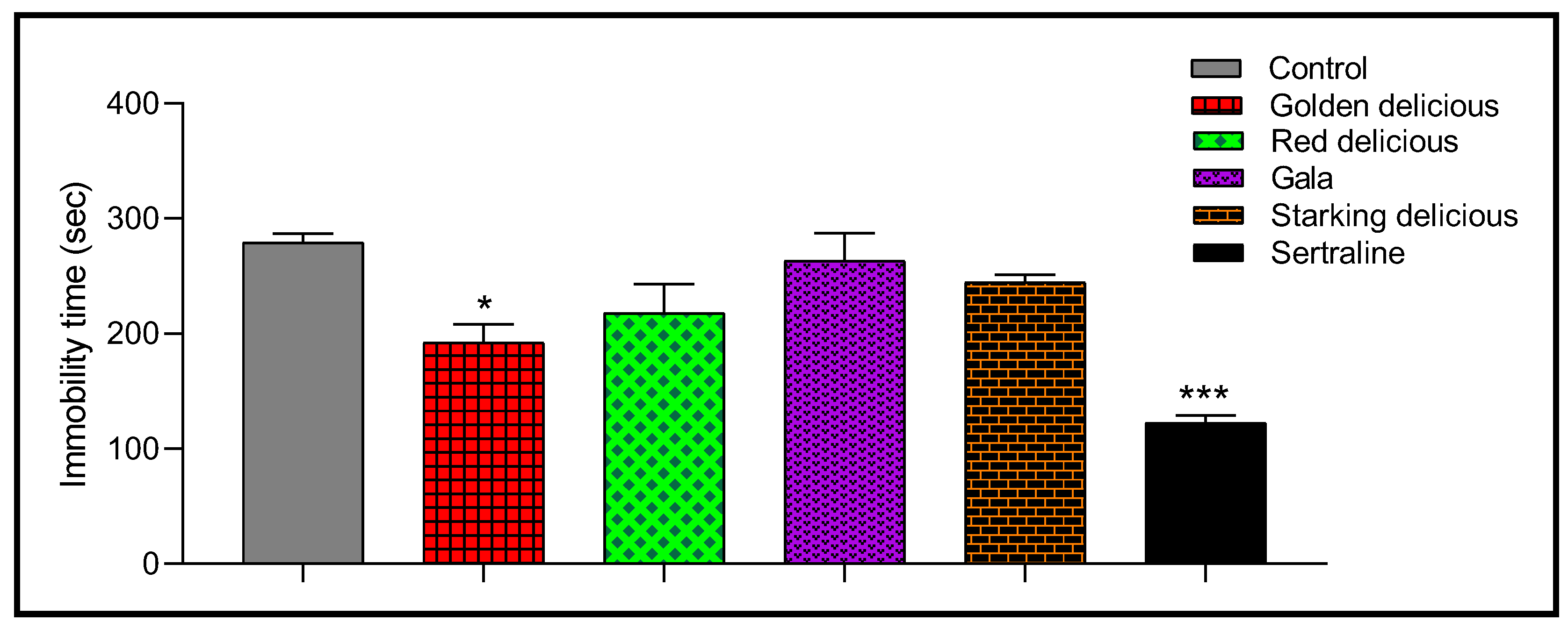
2.6. Antidepressant Activity
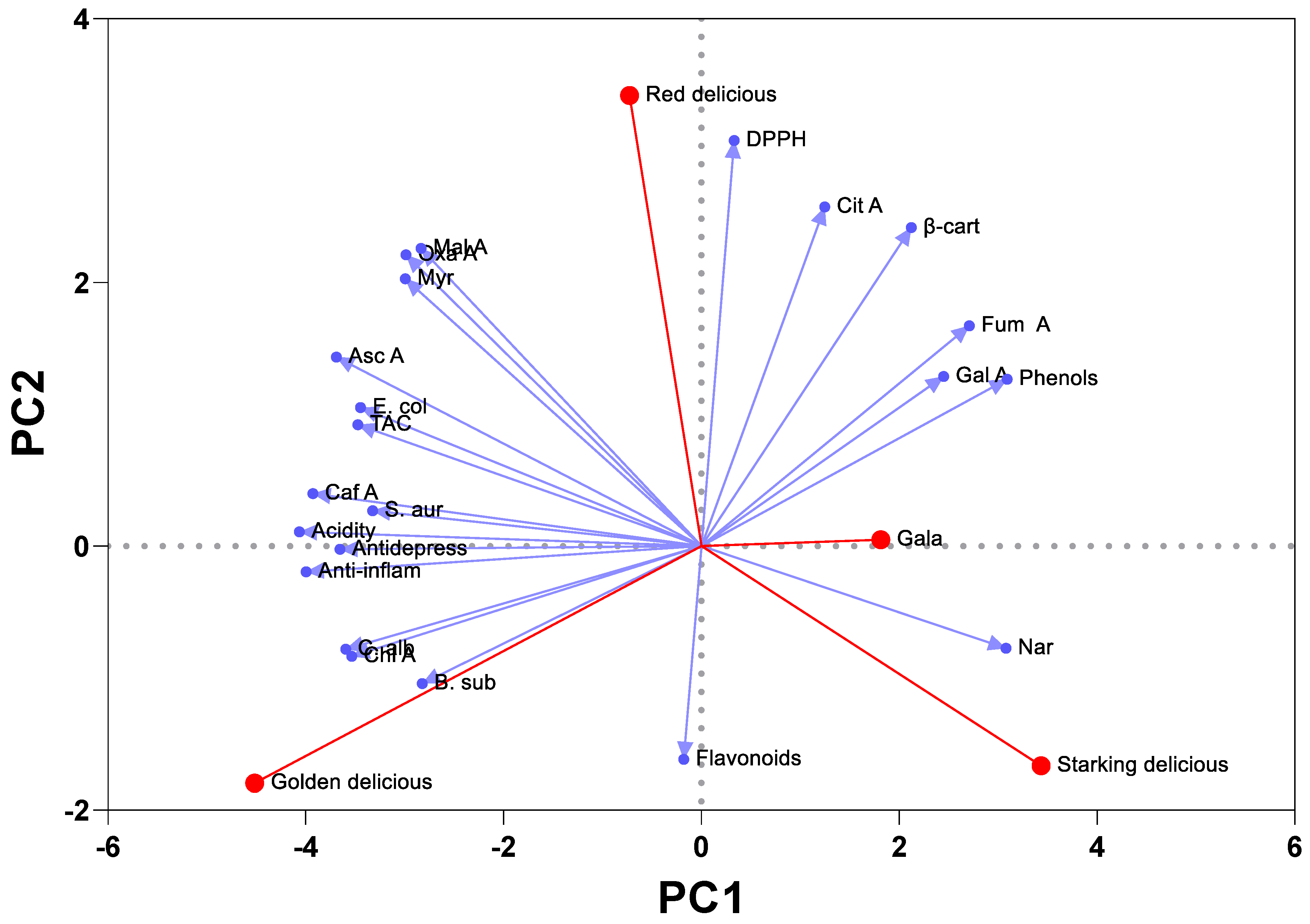
2.7. Correlation and Multivariate Statistical Analysis:
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Apple Vinegar
3.3. Physicochemical Properties
3.4. Total Phenolic and Flavonoid Contents
3.5. HPLC Analysis of Organic Acids
3.6. HPLC-DAD Analysis of Polyphenols
3.7. In Vitro Antioxidant Activity
3.7.1. DPPH Free Radical Scavenging Assay
3.7.2. β-Carotene Discoloration Assay
3.7.3. Total Antioxidant Capacity (TAC) Assay
3.8. Antimicrobial Activity
3.8.1. Growth Medium and Chemicals
3.8.2. Microbial Strains
3.8.3. Preparation of the Microbial Suspensions
3.8.4. Agar Well Diffusion Assay
3.8.5. Determination of the Minimal Inhibitory Concentration (MIC)
3.9. Animal Handling and Housing
3.10. Anti-Inflammatory Test
3.11. Antidepressant Activity
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Kviesis, J.; Salar, F.J.; Moreno, D.A.; Drudze, I. Wild Apple (Malus spp.) by-Products as a Source of Phenolic Compounds and Vitamin C for Food Applications. Food Biosci. 2020, 38, 100744. [Google Scholar] [CrossRef]
- Han, M.L.; Zhao, Y.H.; Meng, J.X.; Yin, J.; Li, H.H. Analysis of Physicochemical and Antioxidant Properties of Malus spp. Petals Reveals Factors Involved in Flower Color Change and Market Value. Sci. Hortic. 2023, 310, 111688. [Google Scholar] [CrossRef]
- Kara, M.; Assouguem, A.; Al Kamaly, O.M.; Benmessaoud, S.; Imtara, H.; Mechchate, H.; Hano, C.; Zerhouni, A.R.; Bahhou, J. The Impact of Apple Variety and the Production Methods on the Antibacterial Activity of Vinegar Samples. Molecules 2021, 26, 5437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Huang, Z.; Zhao, Q.; Fan, J.; Tian, Y.; Huang, A. Isolation, Identification and Characterization of a Novel Antimicrobial Peptide from Moringa Oleifera Seeds Based on Affinity Adsorption. Food Chem. 2023, 398, 133923. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial Activity of Apple Cider Vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; Downregulating Cytokine and Microbial Protein Expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Pianta, L.; Vinciguerra, A.; Bertazzoni, G.; Morello, R.; Mangiatordi, F.; Lund, V.J.; Trimarchi, M. Acetic Acid Disinfection as a Potential Adjunctive Therapy for Non-Severe COVID-19. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2921–2924. [Google Scholar] [CrossRef]
- Gaber, S.N.; Bassyouni, R.H.; Masoud, M.; Ahmed, F.A. Promising Anti-Microbial Effect of Apple Vinegar as a Natural Decolonizing Agent in Healthcare Workers. Alex. J. Med. 2020, 56, 73–80. [Google Scholar] [CrossRef]
- Baldas, B.; Altuner, E.M. The Antimicrobial Activity of Apple Cider Vinegar and Grape Vinegar, Which Are Used as a Traditional Surface Disinfectant for Fruits and Vegetables. Commun. Fac. Sci. Univ. Ankara Ser. C Biol. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Rutala, W.A.; Barbee, S.L.; Aguiar, N.C.; Sobsey, M.D.; Weber, D.J. Antimicrobial Activity of Home Disinfectants and Natural Products Against Potential Human Pathogens. Infect. Control Hosp. Epidemiol. 2000, 21, 33–38. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Huang, Q.; Zhang, L.; Liu, X.; Liu, R.; Lu, Q. Antioxidant and Anti-Inflammatory Activities of Rape Bee Pollen after Fermentation and Their Correlation with Chemical Components by Ultra-Performance Liquid Chromatography-Quadrupole Time of Flight Mass Spectrometry-Based Untargeted Metabolomics. Food Chem. 2023, 409, 135342. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Ousaaid, D.; Imtara, H.; Laaroussi, H.; Lyoussi, B.; Elarabi, I. An Investigation of Moroccan Vinegars: Their Physicochemical Properties and Antioxidant and Antibacterial Activities. J. Food Qual. 2021, 2021, 6618444. [Google Scholar] [CrossRef]
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT-Food Sci. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; ElGhouizi, A.; Aboulghazi, A.; Lyoussi, B.; ElArabi, I. Beneficial Effects of Apple Vinegar on Hyperglycemia and Hyperlipidemia in Hypercaloric-Fed Rats. J. Diabetes Res. 2020, 2020, 9284987. [Google Scholar] [CrossRef]
- Tripathi, S.; Mazumder, P.M. Apple Cider Vinegar (ACV) and Their Pharmacological Approach towards Alzheimer’s Disease (AD): A Review. Indian J. Pharm. Educ. Res. 2020, 54, S67–S74. [Google Scholar] [CrossRef]
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, Antimicrobial, Mineral, Volatile, Physicochemical and Microbiological Characteristics of Traditional Home-Made Turkish Vinegars. LWT-Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberič, R.; Trobec, M.; Toplak, H.; Štampar, F.; Keppel, H.; Grill, D. Sugar-, Acid- and Phenol Contents in Apple Cultivars from Organic and Integrated Fruit Cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136–1140. [Google Scholar] [CrossRef]
- Ergün, Z. Determination of Biochemical Contents of Fresh, Oven-Dried, and Sun-Dried Peels and Pulps of Five Apple Cultivars (Amasya, Braeburn, Golden Delicious, Granny Smith, and Starking). J. Food Qual. 2021, 2021, 9916694. [Google Scholar] [CrossRef]
- Duan, W.; Xia, T.; Zhang, B.; Li, S.; Zhang, C.; Zhao, C.; Song, J.; Wang, M. Changes of Physicochemical, Bioactive Compounds and Antioxidant Capacity during the Brewing Process of Zhenjiang Aromatic Vinegar. Molecules 2019, 24, 3935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, C.; Guo, Y.; Wang, X.; Meng, Y. Polyphenols in Fermented Apple Juice: Beneficial Effects on Human Health. J. Funct. Foods 2021, 76, 104294. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Evolution of Polyphenols and Organic Acids during the Fermentation of Apple Cider. J. Sci. Food Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, H.A.; Tutun, H.; Keyvan, E.; Balkan, B.M. Bioactive Components, Antibacterial and Antiradical Properties of Home-Made Apple and Grape Vinegar. Ank. Üniversitesi Vet. Fakültesi Derg. 2022, 69, 139–148. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of Chemical Composition and Sensorial Properties of Ciders Fermented with Different Non-Saccharomyces Yeasts in Pure and Mixed Fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of Organic Acids and Phenolic Compounds of Cereal Vinegars and Fruit Vinegars in China. J. Food Process. Preserv. 2017, 41, e12937. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H. Bin Comparison of Antioxidant Activities of Different Grape Varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Denis, M.C.; Furtos, A.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple Peel Polyphenols and Their Beneficial Actions on Oxidative Stress and Inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural Polyphenols in the Management of Major Depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef]
- Seydim, A.C.; Guzel-Seydim, Z.B.; Doguc, D.K.; Savas, M.C.; Budak, H.N. Effects of Grape Wine and Apple Cider Vinegar on Oxidative and Antioxidative Status in High Cholesterol-Fed Rats. Funct. Foods Health Dis. 2016, 6, 569–577. [Google Scholar] [CrossRef]
- EL Moussaoui, A.; Bourhia, M.; Jawhari, F.Z.; Salamatullah, A.M.; Ullah, R.; Bari, A.; Majid Mahmood, H.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar] [CrossRef]
- El-Sayed, T.S.; Nour El-Deen, M.M.; Rokaya, M.E.; Sherif, M.M. Evaluation of the Antibacterial Effect of Apple Vinegar as a Root Canal Irrigant Using Endovac Irrigation System. Al-Azhar Dent. J. Girls 2019, 6, 53–59. [Google Scholar] [CrossRef][Green Version]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, Antioxidant and Antibacterial Activities of Two Moroccan Teucrium Polium L. Subspecies: Preventive Approach against Nosocomial Infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak Organic Acids: A Panoply of Effects on Bacteria. Sci. Prog. 2003, 86, 245–269. [Google Scholar] [CrossRef] [PubMed]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The Effect of Food Preservatives on PH Homeostasis in Escherichia coli. J. Gen. Microbiol. 1984, 130, 2845–2850. [Google Scholar] [CrossRef]
- Bjornsdottir, K.; Breidt, F.; McFeeters, R.F. Protective Effects of Organic Acids Oil Survival of Escherichia coli O157:H7 in Acidic Environments. Appl. Environ. Microbiol. 2006, 72, 660–664. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Kelebek, H.; Kadiroğlu, P.; Demircan, N.B.; Selli, S. Screening of Bioactive Components in Grape and Apple Vinegars: Antioxidant and Antimicrobial Potential. J. Inst. Brew. 2017, 123, 407–416. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; Brenes, M.; De Castro, A. Antimicrobial Activity of Olive Oil, Vinegar, and Various Beverages against Foodborne Pathogens. J. Food Prot. 2007, 70, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.M.S.; Neves, A.C.C.; Leão, M.V.P.; Jorge, A.O.C. Vinegar as an Antimicrobial Agent for Control of Candida Spp. in Complete Denture Wearers. J. Appl. Oral Sci. 2008, 16, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Tekulu, G.H.; Hiluf, T.; Brhanu, H.; Araya, E.M.; Bitew, H.; Haile, T. Anti-Inflammatory and Anti-Nociceptive Property of Capparis Tomentosa Lam. Root Extracts. J. Ethnopharmacol. 2020, 253, 112654. [Google Scholar] [CrossRef] [PubMed]
- Nwidu, L.L.; Airhihen, B.; Ahmadu, A. Anti-Inflammatory and Anti-Nociceptive Activities of Stem-Bark Extracts and Fractions of Carpolobia lutea (Polygalaceae). J. Basic Clin. Pharm. 2016, 8, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Murthuza, S.; Manjunatha, B.K. In Vitro and In Vivo Evaluation of Anti-Inflammatory Potency of Mesua ferrea, Saraca asoca, Viscum album & Anthocephalus cadamba in Murine Macrophages Raw 264.7 Cell Lines and Wistar Albino Rats. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 719–723. [Google Scholar] [CrossRef]
- Adnan, M.; Nazim Uddin Chy, M.; Mostafa Kamal, A.T.M.; Barlow, J.W.; Faruque, M.O.; Yang, X.; Uddin, S.B. Evaluation of Anti-Nociceptive and Anti-Inflammatory Activities of the Methanol Extract of Holigarna caustica (Dennst.) Oken Leaves. J. Ethnopharmacol. 2019, 236, 401–411. [Google Scholar] [CrossRef]
- Jing, L.; Yanyan, Z.; Junfeng, F. Acetic Acid in Aged Vinegar Affects Molecular Targets for Thrombus Disease Management. Food Funct. 2015, 6, 2845–2853. [Google Scholar] [CrossRef]
- Asejeje, F.O.; Abiola, M.A.; Ben-Azu, B.; Adeosun, A.M.; Ighodaro, O.M.; Ajayi, A.M. Apple Cider Vinegar Attenuates Lipopolysaccharide-Induced Neurobehavioral Deficits in Mice. Nutrire 2020, 45, 23. [Google Scholar] [CrossRef]
- Shen, F.; Feng, J.; Wang, X.; Qi, Z.; Shi, X.; An, Y.; Zhang, Q.; Wang, C.; Liu, M.; Liu, B.; et al. Vinegar Treatment Prevents the Development of Murine Experimental Colitis via Inhibition of Inflammation and Apoptosis. J. Agric. Food Chem. 2016, 64, 1111–1121. [Google Scholar] [CrossRef]
- Sureda, A.; Tejada, S. Polyphenols and Depression: From Chemistry to Medicine. Curr. Pharm. Biotechnol. 2015, 16, 259–264. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. A Hydroxyl Group of Flavonoids Affects Oral Anti-Inflammatory Activity and Inhibition of Systemic Tumor Necrosis Factor-α Production. Biosci. Biotechnol. Biochem. 2004, 68, 119–125. [Google Scholar] [CrossRef]
- Felger, J.C. Role of Inflammation in Depression and Treatment Implications. Handb. Exp. Pharmacol. 2019, 250, 255–286. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-Induced Depressive-like Behavior Is Mediated by Indoleamine 2,3-Dioxygenase Activation in Mice. Mol. Psychiatry 2008, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The Role of 5-HT Receptors in Depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef]
- Ali, S.; Corbi, G.; Maes, M.; Scapagnini, G.; Davinelli, S. Exploring the Impact of Flavonoids on Symptoms of Depression: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1644. [Google Scholar] [CrossRef]
- Khan, H.; Perviz, S.; Sureda, A.; Nabavi, S.M.; Tejada, S. Current Standing of Plant Derived Flavonoids as an Antidepressant. Food Chem. Toxicol. 2018, 119, 176–188. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, Y.J.; Koh, K.H. Investigation into Factors Influencing Antioxidant Capacity of Vinegars. Appl. Biol. Chem. 2016, 59, 495–509. [Google Scholar] [CrossRef]
- Mat Isham, N.K.; Mokhtar, N.; Fazry, S.; Lim, S.J. The Development of an Alternative Fermentation Model System for Vinegar Production. LWT 2019, 100, 322–327. [Google Scholar] [CrossRef]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- El Abdali, Y.; Beniaich, G.; Mahraz, A.M.; El Moussaoui, A.; Bin Jardan, Y.A.; Akhazzane, M.; Chebaibi, M.; Nafidi, H.-A.; Eloutassi, N.; Bourhia, M.; et al. Antibacterial, Antioxidant, and in Silico NADPH Oxidase Inhibition Studies of Essential Oils of Lavandula dentata against Foodborne Pathogens. Evid.-Based Complement. Altern. Med. 2023, 2023, 9766002. [Google Scholar] [CrossRef]
- El Abdali, Y.; Mahraz, A.M.; Beniaich, G.; Mssillou, I.; Chebaibi, M.; Jardan, Y.A.B.; Lahkimi, A.; Nafidi, H.-A.; Aboul-Soud, M.A.M.; Bourhia, M.; et al. Essential Oils of Origanum compactum Benth: Chemical Characterization, In Vitro, In Silico, Antioxidant, and Antibacterial Activities. Open Chem. 2023, 21, 20220282. [Google Scholar] [CrossRef]
- Agour, A.; Mssillou, I.; Saghrouchni, H.; Bari, A.; Lyoussi, B.; Derwich, E. Chemical Composition, Antioxidant Potential and Antimicrobial Properties of the Essential Oils of Haplophyllum tuberculatum (Forsskal) A. Juss from Morocco. Trop. J. Nat. Prod. Res. 2020, 4, 1108–1115. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Chavez-Esquivel, G.; Cervantes-Cuevas, H.; Ybieta-Olvera, L.F.; Castañeda Briones, M.T.; Acosta, D.; Cabello, J. Antimicrobial Activity of Graphite Oxide Doped with Silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by Agar Well Diffusion Test: Synthesis and Characterization. Mater. Sci. Eng. C 2021, 123, 111934. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.; Alshawwa, S.Z.; Kamaly, O.A.; El Moussaoui, A.; Lyoussi, B.; Derwich, E. Ointment-Based Combination of Dittrichia viscosa L. and Marrubium vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Morozova, A.Y.; Zubkov, E.A.; Storozheva, Z.I.; Kekelidze, Z.I.; Chekhonin, V.P. Effect of Ultrasonic Irradiation on the Development of Symptoms of Depression and Anxiety in Rats. Bull. Exp. Biol. Med. 2013, 154, 740–743. [Google Scholar] [CrossRef]

| Kinds of Vinegar | Acetic Acid (%) | Density (g/cm3) | pH | Conductivity (mS/cm) | Total Phenols (mg GAE/g) | Flavonoids (mg QE/g) |
|---|---|---|---|---|---|---|
| Golden Delicious | 2.56 ± 0.14 a | 1.018 | 3.11 ± 0.02 b | 3.33 ± 0.01 b | 3.72 ± 0.15 c | 0.294 ± 0.009 a |
| Red Delicious | 1.54 ± 0.07 b | 1.002 | 3.10 ± 0.02 b | 3.48 ± 0.02 a | 5.78 ± 0.08 a | 0.254 ± 0.008 a |
| Gala | 0.74 ± 0.09 c | 1.014 | 2.84 ± 0.01 c | 2.96 ± 0.02 d | 4.35 ± 0.02 b | 0.269 ± 0.010 a |
| Starking Delicious | 0.28 ± 0.06 d | 0.962 | 3.82 ± 0.04 a | 3.19 ± 0.02 c | 6.12 ± 0.02 a | 0.296 ± 0.012 a |
| Chemical Class | Compounds | Vinegar | |||
|---|---|---|---|---|---|
| GD | RD | GA | SD | ||
| Organic acids (mg/100 g) | L-Ascorbic acid | 15.78 ± 1.10 a | 15.33 ± 1.27 a | 10.94 ± 1.12 b | 7.78 ± 0.20 c |
| Oxalic acid | 38.41 ± 2.10 b | 45.92 ± 2.11 a | 30.01 ± 3.10 c | 9.91 ± 1.08 d | |
| Citric acid | 193.63 ± 12.23 c | 820.62 ± 20.31 a | 246.50 ± 22.30 c | 532.86 ± 31.41 b | |
| Malic acid | 241.10 ± 10.40 b | 280.16 ± 11.10 a | 181.25 ± 10.01 c | 174.57 ± 9.11 c | |
| Fumaric acid | 0.39 ± 0.10 d | 39.74 ± 5.02 b | 67.32 ± 7.11 a | 23.01 ± 4.00 c | |
| Polyphenols (µg/g) | Gallic acid | 54.40 ± 4.20 d | 157.37 ± 10.10 b | 285.70 ± 14.10 a | 110.81 ± 9.11 c |
| Caffeic acid | 6.44 ± 0.31 a | 4.17 ± 0.21 b | - | - | |
| Chlorogenic acid | 2.65 ± 0.11 a | - | - | - | |
| Myricetin | 15.42 ± 1.41 b | 22.24 ± 2.12 a | - | - | |
| Naringenin | - | - | - | 1.84 ± 0.21 a | |
| Samples | DPPH (IC50 mg/mL) | β-Carotene Antiradical Activity (%) | TAC (mg AAE/g) |
|---|---|---|---|
| Golden Delicious | 596.00 ± 29.50 e | 62.50 ± 2.40 d | 26.45 ± 0.82 c |
| Red Delicious | 65.20 ± 13.00 b | 81.67 ± 3.02 b | 25.64 ± 0.36 c |
| Gala | 358.60 ± 26.30 d | 85.83 ± 2.13 ab | 16.58 ± 0.18 e |
| Starking Delicious | 187.70 ± 18.10 c | 69.17 ± 2.03 c | 18.73 ± 0.34 d |
| BHT | 0.15 ± 0.04 a | 91.06 ± 0.82 a | 46.87 ± 0.72 a |
| Quercetin | - | - | 28.06 ± 0.29 b |
| Inhibition Diameter (mm) | ||||
|---|---|---|---|---|
| Vinegars | Yeast | Gram (−) Bacteria | Gram (+) Bacteria | |
| C. albicans | E. coli | B. subtilis | S. aureus | |
| Golden Delicious | 11.50 ± 1.50 b | 15.00 ± 1.00 b | 25.00 ± 3.00 b | 14.50 ± 0.50 b |
| Red Delicious | 7.50 ± 0.50 c | 12.00 ± 3.00 b | 10.00 ± 2.00 c | 8.50 ± 1.50 d |
| Gala | 7.75 ± 0.25 c | 10.50 ± 2.50 b | 17.00 ± 2.00 c | 10.75 ± 0.25 c |
| Starking Delicious | 7.25 ± 0.75 c | Resistant | 12.00 ± 4.00 c | Resistant |
| AMP | - | 34.67 ± 2.08 a | 53.83 ± 1.04 a | 34.17 ± 0.28 a |
| FLU | 21.00 ± 1.04 a | - | - | - |
| Minimal Inhibitory Concentration (mg/mL) | ||||
|---|---|---|---|---|
| Vinegar | Yeast | Gram (−) Bacteria | Gram (+) Bacteria | |
| C. albicans | E. coli | B. subtilis | S. aureus | |
| Golden Delicious | 31.81 | 127.25 | 31.81 | 31.81 |
| Red Delicious | 125.25 | 62.62 | 62.62 | 62.62 |
| Gala | 126.75 | 126.75 | 126.75 | 253.5 |
| Starking Delicious | 481 | Resistant | 481 | Resistant |
| AMP | - | Resistant | Resistant | Resistant |
| FLU | 0.4 | - | - | - |
| DPPH (IC50) | β-Carotene | TAC | C. albicans (ID) * | E. coli (ID) | B. subtilis (ID) | S. aureus (ID) | Anti-Inflammatory Activity (24 h) | Antidepressant Activity (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Phenols | −0.919 | 0.205 | −0.177 | −0.802 | −0.731 | −0.930 | −0.885 | −0.788 | −0.315 |
| Flavonoids | 0.558 | −0.852 | −0.086 | 0.459 | −0.382 | 0.508 | −0.206 | 0.111 | 0.182 |
| pH | −0.323 | −0.515 | −0.133 | −0.266 | −0.848 | −0.361 | −0.853 | −0.508 | −0.045 |
| Acetic acid | 0.577 | −0.439 | 0.867 | 0.868 | 0.851 | 0.669 | 0.812 | 0.976 | 0.903 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Abdali, Y.; Saghrouchni, H.; Kara, M.; Mssillou, I.; Allali, A.; Jardan, Y.A.B.; Kafkas, N.E.; El-Assri, E.-M.; Nafidi, H.-A.; Bourhia, M.; et al. Exploring the Bioactive Compounds in Some Apple Vinegar Samples and Their Biological Activities. Plants 2023, 12, 3850. https://doi.org/10.3390/plants12223850
El Abdali Y, Saghrouchni H, Kara M, Mssillou I, Allali A, Jardan YAB, Kafkas NE, El-Assri E-M, Nafidi H-A, Bourhia M, et al. Exploring the Bioactive Compounds in Some Apple Vinegar Samples and Their Biological Activities. Plants. 2023; 12(22):3850. https://doi.org/10.3390/plants12223850
Chicago/Turabian StyleEl Abdali, Youness, Hamza Saghrouchni, Mohammed Kara, Ibrahim Mssillou, Aimad Allali, Yousef A. Bin Jardan, Nesibe Ebru Kafkas, El-Mehdi El-Assri, Hiba-Allah Nafidi, Mohammed Bourhia, and et al. 2023. "Exploring the Bioactive Compounds in Some Apple Vinegar Samples and Their Biological Activities" Plants 12, no. 22: 3850. https://doi.org/10.3390/plants12223850
APA StyleEl Abdali, Y., Saghrouchni, H., Kara, M., Mssillou, I., Allali, A., Jardan, Y. A. B., Kafkas, N. E., El-Assri, E.-M., Nafidi, H.-A., Bourhia, M., Almaary, K. S., Eloutassi, N., & Bouia, A. (2023). Exploring the Bioactive Compounds in Some Apple Vinegar Samples and Their Biological Activities. Plants, 12(22), 3850. https://doi.org/10.3390/plants12223850








