Phytochemical Properties of Croton gratissimus Burch (Lavender Croton) Herbal Tea and Its Protective Effect against Iron-Induced Oxidative Hepatic Injury
Abstract
1. Introduction
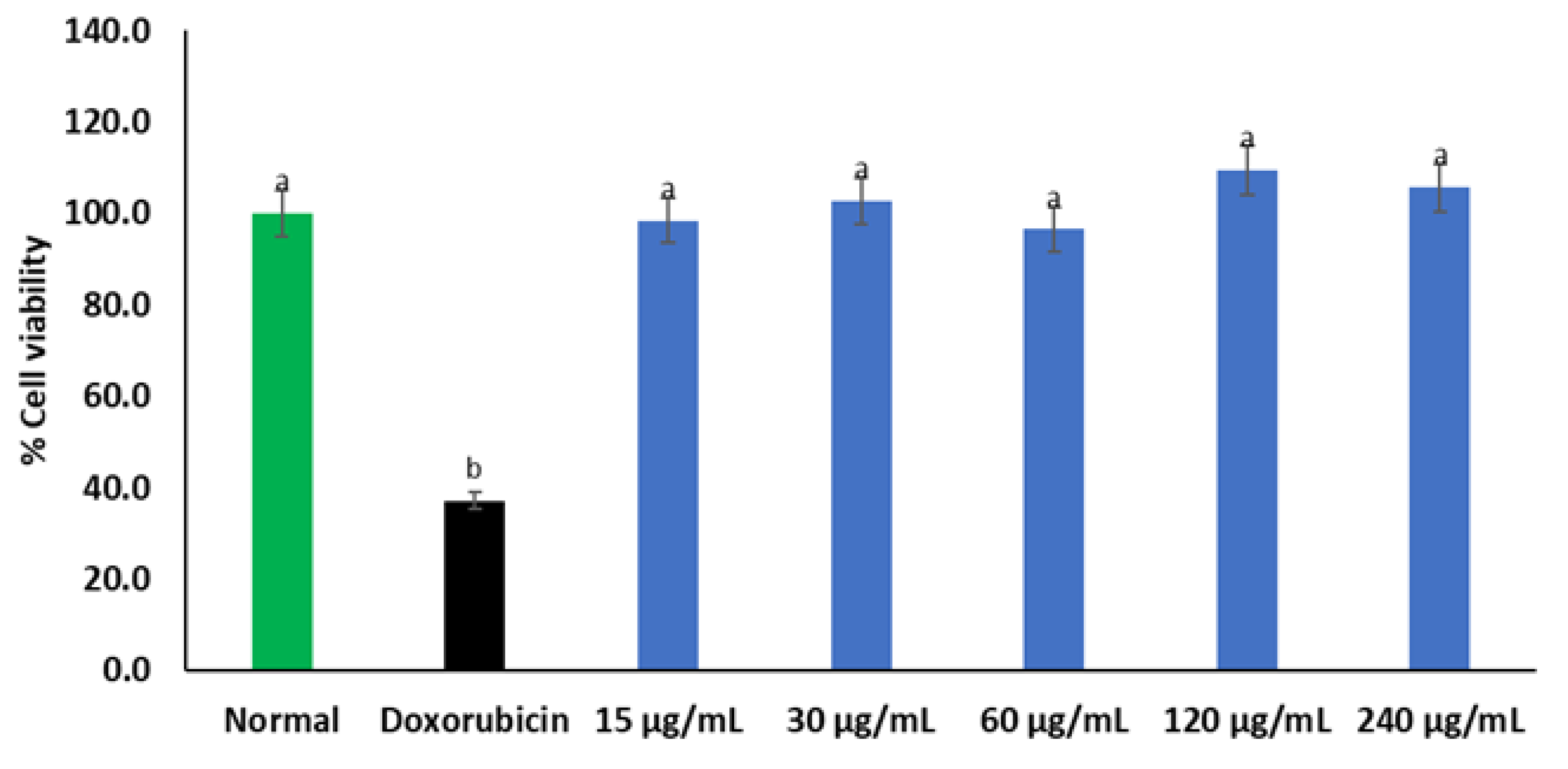
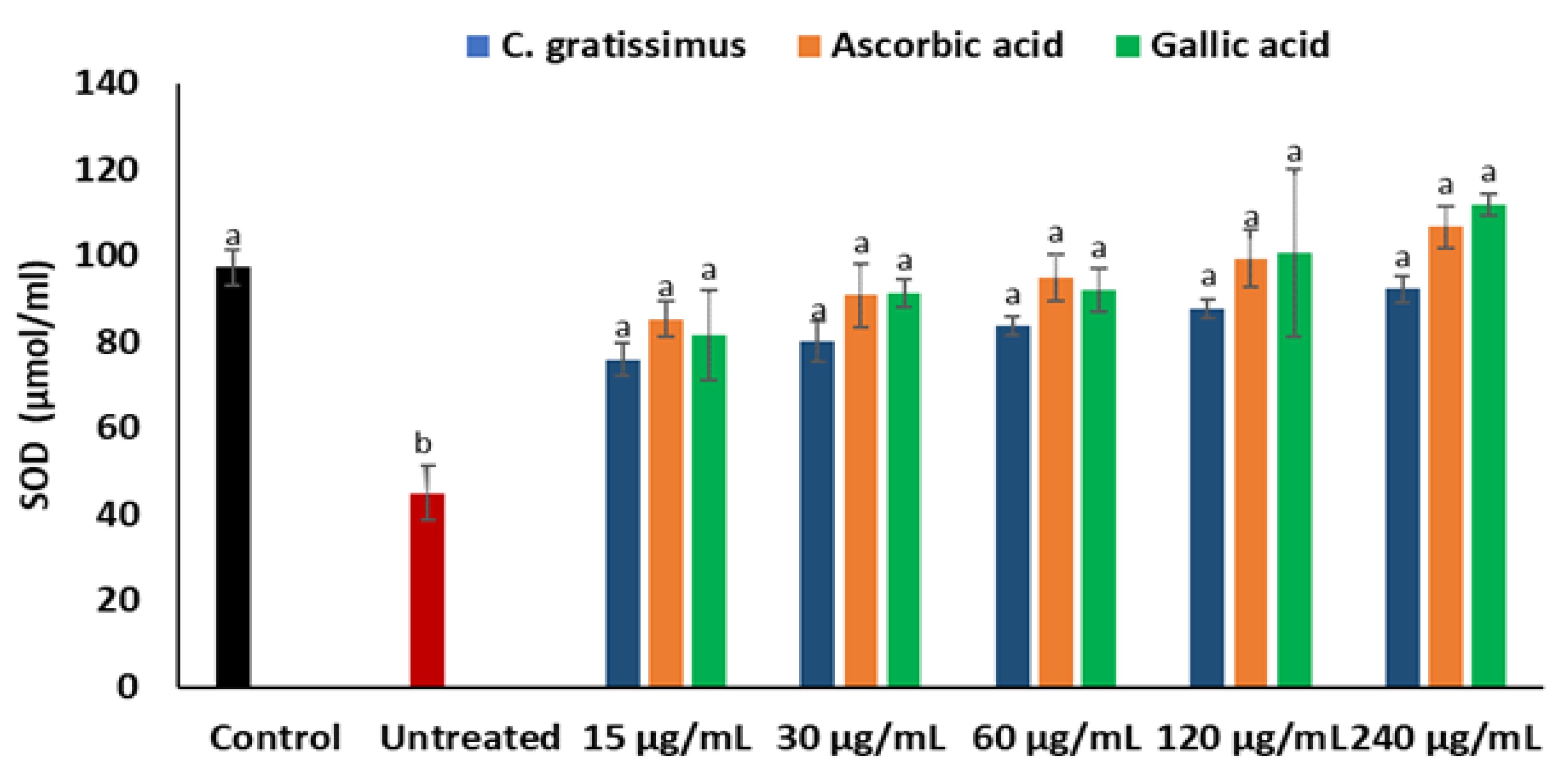
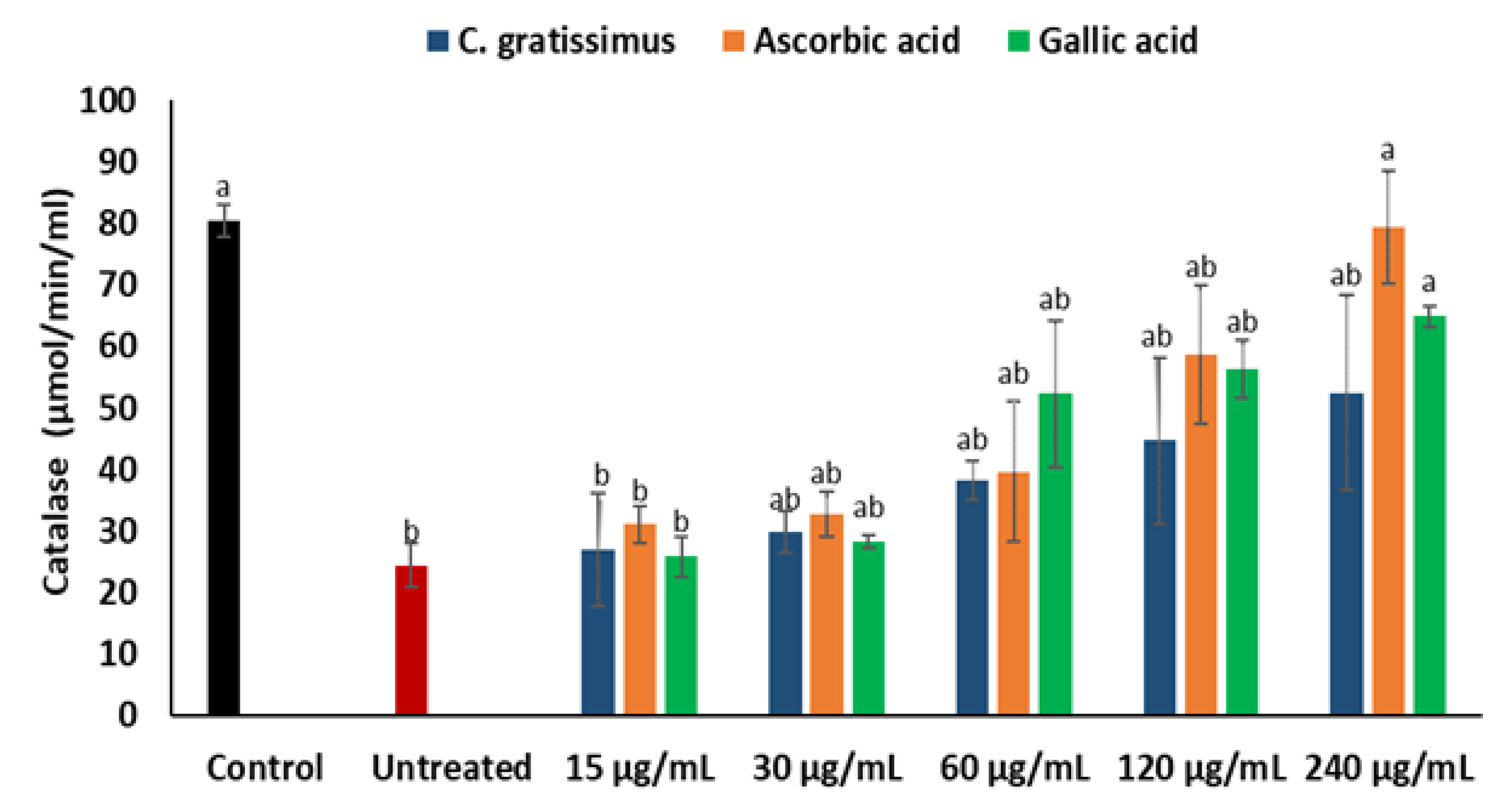
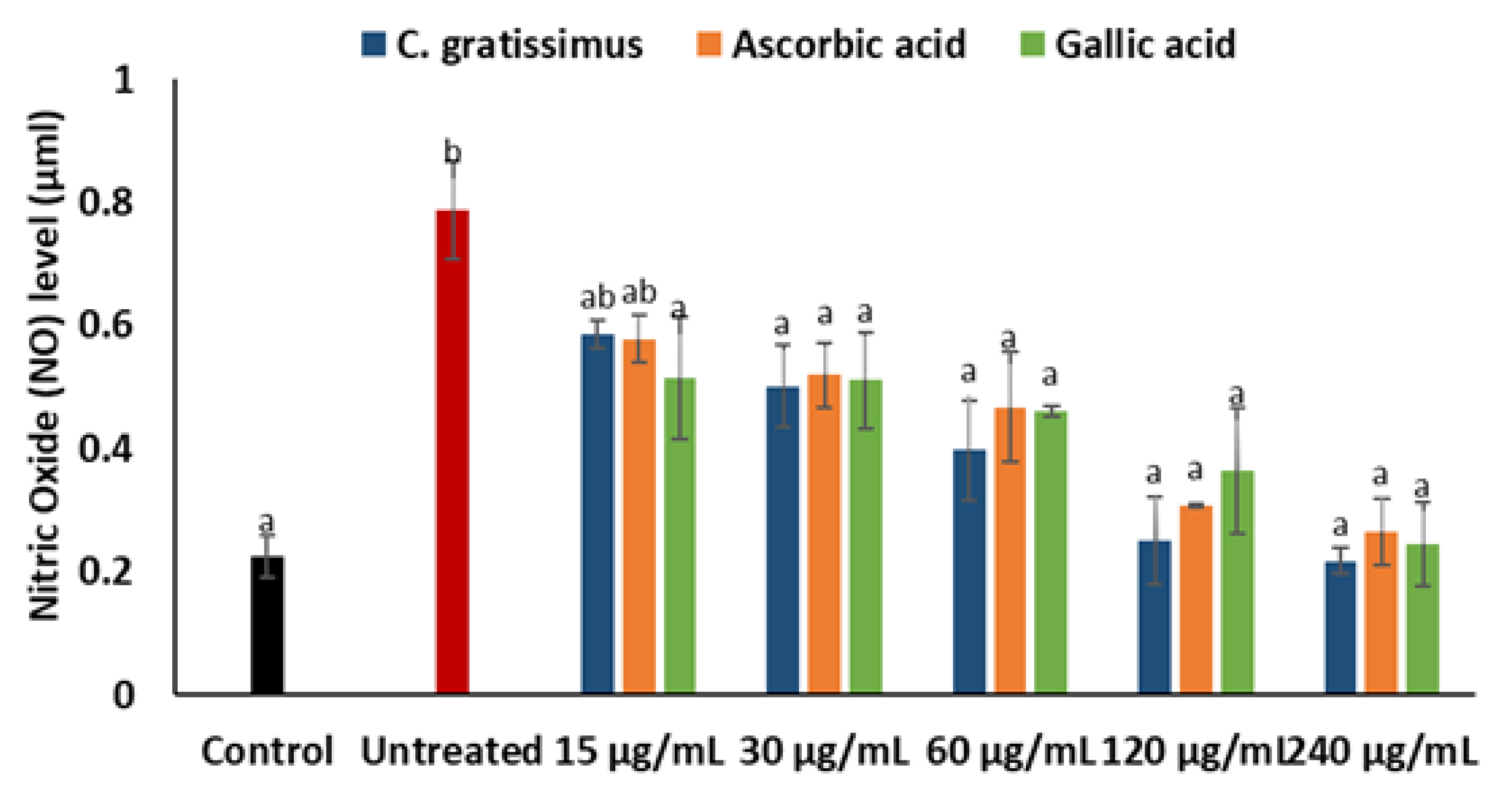
2. Results
3. Discussion
4. Materials and Methods
4.1. Procurement of Herbal Tea Bags and Extraction
4.2. Phytochemical Screening and Characterization
4.2.1. Estimation of Total Phenolic Content
4.2.2. Estimation of Total Flavonoid Content
4.3. Characterization of C. gratissimus Phytoconstituents with High-Performance Liquid Chromatography-Mass Spectrophotometry (HPLC-MS)
4.4. Antioxidant In-Vitro Screening of Herbal the Infusion
4.4.1. Diphenyl-2-Picryl-Hydrazyl (DPPH) Radical Scavenging Activity
4.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.5. Cell Culture and Cytotoxicity Screening
4.5.1. Cell Maintenance
4.5.2. MTT Assay
4.6. Pre-Treatment and Induction of Oxidative Injury on Chang Liver Cells
4.7. Determination of Reduced Glutathione (GSH) Level
4.8. Determination of Superoxide Dismutase (SOD) Activity
4.9. Determination of Catalase Activity
4.10. Determination of Malondialdehyde (MDA) Levels
4.11. Determination of Nitric Oxide (NO) Level
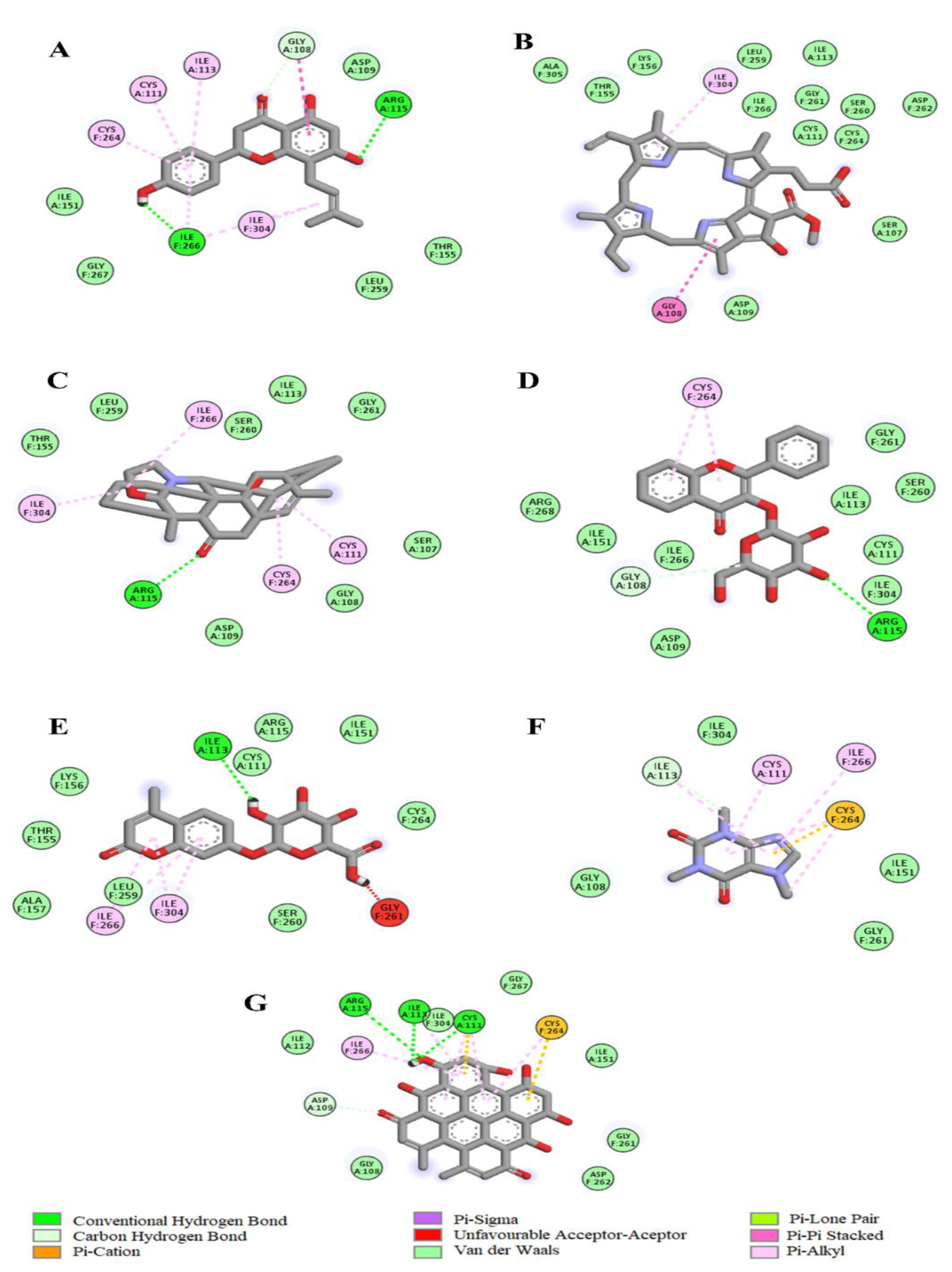
4.12. Molecular Docking Analysis of C. gratissimus Phytochemicals with Enzymatic Antioxidant Protein Targets
4.13. Pharmacokinetics and Drug-Likeness Analysis of C. gratissimus Phytochemical Constituents
4.14. Toxicity Prediction of C. gratissimus Phytochemical Constituents
4.15. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govind, P. Medicinal plants against liver diseases. Int. Res. J. Pharm. 2011, 2, 115–121. [Google Scholar]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Goering, P.; Barber, D. Hepatotoxicity of copper, iron, cadmium, and arsenic. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 501–526. [Google Scholar] [CrossRef]
- Mehta, K. Oxidative Stress in Iron Toxicity of the Liver. In The Liver; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–54. [Google Scholar] [CrossRef]
- Mfotie Njoya, E.; Eloff, J.N.; McGaw, L.J. Croton gratissimus leaf extracts inhibit cancer cell growth by inducing caspase 3/7 activation with additional anti-inflammatory and antioxidant activities. BMC Complement. Altern. Med. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Magwilu, K.D.; Nguta, J.M.; Mapenay, I.; Matara, D. Phylogeny, Phytomedicines, Phytochemistry, Pharmacological Properties, and Toxicity of Croton gratissimus Burch (Euphorbiaceae). Adv. Pharm. Pharm. Sci. 2022, 2022, 1238270. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Langat, M.K.; Crouch, N.R.; Coley, H.M.; Mutambi, E.M.; Nuzillard, J.-M. Cembranolides from the stem bark of the southern African medicinal plant, Croton gratissimus (Euphorbiaceae). Phytochemistry 2010, 71, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Moremi, M.P.; Makolo, F.; Viljoen, A.M.; Kamatou, G.P. A review of biological activities and phytochemistry of six ethnomedicinally important South African Croton species. J. Ethnopharmacol. 2021, 280, 114416. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Aderogba, M.A.; Ncube, B.; Van Staden, J. Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related Croton species. Molecules 2013, 18, 1916–1932. [Google Scholar] [CrossRef]
- Mthethwa, N.S.; Oyedeji, B.A.; Obi, L.C.; Aiyegoro, O.A. Anti-staphylococcal, anti-HIV and cytotoxicity studies of four South African medicinal plants and isolation of bioactive compounds from Cassine transvaalensis (Burtt. Davy) codd. BMC Complement. Altern. Med. 2014, 14, 512. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Hu, X.; Liu, Z.; Chen, J.; Lu, Y.; Liu, J.; Liao, S.; Zhang, Y.; Liang, R. The hepatoprotective role of reduced glutathione and its underlying mechanism in oxaliplatin-induced acute liver injury. Oncol. Lett. 2018, 15, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Schuppan, D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007, 39, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Liu, W.-Y.; Liang, Q. Chemical constituents from Croton species and their biological activities. Molecules 2018, 23, 2333. [Google Scholar] [CrossRef] [PubMed]
- Nagulendran, K.; Velavan, S.; Mahesh, R.; Begum, V.H. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. J. Chem. 2007, 4, 440–449. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.; Ali, H.; Sarwat, M.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Han, D.; Hanawa, N.; Saberi, B.; Kaplowitz, N. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1–G7. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, H.; Thompson, D.; Shertzer, H.; Nebert, D.; Vasiliou, V. Glutathione defense mechanism in liver injury: Insights from animal models. Food Chem. Toxicol. 2013, 60, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Erukainure, O.L.; Mopuri, R.; Oyebode, O.A.; Koorbanally, N.A.; Islam, M.S. Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed. Pharmacother. 2017, 96, 37–47. [Google Scholar] [CrossRef]
- Mathan Kumar, S.; Dey, A. Regulation of Glutathione in Health and Disease with Special Emphasis on Chronic Alcoholism and Hyperglycaemia Mediated Liver Injury: A Brief Perspective. Springer Sci. Rev. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver injury induced by carbon tetrachloride in mice is prevented by the antioxidant capacity of Anji white tea polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Thornley-Brown, D.; Freeman, B.A. Reactive species in sickle cell disease. Ann. N. Y. Acad. Sci. 2000, 899, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Mihas, A.A.; Kanji, V.K.; Mihas, T.A.; Joseph, R.M.; Markov, A.K.; Heuman, D.M. Fructose diphosphate attenuates the acetaminophen-induced liver injury in the rat evidence for involvement of nitric oxide. Res. Commun. Mol. Pathol. Pharmacol. 2003, 113, 253–266. [Google Scholar]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Azadmanesh, J.; Borgstahl, G.E. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Cytotoxicity of African medicinal plants against normal animal and human cells. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–233. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Mahgoub, R.E.; Atatreh, N.; Ghattas, M.A. Using filters in virtual screening: A comprehensive guide to minimize errors and maximize efficiency. Ann. Rep. Med. Chem. 2022, 59, 99–136. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Beseni, B.K.; Olofinsan, K.A.; Salau, V.F.; Erukainure, O.L.; Islam, M.S. Rhus longipes (Engl.) infusions improve glucose metabolism and mitigate oxidative biomarkers in ferrous sulfate-induced renal injury. Asian Pac. J. Trop. Biomed. 2022, 12, 453. [Google Scholar] [CrossRef]
- Ma, Y.; Hashi, Y.; Ji, F.; Lin, J.M. Determination of phthalates in fruit jellies by dispersive SPE coupled with HPLC-MS. J. Sep. Sci. 2010, 33, 251–257. [Google Scholar] [CrossRef]
- Olawale, F.; Olofinsan, K.; Ogunyemi, O.M.; Karigidi, K.O.; Gyebi, G.A.; Ibrahim, I.M.; Iwaloye, O. Deciphering the therapeutic role of Kigelia africana fruit in erectile dysfunction through metabolite profiling and molecular modelling. Inform. Med. Unlocked 2023, 37, 101190. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Rademan, S.; Erhabor, J.O.; Chukwuma, C.I.; Nde, A.L.; Matsabisa, M.G. Cannabis sativa L. protects against oxidative injury in kidney (vero) cells by mitigating perturbed metabolic activities linked to chronic kidney diseases. J. Ethnopharmacol. 2022, 293, 115312. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar] [PubMed]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief. 2016, 6, 194–199. [Google Scholar] [CrossRef]
- Chowdhury, P.; Soulsby, M. Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Ann. Clin. Lab. Sci. 2002, 32, 188–192. [Google Scholar]
- Tsikas, D. Review Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Olofinsan, K.A.; Erukainure, O.L.; Brian, B.K.; Islam, M.S. Harpephyllum caffrum stimulates glucose uptake, abates redox imbalance and modulates purinergic and glucogenic enzyme activities in oxidative hepatic injury. Pac. J. Trop. Biomed. 2022, 12, 9–19. [Google Scholar] [CrossRef]




| Antioxidative Activity | C. gratissimus | Ascorbic Acid | Gallic Acid |
|---|---|---|---|
| DPPH | 0.01 | 0.01 | 0.02 |
| FRAP | >1000 | 229.82 | 42.12 |
| Compounds | [M + H] m/z | Ion Intensity (Area) | Molecular Weight | Molecular Formular |
|---|---|---|---|---|
| 8-Prenylnaringenin | 341.1 | 6,745,309.5 | 340.3 | C20H20O5 |
| Pheophorbide a | 593.2 | 131,495.5 | 592.6 | C35H36N4O5 |
| Spirasine I | 355.4 | 1,006,042.8 | 355.4 | C22H29NO3 |
| Flavonol 3-O-D-galactoside | 401.1 | 941,414.5 | 400.3 | C21H20O8 |
| 4-Methylumbelliferone glucuronide | 353.0 | 117,078,415.1 | 352.3 | C16H16O9 |
| Caffeine | 195.1 | 21,930,284.6 | 195.1 | C8H10N4O2 |
| Hypericin | 505.1 | 3,182,449.0 | 504.4 | C30H16O8 |
| LD50 (mg/kg) | Toxicity Class | Hepatotoxicity | Nrf/ARE | Catalase | SOD | |
|---|---|---|---|---|---|---|
| 8-Prenylnaringenin | 2000 | 4 | No | No | −10.6 | −6.6 |
| Pheophorbide-a | 40 | 2 | No | No | −12.6 | −4.7 |
| Spirasine I | 260 | 3 | No | No | −6.0 | −7.0 |
| Flavonol 3-O-D-galactoside | 5000 | 5 | No | No | −9.0 | −6.6 |
| 4-Methylumbelliferone glucuronide | 5000 | 5 | No | No | −10.3 | −7.6 |
| Caffeine | 127 | 3 | No | No | −7.5 | −4.6 |
| Hypericin | 1000 | 4 | No | No | −10.3 | −6.7 |
| TPSA | LOGP | GIA | CYP1A2 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | No of Lipinski Violations | No of Veber Violations | No of PAINS Alerts | No of Leadlikeness Violations | |
|---|---|---|---|---|---|---|---|---|---|---|
| 8-Prenylnaringenin | 86.99 | 2.59 | High | Yes | Yes | Yes | 0 | 0 | 0 | 1 |
| Pheophorbide-a | 132.94 | 3.31 | Low | No | No | No | 1 | 0 | 0 | 1 |
| Spirasine I | 49.77 | 2.96 | High | No | No | No | 0 | 0 | 0 | 1 |
| Flavonol 3-O-D-galactoside | 129.59 | 1.71 | High | No | No | No | 0 | 0 | 0 | 1 |
| 4-Methylumbelliferone glucuronide | 146.66 | 1.04 | Low | No | No | No | 0 | 1 | 0 | 1 |
| Caffeine | 61.82 | 1.79 | High | No | No | No | 0 | 0 | 0 | 1 |
| Hypericin | 155.52 | 3.10 | Low | No | No | No | 2 | 1 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncume, P.V.; Salau, V.F.; Mtshali, S.; Olofinsan, K.A.; Erukainure, O.L.; Matsabisa, M.G. Phytochemical Properties of Croton gratissimus Burch (Lavender Croton) Herbal Tea and Its Protective Effect against Iron-Induced Oxidative Hepatic Injury. Plants 2023, 12, 2915. https://doi.org/10.3390/plants12162915
Ncume PV, Salau VF, Mtshali S, Olofinsan KA, Erukainure OL, Matsabisa MG. Phytochemical Properties of Croton gratissimus Burch (Lavender Croton) Herbal Tea and Its Protective Effect against Iron-Induced Oxidative Hepatic Injury. Plants. 2023; 12(16):2915. https://doi.org/10.3390/plants12162915
Chicago/Turabian StyleNcume, Paul V., Veronica F. Salau, Sibahle Mtshali, Kolawole A. Olofinsan, Ochuko L. Erukainure, and Motlalepula G. Matsabisa. 2023. "Phytochemical Properties of Croton gratissimus Burch (Lavender Croton) Herbal Tea and Its Protective Effect against Iron-Induced Oxidative Hepatic Injury" Plants 12, no. 16: 2915. https://doi.org/10.3390/plants12162915
APA StyleNcume, P. V., Salau, V. F., Mtshali, S., Olofinsan, K. A., Erukainure, O. L., & Matsabisa, M. G. (2023). Phytochemical Properties of Croton gratissimus Burch (Lavender Croton) Herbal Tea and Its Protective Effect against Iron-Induced Oxidative Hepatic Injury. Plants, 12(16), 2915. https://doi.org/10.3390/plants12162915






