Abstract
The present study aimed to explore the phytochemical profile, and evaluate the antioxidant, antimicrobial, and insecticidal properties, of Moroccan Mentha longifolia L. essential oil (ML-EO) using in vitro and in silico assays. Noteworthily, as chromatography (GC-MS/MS) revealed that ML-EO is majorly composed of piperitenone oxide (53.43%), caryophyllene (20.02%), and (−) germacrene D (16.53%). It possesses excellent antioxidant activity with an IC50 of 1.49 ± 0.00 for DPPH and 0.051 ± 0.06 μg/mL for ABTS. Moreover, the RP and TAC activities were 0.80 ± 0.01 μg/mL and 315.532 ± 0.00 mg EAA/g, respectively. ML-EO exhibited a potent antimicrobial effect, specifically against Pseudomonas aeruginosa. It also exhibited strong antifungal ability, especially against Candida albicans. Regarding insecticidal activity, for ML-EO, a dose of 20 µL/mL produced a complete reduction in fecundity, fertility, and emergence of adult C. maculatus with mortality rates reaching 100%. In silico results showed that the antioxidant activity is mostly attributed to α-Cadinol, the antibacterial efficiency is attributed to piperitenone oxide, and antifungal capacity is related to cis-Muurola-4(15),5-diene and piperitenone oxide. Accordingly, ML-EO has high potential to be used as an alternative for preserving food and stored grain and protecting them against microbes and insect pests in the food and pharmaceutical sectors.
1. Introduction
Aromatic medicinal plants (AMP) have a long medicinal history and are still used as an important health primary care of several populations worldwide [1]. They have a large spectrum of biological properties attributed to their dense chemical composition highly affected by environmental conditions [2]. Medicinal plants possess biological activities, including antioxidant, antimicrobial, antidiabetic, anti-obesity, anti-inflammatory effects, and so on [3].
Among medicinal plants, mint (Mentha species) belongs to the Lamiaceae (Labiatae) family and comprises 25 to 30 species. It grows in the moderate regions of Africa, Europe, Australia, Asia, and North America [4]. Mentha longifolia L. or Himalayan silver mint is well-known in Morocco by its vernacular name “naana touil”, and is considered a perennial plant belonging to the genus Mentha. The herb is widely used in folkloric medicine of different countries, including Iraq, Iran, Pakistan, Turkey, and Arab countries as a natural remedy for several diseases, such as gastrointestinal diseases (gas expeller, indigestion, intestinal colic, intestinal ulcer, antidiarrheal, gut spasm, stomach problems, ulcerative colitis) [5], respiratory disorders, (asthma, colds, bronchitis, tuberculosis, sinusitis, and cough) [6] infectious diseases, inflammatory diseases, and menstrual disorders [7]. Furthermore, the plant has considerable economic importance as natural raw matter for the food and pharmaceutical industries [8]. Robust scientific evidence has confirmed multiple pharmacological properties of M. longifolia including antihemolytic, anti-inflammatory, antibacterial, hepatoprotective, anti-cancer, and gastroprotective effects [9]. In addition, M. longifolia is part of a considerable inventory of essential oils with many therapeutic applications, such as allelopathic properties [10]. Prior research has indicated that the M. longifolia essential oil is a potent free-radical scavenger and an effective antimicrobial agent against a broad range of pathogenic microbes [11]. The delve into the phytochemistry of M. longifolia revealed the existence of several active biomolecules, including chlorogenic acid, catechin, gallic acid, naringenin, ellagic acid, rutin, daidzein, cinnamic acid, and hesperidin [12]. Ghoulami et al. found that piperitenone oxide and piperitone oxide were characteristic bioactive compounds of M. longifolia EO [13]. The phytochemistry of plants is highly affected by several factors, such as ecological ones. Unfortunately, few researchers have evaluated the effectiveness of Moroccan M. longifolia EO in fighting nosocomial pathogens and drug-resistant bacteria.
To the best of our knowledge, there is no sufficient research that studied the chemical constituent and in vitro capabilities of EO isolated from M. longifolia growing in Morocco. Thus, we aimed to investigate the volatile profile of Moroccan M. longifolia EO using GC-MS/MS and study its antioxidant, antimicrobial, and insecticidal activities by use of in vitro and in silico assays.
2. Results and Discussion
2.1. Volatile Profile of Essential Oil
The Mentha longifolia EO yield was approximately 0.79 ± 0.265% (v/w), with seven compounds identified using an HP-5MS capillary column. The Gas Chromatographic examination indicates that the monoterpenoids compounds (Figure 1 and Table 1) predominated in the M. longifolia EO, including piperitenone oxide (53.43%) and bornyl acetate (4.47%), followed by the chemical class of sesquiterpene hydrocarbons, which are caryophyllene (20.02%) and (−) germacrene D (16.53%) (Figure 2). These results are in line with those reported by previous studies [14]. In the same context, M. longifolia from Brazil was discovered to be rich in piperitenone oxide (60.79%) [14]. Indian M. longifolia was shown to be also abundant in piperitenone oxide (54.23%) and trans-piperitone oxide (24.06%) [15].
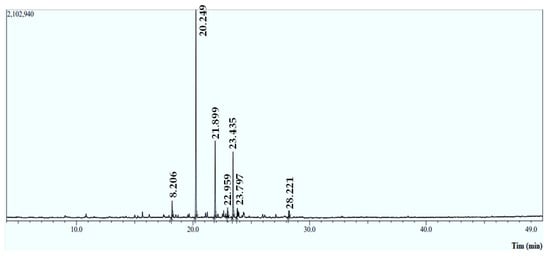
Figure 1.
Chromatographic profile of the essential oil (EO) extracted from M. longifolia leaves using GC-MS/MS.

Table 1.
Phytochemical constituents contained in the EO extracted from Moroccan M. longifolia.
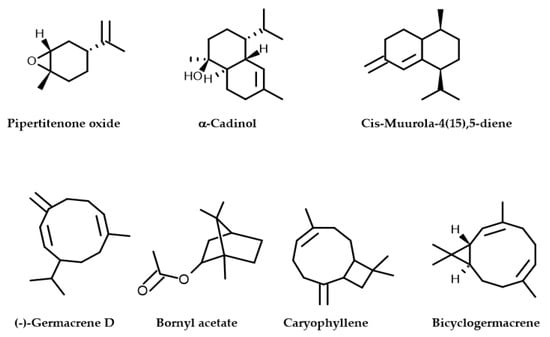
Figure 2.
Molecular structures of phytochemical composition in M. longifolia EO.
The variability in the chemical constituents of EO depends on environmental factors. Indeed, the chemical composition of M. longifolia oils may change based on the used part of the plant, the season of the vegetative cycle, the maturity of the plant, the harvesting period, and even plant genetic characteristics [16].
The phytoconstituents of EOs, such as monoterpenes hydrocarbons (MH), oxygenated monoterpenoids, and sesquiterpenes hydrocarbons from various medicinal herbs have been identified to possess significant biological and pharmacological capacity, including antioxidant, antimicrobial, insecticidal, and cytotoxic potential [9].
2.2. Antioxidant Activity
The antioxidant ability of M. longifolia EO was assessed using DPPH, ABTS, RP, and TAC tests and results are summarized in Table 2. A potent antioxidant activity was obtained to scavenge the ABTS•+ radical cation (Table 2). As shown in Table 2, the EO of M. longifolia exhibited maximum antioxidant capacity with IC50 values of 1.49 ± 0.00 µg/mL for the DPPH test and 0.051 ± 0.06 µg/mL for the ABTS assay compared to BHT and Trolox as a standard (42 ± 0.01 and 24.14 ± 0.19 µg/mL), respectively.

Table 2.
Antioxidant activities of M. longifolia essential oil.
Our data were higher than those reported by Barros and Z. Bouassida and coworkers, who reported IC50 values were 0.86 ± 0.01 mg/mL and 100 µg/mL for their M. longifolia essential oil, respectively [17].
To the best of our knowledge, and based on the literature studies, M. longifolia antioxidant ability has not yet been reported using the ABTS assay; however, a few studies have been carried out to assess its antioxidant capacity using different test methods, including DPPH and reducing power (RP). The antioxidant ability of ML-EO from Yugoslavia was examined by DPPH scavenging capacity assay [18], a DPPH test used in Saudi Arabia, Bosnia, and Herzegovina that displays potent activity for ML-EO [19].
Regarding the reducing power (RP) of the studied EO, the results are represented in (Table 2) and indicate that the EC50 value is relatively high (0.80 ± 0.01 µg/mL) in comparison to the ascorbic acid as standard (31 ± 0.07 µg/mL). Our results are significantly more important than those reported by Bardaweel et al. for Algerian Mentha spicata L. with an EC50 of 215 ± 4.50 µg/mL [20].
The ammonium phosphomolybdate assay was achieved to determine the total antioxidant capacity of our M. longifolia EO. Results were represented in (Table 2) and showed that our EO had a TAC value of 315.53 ± 0.01 mg AAE/mL of EO. These results are stronger than those reported by Mhiri et al. for Tunisian Mentha piperita, where the TAC value was 177.00 ± 0.30 mg/g of DW. Another study made by Salamatullah et al. for the EO of Mentha rotundifolia var demonstrated that their TAC value was 697.45 ± 1.07 mg AAE/g [21], these findings were higher than those obtained for our essential oil.
Indeed, a previous study of the chemical constitution of M. longifolia EO revealed that these EOs contained a wide spectrum of monoterpenoids such as piperitone, piperitenone oxide, carvone, pulegone, β-caryophyllene, menthol, 1,8-cineole, menthone, and limonene [22]. However, several naturally occurring substances such as piperitenone oxide and germacrene D might have contributed to the strong antioxidant capacity [23].
2.3. Antibacterial Activities of M. longifolia Essential Oil
The antibacterial potency of M. longifolia EO and the minimal inhibitory concentration against five microbe species are shown in Table 3. The EO derived from M. longifolia leaves showed significant antibacterial activities depending on the concentration and the antibiotic used (streptomycin sulfate or erythromycin). M. longifolia EO showed the strongest antibacterial effect against Pseudomonas aeruginosa, with an inhibition diameter of 24.50 ± 0.71 mm and an MIC of 0.005 ± 0.00 µg/mL. In contrast, showed an inhibition diameter of 16.00 ± 1.41 mm and an MIC of 0.015 ± 0.007 µg/mL against Bacillus subtilis, an inhibition diameter of 7.55 ± 0.64 and an MIC of 0.010 ± 0.01 against Escherichia coils, and an inhibition diameter of 7.50 ± 0.71 mm and an MIC of 0.011 ± 0.00 µg/mL against Staphylococcus aureus.

Table 3.
Diameter of the inhibition zone (mm), and Minimum inhibitory concentration (MIC in µg/mL) from EO of M. longifolia in comparison with antibiotics.
Our data found that the investigated bacterial strains, whether Gram-negative or Gram-positive, were absolutely resistant to Erythromycin and moderately resistant to Streptomycin. These findings are consistent with previous studies, which demonstrated that the most dangerous drug-resistant microbes, were S. aureus, P. aeruginosa, E. coli, K. pneumoniae, A. baumannii, and Enterobacter spp. strains [24].
Our findings demonstrated that Gram-positive and Gram-negative bacteria were most sensitive to the studied EO, which was consistent with the results found by Hajlaoui et al. who discovered that their M. longifolia EO had the same positive effect against all tested Gram (−) and Gram (+) pathogens [25]. In the present study, the examined EO revealed a stronger antibacterial effect against Pseudomonas aeruginosa with an inhibition diameter of 24.50 ± 0.71 mm. These results were in line with those of Niki and co-workers, which revealed that their M. longifolia essential oil has a considerable antibacterial effect against Pseudomonas aeruginosa (ATCC 9027), with an inhibition zone of 25 mm [26]. In addition, the M. longifolia folium EO gathered in Bosnia and Herzegovina also demonstrated important antibacterial activity against Escherichia coli (ATCC 8739) and Salmonella enterica. ABONY subsp. enterica (NCTC 6017) with inhibitory diameters ranging from 7 ± 0.33 to 19 ± 0.71 mm and 10 ± 0.99 to 20 ± 0.33 mm, respectively [26]. Furthermore, our M. longifolia EO also indicated a high growth inhibition of Gram-positive bacteria particularly Bacillus subtilis, with an inhibition diameter of 16.00 ± 1.41 mm. Our results contrasted those found by Gulluce et al. who showed a slight inhibition of 8 mm of Bacillus subtilis growth by Mentha longifolia L. EO. Gulluce et al. suggested that the shown effect was correlated to the high concentration of cis-piperine epoxide (18.4%), pulegone (15.5%), and piperitenone oxide (14.7%) [27]. In our study, the bacteria P. aeruginosa and E. coli were shown to be resistant to the antibiotics streptomycin and erythromycin. This resistance can be explained by the barrier effect of these species, where the membrane of Gram-negative bacteria is more complex with a smaller peptidoglycan layer and an outer membrane comprised of a double layer of phospholipids, which block antibiotic diffusion into the cells [28].
Previous reports have described several mechanisms of action of EO antimicrobial effects, including penetrating the bacterial cell wall, cytoplasmic membrane proteins attributed to their lipophilic nature causing ions loss, membrane potential decrease, the collapse of the proton pump, and ATP pool overexploitation [29]. As a result, they induce lipid partitioning of bacterial cell membranes and mitochondria, causing cell lysis and release of cell contents. Altered bacterial enzymatic systems may be a possible antibacterial mechanism [30].
Indeed, the mechanisms of action of EOs depend on their chemical compositions, concentration, structure, aromatic nuclei, and placement of functional groups [31]. The M. longifolia EO is high in terpene compounds, particularly oxygenated monoterpenes such as piperitenone oxide and oxygenated sesquiterpenes such as caryophyllene and (−) germacrene D, which are now widely known to have high antimicrobial properties against a variety of microorganism strains [32]. Furthermore, the antibacterial mechanism of action of terpene is not yet clear, our findings indicated that lipophilia, terpene hydrophobicity, and the existence of hydroxyl groups (-OH) in the constituents all play significant roles in the mechanism of antibacterial activity [33].
In the same context, our result indicates that terpenes, a major category in our M. longifolia EO, are now widely recognized to show strong antimicrobial activity, which our research also confirmed.
2.4. Antifungal Activities of M. longifolia Essential Oil
The disc diffusion approach was used to test the antifungal capacity of our EO against the yeast and fungus Aspergillus niger (MTCC 282) and Candida albicans (ATCC 10231). Results of the antifungal activity are represented in (Table 3) and indicate that M. longifolia EO has a strong effect against both tested strains. The results demonstrate that a high EO inhibition was found for C. albicans with an inhibition zone of 20.00 ± 0.00 mm and MIC values of 0.005 ± 0.00 mg/mL, showing a noticeable effect against A. niger with a percent of inhibition of 1.50 ± 0.00% and an MIC value of 0.005 ± 0.00 mg/mL (Table 4).

Table 4.
Results of the antifungal ability of the EO of M. longifolia diameter of the inhibition zone (mm) and MIC (MIC in mg/mL).
Yeast infections are very frequent among patients in hospitals across the world, and there are several risk factors linked with poor outcomes. Multiple epidemiological studies on fungal infections have displayed that Candida is responsible for a wide range of disorders. Additionally, the fourth highest frequent source of nosocomial bloodstream infection is a Candida fungus, computing for 8–10% of infections [34]. The Aspergillus spp. can induce invasive infections, causing mortality and serious diseases in various types of patients, including those in serious attention units [35].
Findings indicate that our EO has a potent antifungal capacity against C. albicans with an inhibition zone of 20.00 ± 0.01, more than A. niger with an inhibition percentage of 1.50 ± 0.02 compared to an antibiotic (fluconazole). The result is consistent with those reported by Mkaddem and coworkers, which indicated that the EO of M. longifolia collected from Tunisia revealed antifungal efficiency against Candida albicans (IPA 200) with an inhibition diameter of 19 mm [36]. The EO of M. longifolia collected from Saudi Arabia showed moderate antifungal ability against A. niger with a percent inhibition ranging between 2.2 and 3.3% [19]. Similarly, the findings of another study showed notable antifungal activity, especially against Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, and Fusarium culmorum with 100% fungal mycelial inhibition development at the concentration of 500 µL/mL [37].
The volatile profile of M. longifolia EO could express its antifungal activity [26]. The antifungal effect of M. longifolia essential oil may be attributed mostly to its chemical components; it is particularly rich in piperitenone oxide, which is widely recognized for its antimicrobial activity, especially its antifungal activity [38].
According to several studies, the toxicity impact of EO and their derivatives is associated with their capacity to block or inactivate fungal cell walls and cell membrane, coagulate the cytoplasm, consequently damage cell components, and allow macromolecules to leave [39]. The hydrophobic components in EO could affect the permeability of microbial cell membranes to cations such as H+ and K+, altering the passage of protons, controlling cellular pH, and affecting the chemical structure and function of the cells [40].
2.5. Insecticidal Activity
In many countries of the globe, it is an ancient tradition to apply natural sources of plant materials, specifically focusing on EOs, to protect agricultural products from various insect pests. Callosobruchus maculatus is one of the most significant storage insect pests of cowpea grains [41].
In the present work, we examined the toxic potential of M. longifolia EO against C. maculatus over 24 h at different doses using fumigation and repellency tests. The lethal concentration (LC50) was also computed for each EO concentration at the appropriate treatment durations.
2.6. Fumigation Bioassay
2.6.1. Effect of ML-EO on Adult Mortality
The insecticide efficiency of ML-EO is represented in (Table 5). Our finding demonstrated that the fumigation bioassay variability is due to the oil doses, plant species, and exposition period. ML-EO is more toxic in all doses tested, showing 100% mortality against C. maculatus at doses of 12.0 and 20.0 μL/L after 18 h of treatment. The lethal dose (LC50) for M. longifolia essential oil was 29.42 and 2.24 µL/L in air at 3 h and 12 h postexposure, with respective 95 percent confidence intervals (19.96–309.41; 0.0–4.68). In contrast, the LC90 ranged from 73.09 to 18.74 µL/L air (Table 6). According to previous reports, essential oil from M. longifolia examined during a fumigation test showed moderate fumigation toxicity against Sitophilus zeamais at concentrations of (24 and 32 µL/L in air) [42]. The efficiency of ML-EO was in line with the outcome reported by Khani and coworkers, who reported that the ML-EO exhibited potent insecticidal activity against Callosobruchus maculatus with an LC50 of 13.05 μL/L air [43]. The presence of major monoterpenes and sesquiterpenes notably piperitenone oxide, germacrene D, in the studied EO, could be attributed to their strongest insecticidal activity since this compound had been reported to possess a strong insecticidal efficiency [44].

Table 5.
Effect of ML-EO on C. maculatus mortality as a function of concentration and exposure durations based on fumigation test.

Table 6.
Lethal concentration, and chi-square (χ2) values of M. longifolia and M. aquatica essential oil tested by fumigation assay on C. maculatus.
2.6.2. Effect of ML-EO on Fecundity
The insecticidal effects of the tested EOs affect significantly the fecundity of C. maculatus females. The obtained findings demonstrated a considerable reduction in the number of eggs deposited by females compared to the control after being exposed to the vapor of ML-EO (Figure 3 and Table 7). A dose of 16 µL/L of ML-EO completely reduces the fecundity of female C. maculatus. The ML-EO inhibited female fecundity in comparison with control values of 196.66 ± 11.55 at the highest test concentration (20 µL/10 g seeds). Statistical analysis showed that the EO-induced toxicity against C. maculatus fecundity was extremely significant for the different concentrations (ANOVA: F = 742.7; df = 4, 10; p < 0.0001). Previous research suggested that various plant derivatives, such as EOs and their components, decreased insect fecundity [45]. Based on the literature researchers, this is the first study on the efficiency of ML-EO on the fecundity of C. maculatus grain cowpea pest. In contrast, the efficiency of essential oils of Mentha species on fecundity had been studied against various pests, including the effect of M. × piperita L. essential oil on reduction in fecundity of Acanthoscelides obtectus, reduction in fecundity/female/day was 3.1 ± 2.0 [46]. M. pulegium L. essential oil reduces 82–88% in female fecundity of C. maculates at a concentration of (0.25–1%) [47]. In the seam context, Saxena and Mathur hypothesized that the reduced fecundity by the plant derivatives may be related to the disruption of regulatory processes as opposed to a direct impact on the ovarian tissue [48]. Previous reports illustrate that plant EOs have important effects on oogenesis, causing a reduction in oviposition, and appear to have juvenomimetic activity similar to juvenile and molting hormones [49].
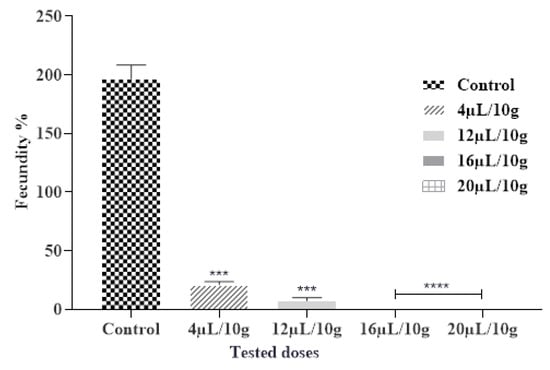
Figure 3.
Fecundity % of C. maculatus in the fumigation test by the exposure to the ML-EO at different doses (Control, 4, 12, 16, 20 μL/10 g). The outcomes are presented as mean ± SD. (*) comparison between the control group and all remaining concentrations. (The significance initiates at *** p < 0.001; **** p < 0.0001).

Table 7.
Effect of EOs on different biological parameters of C. maculatus (mean ± SD).
2.6.3. Effect on Fertility
Our findings also showed that the tested EO had an inhibitory effect on the fertility of C. maculatus. Results obtained expressed that each ML-EO significantly decreased the egg production in a dose and time-dependent approach as compared to the control (Figure 4 and Table 7). In addition, ML-EO exhibited a potent hatching egg (fertility) inhibitory effect at a dose of 16 μL/10 g compared to the control. The insecticidal efficacy of the essential oils examined showed that the fertility of C. maculatus females was extremely significant between the different concentrations (F = 548.5; df = 4, 10; p < 0.0001). In our study, the lowest used dose of ML-EO, the female C. maculatus eggs were prevented from hatching. We can thus deduce that our outcomes were stronger than those reported by Allali and coworkers, especially at a dose of 20 µL/L of essential oil extracted from M. pulegium L., which inhibited 100% of the fertility of C. maculatus [50].
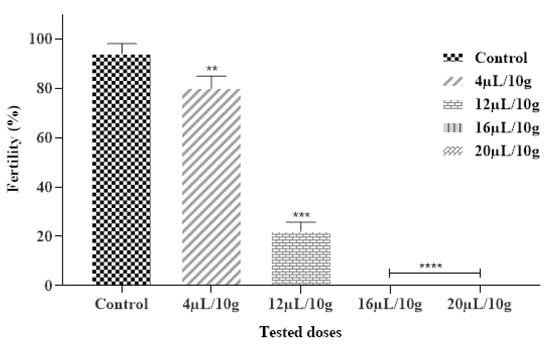
Figure 4.
Fertility % of C. maculatus in the fumigation test by the exposure to the ML-EO at different doses (Control, 4, 12, 16, 20 μL/10 g). The outcomes are presented as mean ± SD. (*) comparison between the control group and all remaining concentrations. (The significance initiates at: ** p < 0.01; *** p < 0.001; **** p < 0.0001).
2.6.4. Effect on Adult Emergence
The achieved findings demonstrated that no C. maculatus adults emerged in Cowpea Seeds that were provisory treated with ML-EO starting at a concentration of 12 µL/10 g (Figure 5 and Table 7). According to previous research, the EO from Mentha species had the greatest effect against a range of insect pests [44]. In contrast, EO extracted from M. pulegium L. assessed by inhalation against C. maculatus reduced the emergence of larvae with 100% death after 24 h of application with doses of 20.0 µL/L air using contact assay [50].
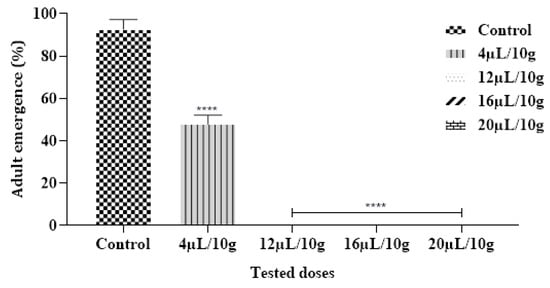
Figure 5.
Adult emergence % of C. maculatus in the fumigation test by exposure to the ML-EO at different doses (Control, 4, 12, 16, 20 μL/10 g). The outcomes are presented as mean ± SD. (*) comparison between the control group and all remaining concentrations. (The significance starts at **** p < 0.0001).
2.6.5. Repellency Test
The herbal tradition has a great history of using plants as insect repellents, especially aromatic herbs and oils [51]. Various experiments were performed to explore the repellent capabilities of Mentha species against agricultural insect pests [42]. In the present study, the repellent effect is dependent on the plant and used concentration. Indeed, ML-EO showed a potent repellent efficiency. In the filter paper disc assay, all concentrations of ML-EO demonstrated greater than 70% repulsion power against C. maculatus within 24 h of treatment (Table 8). According to the classification by McDonald, 1970, ML-EO had a strong repulsive power of 100% at the high dose of 20 µL/cm2 with an average repulsion percentage of 89.75%. This EO is within the repellant class following McDonald, 1970. The obtained outcome corroborated with those described by Odaymi and coworkers who found that M. longifolia EO completely (100%) repelled adult Sitophilus zeamais at all concentrations [42].

Table 8.
Repellent index of the ML-EO against C. maculatus.
Several studies have been performed on plant extracts, particularly essential oils of Mentha species, to examine their insecticidal activity against pest insects that attack stored green. Our finding demonstrated that the ML-EO was highly efficient against the Callosobruchus maculatus insect pest. These outcomes are consistent with those that were previously reported [42].
Likewise, many plant extracts and essential oils contain volatile chemicals that have insecticidal properties and are constituted of, alcohols, alkanes, aldehydes, and terpenoids, particularly monoterpenoids [52]. Essential oils and their constituents affect biochemical processes in various and different pathways, altering the endocrinologic balance of insects in particular [53]. Monoterpenoids and sesquiterpenoids are extremely volatile compounds that defend plants against insect infestations. Monoterpenoids are lipophilic chemicals, that have been studied for their neurotoxic effect. They can produce symptoms such as convulsions, hyperactivity, and tremors succeeded by paralysis similar to the effect of organophosphate and carbamate insecticides [44]. These compounds operate through several modes of action including the modulation of GABA, blocking neurotransmitters such as acetylcholinesterase [54], and the impact on octopamine synapses [55]. Acetylcholinesterase (AChE) inhibition is a specific enzyme for saved substance insect control agents that can block the neurotransmitter acetylcholine in the synaptic gap [54].
The essential oil of M. longifolia is rich in diverse chemical compounds, however, some components are present in larger quantities, for example, piperitenone oxide, 1,8-cineole, germacrene D, and caryophyllene. Previous research has shown the insecticidal effects of piperitenone oxide, as well as other terpenes, against a variety of pests [56].
Piperitenone oxide and caryophyllene is the major component in our EO. According to Miyazawa et al. piperitenone oxide has insecticidal and acetylcholinesterase (AChE) inhibitory effects [57]. Consequently, Lee et al. suggested that, in addition to blocking AChE, monoterpenes may also act on other vulnerable sites such as cytochrome P450-dependent monooxygenases [58]. Thus, the insect octopamine receptor pathway is more sensitive to some oils compared to AChE. The essential oil affects octopamine receptors to increase heart rate or cAMP levels in insects [59]. Kavallieratos et al. showed that the mechanism of action of the piperitenone oxide against insects has been performed via the coupling of the exocyclic carbonyl with the conjugated C-C double bond, enabling it to penetrate the cuticle rapidly and reach the target sites [56].
Accordingly, the inhibitory effect of essential oil components was induced by synergistic effects around various chemical groups relatively more than by a single powerful inhibitor.
Overall, this study suggests that essential oils derived from Moroccan Mentha longifolia and such species have potential as natural insect repellents for controlling C. maculatus invasion in stored cowpea grains. However, further research is needed to determine the optimal concentration and application technique for these essential oils in a practical pest management context.
2.7. Molecular Docking
Inhibition of NADPH in antioxidant activity plays a significant role in regulating cellular redox balance and antioxidant defense mechanisms. NADPH (nicotinamide adenine dinucleotide phosphate) is a crucial cofactor in several enzymatic reactions that contribute to cellular antioxidant defenses.
Alpha-cadinol and piperitenone oxide are naturally occurring compounds, commonly found in the essential oils of various plants. These compounds have attracted attention in the field of natural products and health research due to some preliminary evidence suggesting that they may possess antioxidant activity [60,61].
Antioxidants are substances that play a crucial role in protecting cells and tissues from oxidative damage caused by free radicals. Free radicals are highly reactive molecules that can harm biological molecules such DNA, proteins, and lipids potentially contributing to aging and various diseases.
The antioxidant activity of alpha-cadinol and piperitenone oxide is thought to be linked to their ability to scavenge free radicals and neutralize their detrimental effects. Essentially, they act as electron donors, stabilizing free radicals by donating electrons and preventing them from causing cellular damage.
In our in silico study, all the molecules identified in the bulk of M. longifolia EO presented a remarkable antioxidant activity represented by binding energy between −3.585 and −6.041 Kcal/mol. The α-cadinol was the most powerful substance to combat NADPH oxidase with a glide score of −6.041 Kcal/mol followed by piperitenone oxide (−5.195 Kcal/mol) (Table 9).

Table 9.
Docking results in ligands in different receptors.
Regarding antimicrobial activity, several studies on the essential oils of plants, in which alpha-cadinol and piperitenone oxide are the major compounds, have shown remarkable antibacterial activity [62,63].
Piperitenone oxide was the most active molecule against E. coli with a glide score of −7.104 Kcal/mol. While α-Cadinol is the most active against S. aureus with a glide score of −5.714 Kcal/mol.
Concerning the antifungal ability, cis-Muurola-4(15),5-diene showed strong capacity against Candida albicans with a glide score of −7.486 Kcal/mol. While piperitenone oxide was the most effective chemical against Aspergillus niger with a glide score of −4.687 Kcal/mol (Table 9).
The 2D and 3D visualization of the interaction between the active site and ligands of NADPH oxidase has shown that α-Cadinol is capable of forming two hydrogen bonds with residues of ASP 282 and LYS 134. In the energetic site of beta-ketoacyl-[acyl carrier protein] synthase from Escherichia coli, piperitenone oxide has established two hydrogen bonds with residues of THR 300 and THR 302. Additionally, piperitenone oxide has formed a single hydrogen bond with residue TYR 309 in the energetic site of beta-1,4-endoglucanase from Aspergillus niger (Figure 6 and Figure 7).
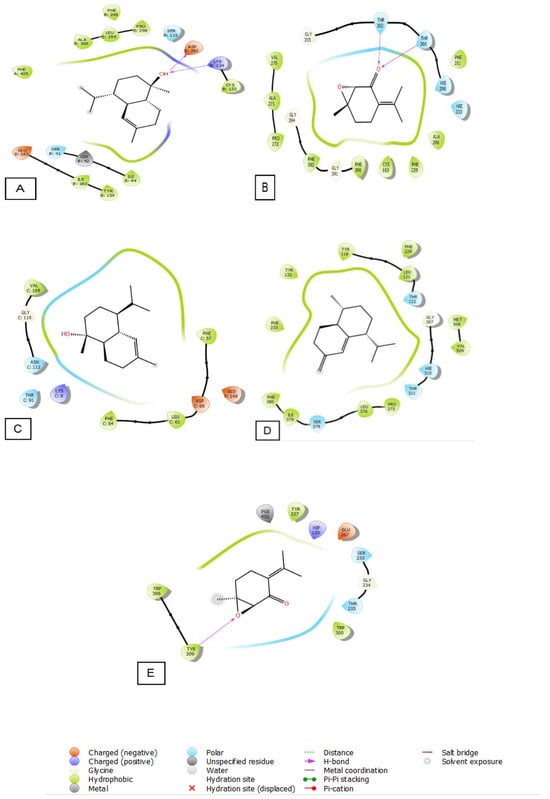
Figure 6.
The 2D viewer of ligands interactions with the active site. (A): α-Cadinol interactions with the active site of NADPH oxidase. (B): piperitenone oxide interactions with the energetic site of beta-ketoacyl-[acyl carrier protein] synthase from Escherichia coli. (C): α-Cadinol interactions with the active site of Staphylococcus aureus nucleoside diphosphate kinase. (D): cis-Muurola-4(15),5-diene interactions with the active site of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans. (E): piperitenone oxide interactions with the energetic site of a beta-1,4-endoglucanase from Aspergillus niger.
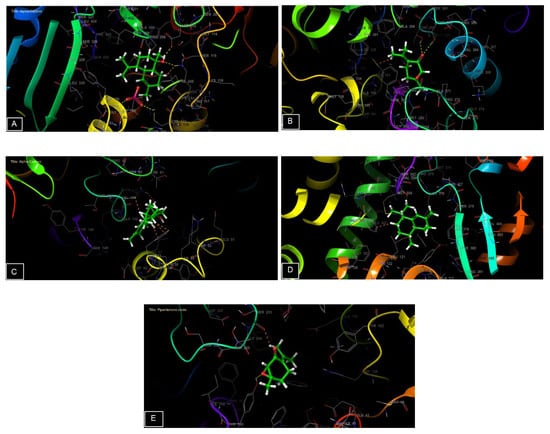
Figure 7.
The 3D viewer of ligands interactions with the active site. (A,C): α-cadinol interactions with the active site of NADPH oxidase and Staphylococcus aureus nucleoside diphosphate kinase. (B,E): piperitenone oxide interactions with the active site of beta-ketoacyl- [acyl carrier protein] synthase from Escherichia coli and a beta-1,4-endoglucanase from Aspergillus niger. (D): cis-Muurola-4(15),5-diene interactions with the active site of sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans.
2.8. Statistical Analysis
Principal Component Analysis
The previously acquired results were treated using the principal component analysis (PCA), and the key outputs are displayed in Figure 8. Notably, principal component analysis is a dimensionality-reduction method that is used to condense a large set of variables into a more manageable set while retaining the majority of the details in the larger set.
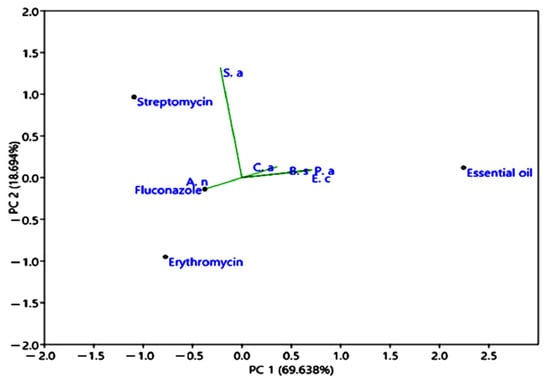
Figure 8.
Principal component analysis (PCA), presents the correlations of antimicrobial properties of M. longifolia EO compared to erythromycin, streptomycin, and fluconazole. Ca: Candida albicans; Sa: Staphylococcus aureus; Ec: Escherichia coli; Bs: Bacillus subtilis; An: Aspergillus niger; Pa: Pseudomonas aeruginosa.
The principal component analysis of M. longifolia EO was based on the correlations of antimicrobial activities of M. longifolia compared to standard medicine (erythromycin, streptomycin, and fluconazole). The first component explained (69.638%), and represented, in its positive part, the inhibition diameter values of Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans, and Escherichia coli and, in its negative part, the inhibition diameters values of Staphylococcus aureus and Aspergillus niger. The second component explained (18.694%) and represented, in the positive part, the inhibition diameter values of Candida albicans, Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa. Whereas in the negative part, we found only the diameter inhibition value of Aspergillus niger.
In addition, Figure 8 showed similar sensitivity to M. longifolia essential oil for the following strains: Candida albicans, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa. Similar outcomes were shown in another study carried out by Lafraxo et al. [64].
It has been shown that the hydrocarbons and oxygenated monoterpenes found in Mentha longifolia essential oil are able to damage the cellular integrity of bacteria, thus impeding respiration and ion transport functions [65]. In addition, it was observed that Gram-positive bacteria are slightly more resistant to the studied essential oil than Gram-negative bacteria. This can be explained by the difference in the bacterial wall composition [66].
3. Material and Methods
3.1. Collection of Plant Materials
Aerial elements of Mentha longifolia were collected from the town of Ifran (Morocco’s Middle Atlas Mountains, latitude 33°31′35″ N; longitude 5°06′ 36″; altitude 1648 m) in June 2021, and it was identified by Professor Amina BARI the botanist of the department of biology, at the Sidi Mohamed Ben Abdellah University. The voucher specimen has been deposited at the faculty herbarium under number: 001MLAV202162. The collected plant was dried in dark conditions for two weeks, and then the aerial parts were removed and powdered using a professional herb grinder. The obtained powder was packed into a glass bottle and stored in a dark and heat-protected place until extraction.
3.1.1. Extraction of the Volatile Oils
The dry (flowering) leaves of M. longifolia, (100 g) were slightly ground, the plant materials were placed in a 2 L flask with 1 L of distilled water, and the combination was heated for 3 h while refluxing using a Clevenger-type apparatus [67]. The EO obtained was separated from water with anhydrous sodium sulfate, stored in a closed glass flask, and kept at 4 °C in the dark conditions until the in vitro test. At leas three separate extractions were performed, and the mean yield and standard error were calculated.
3.1.2. Chemical Characterization of Essential Oil by GC/MS/MS
Gas chromatography with an ion trap mass spectrometry apparatus was used for the GC-MS analysis (Trace GC ULTRA S/N 20062969/Polaris Q Thermo Fischer, Waltham, MA, USA). For the chromatographic separations, an HP-5MS capillary column (60 m × 0.32 mm; coating thickness 0.25 μm) was employed. Scan range: 40–650 amu; scan rate: 3.9 scans/s; temperatures of the transfer line and ionic source: 300 °C and 200 °C, respectively. The oven temperature was programmed to increase from 40 to 280 °C in a 5 °C/min range; the injector temperature was 260 °C; helium was used as the carrier gas at 1 mL/min; and 1 µL of a 10 percent cyclohexane essential oil solution was injected; the split ratio was 1:30. The retention times were compared to those of authentic samples, their indices of linear retention were compared to the series to (C8–C29) alkanes, and computer matching against commercial (NISTMS) and laboratory-developed library mass spectra built up from pure substances and components of known oils and MS literature data were used to determine the components identification [68].
3.2. Antioxidant Ability of Essential Oil
3.2.1. DPPH Scavenging Capacity
The scavenging capacity of free radicals in EO was performed by the scavenging method of 1,1-diphenyl-2-picrylhydrazyl (DPPH) reported by Burits and Bucar [69]. Briefly, 50 µL of EO dissolved in ethanol was used with different concentrations. A total of 825 µL of DPPH solution (60 μM) and an absorbance of 0.7 at 517 nm) was added to every dilution. The absorbance measurements at 517 nm wavelength were conducted after incubation for 60 min at room temperature. The IC50 values were determined from the graph by the percentage of inhibition (PI), according to the following formula.
BHT was chosen as a positive control and the IC50 value was calculated from the percentage of the inhibition curve.
3.2.2. Radical Cation Decolorization (ABTS Assay)
Radical cation (ABTS+) decolorization of the extracts was measured according to the protocol described by RE et al. [70]. For this, 825 μL of 2,2′-casino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS was dissolved in water to a 7 mM concentration. ABTS radical cation (ABTS•1) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration) and allowing the mixture to stand in dark conditions at room temperature for 12–16 h before use) was added to 50 μL of each essential oil, after 30 min of incubation in the dark, the absorbances were measured at 734 nm using a UV/Vis spectrophotometer. The percentages of ABTS inhibition were calculated according to the following formula:
The results were calculated in triplicate and the values were represented in mg/mL.
Trolox was chosen as a positive control and the IC50 value was calculated by use of the percentage of the inhibition curve.
3.2.3. Reducing Power (RP)
The reducing power of EO has been assessed by the procedure described by Parki et al. [71], with slight modification. Briefly, 250 μL of 0.2 M sodium phosphate buffer (pH 6.6), and 250 μL of 1% potassium ferricyanide were added to 50 μL of M. longifolia essential oil, the mixture was incubated at 50 °C for 20 min, then 250 μL of 10% TCA was added and immediately mixed with 250 μL of distilled water and 60 μL of 0.1% ferric chloride. The absorbances were measured at 700 nm with a PerkinElmer Lambda 40 PerkinElmer spectrophotometer.
Ascorbic acid was used as the standard. The results were expressed in EC50 (Half maximal effective concentration), which was calculated by use of the absorbance curve (Y = ax + b; Y = 0.5). The EC50 values were represented in μg/mL.
3.2.4. Total Antioxidant Activity (TAC)
The ammonium phosphomolybdate test was used for the assessment of total antioxidant potential (TAC) according to the protocol described by Prieto et al. [72]. Here, 25 μL of EO or standard (Ascorbic acid) was mixed with 1 mL of reagent solution (sulfuric acid 6 M, sodium phosphate 28 mM, and ammonium molybdate 4 mM). After incubation in a heated bath for 90 min at 95 °C, the sample’s absorbance was determined at 700 nm. The sample’s absorbance was determined at 700 nm against the blank with a PerkinElmer Lambda 40 UV/Vis, (Barcelona, Spain) spectrophotometer. The values obtained were represented as milligrams of ascorbic acid equivalent per gram of EO (mg AAE/g EO) and the standard curve (Y = 2.9009x + 0.0999, R2 = 0.9991).
3.3. Antimicrobial Activities of Essential Oil
The antimicrobial capacity of the EO was examined against five Gram-negative bacteria including Escherichia coli (K12) and Pseudomonas aeruginosa (CIP A22) and Gram-positive bacteria including Staphylococcus aureus (ATCC 6633) and Bacillus subtilis (DSM 6333), and against two fungal strains including Candida albicans; (ATCC 10231), and Aspergillus niger (MTCC 282). All strains examined were isolated clinically from University Hospital Complex, Fez, Morocco. These bacterial and fungal strains were found to be multi-drug-resistant.
3.3.1. Assessment Procedure of the Antimicrobial Capacity
The antibacterial and antifungal performance of M. longifolia EO was examined using the disc diffusion technique [73]. Briefly, 15–20 mL of Mueller–Hinton agar (MH) and malt extract (ME) were poured into Petri plates. Inoculant was primed from fresh cultivars grown in the MH and ME environments in (0.9% NaCl) under sterile conditions. Secondarily, 6 mm diameter Whatman paper discs were imbibed with 10 μL of EO and subsequently placed in Petri dishes inoculated with bacteria (106 to 108 CFU/mL) and yeast. Following 24 and 48 h of inoculating Petri plates with bacteria and yeast at 37 °C and 30 °C, respectively, the diameters of inhibition and the percentages of inhibition were measured for the bacterial, fungi, and yeast strains. Antibiotics: streptomycin, erythromycin, and fluconazole. Each experience was executed in triplicate.
3.3.2. Minimum Inhibitory Concentration (MIC)
The microdilution technique is used to calculate the MIC of the EO of Mentha longifolia against various microbiological strains [74]. This was accomplished using a dilution series of M. longifolia EO (0.097 to 50 mg/mL). One hundred microliters of M. longifolia EO with a 1:10 (v/v) dilution of DMSO (10%) each concentration of such dilution series was mixed in microplate wells with sterile Mueller–Hinton broth and 30 μL of microbial inoculate at a finish microbial concentration of (108 CFU/mL). After an incubation period of 48 h to 7 nights for fungi and 24 h for bacteria at 37 °C [75], the MIC was calculated by the colorimetric mean using resazurin 0.015% [76].
3.4. Insecticidal Potential
3.4.1. Breeding of Insects
The glass should be placed with a temperature of 27 ± 1 °C, relative humidity of 70 ± 5%, and a photoperiod of 14 h (light)/10 h (dark). This setting resembles the natural conditions that Callosobruchus maculatus is used to, and will promote their development and reproduction.
3.4.2. Toxicity of Essential Oils against Callosobruchus maculatus: Fumigation Test
The fumigation test was carried out to evaluate the insecticide activity of the essential oil in the vapor phase. The test was carried out in 1-L jars, and to avoid direct contact with insects, pieces of Whatman No. 1 paper (3 × 3 cm) saturated with various essential oil concentrations of 4 μL, 12 μL, 16 μL, and 20 μL/L of air were affixed to the inside surface of each jar’s cover. Then, 20 adult insects of C. maculatus (10 males and 10 females) aged 0 and 48 h were introduced separately into each jar, and the control was performed without treatment. Three replicates were performed for all tests. Dead individuals were counted daily for each dose for one day. Using a magnifying binocular, the egg-laying capability of C. maculatus females was estimated. The jars (controls and treated), were kept in a culture chamber set at a temperature of 27 ± 1 °C, a relative humidity of 70.5%, and a photoperiod of 14 h (light)/10 h (darkness), until the emergence phase of adults [77].
Mortality of adults: The observed mortality rate is corrected by the Abbott formula [78].
where Pc = % Corrected mortality, Po = the trial’s observed mortality, and Pt = mortality observed under control.
3.4.3. Repellent Influence of Essential Oils
The repulsiveness of essential oils to adults of Callosobruchus maculatus (C. maculatus) has been studied according to the preferred zone methodology used on filter paper with the method described by McDonald et al. [79]. Whatman No. 1 filter paper discs (Diameter 8 cm) were cut into two equal parts, one imprinted with each essential oil tested at different doses of 4 μL, 12 μL, 16 μL, and 20 μL diluted in 0.5 mL of acetone, the other part was imbibed exclusively with the same volume of acetone (Negative control). The two filter paper halves were air-dried and then taped together. Ten adult insects of C. maculatus (5 males and 5 females) were placed in the center of the boxes. Three replicates are performed for each dose. A half-hour after each treatment, the number of individuals on each of the two portions was tallied.
The percent repulsion (PR) of essential oils for adults of C. maculatus was calculated using the formula.
PR = Percentage repulsion, N = Number of individuals present in the acetone portion only. NT = Number of individuals present in the part subjected to the essential oil diluted in acetone solvent.
3.5. Molecular Docking
To theoretically study the role of the identified EO in M. longifolia concerning their potential antioxidant and antimicrobial properties, molecular docking was performed.
3.5.1. Ligand Preparation
To conduct the ligand preparation, the molecules existing in the M. longifolia EO were obtained from the PubChem database in SDF format. Subsequently, the LigPrep tool from the Schrödinger Software program (version 11.5) was employed, using the OPLS3 force field, to prepare the ligands. Each ligand was subjected to ionization state selection at pH 7.0 ± 2.0, resulting in the generation of 32 stereoisomers for each molecule [80].
3.5.2. Protein Preparation
The structure of human NADPH oxidase (PDB ID: 2CDU) [81] and the beta-ketoacyl-[acyl carrier protein] synthase from Escherichia coli (PDB ID: 1FJ4) [82], Staphylococcus aureus nucleoside diphosphate kinase (PDB ID: 3Q8U) [81], a beta-1,4-endoglucanase from Aspergillus niger (PDB ID: 5I77) [83], and sterol 14-alpha demethylase (CYP51) from a pathogenic yeast Candida albicans (PDB ID: 5FSA) [84] were directly obtained from the Protein Data Bank. The optimization process was completed by adding hydrogen atoms, completing bond orders, removing water molecules, assigning hydrogen bonds, fixing the potential of receptor atoms, and energy minimization using the OPLS3 force field [80].
3.6. Statistical Analysis
Average standard deviation values were obtained with GraphPad Prism 8.0.1. The results were compared through a variance analysis (ANOVA) followed by the Tukey test. The difference at p < 0.05 was interpreted as significant. The Principal Component Analysis (PCA) was conducted using historical data.
4. Conclusions
In the current study, we examined the chemical components, antioxidant, antimicrobial, and insecticidal properties of EO extracted from M. longifolia on nosocomial antibiotic-resistant microorganisms and stored grain pests. Our findings indicated that Mentha longifolia L. EO is rich in terpene compounds with a wide range of biological activities including antioxidant, antibacterial, antifungal, and insecticidal potentials against bacteria, yeast, and insect pests. This provides the potentiality of using M. longifolia essential oil and its active components in several biological applications, including the creation of new products in the pharmaceutical industry, and as a preservative agent of fresh food in the food industry instead of chemical preservatives.
This study can be reinforced by more in-depth research, including quantifying numerous new compounds, as well as purifying and molecularly identifying the chemical structures that underlie particular biological properties. The safety of the studied oils needs to be studied using preclinical tests on non-human models before any potential medical use.
Author Contributions
Conceptualization, M.T., E.D. and B.L.; methodology, M.T., A.E.G., H.T., A.B. and M.C.; software, K.F., M.T. and M.B. (Meryem Bakour); formal analysis, M.T., A.E.G. and M.B. (Mohammed Bourhia); data curation, G.N., H.-A.N. and A.E.G.; writing—original draft preparation, K.S.A., M.T. and A.E.G.; writing—review and editing, M.T., D.O., A.E.G. and M.B. (Mohammed Bourhia); supervision, B.L., M.B. (Mohammed Bourhia) and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by the Researchers Supporting Project number (RSP2023R189), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors affirm that they have no known financial or interpersonal conflict of interest.
References
- Laranjo, M.; Fernández-León, A.M.; Agulheiro-Santos, A.C.; Potes, M.E.; Elias, M. Essential Oils of Aromatic and Medicinal Plants Play a Role in Food Safety. J. Food Process. Preserv. 2022, 46, e14278. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon Cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Laranjo, M.; Fernández-León, A.M.; Potes, M.E.; Agulheiro-Santos, A.; Elias, M. Use of Essential Oils in Food Preservation. 2017. Available online: https://api.semanticscholar.org/CorpusID:96440310 (accessed on 29 January 2023).
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, E.; Alpınar, K. An Ethnobotanical Survey of Medicinal Plants in Western Part of Central Taurus Mountains: Aladaglar (Nigde—Turkey). J. Ethnopharmacol. 2015, 166, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Tuzlaci, E. Turkish Folk Medicinal Plants, IX: Ovacik (Tunceli). Marmara Pharm. J. 2010, 3, 136–143. [Google Scholar] [CrossRef]
- Mokaberinejad, R.; Akhtari, E.; Tansaz, M.; Bioos, S.; Kamalinejad, M.; Zafarghandi, N.; Ghobadi, A.; Sohrabvand, F.; Akhbari, A. Effect of Mentha longifolia on FSH Serum Level in Premature Ovarian Failure. Open J. Obstet. Gynecol. 2014, 4, 356–360. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Ghobadi, A.; Farzaei, F.; Najafi, F. Pharmacological Activity of Mentha longifolia and Its Phytoconstituents. J. Tradit. Chin. Med. 2017, 37, 710–720. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Mousavizadeh, S.J.; Mohammadi, H.; Majidi, M.; Amini, S. Allelopathic Effect of Some Essential Oils on Seed Germination of Lathyrus Annuus and Vicia Villosa. J. Biodivers. Environ. Sci. 2013, 3, 67–73. [Google Scholar]
- Džamić, A.M.; Soković, M.D.; Ristić, M.S.; Novaković, M.; Grujić-Jovanović, S.; Tešević, V.; Marin, P.D. Antifungal and Antioxidant Activity of Mentha Longifolia (L.) Hudson (Lamiaceae) Essential Oil. Bot. Serb. 2010, 34, 5. [Google Scholar]
- Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Alharbi, B.; Alshammari, N.; Qahl, S.H.; Alhuthali, H.M.; Bagher, A.A. Phytochemical Characterization of Saudi Mint and Its Mediating Effect on the Production of Silver Nanoparticles and Its Antimicrobial and Antioxidant Activities. Plants 2023, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Ghoulami, S. Phytochemical Study of Mentha longifolia of Morocco. Fitoterapia 2001, 72, 596–598. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical Composition and Functional Properties of Essential Oils from Mentha Species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Mittal, S.; Dogra, K.S.; Yadav, S.; Kohli, R.K. Constituents of Leaf Essential Oil of Mentha longifolia from India. Chem. Nat. Compd. 2008, 44, 528–529. [Google Scholar] [CrossRef]
- Boira, H.; Blanquer, A. Environmental Factors Affecting Chemical Variability of Essential Oils in 1hymus Piperella L. Biochem. Syst. Ecol. 1998, 26, 811–822. [Google Scholar] [CrossRef]
- Zouari-Bouassida, K.; Trigui, M.; Makni, S.; Jlaiel, L.; Tounsi, S. Seasonal Variation in Essential Oils Composition and the Biological and Pharmaceutical Protective Effects of Mentha longifolia Leaves Grown in Tunisia. BioMed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and Antioxidant Activities of Three Mentha Species Essential Oils. Planta Med. 2003, 69, 413–419. [Google Scholar] [CrossRef]
- Anwar, F.; Alkharfy, K.M.; Rehman, N.U.; Adam, E.; Gilani, A. Chemo-Geographical Variations in the Composition of Volatiles and the Biological Attributes of Mentha longifolia (L.) Essential Oils from Saudi Arabia. Int. J. Pharmacol. 2017, 13, 408–424. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Bakchiche, B.; ALSalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of Essential Oil of Mentha Spicata L. (Lamiaceae) from Algerian Saharan Atlas. BMC Complement. Altern. Med. 2018, 18, 201. [Google Scholar] [CrossRef]
- Salamatullah, A.M. Identification of Volatile Compounds and Antioxidant, Antibacterial, and Antifungal Properties against Drug-Resistant Microbes of Essential Oils from the Leaves of Mentha rotundifolia Var. Apodysa Briq. (Lamiaceae). Open Chem. 2022, 20, 484–493. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Afolayan, A.J. Chemical Composition and Antibacterial Activity of the Essential Oil Isolated from South African Mentha longifolia (L.) L. Subsp. Capensis (Thunb.) Briq. J. Essent. Oil Res. 2006, 18, 57–59. [Google Scholar] [CrossRef]
- Sitzmann, J.; Habegger, R.; Schnitzler, W.H.; Grassmann, J. Comparative Analysis of Antioxidant Activities of Fourteen Mentha Essential Oils and Their Components. Chem. Biodivers. 2014, 11, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Blaskovich, M.A.; Cooper, M.A. Antibiotics in the Clinical Pipeline at the End of 2015. J. Antibiot. 2017, 70, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Snoussi, M.; Ben Jannet, H.; Mighri, Z.; Bakhrouf, A. Comparison of Chemical Composition and Antimicrobial Activities of Mentha longifolia L. ssp. longifolia Essential Oil from Two Tunisian Localities (Gabes and Sidi Bouzid). Ann. Microbiol. 2008, 58, 513–520. [Google Scholar] [CrossRef]
- Nikšić, H.; Bešović, E.K.; Makarević, E.; Durić, K. Chemical Composition, Antimicrobial and Antioxidant Properties of Mentha longifolia (L.) Huds. Essential Oil. J. Health Sci. 2012, 2, 192–200. [Google Scholar] [CrossRef]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and Antioxidant Properties of the Essential Oils and Methanol Extract from Mentha longifolia L. Ssp. Longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Guo, L.; Wang, L.; Liu, Y. Antibacterial Mechanism of Rose Essential Oil against Pseudomonas putida Isolated from White Hypsizygus Marmoreus at Cellular and Metabolic Levels. Ind. Crops Prod. 2023, 196, 116523. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Sefu, G.; Satheesh, N.; Berecha, G. Antifungal Activity of Ginger and Cinnamon Leaf Essential Oils on Mango Anthracnose Disease Causing Fungi (C. gloeosporioides). Carpathian J. Food Sci. Technol. 2015, 7, 26–34. [Google Scholar]
- Di Sotto, A.; Di Giacomo, S.; Abete, L.; Božović, M.; Parisi, O.A.; Barile, F.; Vitalone, A.; Izzo, A.A.; Ragno, R.; Mazzanti, G. Genotoxicity Assessment of Piperitenone Oxide: An in Vitro and in Silico Evaluation. Food Chem. Toxicol. 2017, 106, 506–513. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Lugosi, M.; Alberti, C.; Zahar, J.-R.; Garrouste, M.; Lemiale, V.; Descorps-Desclère, A.; Ricard, J.-D.; Goldgran-Tolédano, D.; Cohen, Y.; Schwebel, C.; et al. Aspergillus in the Lower Respiratory Tract of Immunocompetent Critically Ill Patients. J. Infect. 2014, 69, 284–292. [Google Scholar] [CrossRef]
- Mkaddem, M.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Romdhane, M. Chemical Composition and Antimicrobial and Antioxidant Activities of Mentha (Longifolia L. and viridis) Essential Oils. J. Food Sci. 2009, 74, M358–M363. [Google Scholar] [CrossRef]
- Ali, H.M.; Elgat, W.A.A.A.; EL-Hefny, M.; Salem, M.Z.M.; Taha, A.S.; Al Farraj, D.A.; Elshikh, M.S.; Hatamleh, A.A.; Abdel-Salam, E.M. New Approach for Using of Mentha longifolia L. and Citrus reticulata L. Essential Oils as Wood-Biofungicides: GC-MS, SEM, and MNDO Quantum Chemical Studies. Materials 2021, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and Antifungal Activity of Essential Oils of Mentha suaveolens. Phytother. Res. 2002, 16, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Idaomar, M. Cytotoxicity and Gene Induction by Some Essential Oils in the Yeast Saccharomyces cerevisiae. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 585, 1–13. [Google Scholar] [CrossRef]
- Lopes, L.M.; Sousa, A.H.; Santos, V.B.; Silva, G.N.; Abreu, A.O. Development Rates of Callosobruchus maculatus (Coleoptera: Chrysomelidae) in Landrace Cowpea Varieties Occurring in Southwestern Amazonia. J. Stored Prod. Res. 2018, 76, 111–115. [Google Scholar] [CrossRef]
- Odeyemi, O.O.; Masika, P.; Afolayan, A.J. Insecticidal Activities of Essential Oil from the Leaves of Mentha longifolia L. Subsp. Capensis against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Afr. Entomol. 2008, 16, 220–225. [Google Scholar] [CrossRef]
- Khani, A.; Asghari, J. Insecticide Activity of Essential Oils of Mentha Longifolia, Pulicaria Gnaphalodes and Achillea Wilhelmsii Against Two Stored Product Pests, the Flour Beetle, Tribolium Castaneum, and the Cowpea Weevil, Callosobruchus maculatus. J. Insect Sci. 2012, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal Properties of Mentha Species: A Review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Hategekimana, A.; Erler, F. Fecundity and Fertility Inhibition Effects of Some Plant Essential Oils and Their Major Components against Acanthoscelides obtectus say (Coleoptera: Bruchidae). J. Plant Dis. Prot. 2020, 127, 615–623. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Comparison of the Insecticidal Effects of Water Extracted and Intact Aromatic Plants on Acanthoscelides obtectus, a Bruchid Beetle Pest of Kidney Beans. Chemoecology 1994, 5–6, 1–5. [Google Scholar] [CrossRef]
- Aziz, E.E.; Abbass, M.H. Chemical Composition and Efficiency of Five Essential Oils against the Pulse Beetle Callosobruchus maculatus (F.) on Vigna Radiata Seeds. Am. Eurasian J. Agric. Environ. Sci. 2010, 8, 411–419. [Google Scholar]
- Saxena, B.P.; Mathur, A.C. Loss of Fecundity in Dysdercus Koenigii F. Due to Vapours of Acorus calamus L. Oil. Experientia 1976, 32, 315–316. [Google Scholar] [CrossRef]
- Williams, C.M. The Juvenile Hormone of Insects. Nature 1956, 178, 212–213. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid. Based Complement. Altern. Med. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Lee, S.B.; Lee, H.S.; Kim, G.H. Insecticidal and Acaricidal Activity of Carvacrol and Beta-Thujaplicine Derived from Thujopsis Dolabrata Var. Hondai Sawdust. J. Chem. Ecol. 1998, 24, 81–90. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of Action of Insecticidal Secondary Metabolites of Plant Origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Price, D.N.; Berry, M.S. Comparison of Effects of Octopamine and Insecticidal Essential Oils on Activity in the Nerve Cord, Foregut, and Dorsal Unpaired Median Neurons of Cockroaches. J. Insect. Physiol. 2006, 52, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Xefteri, D.N.; Cianfaglione, K.; Perinelli, D.R.; Spinozzi, E.; Bonacucina, G.; Canale, A.; Benelli, G.; et al. Piperitenone Oxide-Rich Mentha longifolia Essential Oil and Its Nanoemulsion to Manage Different Developmental Stages of Insect and Mite Pests Attacking Stored Wheat. Ind. Crops Prod. 2022, 178, 114600. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Umemoto, K.; Kameoka, H. Inhibition of Acetylcholinesterase Activity by Essential Oils of Mentha Species. J. Agric. Food Chem. 1998, 46, 3431–3434. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lee, B.-H.; Choi, W.-S.; Park, B.-S.; Kim, J.-G.; Campbell, B.C. Fumigant Toxicity of Volatile Natural Products from Korean Spices and Medicinal Plants towards the Rice Weevil, Sitophilus oryzae (L). Pest. Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Enan, E. Insecticidal Activity of Essential Oils: Octopaminergic Sites of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Barbieri, N.; Costamagna, M.; Gilabert, M.; Perotti, M.; Schuff, C.; Isla, M.I.; Benavente, A. Antioxidant Activity and Chemical Composition of Essential Oils of Three Aromatic Plants from La Rioja Province. Pharm. Biol. 2016, 54, 168–173. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Koyama, H.; Tawata, S. Antioxidant Activity and Contents of Essential Oil and Phenolic Compounds in Flowers and Seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem. 2007, 104, 1648–1653. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Dokik, E.T.; Panovska, T.K.; Kaftandzieva, A.; Kulevanova, S. Chemical Characterization and Antimicrobial Activity of the Needle Essential Oil of Pinus mugo (Pinaceae) from Macedonian Flora. Planta Med. 2011, 77, PL59. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Menković, N.R.; Zdunić, G.M.; Tasić, S.R.; Ristić, M.S.; Stević, T.R.; Dajić-Stevanović, Z.P. Chemical Composition and Antimicrobial Activity of the Essential Oils of Micromeria thymifolia (Scop.) Fritsch., M. Dalmatica Benth., and Satureja Cuneifolia Ten. and Its Secretory Elements. J. Essent. Oil Res. 2010, 22, 91–96. [Google Scholar] [CrossRef]
- Lafraxo, S.; El Moussaoui, A.; A Bin Jardan, Y.; El Barnossi, A.; Chebaibi, M.; Baammi, S.; Akka, A.A.; Chebbac, K.; Akhazzane, M.; Chelouati, T.; et al. GC-MS Profiling, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Essential Oil of Juniperus thurifera Bark. Evid. Based Complement. Altern. Med. 2022, 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Gouja, H.; Hannachi, H.; Isoda, H.; Neffati, M.; Najjaa, H. Seasonal Changes in Rosemary Species: A Chemotaxonomic Assessment of Two Varieties Based on Essential Oil Compounds, Antioxidant and Antibacterial Activities. PLoS ONE 2022, 17, e0273367. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Tunalier, Z.; Özek, T.; Kürkcüoglu, M.; Baser, K.H.C. A Simple Method to Obtain Essential Oils from Salvia Triloba L. and Laurus Nobilis L. by Using Microwave-Assisted Hydrodistillation. Z. Naturforschung C 2005, 60, 501–504. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Identif. Essent. Oil Compon. Gas Chromatogr. Mass Spectrom. 2007, 24, 245–251. [Google Scholar]
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella sativa Essential Oil. Phytother Res 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Parki, A.; Chaubey, P.; Prakash, O.; Kumar, R.; Pant, A.K. Seasonal Variation in Essential Oil Compositions and Antioxidant Properties of Acorus calamus L. Accessions. Medicines 2017, 4, 81. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- M100Ed32; Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 29 January 2023).
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The Agar Microdilution Method—A New Method for Antimicrobial Susceptibility Testing for Essential Oils and Plant Extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Baghouz, A.; Bouchelta, Y.; Es-safi, I.; Bourhia, M.; Abdelfattah, E.M.; Alarfaj, A.A.; Hirad, A.H.; Nafidi, H.-A.; Guemmouh, R. Identification of Volatile Compounds and Insecticidal Activity of Essential Oils from Origanum compactum Benth. and Rosmarinus officinalis L. against Callosobruchus maculatus (Fab.). J. Chem. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [PubMed]
- McDonald, L.L.; Guy, R.H.; Speirs, R.D. Preliminary Evaluation of New Candidate Materials as Toxicants, Repellents, and Attractants Against Stored-Product Insects; U.S. Agricultural Research Service: Washington, DC, USA, 1970.
- Aboul-Soud, M.A.M.; Ennaji, H.; Kumar, A.; Alfhili, M.A.; Bari, A.; Ahamed, M.; Chebaibi, M.; Bourhia, M.; Khallouki, F.; Alghamdi, K.M.; et al. Antioxidant, Anti-Proliferative Activity and Chemical Fingerprinting of Centaurea calcitrapa against Breast Cancer Cells and Molecular Docking of Caspase-3. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutiérrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.-S. Chemical Constituents, In Vitro Antioxidant Activity and In Silico Study on NADPH Oxidase of Allium sativum L. (Garlic) Essential Oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]
- Bouslamti, M.; Metouekel, A.; Chelouati, T.; El Moussaoui, A.; Barnossi, A.E.; Chebaibi, M.; Nafidi, H.-A.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; et al. Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches. Molecules 2022, 27, 8688. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Li, Y.; Lai, H.-L.; Zheng, Y.; Huang, J.-W.; Chen, C.-C.; Chen, Y.; Jin, J.; Li, H.; et al. Functional and Structural Analysis of Pichia Pastoris-Expressed Aspergillus niger 1,4-β-Endoglucanase. Biochem. Biophys. Res. Commun. 2016, 475, 8–12. [Google Scholar] [CrossRef]
- Talukdar, R.; Padhi, S.; Rai, A.K.; Masi, M.; Evidente, A.; Jha, D.K.; Cimmino, A.; Tayung, K. Isolation and Characterization of an Endophytic Fungus Colletotrichum Coccodes Producing Tyrosol from Houttuynia Cordata Thunb. Using ITS2 RNA Secondary Structure and Molecular Docking Study. Front. Bioeng. Biotechnol. 2021, 9, 650247. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).