Impacts of Micro(nano)plastics on Terrestrial Plants: Germination, Growth, and Litter
Abstract
:1. Introduction
2. The Impact of MNP on Plant Seed Germination
3. Effects of MNP on Plant Growth
3.1. Impact on Plant Photosynthesis
3.2. Impact on Plant Biomass
| Test Object | MNP | Exposure Time | Effect | Mechanism | References | ||
|---|---|---|---|---|---|---|---|
| Type | Size | Concentration | |||||
| strawberry | HDPE | 2–5 mm | 0.2 g/kg | 5 m | HDPE affects plant height, stem thickness, biomass, root volume, and surface area. | The synthesis of chlorophyll and its stable binding with proteins is inhibited. | [52] |
| corn | PMFs | 2.87 mm | 0.5% | 5 m | PMFs lead to a reduction in plant nitrogen uptake and biomass production. | Soil macro- and micro-porosity are influenced by PMFs. | [53] |
| plant community | EPS | 200 μm | 0.1% 0.2% | 6 m | When EPS concentration is high, the total biomass and root biomass of the plant community are significantly lower than the control. | Exacerbated the generation of ROS and induced genetic toxicity in cells. | [54] |
| sweet potato | PVC | 6.5 μm | 100 mg/L 200 mg/L | 14 m | The combined application with Cr(VI) reduces plant height, per plant fresh biomass, and chlorophyll content, among others. | MNP enhance the accumulation of Cr(VI) and its toxic effects on the physiological and biochemical characteristics of sweet potato plants. | [55] |
| wheat | PS | 100 nm | 0.01–10 mg/L | 21 m | PS causes a reduction in the stem-to-root biomass ratio (S:R) in wheat seedlings. | Plants experiencing low nutrient supply exhibit an increased allocation to the root system. | [56] |
3.3. Impact on Plant Antioxidant Characteristics and Cellular Toxicity
3.4. The Impact on Plant Nutrient Uptake
3.5. The Impact on Plant Gene Expression and Genetics
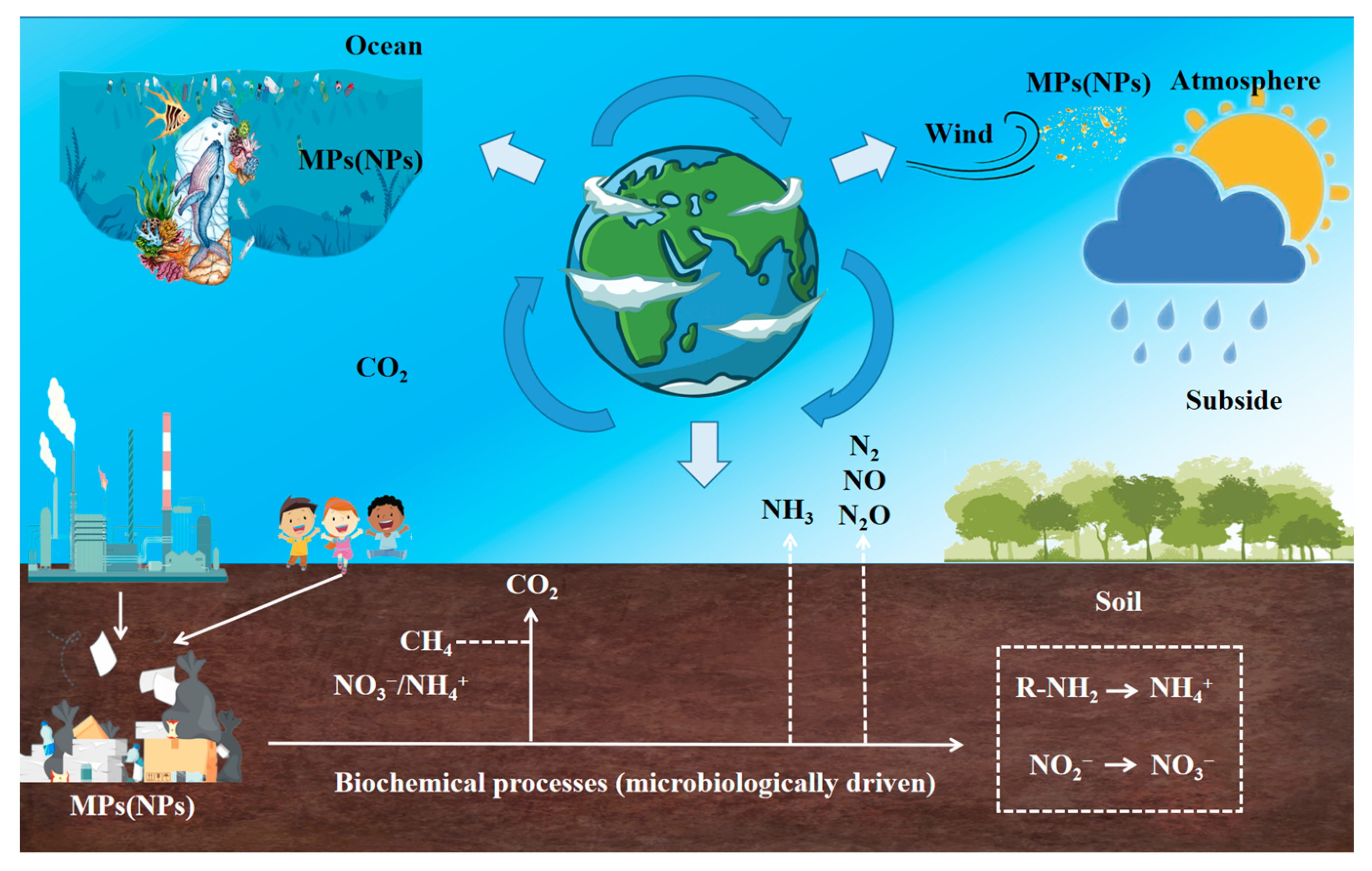
4. The Impact of MNP on the Decomposition of Plant Litter
5. Conclusions and Perspectives
- (1)
- In order to gain a more comprehensive understanding of the impact of MNP, it is necessary to expand the scope of research and investigate the effects of various types of MNP on different plant species. MNP have diverse sources and types, but current research often relies on primary MNP for indoor experiments, and most MNP are artificially produced. However, MNP in the environment mostly exist in the form of secondary MNP, with differences in their physicochemical properties and the environment they inhabit compared to those used in laboratory experiments. This fails to reflect their actual state in natural soils. In the future, it is essential to delve deeper into the actual effects of MNP with different sources and characteristics on soil plants in real environmental conditions. Additionally, while experiments have been conducted on a limited number of plant species at the individual level, there is a lack of research on their effects on plant community structure, diversity, and primary production changes.
- (2)
- To reveal the toxicological mechanisms of MNP on plants, further in-depth research is required to elucidate their effects at the molecular and genetic levels. Current research primarily focuses on the physiological effects of MNP on plants, with limited understanding of their toxic effects at the molecular and genetic levels. Advanced research techniques such as high-throughput genomics can be employed to further uncover the toxic mechanisms of MNP at the molecular and genetic levels.
- (3)
- MNP not only affect plants but also exert influences on microorganisms, and there is an interaction between all three of them. Since microorganisms share similarities with NP, such as small size and a large surface area, they can come into direct contact with the external environment. They are also widely distributed in the environment, abundant in number, and diverse in species. It is crucial to elucidate the intrinsic mechanisms of the interaction between MNP, plants, and microorganisms. However, current research on the impact of MNP on endophytic microbial communities in plants and phyllosphere microbial communities is relatively limited. Further in-depth research is needed to investigate the effects of MNP on the structure and function of these microbial communities, as well as the underlying mechanisms of interaction among the three.
- (4)
- During the decomposition of litter, microorganisms can form biofilms on the surface of MNP, and MNP may potentially serve as “electron shuttles”, participating in microbial metabolism as electron sinks or sources. However, the mechanisms by which MNP mediate the extracellular electron transfer by microorganisms remain unclear. Furthermore, soils contain a significant amount of natural “electron shuttles”, such as humic substances, and research on the interaction between MNP and these natural “electron shuttles” is currently limited. A deeper understanding of the relationship between MNP and “electron shuttles”, as well as their impact on microbial decomposition activities, is crucial in addressing the challenges posed by plastic pollution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.H.; Liu, D.; Lin, J.J.; Kumar, A.; Jia, K.; Tian, X.; Yu, Z.; Zhu, B. Priming effects induced by degradable microplastics in agricultural soils. Soil Biol. Biochem. 2023, 180, 109006. [Google Scholar] [CrossRef]
- Li, H.; Chang, X.; Zhang, J.; Wang, Y.; Zhong, R.; Wang, L.; Wei, J.; Wang, Y. Uptake and distribution of microplastics of different particle sizes in maize (Zea mays) seedling roots. Chemosphere 2022, 313, 137491. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination—A critical review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, X.; Liu, Y.; Adams, C.A.; Sun, Y.; Zhang, S. Micro(nano)plastics and terrestrial plants: Up-to-date knowledge on uptake, translocation, and phytotoxicity. Resour. Conserv. Recycl. 2022, 185, 106503. [Google Scholar] [CrossRef]
- Yang, H.R.; Yumeng, Y.M.; Yu, Y.K.; Yinglin, H.; Fu, B.; Wang, J. Distribution, sources, migration, influence and analytical methods of microplastics in soil ecosystems. Ecotox Environ. Safe 2022, 243, 114009. [Google Scholar] [CrossRef]
- Chen, R.H.; Yu, Y.; Huang, S.; Chen, R.L.; Jia, X.K.; Chen, Y.H.; Xue, S.; Liu, M.J.; Yang, X.M. Effect of Polyethylene Microplastic Concentration on the Characteristics and Stability of Black Soil Aggregates. Soil Sci. 2023, 54, 56–66. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Zuo, Z.J.; Hao, W.L.; Deng, L.; Xu, M. Effects of microplastics on soil aggregate stability and soil organic carbon mineralization. J. Northwest AF Univ. (Nat. Sci. Ed.) 2023, 51, 91–100. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Zheng, L.Z.; Huang, H.; Tian, Y.-C.; Gong, Z.; Liu, P.; Wu, X.; Li, W.-T.; Gao, S. Formation of Nano- and Microplastics and Dissolved Chemicals During Photodegradation of Polyester Base Fabrics with Polyurethane Coating. Environ. Sci. Technol. 2023, 57, 1894–1906. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic Disguising As Soil Carbon Storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef] [PubMed]
- Zantis, L.J.; Borchi, C.; Vijver, M.G.; Peijnenburg, W.; Di Lonardo, S.; Bosker, T. Nano- and microplastics commonly cause adverse impacts on plants at environmentally relevant levels: A systematic review. Sci. Total Environ. 2023, 867, 161211. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Lopez, M.D.; Toro, M.T.; Riveros, G.; Illanes, M.; Noriega, F.; Schoebitz, M.; García-Viguera, C.; Moreno, D. Brassica sprouts exposed to microplastics: Effects on phytochemical constituents. Sci. Total Environ. 2022, 823, 153796. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Tsui, M.; Chow, A.; Chen, H.; Williams, C.; Ligaba-Osena, A. Micro(nano)plastic pollution in terrestrial ecosystem: Emphasis on impacts of polystyrene on soil biota, plants, animals, and humans. Environ Monit Assess. 2023, 195, 252. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Li, W.; Yang, W.; Jing, S. Effects of microplastics on the water characteristic curve of soils with different textures. Chemosphere 2023, 317, 137762. [Google Scholar] [CrossRef]
- Liang, L.; Wong, S.C.; Lisak, G. Effects of plastic-derived carbon dots on germination and growth of pea (Pisum sativum) via seed nano-priming. Chemosphere 2023, 316, 137868. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, K.; Benzarti, M.; Debez, A.; Bailly, C.; Savouré, A.; Abdelly, C. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 2015, 174, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhao, F.; Tian, L.; Ni, K.; Lu, Y.; Borah, P. Effects of polystyrene microplastics on the seed germination of herbaceous ornamental plants. Sci. Total Environ. 2022, 809, 151100. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, X.F.; Zhao, R.; Cui, Z. Stress response to nanoplastics with different charges in Brassica napus L. during seed germination and seedling growth stages. Front. Env. Sci. Eng. 2023, 17, 43. [Google Scholar] [CrossRef]
- Azhagesan, A.; Chandrasekaran, N.; Mukherjee, A. Multispectroscopy analysis of polystyrene nanoplastic interaction with diastase α-amylase. Ecotox Environ. Safe 2022, 247, 114226. [Google Scholar] [CrossRef]
- Yu, Y.; Dai, W.; Luan, Y. Bio- and eco-corona related to plants: Understanding the formation and biological effects of plant protein coatings on nanoparticles. Environ. Pollut. 2023, 317, 120784. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Gabellieri, E.; Cioni, P.; Paccagnini, E.; Faleri, C.; Lupetti, P.; Corsi, I.; Morelli, E. Interplay between extracellular polymeric substances (EPS) from a marine diatom and model nanoplastic through eco-corona formation. Sci. Total Environ. 2020, 725, 138457. [Google Scholar] [CrossRef]
- Lakshmikanthan, D.; Chandrasekaran, N. The Effect of Humic Acid and Polystyrene Fluorescence Nanoplastics on Solanum lycopersicum Environmental Behavior and Phytotoxicity. Plants 2022, 11, 3000. [Google Scholar] [CrossRef]
- Xu, Z.M.; Zhang, Y.X.; Lin, L.P.; Wang, L.; Sun, W.; Liu, C.; Yu, G.; Yu, J.; Lv, Y.; Chen, J.; et al. cToxic effects of microplastics in plants depend more by their surface functional groups than just accumulation contents. Sci. Total Environ. 2022, 833, 155097. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Dong, Y.M.; Gao, M.L.; Qiu, W.W.; Song, Z. Uptake of microplastics by carrots in presence of As (III): Combined toxic effects. J. Hazard. Mater. 2021, 411, 125055. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Su, Z.; Chen, J.; Zou, J.; Liu, Z.; Li, Y.; Wang, J.; Wu, L.; Wei, H.; Zhang, J. Polyethylene microplastics attenuate soil carbon sequestration by reducing plant photosynthetic carbon assimilation and transfer: Evidence from a 13C-labeling mesocosm study. J. Clean. Prod. 2022, 385, 135558. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 135558. [Google Scholar] [CrossRef]
- Yu, H.; Qi, W.; Cao, X.; Wang, Y.; Li, Y.; Xu, Y.; Zhang, X.; Peng, J.; Qu, J. Impact of microplastics on the foraging, photosynthesis and digestive systems of submerged carnivorous macrophytes under low and high nutrient concentrations. Environ. Pollut. 2022, 292, 118220. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wei, Y.; Yang, C.; He, Z. Interactions of microplastics and soil pollutants in soil-plant systems. Environ. Pollut. 2022, 315, 120357. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, J.; Wang, L.; Liu, Q. Microplastics in soil-plant system: Effects of nano/microplastics on plant photosynthesis, rhizosphere microbes and soil properties in soil with different residues. Plant Soil 2021, 462, 561–576. [Google Scholar] [CrossRef]
- Dong, R.; Liu, R.; Xu, Y.; Liu, W.; Sun, Y. Effect of foliar and root exposure to polymethyl methacrylate microplastics on biochemistry, ultrastructure, and arsenic accumulation in Brassica campestris L. Environ. Res. 2022, 215, 114402. [Google Scholar] [CrossRef]
- Lee, T.Y.; Kim, L.; Kim, D.; An, S.; An, Y.-J. Microplastics from shoe sole fragments cause oxidative stress in a plant (Vigna radiata) and impair soil environment. J. Hazard. Mater. 2022, 429, 128306. [Google Scholar] [CrossRef]
- Dey, S.; Guha, T.; Barman, F.; Natarajan, L.; Kundu, R.; Mukherjee, A.; Paul, S. Surface functionalization and size of polystyrene microplastics concomitantly regulate growth, photosynthesis and anti-oxidant status of Cicer arietinum L. Plant Physiol. Bioch 2023, 194, 41–51. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Zhang, Q.Y.; Liu, P.; Zhang, Y. Effects of Polyethylene and Heavy Metal Cadmium on the Growth and Development of Brassica chinensis var. chinensis. Water Air Soil Poll. 2022, 233, 426. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Dong, Y.; Song, Z. Effect of polyethylene particles on dibutyl phthalate toxicity in lettuce (Lactuca sativa L.). J. Hazard. Mater. 2021, 401, 123422. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.A.D.S.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.Y.; Liu, W.T.; Lian, Y.H.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manag. 2022, 317, 115441. [Google Scholar] [CrossRef]
- Avellan, A.; Schwab, F.; Masion, A.; Chaurand, P.; Borschneck, D.; Vidal, V.; Rose, J.; Santaella, C.; Levard, C. Nanoparticle Uptake in Plants: Gold Nanomaterial Localized in Roots of Arabidopsis thaliana by X-ray Computed Nanotomography and Hyperspectral Imaging. Environ. Sci. Technol. 2017, 51, 8682–8691. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, F.; Ding, L.; Zhang, G.; Bai, B.; Han, Y.; Xiao, L.; Song, Y.; Li, Y.; Wan, S.; et al. Microplastics reduce nitrogen uptake in peanut plants by damaging root cells and impairing soil nitrogen cycling. J. Hazard. Mater. 2023, 443, 130384. [Google Scholar] [CrossRef]
- Karalija, E.; Carbo, M.; Coppi, A.; Colzi, I.; Dainelli, M.; Gašparović, M.; Grebenc, T.; Gonnelli, C.; Papadakis, V.; Pilić, S.; et al. Interplay of plastic pollution with algae and plants: Hidden danger or a blessing? J. Hazard. Mater. 2022, 438, 129450. [Google Scholar] [CrossRef]
- Zang, H.; Zhou, J.; Marshall, M.R.; Chadwick, D.R.; Wen, Y.; Jones, D.L. Microplastics in the agroecosystem: Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, J.T.; Chang, Y.S.; Kim, E.-J. Fragmentation of nanoplastics driven by plant-microbe rhizosphere interaction during abiotic stress combination. Environ. Sci.-Nano 2021, 8, 2802–2810. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; Lopez, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants 2022, 11, 536. [Google Scholar] [CrossRef]
- Ingraffia, R.; Amato, G.; Iovino, M.; Rillig, M.C.; Giambalvo, D.; Frenda, A.S. Polyester microplastic fibers in soil increase nitrogen loss via leaching and decrease plant biomass production and N uptake. Environ. Res. Lett. 2022, 17, 054012. [Google Scholar] [CrossRef]
- Zhang, X.M.; Cao, X.X.; He, L.X.; Xue, W.; Gao, J.-Q.; Lei, N.-F.; Chen, J.-S.; Yu, F.-H.; Li, M.-H. Soil heterogeneity in the horizontal distribution of microplastics influences productivity and species composition of plant communities. Front. Plant Sci. 2022, 13, 1075007. [Google Scholar] [CrossRef]
- Khan, M.A.; Kumar, S.; Wang, Q.; Wang, M.; Fahad, S.; Nizamani, M.M.; Chang, K.; Khan, S.; Huang, Q.; Zhu, G. Influence of polyvinyl chloride microplastic on chromium uptake and toxicity in sweet potato. Ecotox Environ. Safe 2023, 251, 114526. [Google Scholar] [CrossRef]
- Lian, J.P.; Wu, J.N.; Xiong, H.X.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Deng, T.; Cloquet, C.; Tang, Y.; Sterckeman, T.; Echevarria, G.; Estrade, N.; Morel, J.L.; Qiu, R.L. Nickel and zinc isotope fractionation in hyperaccumulating and nonaccumulating plants. Environ. Sci. Technol. 2014, 48, 11926–11933. [Google Scholar]
- Spano, C.; Muccifora, S.; Castiglione, M.R.; Bellani, L.; Bottega, S.; Giorgetti, L. Polystyrene nanoplastics affect seed germination, cell biology and physiology of rice seedlings in-short term treatments: Evidence of their internalization and translocation. Plant Physiol. Bioch 2022, 172, 158–166. [Google Scholar] [CrossRef]
- Ogo, H.A.; Tang, N.; Li, X.; Gao, X.; Xing, W. Combined toxicity of microplastic and lead on submerged macrophytes. Chemosphere 2022, 295, 133956. [Google Scholar] [CrossRef]
- Bandmann, V.; Mueller, J.D.; Koehler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef]
- Mondal, N.K.; Kundu, S.; Debnath, P.; Mondal, A.; Sen, K. Effects of polyethylene terephthalate microplastic on germination, biochemistry and phytotoxicity of Cicer arietinum L. and cytotoxicity study on Allium cepa L. Environ. Toxicol. Pharmacol. 2022, 94, 103908. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, X.; Liu, Q.; Li, L.; Zhao, Y.; Zhao, Y. Different effecting mechanisms of two sized polystyrene microplastics on microalgal oxidative stress and photosynthetic responses. Ecotox Environ. Safe 2022, 244, 114072. [Google Scholar] [CrossRef]
- Radic, T.M.; Vukosav, P.; Komazec, B.; Formosa-Dague, C.; Jurašin, D.D.; Štefanić, P.P.; Čačković, A.; Juraić, K.; DeNardis, N.I. Nanoplastic-Induced Nanostructural, Nanomechanical, and Antioxidant Response of Marine Diatom Cylindrotheca closterium. Water 2022, 14, 2163. [Google Scholar] [CrossRef]
- Thompson, J.; Wilder, L.; Crooks, R. Filtering and continuously separating microplastics from water using electric field gradients formed electrochemically in the absence of buffer. Chem. Sci. 2021, 12, 13744–13755. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotox Environ. Safe 2021, 211, 111899. [Google Scholar] [CrossRef]
- Experimental Investigation of Water-Retaining and Unsaturated Infiltration Characteristics of Loess Soils Imbued with Microplastics. Available online: https://www.mdpi.com/2071-1050/15/1/62 (accessed on 2 October 2023).
- Lian, J.P.; Liu, W.T.; Meng, L.Z.; Wu, J.; Zeb, A.; Cheng, L.; Lian, Y.; Sun, H. Effects of microplastics derived from polymer-coated fertilizer on maize growth, rhizosphere, and soil properties. J. Clean. Prod. 2021, 318, 128571. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, H.; Gong, J. The Arabidopsis Ethylene/Jasmonic Acid-NRT Signaling Module Coordinates Nitrate Reallocation and the Trade-Off between Growth and Environmental Adaptation. Plant Cell 2014, 26, 3984–3998. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, T.Y.; Guo, J.H.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef]
- Movahedi, A.; Aghaei-Dargiri, S.; Barati, B.; Kadkhodaei, S.; Wei, H.; Sangari, S.; Yang, L.; Xu, C. Plant Immunity Is Regulated by Biological, Genetic, and Epigenetic Factors. Agronomy 2022, 12, 2790. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X. Impact of microplastics from polyethylene and biodegradable mulch films on rice (Oryza sativa L.). Sci. Total Environ. 2022, 828, 154579. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, T.; Xu, G.; Teng, H.; Liu, B.; Yu, Y. Effects of micro- and nano-plastics on accumulation and toxicity of pyrene in water spinach (Ipomoea aquatica Forsk). Environ. Sci. Pollut. Res. 2022, 30, 956–965. [Google Scholar] [CrossRef]
- Kaur, M.; Xu, M.; Wang, L. Cyto-Genotoxic Effect Causing Potential of Polystyrene Micro-Plastics in Terrestrial Plants. Nanomaterials 2022, 12, 2024. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Z.; Werner, K.M.; Gollin, S.M.; Saunders, W.S. Cigarette smoke induces anaphase bridges and genomic imbalances in normal cells. Mutat. Res./Fund. Mol. Mech. Mutagen. 2004, 554, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, M.; Li, G.; Kanji, Z.A.; Mitrano, D.M. Potential impacts of atmospheric microplastics and nanoplastics on cloud formation processes. Nat. Geosci. 2022, 15, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; He, Q.; Chen, Y. What Roles Are Terrestrial Plants Playing in Global Microplastic Cycling? Environ. Sci. Technol. 2020, 54, 5325–5327. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, S.; Li, H.; Jian, S.; Liu, F.; Li, X. Reprogramming of microbial community in barley root endosphere and rhizosphere soil by polystyrene plastics with different particle sizes. Sci. Total Environ. 2023, 866, 161420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Gao, Y.; Ren, A. Plant endophytes and arbuscular mycorrhizal fungi alter the decomposition of Achnatherum sibiricum litter. Appl. Soil Ecol. 2022, 180, 104616. [Google Scholar] [CrossRef]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2019, 16, 3–7. [Google Scholar] [CrossRef]
- Kim, S.W.; Liang, Y.; Lozano, Y.M.; Rillig, M.C. Microplastics Reduce the Negative Effects of Litter-Derived Plant Secondary Metabolites on Nematodes in Soil. Front. Environ. Sci. 2021, 9, 790560. [Google Scholar] [CrossRef]
- Zhao, M.T.; Qin, Y.Y.; Qiu, Y.; Zhu, M.; Chen, W. Environmental aging of microplastic: Processes, mechanisms and implications. Environ. Chem. 2022, 41, 1–13. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, H.; Zhou, S.; Sun, L.; Lu, H. Enhanced performance and electron transfer of sulfur-mediated biological process under polyethylene terephthalate microplastics exposure. Water Res. 2022, 223, 119038. [Google Scholar] [CrossRef]
- Ding, L.; Luo, Y.Y.; Yu, X.Q.; Ouyang, Z.; Liu, P.; Guo, X. Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter. Sci. Total Environ. 2022, 824, 153883. [Google Scholar] [CrossRef]
- Huang, Y.; Dang, F.; Yin, Y.; Fang, G.; Wang, Y.; Yu, G.; Zhou, D.; Xing, B. Weathered Microplastics Induce Silver Nanoparticle Formation. Environ. Sci. Tech. Let. 2022, 9, 179–185. [Google Scholar] [CrossRef]


| Test Object | MNP | Exposure Time | Position | Effect | References | ||
|---|---|---|---|---|---|---|---|
| Type | Size | Concentration | |||||
| cabbage | PS | 5 μm 70 nm | 10 mg/kg | 2 d | leaf surface | Plant photosynthesis and growth are affected, with chlorophyll a being more susceptible to influence than chlorophyll b. | [37] |
| oilseed seedlings | PMMA | 63.3 ± 17.9 nm | 0.05 g/L 0.5 g/L | 6 d | leaf surface; root system | PMMA, when exposed through the roots, enters the stems and roots of canola seeds, with a greater impact on root tip cells and chloroplasts compared to leaf surface exposure. | [38] |
| mung bean | - | 57–229 μm | 0.1% 1.1% | 28 d | leaf surface | MNP alter the flavonoid content and photosynthetic factors, resulting in a reduction in the amount of light absorbed by the plants. | [39] |
| madder | PS | 1 μm 12 μm | 10, 50 and 100 mg/L | 10 d | seed | MNP with both 1 μm and 12 μm significantly increase the chlorophyll content and fluorescence in all treatment groups. | [40] |
| oilseed rape | PE | 0.15 mm | 0, 18 and 36 g/kg | 3 m | surrounding the seed | PE affects the synthesis of chlorophyll b, with the most significant impact on chlorophyll b content; it reduces the ability of leaves to capture and convert light energy. | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, R.; Dai, W.; Luan, Y.; Li, J. Impacts of Micro(nano)plastics on Terrestrial Plants: Germination, Growth, and Litter. Plants 2023, 12, 3554. https://doi.org/10.3390/plants12203554
Li X, Wang R, Dai W, Luan Y, Li J. Impacts of Micro(nano)plastics on Terrestrial Plants: Germination, Growth, and Litter. Plants. 2023; 12(20):3554. https://doi.org/10.3390/plants12203554
Chicago/Turabian StyleLi, Xiaodong, Rongyu Wang, Wei Dai, Yaning Luan, and Jing Li. 2023. "Impacts of Micro(nano)plastics on Terrestrial Plants: Germination, Growth, and Litter" Plants 12, no. 20: 3554. https://doi.org/10.3390/plants12203554
APA StyleLi, X., Wang, R., Dai, W., Luan, Y., & Li, J. (2023). Impacts of Micro(nano)plastics on Terrestrial Plants: Germination, Growth, and Litter. Plants, 12(20), 3554. https://doi.org/10.3390/plants12203554






