Abstract
Obesity has become a worldwide epidemic and its prevalence continues to increase at an alarming rate. It is considered a major risk factor for the development of several comorbidities, including type 2 diabetes, stroke, other cardiovascular diseases and even cancer. Conventional treatments for obesity, such as dietary interventions, exercise and pharmacotherapy, have proven to have limited effectiveness and are often associated with undesirable side effects. Therefore, there is a growing interest in exploring alternative therapeutic approaches. Nigella sativa (NS), a medicinal plant with multiple pharmacological properties, has gained attention due to its potential role in the treatment of obesity and its associated complications. The aim of this review is therefore to assess the effects of NS on obesity and its complications and to provide insights into the underlying mechanisms. From this review, NS appears to play a complementary or supportive role in the treatment of obesity and its complications. However, future studies are needed to verify the efficacy of NS in the treatment of obesity and its complications and to prove its safety so that it can be introduced in patients with obesity.
1. Introduction
In recent years, obesity rates have risen alarmingly worldwide, in both developed and developing countries [1]. It currently affects more than one-third of the world’s population, including both overweight and obese people [2]. If the present patterns persist, it is projected that by 2030 approximately 38% of global adult population will be overweight and another 20% will fall into the obese category [3]. If not properly addressed, the problem could lead to many dreaded complications that increase mortality and morbidity in the population. Obesity is considered an important risk factor for the development of several comorbidities, including type 2 diabetes, stroke, other cardiovascular diseases and even cancer [4,5]. Prevention and management of this problem are crucial to minimise the impact on the global community.
Current treatment strategies for obesity focus primarily on lifestyle changes, including dietary interventions, physical activity, behavioural therapy and pharmacotherapeutic interventions [6]. However, it has been proven difficult to maintain long-term weight loss, as only a small percentage of people (5–10%) are able to sustain their weight loss over time [7,8]. Adopting a plant-based diet that targets lower calorie density has also shown encouraging results in relation to weight loss, leading to lower cholesterol levels and improved insulin sensitivity [9,10]. However, for some people this approach is challenging and cannot be sustained over a long period of time. In addition, there are a variety of pharmaceutical interventions to combat obesity, most of which focus on appetite suppression mechanisms to increase energy expenditure and limit calorie intake [11,12,13]. However, anti-obesity drugs are often associated with various adverse effects and their efficacy decreases after prolonged use [14]. The multifactorial aetiology of obesity requires multiple intervention strategies beyond traditional drug therapies that are better accepted by patients [15]. Suboptimal outcomes have also been observed after discontinuation of lifestyle modifications or pharmacotherapy, highlighting the need for alternative or complementary therapeutic options to achieve better and more durable weight loss outcomes in obesity [16].
Among the modalities of complementary and alternative medicine (CAM), herbal supplements and nutritional therapies have emerged as prominent modalities related to obesity interventions [17]. Extensive research on natural products and medicinal plants, including crude extracts and isolated compounds, has shown that they have the potential to induce weight loss and prevent obesity [18,19,20]. Their efficacy can be attributed to the different phytochemical components they contain, each of which has different effects against obesity and as antioxidants by modulating body metabolism and fat oxidation [21,22]. In addition, medicinal herbs are more accessible, less expensive and generally have fewer side effects than synthetic drugs. One such herb that has shown promise in weight loss and combating obesity is Nigella sativa (NS) [23].
NS commonly known as black cumin, is a medicinal plant belonging to the Ranunculaceae family [24]. It is mainly cultivated in the countries of the Middle East and Southwest Asia and has been used in traditional medicine for centuries due to its diverse medicinal properties. NS has been used to treat various diseases for 2000 years [25]. In traditional Arab and Indian culture, it is often used as food and medicine [26,27,28]. In Islamic culture it is called “El Habba Saouda (Seeds of Blessing)” and is known as a medicinal plant traditionally used for all diseases except death [29]. Avicenna (Ibn Sina), a famous 10th century physician and the father of early modern medicine, described several benefits of NS in increasing body energy [23].
Numerous studies have NS potential therapeutic effects in diseases such as diabetes mellitus [30,31], cancer [32,33] and neuroprotective activity [34]; and gastroprotective activity [35], cardioprotective activity [36,37], anti-dyslipidaemia and anti-obesity activity [38], to name a few. In addition, NS has anti-inflammatory activity, inhibits proinflammatory cytokines and eosinophils in rheumatoid arthritis [39] and responds well to the treatment of psoriasis [40]. In terms of antioxidant activity, the fatty acid compound in NS is able to suppress the excessive formation of ROS [41]. NS has also responded well to anti-cancer activity, as all agents have a positive anti-cancer effect [33]. It has induced apoptosis in blood cancers [42] and it has significantly reduced the viability of breast cancer cells [43]. There are several bioactive compounds present in NS, including thymoquinone (TQ), thymohydroquinone and dithymoquinone, which have anti-inflammatory and antioxidant properties and contribute to NS various pharmacological potentials [24]. These compounds target multiple signalling pathways involved in adipogenesis [44], lipid metabolism [45] and appetite regulation [46], making NS a potential alternative intervention for obesity.
While several reviews have examined the broad pharmacological effects of NS, there is little literature that specifically addresses its effects on obesity and its associated complications. To address this research gap, the aim of this study is to comprehensively review the existing literature on the potential therapeutic benefits of NS on obesity and its associated complications. This includes a comprehensive analysis of data derived from various animal models, clinical trials and in vitro studies. This review will contribute to the existing body of knowledge by consolidating the available evidence and providing a basis for future studies aimed at harnessing the therapeutic potential of NS in the fight against obesity. Understanding the potential benefits and limitations of NS as a complementary or alternative treatment for obesity could pave the way for the development of new therapeutic strategies.
2. Methods
A literature search was conducted to identify and present relevant articles related to the effects of NS on obesity. Peer-reviewed full-text English articles up to May 2023 were collected from electronic databases, including Scopus, MEDLINE via EBSCOhost and Google Scholar. The following keywords were used: (1) “Nigella sativa” or “Nigella sativa oil” or “Nigella sativa extracts” and (2) “obesity”. The literature search was supplemented with references to related review articles and scientific reports found in the search results. All studies, including in vitro, in vivo and human studies, reporting the effects of NS or NS oil or NS seeds on parameters related to obesity and its associated complications were included in this review. On the other hand, studies on NS, which did not address parameters of obesity and related complications were excluded from this study.
3. Results and Discussions
3.1. Chemical Composition of Nigella sativa
Numerous chemical compounds have been identified in NS. The compounds may vary depending on the area of cultivation, degree of ripeness, processing methods and isolation techniques. Different extraction methods or solvents, such as in oil samples extracted with a cold press, hexane, tetrahydrofuran, ethanol, dichloromethane and methanol, yielded different amounts of chemical compounds as the methods used affected the oil quality [47]. NS consisted of significant amounts of iron, copper, zinc, phosphorus, calcium, thiamine, niacin, pyridoxine, and folic acid [27,48,49]. In addition, it had maximum nutritional value with a significant amount of 20–85% vegetable protein, 7–94% fibre, 38.02% fat and 31.94% carbohydrate [50]. Apart from these, NS also contains bioactive phytochemicals such as terpenes and terpenoids, phytosterols, alkaloids, tocols and polyphenols [23]. Table 1 lists the chemical composition of NS.
The main constituent of NS, which is considered to have to medicinal value, is TQ [24]. TQ (2-isopropyl-5-methylbenzo-1, 4-quinone) is the main constituent of the volatile oil of the NS seeds [51]. TQ can be found in tautomeric forms, such as the enol form, the keto form, and in combination. The pharmacological properties of this compound are due to the keto form, which constitutes the major part (about 90%) of the compound [51]. TQ is a promising compound for modern pharmacology because it has been shown to act as a modulator in several pharmacological pathways involving inflammatory responses, oxidative markers, apoptosis, peroxisome proliferator-activated receptors (PPARs) and transcription factors [52].
The bioactive compounds in NS, especially TQ and polyphenols such as gallic acid, p-coumaric acid, naringenin and quercetin, are thought to play a role in the inhibitory effect of NS on the digestive enzyme α-amylase and glucose uptake in the intestine, which could explain its properties as an anti-obesity agent [53,54]. Further studies could focus on the chemical compounds in NS that mediate the anti-obesity effect, with a view to possibly extracting them and using them as an anti-obesity agent.

Table 1.
Lists of the chemical composition of NS.
Table 1.
Lists of the chemical composition of NS.
| Component | Composition | References |
|---|---|---|
| Fatty acid | Linoleic acid, oleic acid, lauric acid, stearic acid, linolenic acid | [55,56] |
| Vitamin | Ascorbic acid, tiamin, riboflavin, pyridoxine, niacin | [56,57] |
| Mineral | Calcium, magnesium, potassium, phosphorus and iron | [58] |
| Alkaloids | Nigellidine, nigeglanine, nigelanoid, 17-O-(β-d-glucopyranosyl)-4-O-methylnigellidine, 4-O-methylnigeglanine | [59] |
| Terpenes and Terpenoids | Thymoquinone, thymohydroquinone, dithymoquinone, p-cymene, sesquiterpene longifolene | [23,55] |
| Polyphenols | Apigenin, naringenin, gallic acid, rutin, quercetin, kaempferol | [60] |
| Phytosterols | Campesterol, stigmasterol, β-sitosterol | [61] |
| Tocols | β-tocotrienol, γ-tocopherol isomer, β-sitosterol | [55] |
| Saponin | Alpha-hederin (α-HN), 3-O-(β-d-xylopyranosyl-(1-3)-α-l-rhamnopyrnaosyl-(1-2)-α-l-arabinopyranosyl]-28-O-(α-l-rhamno-pyranosyl-(1-4)-β-d-glucopyranosyl-(1-6)-β-d-glucopyranosyl] hederagenin, 3-O-[α-L-rhamnopyranosyl-(1-2)-α-L-arabinopyranosyl]-28-O-[α-L-rhamnopyranosyl-(1-4)-β-D-glucopyranosyl-(1-6)-\β-D-glucopyranosyl]-hederagenin and 3-O-[β-D-xylopyranosyl-(1-3)-α-L-rhamnopyranosyl-(1-2)-α-L-arabinopyranosyl]-hederagenin | [62,63,64] |
3.2. Effects of NS on Obesity and Its Associated Complications
3.2.1. Preclinical Studies
The prevalence of obesity is rapidly increasing worldwide. It is generally accepted that natural products that have been shown to be safe can be used to combat obesity [65]. A previous in vivo study showed that rats treated with NS oils had a significant decrease in body weight (BW) and a lower risk of developing hyperglycaemia and hyperlipidaemia (serum total cholesterol and triglyceride (TG) levels compared to high-fat-diet (HFD) rats [66]. Rats receiving petroleum ether extract from NS for four weeks also showed a 25% decrease in food intake, resulting in transient weight loss. Remarkably, the animals fed NS had lower fasting plasma levels of insulin and TG and higher HDL cholesterol compared to control rats [67]. Another in vivo study demonstrated the therapeutic and protective effects of various extracts of NS (TQ, hydroalcoholic and hexane extracts) against oxidative stress induced by a HFD. The hydroalcoholic and hexane extracts were found to induce weight loss by having a positive effect on UCP-1, the index protein of brown adipose tissue, at gene and protein levels [68].
Treatment with NS also reduced BW and food intake without affecting water intake. There is evidence that NS may reduce BW through the mechanism of appetite suppression. The suppression of appetite might be associated with the neuronal circuits responsible for regulating the catecholaminergic, serotonergic and peptidergic systems [69]. Furthermore, the effect could be mediated via the signalling of the hormone leptin in the satiety centre of the brain, leading to hypophagic effects and subsequent weight loss in experimental animals [70]. Previous research has also reported that consuming NS could lead to an increase in ghrelin, one of the peptides that regulate appetite [71].
At the same time, HFD intake leads to obesity by promoting positive energy balance and the deposition of visceral fat [20]. In in vivo studies, 6-week administration of NS to obese male albino rats fed HFD resulted in a significant improvement in lipid profile to normal levels and a reduction in final body size, serum atherogenic index (AI) and levels of ALT. Histopathological analysis revealed that treatment with NS significantly improved liver lesions in the livers of HFD rats that previously exhibited steatosis features [72]. It was postulated that oxidative damage to hepatocellular proteins and hepatocellular necrosis leading to abnormal hepatocytes is responsible for the altered liver architecture [73]. The beneficial effects of NS in this study may be due to TQ, the main active ingredient of NS. In addition, a previous study has shown that NS fixed oil has low toxicity with a high LD50 and does not cause histopathological changes in the heart, liver, kidney and pancreas tissues of treated rats [74]. In another study, administration of TQ, the major bioactive quinone produced by NS, in combination with omega-3-ω3 fish oil was shown to reduce obesity-associated insulin resistance and the chronic inflammatory state of obesity in mice with HFD [75].
In another study, obese rats that received an extract of NS black cumin for six weeks had significantly lower blood glucose, serum cholesterol, TG and low-density lipoprotein (LDL) levels and significantly higher levels of high-density lipoprotein (HDL) compared to controls [69]. It is plausible that NS may have contributed to lower blood glucose levels via two potential mechanisms: restoration of glucose homeostasis and improvement of insulin sensitivity of liver cells [67].
Obesity is often associated with the build-up of adipose tissue, which is widely recognised as a common phenomenon [76,77,78]. Complex diseases such as obesity and insulin resistance are due to alterations in the production of adipokines by adipose tissue [79]. In a recent study, treatment with NS seed extract at various doses of 100–400 mg/kg showed dependent amelioration of high-fat-diet (HFD)-induced obesity in mice compared to metformin (250 mg/kg) [80]. Fat formation and adipocyte hypertrophy were significantly and dose-dependently reduced by all three doses of the extracts from the seeds of NS. In particular, administration of the extract from the seeds of NS at a dose of 200 mg/kg showed significant inhibition of fat formation and adipocyte hypertrophy induced by a high-fat diet compared to the metformin (250 mg/kg) treated group. These results thus suggest that oral administration of NS seed extracts (400, 200 and 100 mg/kg) shows a dose-dependent trend in improving obesity compared to metformin (250 mg/kg).
An in vitro study has shown that the lipase inhibitor cocktail RAYstat4ns, isolated and purified from the seeds of NS, exerts good lipase inhibitory activity on pancreatic lipase with mixed types of inhibition as well as inhibition of hormone-sensitive lipase. These NS-based lipase inhibitors have the potential to treat obesity and prevent type 2 diabetes by controlling lipolysis and insulin resistance. They also suppress the uptake of dietary fats into the living system [81]. Table 2 shows the summary of preclinical studies of NS against obesity and its complications.

Table 2.
Summary of preclinical studies of NS against obesity and its complications.
3.2.2. Clinical Studies
BW and waist circumference are the ideal anthropometric predictors of abdominal obesity that can be used to predict cardiovascular disease, type 2 diabetes mellitus, metabolic syndrome and other chronic diseases. In a clinical trial of obese women who followed a low-calorie diet and received 3 g/day NS oil, BW, TG and very low-density lipoprotein (VLDL) levels and waist circumference (WC) decreased significantly at the end of the 8-week study period compared to the placebo group [82]. It is possible that NS produces these effects due to its high unsaturated fatty acid content, antioxidant and anti-inflammatory components. Previously, it was shown that the hypotriglyceridaemic effect of NS could be due to the presence of unsaturated fatty acids [83]. Unsaturated fatty acids may modulate the levels of TG and VLDL by affecting the synthesis and degradation of TG-rich lipoproteins. Moreover, NS antioxidants such as TQ and ter-butylhydroquinone (TBHQ) could prevent lipid peroxidation and improve enzyme function in lipid metabolism [84,85]. Meanwhile, a follow-up study showed that the intake of NS oil (3 g/day) in obese women led to a reduction in body fat mass (BFM) and insulin levels, while adiponectin levels increased compared to the placebo group. Adiponectin is an adipokine that is mainly secreted by adipose tissue and has anti-inflammatory and insulin-sensitising properties. However, no significant changes were observed in body mass index (BMI), insulin sensitivity and nuclear receptor PPAR-γ [86]. Liver enzymes also did not change significantly after the intervention, and none of the participants experienced severe side effects, demonstrating the safety of NS. The findings of this study show that taking NS oil in combination with a low-calorie diet can influence hormone secretion and body composition in obese women.
As shown in animal studies, the potential mechanisms of NS against obesity could include the reduction in appetite and food intake, as well as the active components of NS, such as TQ and thymol, which can influence fat metabolism and insulin secretion [87]. In addition, phytochemicals have been shown to have the ability to manifest their anti-obesity properties through many pathways, including inhibiting the activity of digestive enzymes such as pancreatic lipase and amylase, thereby modulating appetite and reducing white adipose tissue production [88].
Previous research has shown that central obesity is a risk factor that increases the likelihood of cardiovascular events and is associated with metabolic syndrome, insulin resistance and other metabolic disorders. According to a study of centrally obese men, a daily intake of 3000 mg NS resulted in significant reductions in BW, WC and systolic BP. Although not statistically different from the control group, several biochemical measures, including serum free testosterone, diastolic BP, fasting blood glucose (FBG), TG and HDL cholesterol, uric acid and hs-CRP were reduced [89]. To achieve better results, it has been suggested that the dose should be increased and the duration of the treatment should be prolonged. Meanwhile, in a study on obesity, diabetes and dyslipidaemia, patients treated with 6 weeks of standard treatment (atorvastatin 10 mg/day, metformin 500 mg/twice daily in tablet form) were compared with an experimental group receiving the same treatment with NS oil 2.5 mL/twice daily for the same period. In this study, the experimental group showed significantly better results in total cholesterol, low-density lipoproteins (LDL) and FBG levels. In addition, the NS group also showed a decrease in BW, BMI and abdominal circumference, although the difference was not significant [83]. In a comparative study between obese prediabetics taking NS oil soft gelatine capsules 450 mg twice daily and those receiving metformin 500 mg tablets twice daily, anthropometric (BW, BMI), glycaemic, lipid and inflammatory markers were assessed before and six months after the interventions. It was found that both groups showed equivalent improvements in anthropometric (BW, BMI) and glycaemic indicators. In particular, NS improved the lipid panel and showed a significant reduction in TNF-α levels, in contrast to the metformin group [90]. To determine the exact effect of NS on obesity, further studies should be conducted with more participants, longer treatment periods and different NS doses in both sexes [46].
A recent study showed that anthropometric and body composition indices, including BW, BMI, WC, body fat percentage (BFP), BFM and visceral fat area, as well as appetite and satiety were improved in obese and overweight women through the daily consumption of 2000 mg of NS oil for two periods of eight weeks [46]. The anti-obesity effects of NS have been shown to be due to its bioactive constituents, including TQ, thymol, the lipase enzyme, and various unsaturated fatty acids, such as linolenic acid, linoleic acid, oleic acid, arachidonic acid, and eicosadienoic acid [67,91]. Meanwhile, numerous studies have shown that supplementation of NS can improve insulin sensitivity and metabolic status, leading to an improvement in metabolic profile and a significant reduction in BW [66,92,93]. Thus, the findings may suggest that the consumption of NS is a safe adjuvant therapy for the treatment of obesity in adult women.
In obese people, the level of reactive oxygen species (ROS) is increased and at the same time antioxidant defence mechanisms are reduced. Obesity is associated with increased oxidative stress, which can lead to the initiation and progression of inflammatory processes [94]. It should also be noted that the hormone leptin, which is produced by fat cells, also contributes to the triggering of oxidative stress [95]. Weight loss has been shown to strengthen antioxidant defences [96,97]. A study by Namazi et al. (2015) showed that an eight-week low-calorie diet supplemented with 3 g/day NS oil resulted in a significant reduction in BW and an increase in antioxidant superoxidase dismutase (SOD) in red blood cells in obese women compared to the placebo group [98]. This could be due to the fact that NS has a protective effect against free radical attacks [98]. A previous study showed that treatment of rats with hepatotoxicity-induced lipid peroxidation with 10 mg/kg TQ orally for 5 weeks decreased lipid peroxidation and increased SOD activity in liver tissue [99]. There is also evidence that the synergistic action of the antioxidant components in NS may protect tissues from lipid peroxidation and oxidative stress. The regulation and expression of antioxidant enzyme genes to defend against free radical attack has been proposed as another possible mechanism for the antioxidant effects of NS. In rats suffering from lipid peroxidation caused by hepatotoxicity, Wahab et al. found that TQ (10 mg/kg orally for 5 weeks) increased SOD activity in liver tissue and decreased lipid peroxidation. It has been postulated that TQ has the ability to maintain cell membrane integrity and attenuate the enhanced process of lipid peroxidation [99].
Another study found that consuming NS supplements helps obese people prevent cardiovascular diseases (CVD). Healthy overweight and obese women showed an overall improvement in their cardiovascular disease risk variables after consuming 2000 mg/day of NS for 8 weeks. Specifically, supplementation of NS also lowered the following parameters: systolic blood pressure (BP), TC/HDL-C ratio, serum glutamic-oxaloacetic transaminase, low-density lipoprotein cholesterol and elevated serum high-density lipoprotein cholesterol [100]. The overall improvement in cardiovascular disease risk factors showed that NS supplements may help prevent possible cardiovascular disease in adults with obesity. A more recent study with a similar subject group and intervention demonstrated that supplementation with NS oil had a positive effect on BW and improved important parameters of adipogenesis and obesity, such as the reduction in transcription levels and blood levels of TNF-α; the significant increase in AdipoR1 expression, serum adiponectin, gene expression and serum levels of PPAR-γ [44]; the increase in serum total antioxidant capacity (TAC) and the significant decrease in serum malondialdehyde (MDA) levels [101]. Table 3 shows a summary of clinical studies of NS against obesity and its complications. Meanwhile, Figure 1 summarises the effects of NS against obesity and its complications in animal and clinical studies.

Table 3.
Summary of clinical studies of NS against obesity and its complications.
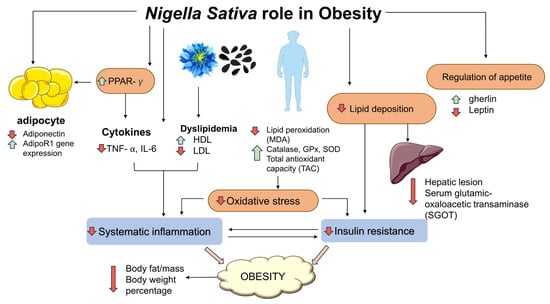
Figure 1.
Effects of N. sativa in obesity. Solid black lines indicate a mechanism associated with development of obesity, including oxidative stress, systemic inflammation and insulin resistance. The green arrows indicate increased effects and red arrows indicate decreased effects following NS administration. Malondialdehyde (MDA); superoxide dismutase (SOD); glutathione peroxidase (GPx; interleukin (IL); tumour necrosis factor-alpha (TNF-⍺); high density lipoprotein (HDL); low density lipoprotein (LDL).
3.3. Strength and Limitations of Study
The strength of the current review lies in its comprehensive approach, including both experimental and clinical studies, to establish NS as a potential intervention in the treatment of obesity. Nevertheless, it should be remembered that the omission of studies on metabolic syndrome and, to some extent, type 2 diabetes mellitus, which are often associated with obesity, may be a limitation in this review. However, as this review was intended to focus on the experimental model and the topic of obesity, the aforementioned limitation can be considered minor in the context of the defined scope and objectives of the review.
3.4. Future Perspective
To verify the effectiveness of NS in the treatment of obesity and its complications, future studies with a larger sample are needed to provide more accurate results and include both sexes. This will provide proof of its safety so that it can be introduced to patients with obesity. Further studies are needed to establish the mechanism of action of NS and its phytochemical compounds. This includes identifying the composition of NS sources, dosage, duration of intervention and the best method of administration to achieve optimal therapeutic benefit. Drug–herb interaction is another key factor to ensure safe administration of NS as a therapeutic intervention for the treatment of obesity. The consideration of these parameters will bring us one step closer to the safe introduction of NS as one of the alternative interventions in the treatment of obesity and its complications.
4. Conclusions
In summary, several preclinical studies have demonstrated the anti-obesity effects of NS and its compounds. In animal models treated with extracts of NS or its active constituents, a reduction in body weight, adipose tissue mass and serum fat levels was observed. These effects are thought to be mediated through modulation of adipocyte differentiation, lipolysis, fatty acid synthesis and energy expenditure. In addition, NS has been reported to regulate appetite by affecting neuropeptides involved in the signalling of hunger and satiety. Similarly, clinical studies show that NS has the potential to improve obesity by improving anthropometric parameters, restoring dyslipidaemia and reducing inflammatory markers TNF-a and SOD. Therefore, NS appears to play a complementary or supportive role in the treatment of obesity and its complications.
Author Contributions
Conceptualization, M.H.M., S.H.A. and I.F.A.; methodology, M.H.M., M.I.A.M.K. and D.A.M.K.; writing—original draft preparation, M.H.M., S.H.A., I.F.A., D.A.M.K., M.I.A.M.K. and A.F.; writing—review and editing, M.H.M., S.H.A. and A.F.; visualization, I.F.A. and D.A.M.K.; project administration, M.H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was funded by the Faculty of Medicine, Universiti Kebangsaan Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Singh, G.M.; Lu, Y.; Danaei, G.; Lin, J.K.; Finucane, M.M.; Bahalim, A.N.; McIntire, R.K.; Gutierrez, H.R.; Cowan, M.; et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Tronieri, J.S.; Butryn, M.L. Lifestyle modification approaches for the treatment of obesity in adults. Am. Psychol. 2020, 75, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.N. The historical development, efficacy and safety of very-low-calorie diets. Int. J. Obes. 1981, 5, 195–208. [Google Scholar]
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes Metab. Syndr. Obes. 2016, 9, 37–46. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Natural Dietary and Medicinal Plants with Anti-Obesity Therapeutics Activities for Treatment and Prevention of Obesity during Lock Down and in Post-COVID-19 Era. Appl. Sci. 2021, 11, 7889. [Google Scholar] [CrossRef]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Canuto, R.; Garcez, A.; de Souza, R.V.; Kac, G.; Olinto, M.T.A. Nutritional intervention strategies for the management of overweight and obesity in primary health care: A systematic review with meta-analysis. Obes. Rev. 2021, 22, e13143. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Kang, S.M.; Kang, J.H.; Kang, S.Y.; Kim, K.K.; Kim, K.B.; Kim, B.; Kim, S.J.; Kim, Y.H.; Kim, J.H.; et al. 2020 Korean Society for the Study of Obesity Guidelines for the Management of Obesity in Korea. J. Obes. Metab. Syndr. 2021, 30, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A systematic review of anti-obesity medicinal plants-an update. J. Diabetes Metab. Disord. 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, M.; Yao, H.; Liu, Y.; Gao, R. Herbal Medicine for the Treatment of Obesity: An Overview of Scientific Evidence from 2007 to 2017. Evid.-Based Complement. Altern. Med. 2017, 2017, 8943059. [Google Scholar] [CrossRef] [PubMed]
- Bertisch, S.M.; Wee, C.C.; McCarthy, E.P. Use of complementary and alternative therapies by overweight and obese adults. Obesity 2008, 16, 1610–1615. [Google Scholar] [CrossRef]
- Sun, N.N.; Wu, T.Y.; Chau, C.F. Natural Dietary and Herbal Products in Anti-Obesity Treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Ugusman, A.; Shahrin, S.A.S.; Azizan, N.H.; Pillai, S.B.; Krishnan, K.; Salamt, N.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Mokhtar, M.H. Role of Honey in Obesity Management: A Systematic Review. Front. Nutr. 2022, 9, 924097. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.D.; Lakhawat, S.S.; Yasmeen, N.; Pandey, A.; Singla, R.K. Biogenic Phytochemicals Modulating Obesity: From Molecular Mechanism to Preventive and Therapeutic Approaches. Evid. Based Complement. Altern. Med. 2022, 2022, 6852276. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing obesity through natural polyphenols: A review. Future Foods 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef] [PubMed]
- Forounzar, F.; Bazzaz, B.S.F.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its constituent (thymoquinone): A review on antimicrobial effects. Iran. J. Basic Med. Sci. 2018, 17, 929–938. [Google Scholar]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Shabana, A.; El-Menyar, A.; Asim, M.; Al-Azzeh, H.; Al Thani, H. Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc. Toxicol. 2013, 13, 9–21. [Google Scholar] [CrossRef]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical Profile and Antioxidant Activity of Nigella sativa L. Growing in Morocco. Sci. World J. 2021, 2021, 6623609. [Google Scholar] [CrossRef]
- Adam, S.H.; Mohd Nasri, N.; Kashim, M.; Abd Latib, E.H.; Ahmad Juhari, M.A.A.; Mokhtar, M.H. Potential health benefits of Nigella sativa on diabetes mellitus and its complications: A review from laboratory studies to clinical trials. Front. Nutr. 2022, 9, 1057825. [Google Scholar] [CrossRef]
- Hamdan, A.; Haji Idrus, R.; Mokhtar, M.H. Effects of Nigella sativa on Type-2 Diabetes Mellitus: A Systematic Review. Int. J. Env. Res. Public. Health 2019, 16, 4911. [Google Scholar] [CrossRef]
- Torres, M.P.; Ponnusamy, M.P.; Chakraborty, S.; Smith, L.M.; Das, S.; Arafat, H.A.; Batra, S.K. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: Implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010, 9, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Chen, H.C.; Tania, M.; Zhang, D.Z. Anticancer activities of Nigella sativa (black cumin). Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Coskun, O.; Uysal, H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch. Toxicol. 2006, 80, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F.; Rais Mustafa, M.; Jaarin, K. Nigella sativa and Its Protective Role in Oxidative Stress and Hypertension. Evid. Based Complement. Altern. Med. 2013, 2013, 120732. [Google Scholar] [CrossRef]
- Fallah Huseini, H.; Amini, M.; Mohtashami, R.; Ghamarchehre, M.E.; Sadeqhi, Z.; Kianbakht, S.; Fallah Huseini, A. Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2013, 27, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J. Endocrinol. Investig. 2014, 37, 1031–1040. [Google Scholar] [CrossRef]
- Arjumand, S.; Shahzad, M.; Shabbir, A.; Yousaf, M.Z. Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-alpha, IL-1, and NFkappaB expression levels. Biomed. Pharmacother. 2019, 111, 958–963. [Google Scholar] [CrossRef]
- Dwarampudi, L.P.; Palaniswamy, D.; Nithyanantham, M.; Raghu, P.S. Antipsoriatic activity and cytotoxicity of ethanolic extract of Nigella sativa seeds. Pharmacogn. Mag. 2012, 8, 268–272. [Google Scholar] [CrossRef]
- Mubinov, A.R.; Avdeeva, E.V.; Kurkin, V.A.; Latypova, G.M.; Farkhutdinov, R.R.; Kataev, V.A.; Ryazanova, T.K. Fatty Acid Profile and Antioxidant Activity of Nigella sativa Fatty Oil. Pharm. Chem. J. 2021, 55, 798–802. [Google Scholar] [CrossRef]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.E.; Wani, G.; Wani, A.A. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer 2005, 117, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem 2016, 31, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Safi, S.; Nadjarzadeh, A.; Salehi-Abargouei, A.; Mazaheri, M.; Mirmiran, P.; Meyre, D. Effects of Nigella sativa supplementation on blood concentration and mRNA expression of TNF-α, PPAR-γ and adiponectin, as major adipogenesis-related markers, in obese and overweight women: A crossover, randomised-controlled trial. Br. J. Nutr. 2022, 129, 1–27. [Google Scholar] [CrossRef]
- Haas, M.J.; Naem, E.; Almdallaleh, S.; Mooradian, A.D. The effect of black seed (Nigella sativa) extract on lipid metabolism in HepG2 cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159155. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.; Razmpoosh, E.; Fallahzadeh, H.; Mazaheri, M.; Abdollahi, N.; Nazari, M.; Nadjarzadeh, A.; Salehi-Abargouei, A. The effect of Nigella sativa on appetite, anthropometric and body composition indices among overweight and obese women: A crossover, double-blind, placebo-controlled, randomized clinical trial. Complement. Ther. Med. 2021, 57, 102653. [Google Scholar] [CrossRef] [PubMed]
- Alrashidi, M.; Derawi, D.; Salimon, J.; Firdaus Yusoff, M. An investigation of physicochemical properties of Nigella sativa L. Seed oil from Al-Qassim by different extraction methods. J. King Saud Univ.-Sci. 2020, 32, 3337–3342. [Google Scholar] [CrossRef]
- Takruri, H.R.H.; Dameh, M.A.F. Study of the Nutritional Value of Black Cumin Seeds (Nigella sativa L.). J. Sci. Food Agric. 1998, 76, 404–410. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic Potential and Pharmaceutical Development of Thymoquinone: A Multitargeted Molecule of Natural Origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Daoudi, N.E.; Azizi, S.-e.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical Composition Analysis Using HPLC-UV/GC-MS and Inhibitory Activity of Different Nigella sativa Fractions on Pancreatic α-Amylase and Intestinal Glucose Absorption. BioMed Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef]
- Kiralan, M.; Kiralan, S.S.; Ozkan, G.; Ramadan, M.F. Composition and Functionality of Nigella sativa Fixed Oil. In Black cumin (Nigella sativa) Seeds: Chemistry, Technology, Functionality, and Applications; Fawzy Ramadan, M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 319–333. [Google Scholar]
- Fidan, H.; Stankov, S.; Daraba, A.; Doğan, H.; Alexieva, I.; Stoyanova, A.; Ercisli, S. Phytochemical composition of black cumin (Nigella sativa L.) seeds from Turkey as an unconventional source for the food industry. J. Braz. Chem. Soc. 2019, 1, 1–9. [Google Scholar]
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Meor Hussin, A.S. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa L.) Oil. Evid.-Based Complement. Altern. Med. 2016, 2016, 6273817. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqeep, G.; Ismail, M.; Al-Zubairi, A.; Esa, N. Nutrients composition and minerals content of three different samples of Nigella sativa L. cultivated in Yemen. Asian J. Biol. Sci. 2009, 2, 43–48. [Google Scholar] [CrossRef]
- Yuan, T.; Nahar, P.; Sharma, M.; Liu, K.; Slitt, A.; Aisa, H.A.; Seeram, N.P. Indazole-type alkaloids from Nigella sativa seeds exhibit antihyperglycemic effects via AMPK activation in vitro. J. Nat. Prod. 2014, 77, 2316–2320. [Google Scholar] [CrossRef]
- Omar, M.; Segni, L.; Nedjimi, M.S.; Belfar, M.; Moussaoui, Y. Determination of polyphenols content, antioxidant and antibacterial activity of Nigella sativa L. Seed phenolic extracts. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 411. [Google Scholar]
- Cheikh-Rouhou, S.; Besbes, S.; Lognay, G.; Blecker, C.; Deroanne, C.; Attia, H. Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils. J. Food Compos. Anal. 2008, 21, 162–168. [Google Scholar] [CrossRef]
- Adamska, A.; Stefanowicz-Hajduk, J.; Ochocka, J.R. Alpha-Hederin, the Active Saponin of Nigella sativa, as an Anticancer Agent Inducing Apoptosis in the SKOV-3 Cell Line. Molecules 2019, 24, 2958. [Google Scholar] [CrossRef] [PubMed]
- Taşkin, M.K.; Alankus-Caliskan, O.; Anil, H.; Abou-Gazar, H.; Khan, I.; Bedir, E. Triterpene Saponins from Nigella sativa L. Turk. J. Chem. 2005, 29, 561–569. [Google Scholar]
- Parveen, A.; Farooq, M.A.; Kyunn, W.W. A New Oleanane Type Saponin from the Aerial Parts of Nigella sativa with Anti-Oxidant and Anti-Diabetic Potential. Molecules 2020, 25, 2171. [Google Scholar] [CrossRef] [PubMed]
- Shaik Mohamed Sayed, U.F.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef] [PubMed]
- Didi, A.; Meziane, R.K.; Amamou, F.; Benhmidat, A.; Sari, D.C. Metabolic and antioxidant effects of Nigella sativa oil on prevention of obesity development in rats feed high fat diet. Eur. Chem. Bull. 2014, 3, 888–896. [Google Scholar]
- Le, P.M.; Benhaddou-Andaloussi, A.; Elimadi, A.; Settaf, A.; Cherrah, Y.; Haddad, P.S. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J. Ethnopharmacol. 2004, 94, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Ghatreh Samani, K.; Farrokhi, E.; Heidarian, E. Effects of Nigella sativa Extracts on the Lipid Profile and Uncoupling Protein-1 Gene Expression in Brown Adipose Tissue of Mice. Adv. Biomed. Res. 2018, 7, 121. [Google Scholar] [CrossRef]
- Bano, F.; Wajeeh, M.; Baig, N.; Naz, H.; Akhtar, N. Antiobesity, antihyperlipidemic and hypoglycemic effects of the aqueous extract of Nigella sativa seeds (Kalongi). Pak. J. Biochem. Mol. Biol. 2009, 42, 136–140. [Google Scholar]
- Brennan, A.M.; Mantzoros, C.S. Drug Insight: The role of leptin in human physiology and pathophysiology--emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 318–327. [Google Scholar] [CrossRef]
- Al Asoom, L.; Alassaf, M.A.; AlSulaiman, N.S.; Boumarah, D.N.; Almubireek, A.M.; Alkaltham, G.K.; Alhawaj, H.A.; Alkhamis, T.; Rafique, N.; Alsunni, A.; et al. The Effectiveness of Nigella sativa and Ginger as Appetite Suppressants: An Experimental Study on Healthy Wistar Rats. Vasc. Health Risk Manag. 2023, 19, 1–11. [Google Scholar] [CrossRef]
- Esmail, M.; Anwar, S.; Kandeil, M.; El-Zanaty, A.M.; Abdel-Gabbar, M. Effect of Nigella sativa, atorvastatin, or L-Carnitine on high fat diet-induced obesity in adult male Albino rats. Biomed. Pharmacother. 2021, 141, 111818. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.F.; Soliman, G.M.; Okasha, E.F.; Shalaby, A.M. Histological, Immunohistochemical, and Biochemical Study of Experimentally Induced Fatty Liver in Adult Male Albino Rat and the Possible Protective Role of Pomegranate. J. Microsc. Ultrastruct. 2018, 6, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Al Mofleh, I.A.; Alhaider, A.A.; Mossa, J.S.; Al-Sohaibani, M.O.; Al-Yahya, M.A.; Rafatullah, S.; Shaik, S.A. Gastroprotective effect of an aqueous suspension of black cumin Nigella sativa on necrotizing agents-induced gastric injury in experimental animals. Saudi J. Gastroenterol. 2008, 14, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Peterson, S.J.; Bellner, L.; Choudhary, A.; Levy, L.; Gancz, L.; Sasson, A.; Trainer, J.; Rezzani, R.; Resnick, A.; et al. Cold-Pressed Nigella sativa Oil Standardized to 3% Thymoquinone Potentiates Omega-3 Protection against Obesity-Induced Oxidative Stress, Inflammation, and Markers of Insulin Resistance Accompanied with Conversion of White to Beige Fat in Mice. Antioxidants 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, Y.S.; Seol, D.J.; Cho, I.J.; Ku, S.K.; Choi, J.S.; Lee, H.J. Anti-obesity and fatty liver-preventing activities of Lonicera caerulea in high-fat diet-fed mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kim, H.J.; Lee, Y.J.; Ku, S.K. Anti-Diabetic Obesity Effects of Wasabia Japonica Matsum Leaf Extract on 45% Kcal High-Fat Diet-Fed Mice. Nutrients 2020, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-H.; Chun, Y.-S.; Kim, J.; Ku, S.-K.; Jeon, S.; Park, T.-S.; Shim, S.-M. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes. J. Funct. Foods 2020, 72, 104072. [Google Scholar] [CrossRef]
- Mitchell, M.; Armstrong, D.T.; Robker, R.L.; Norman, R.J. Adipokines: Implications for female fertility and obesity. Reproduction 2005, 130, 583–597. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Kim, J.W.; Kim, J.K.; Chun, Y.S.; Choi, J.S.; Ku, S.K. Efficacy Confirmation Test of Black Cumin (Nigella sativa L.) Seeds Extract Using a High-Fat Diet Mouse Model. Metabolites 2023, 13, 501. [Google Scholar] [CrossRef]
- Shamsiya, T.K.; Manjunatha, J.R.; Manonmani, H.K. Lipase inhibitors from Nigella sativa and Punica granatum as an effective approach towards controlling obesity. LIFE Int. J. Health Life-Sci. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: A randomized controlled clinical trial. Food Funct. 2015, 6, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Nasiruddin, M.; Khan, R.A.; Haque, S.F. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int. J. Diabetes Dev. Ctries. 2008, 28, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Al-Logmani, A.; Zari, T. Long-term effects of Nigella sativa L. oil on some physiological parameters in normal and streptozotocin-induced diabetic rats. J. Diabetes Mellit. 2011, 1, 46–53. [Google Scholar] [CrossRef][Green Version]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Alizadeh, M.; Namazi, N.; Farajnia, S. Changes of body composition and circulating adipokines in response to Nigella sativa oil with a calorie restricted diet in obese women. J. Herb. Med. 2016, 6, 67–72. [Google Scholar] [CrossRef]
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Datau, E.A.; Wardhana; Surachmanto, E.E.; Pandelaki, K.; Langi, J.A. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med. Indones. 2010, 42, 130–134. [Google Scholar]
- Mostafa, T.M.; Hegazy, S.K.; Elnaidany, S.S.; Shehabeldin, W.A.; Sawan, E.S. Nigella sativa as a promising intervention for metabolic and inflammatory disorders in obese prediabetic subjects: A comparative study of Nigella sativa versus both lifestyle modification and metformin. J. Diabetes Its Complicat. 2021, 35, 107947. [Google Scholar] [CrossRef]
- Akova, A.; Ustun, G. Activity and adsorption of lipase from Nigella sativa seeds on Celite at different pH values. Biotechnol. Lett. 2000, 22, 355–359. [Google Scholar] [CrossRef]
- Hussain, M.; Tunio, A.G.; Arain, L.A.; Shaikh, G.S. Effects of Nigella sativa on various parameters in Patients of non-alcoholic fatty liver disease. J. Ayub Med. Coll. Abbottabad 2017, 29, 403–407. [Google Scholar] [PubMed]
- Amin, S.; Mir, S.R.; Kohli, K.; Ali, B.; Ali, M. A study of the chemical composition of black cumin oil and its effect on penetration enhancement from transdermal formulations. Nat. Prod. Res. 2010, 24, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Khutami, C.; Sumiwi, S.A.; Khairul Ikram, N.K.; Muchtaridi, M. The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. Int. J. Mol. Sci. 2022, 23, 2056. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef]
- Santilli, F.; Guagnano, M.T.; Vazzana, N.; La Barba, S.; Davi, G. Oxidative stress drivers and modulators in obesity and cardiovascular disease: From biomarkers to therapeutic approach. Curr. Med. Chem. 2015, 22, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Angelico, F.; Loffredo, L.; Pignatelli, P.; Augelletti, T.; Carnevale, R.; Pacella, A.; Albanese, F.; Mancini, I.; Di Santo, S.; Del Ben, M.; et al. Weight loss is associated with improved endothelial dysfunction via NOX2-generated oxidative stress down-regulation in patients with the metabolic syndrome. Intern. Emerg. Med. 2012, 7, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Mahdavi, R.; Alizadeh, M.; Farajnia, S. Oxidative Stress Responses to Nigella sativa Oil Concurrent with a Low-Calorie Diet in Obese Women: A Randomized, Double-Blind Controlled Clinical Trial. Phytother. Res. 2015, 29, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, W.M. Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J. Basic Appl. Zool. 2013, 66, 263–270. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Safi, S.; Nadjarzadeh, A.; Fallahzadeh, H.; Abdollahi, N.; Mazaheri, M.; Nazari, M.; Salehi-Abargouei, A. The effect of Nigella sativa supplementation on cardiovascular risk factors in obese and overweight women: A crossover, double-blind, placebo-controlled randomized clinical trial. Eur. J. Nutr. 2021, 60, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, N.; Nadjarzadeh, A.; Salehi-Abargouei, A.; Fallahzadeh, H.; Razmpoosh, E.; Lorzaedeh, E.; Safi, S. The effect of Nigella sativa on TAC and MDA in obese and overweight women: Secondary analysis of a crossover, double blind, randomized clinical trial. J. Diabetes Metab. Disord. 2022, 21, 171–179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).