Hidden Tenants: Microbiota of the Rhizosphere and Phyllosphere of Cordia dodecandra Trees in Mayan Forests and Homegardens
Abstract
1. Introduction
2. Results
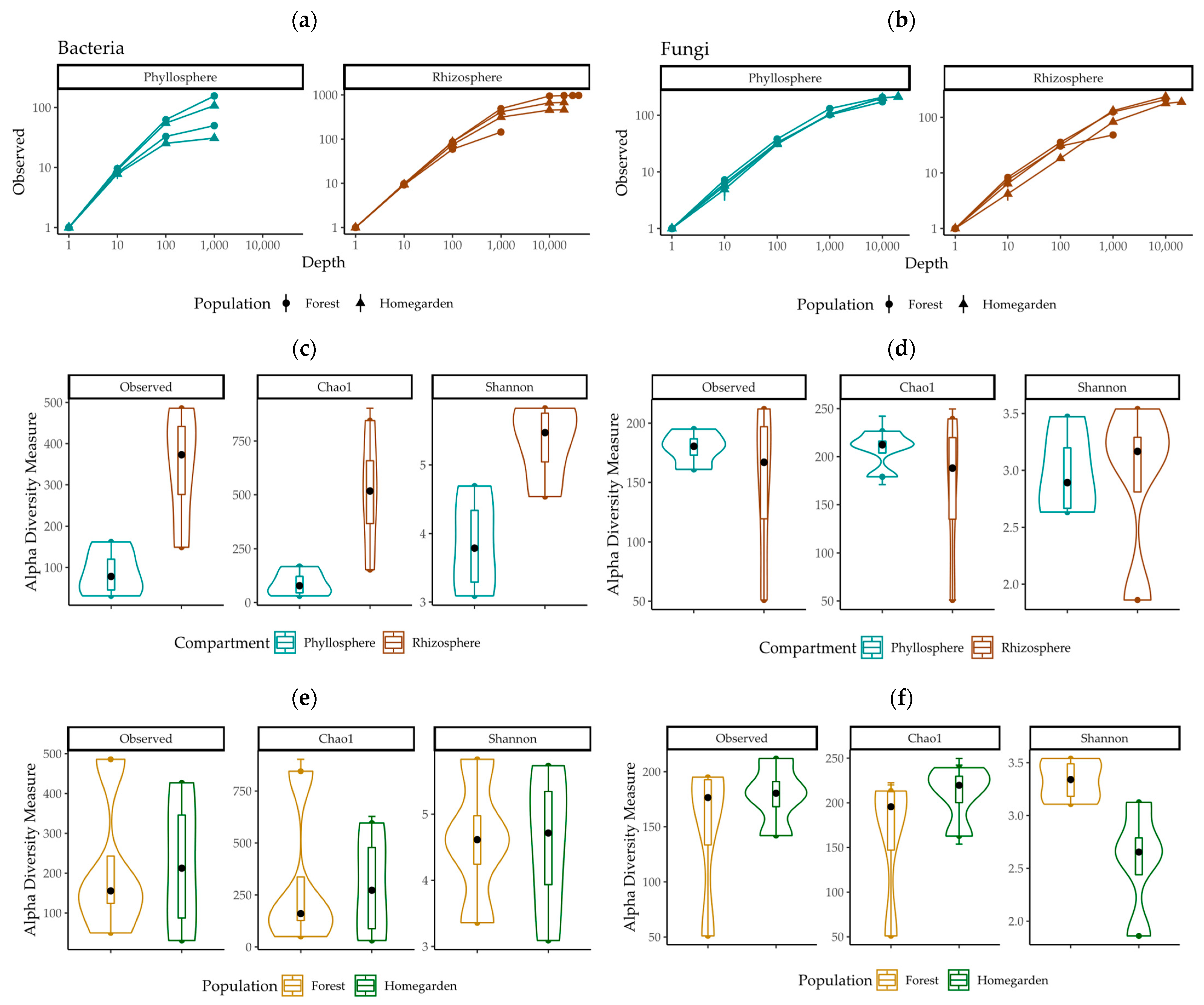
2.1. Sequence Characteristics and Alpha Diversity
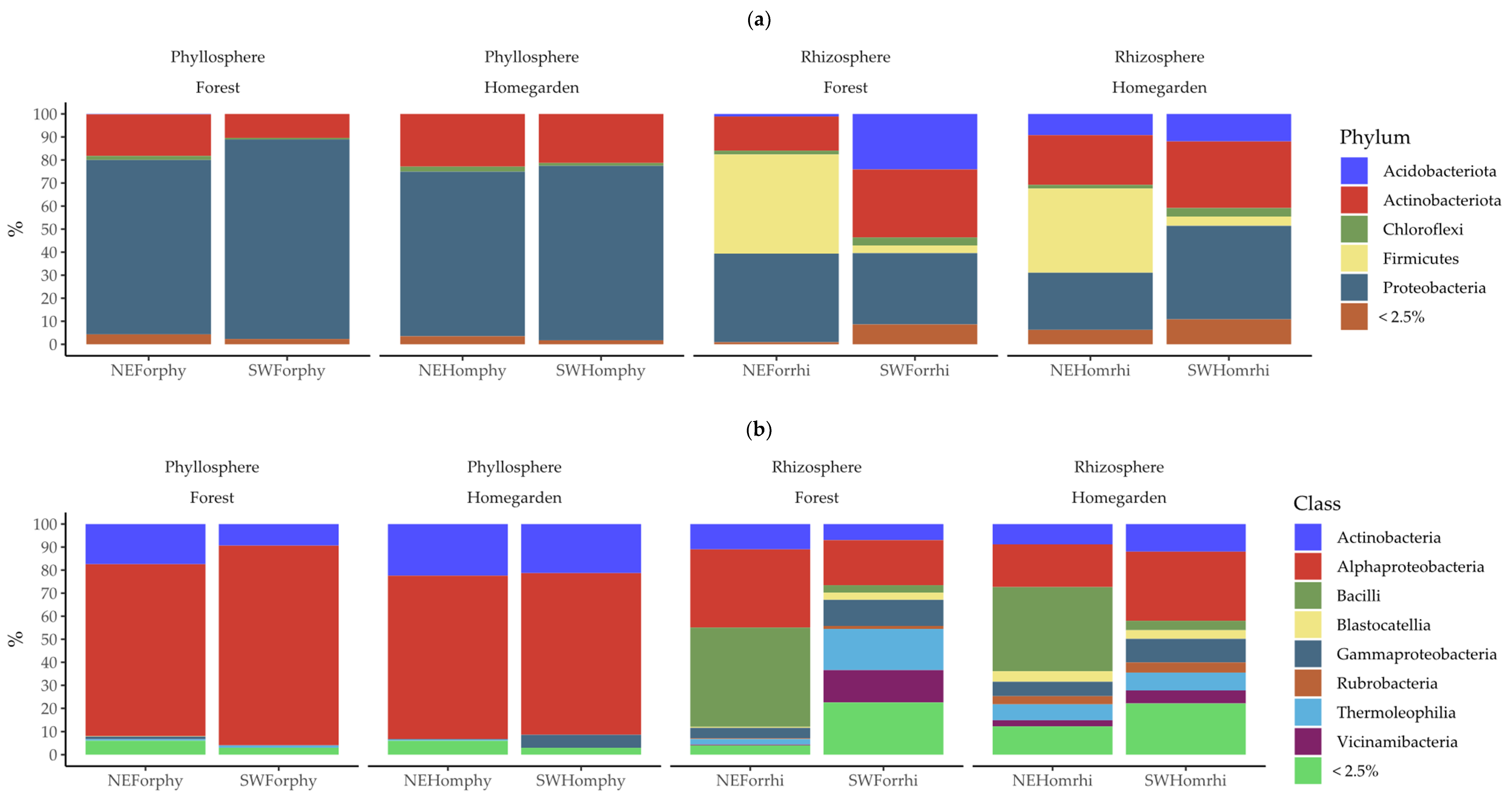
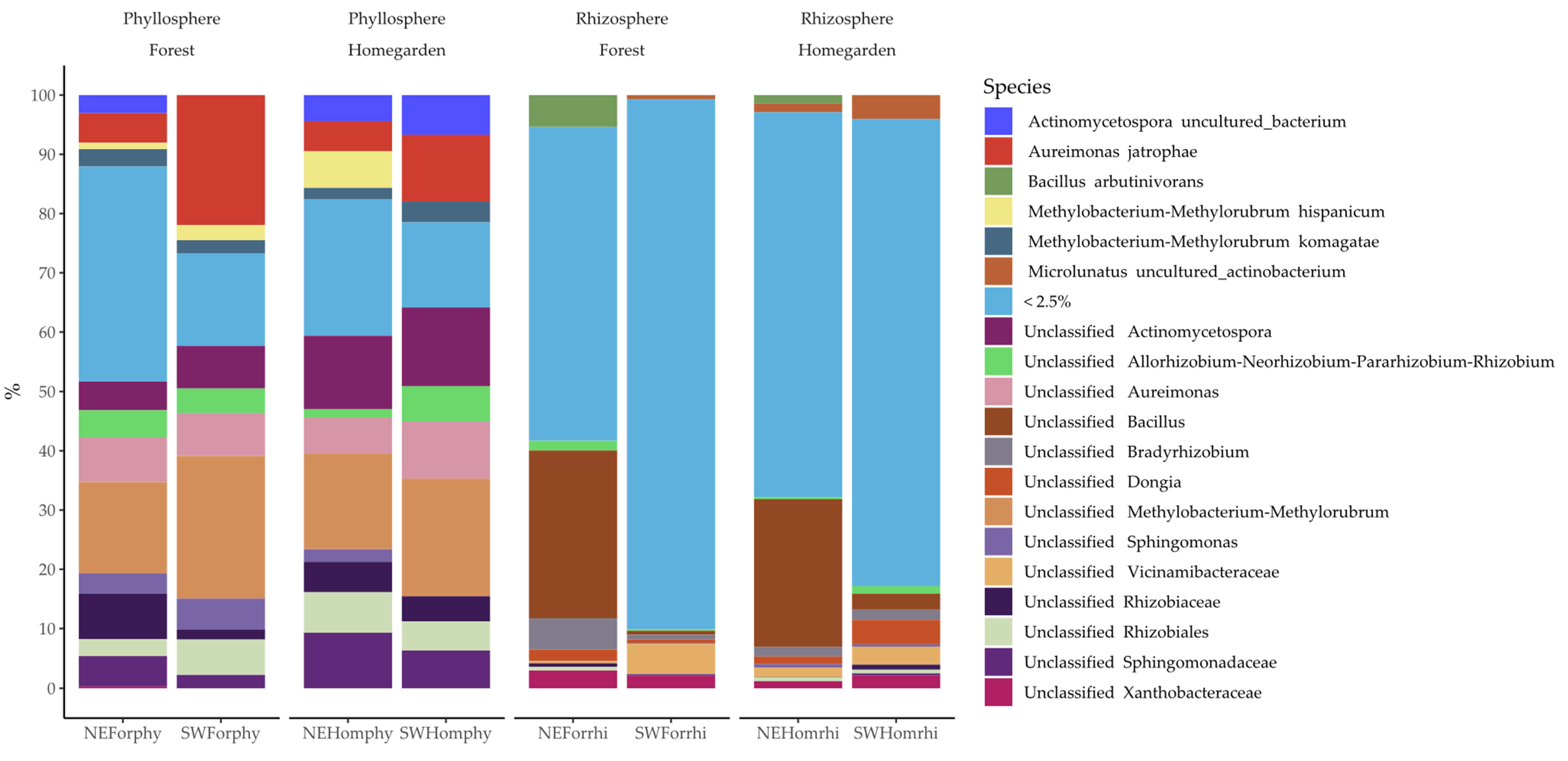
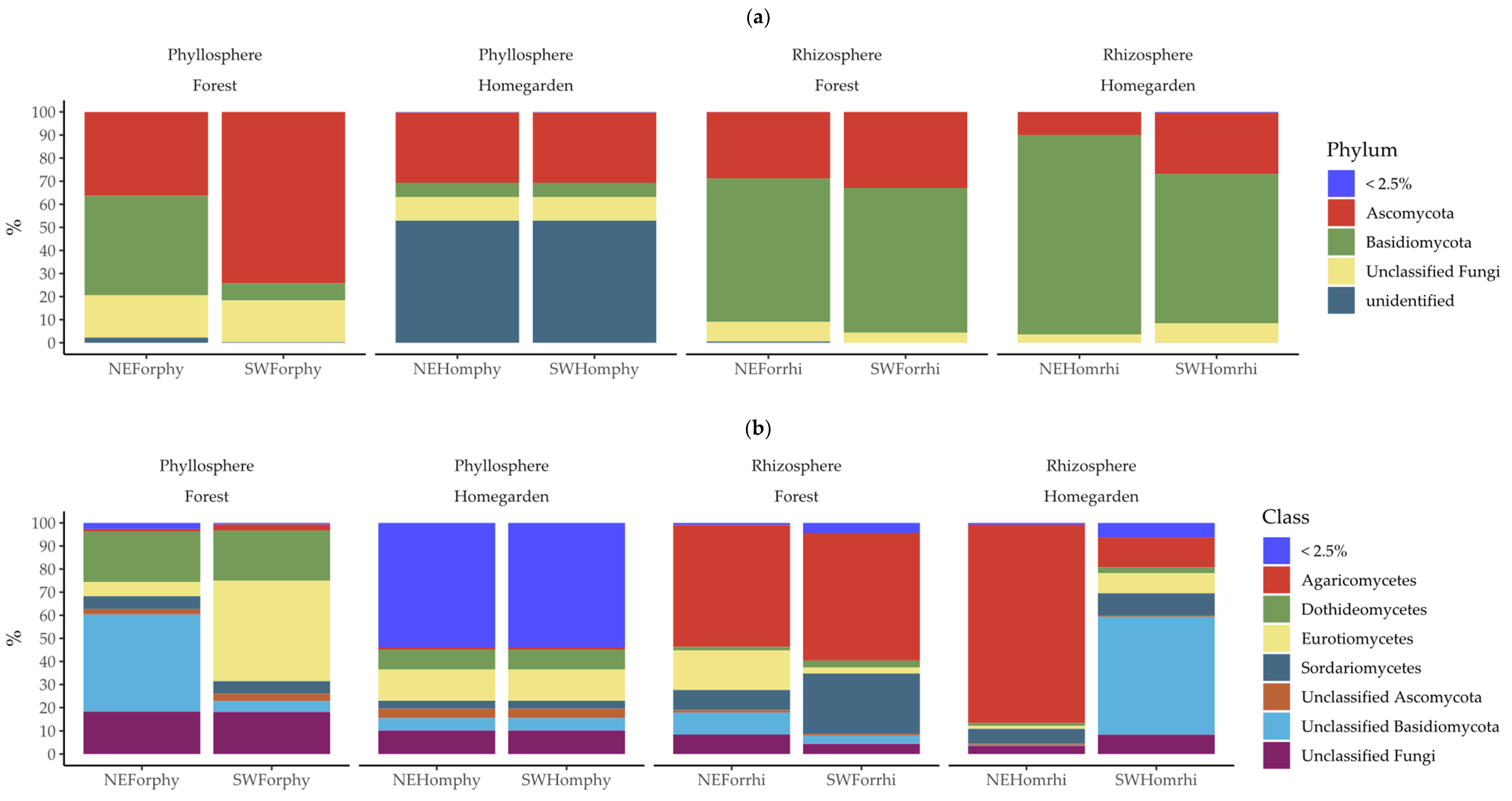
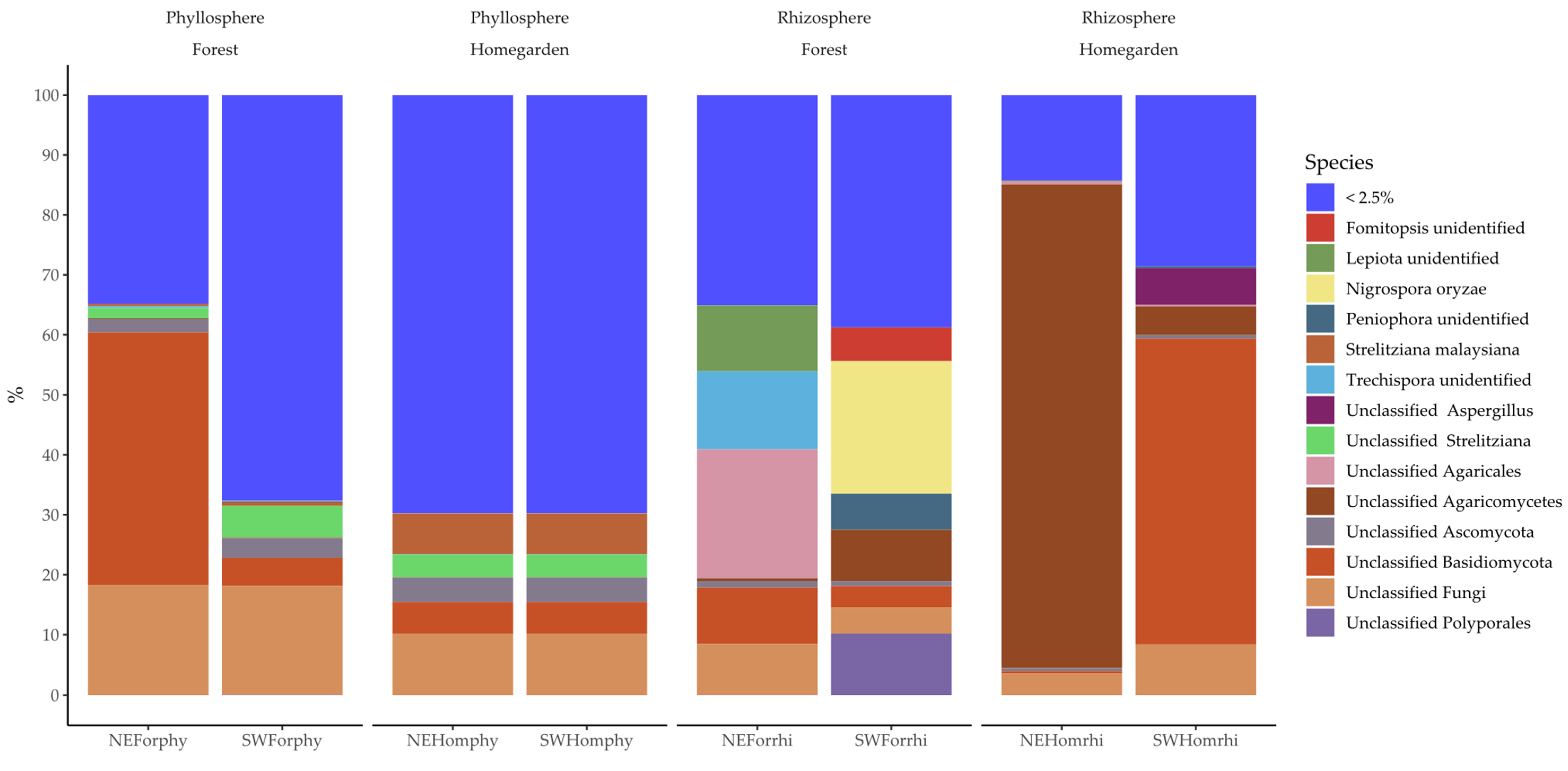
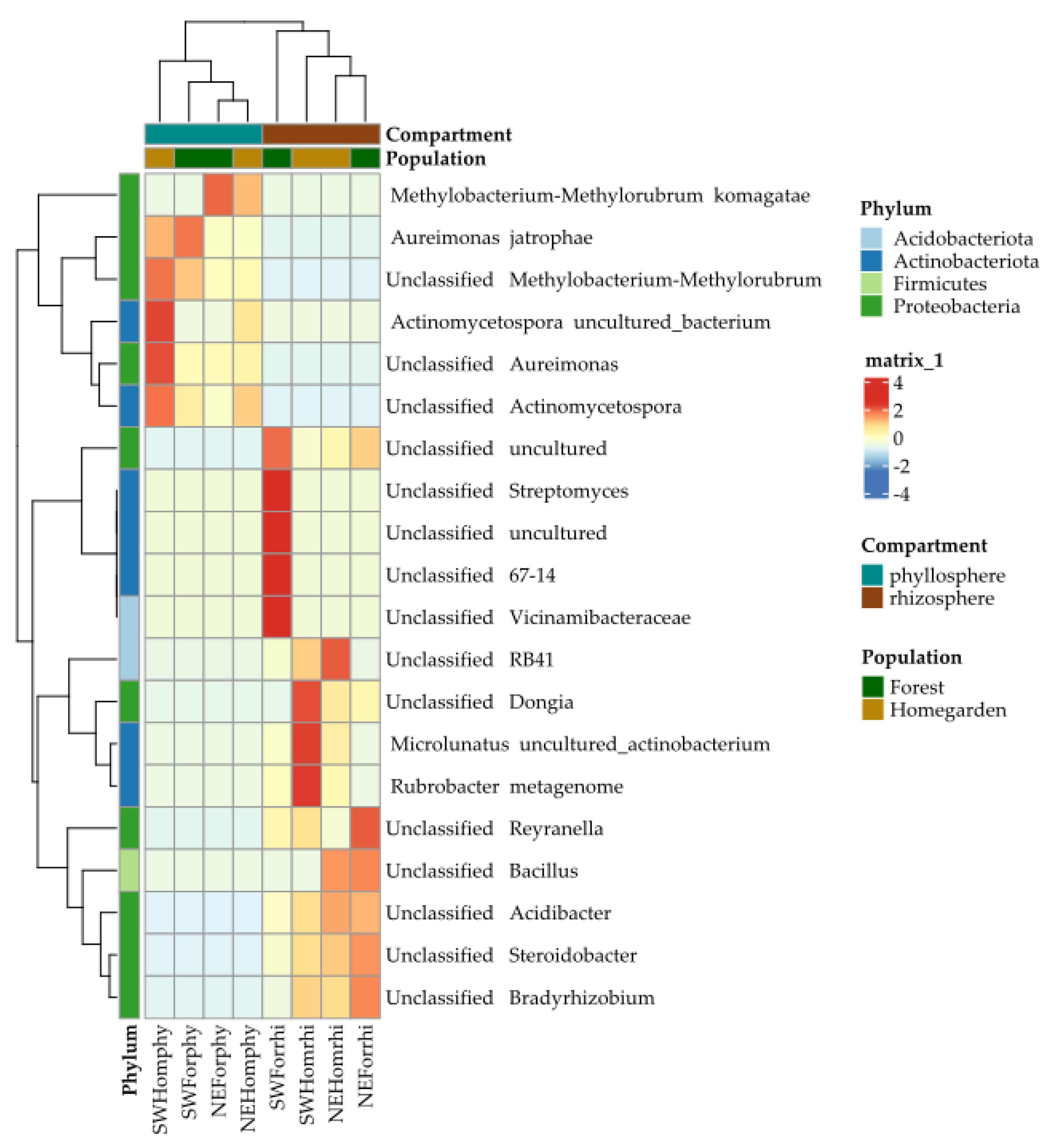
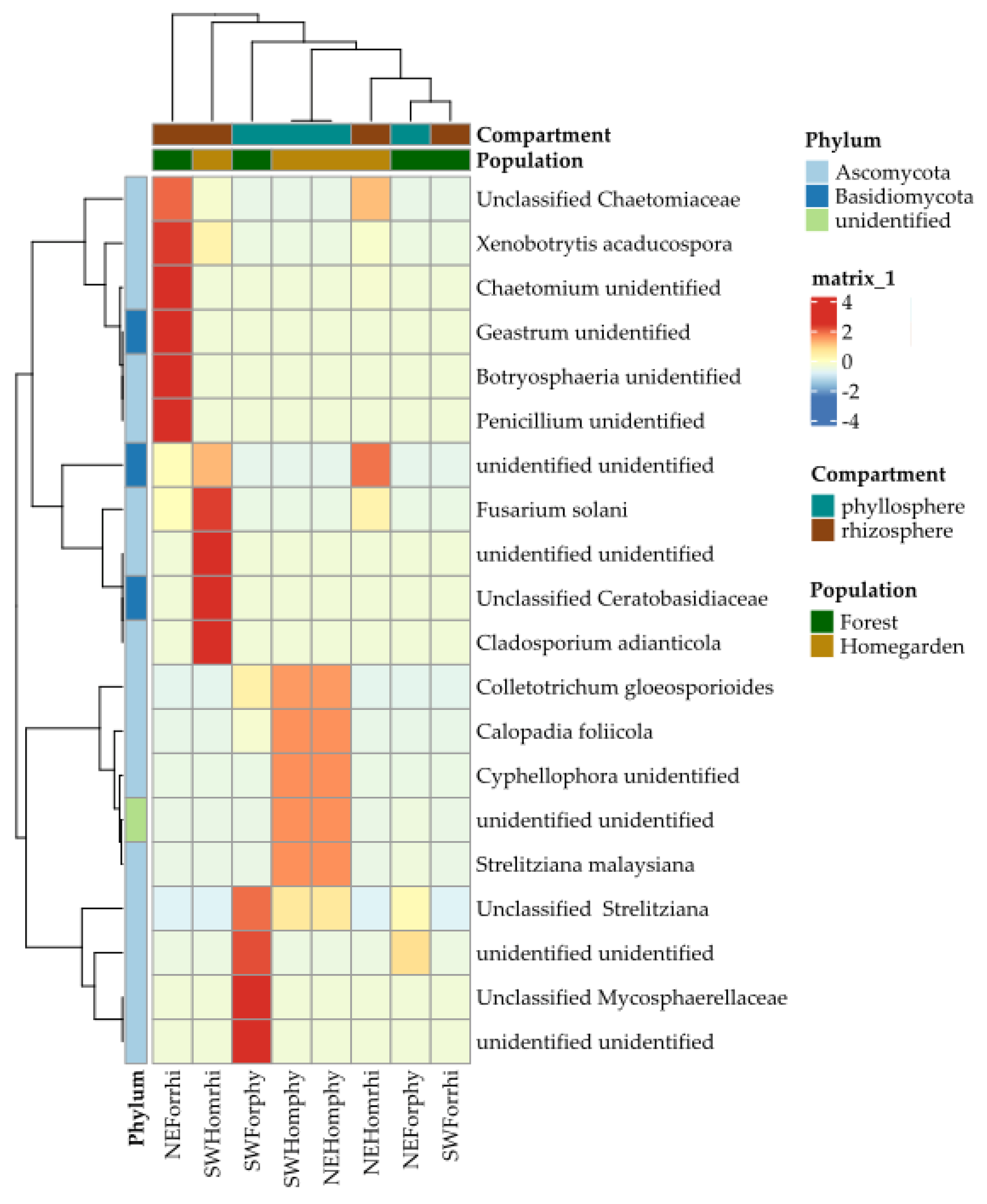
2.2. Taxonomic Assignation
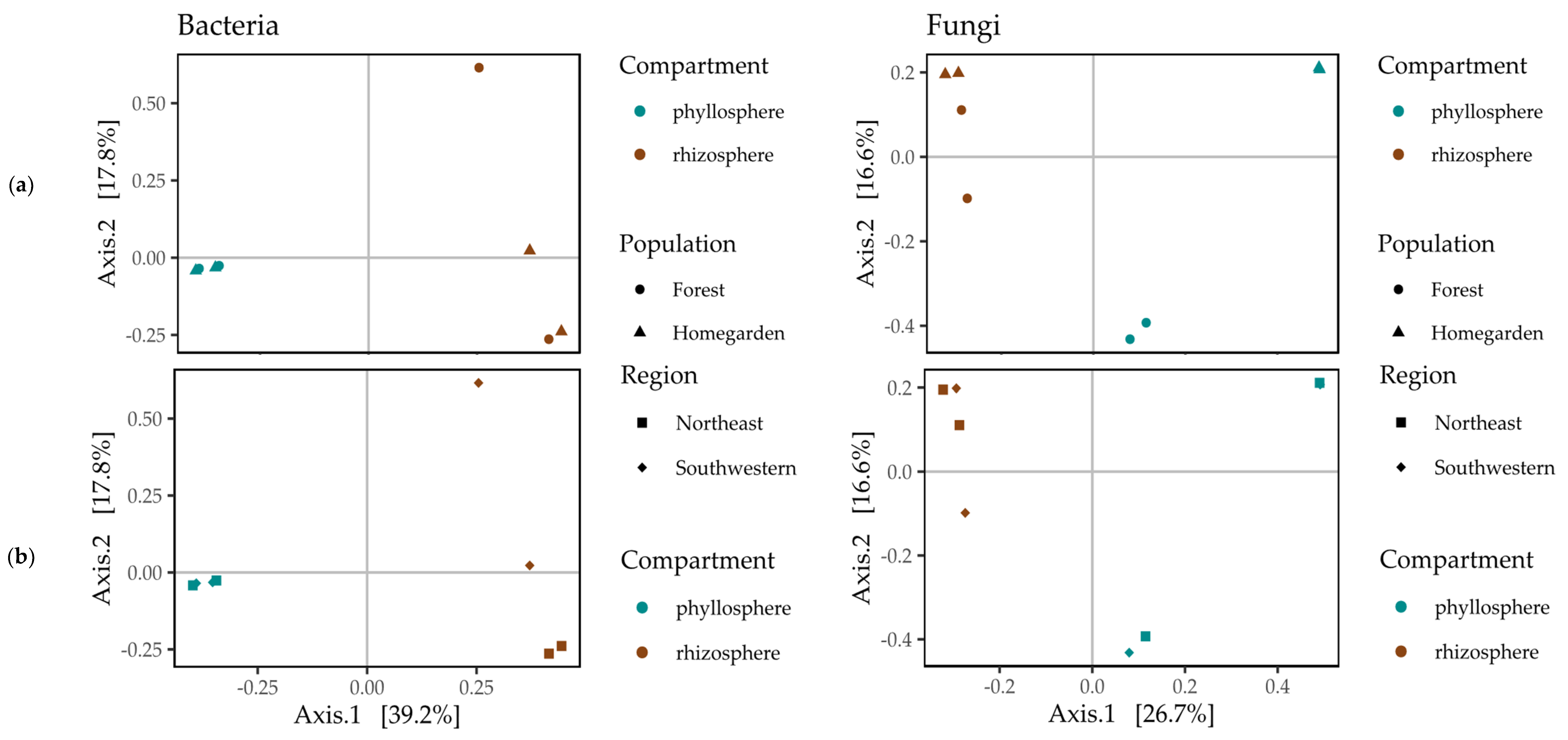
2.3. Beta Diversity of the Microbiota
3. Discussion
4. Materials and Methods
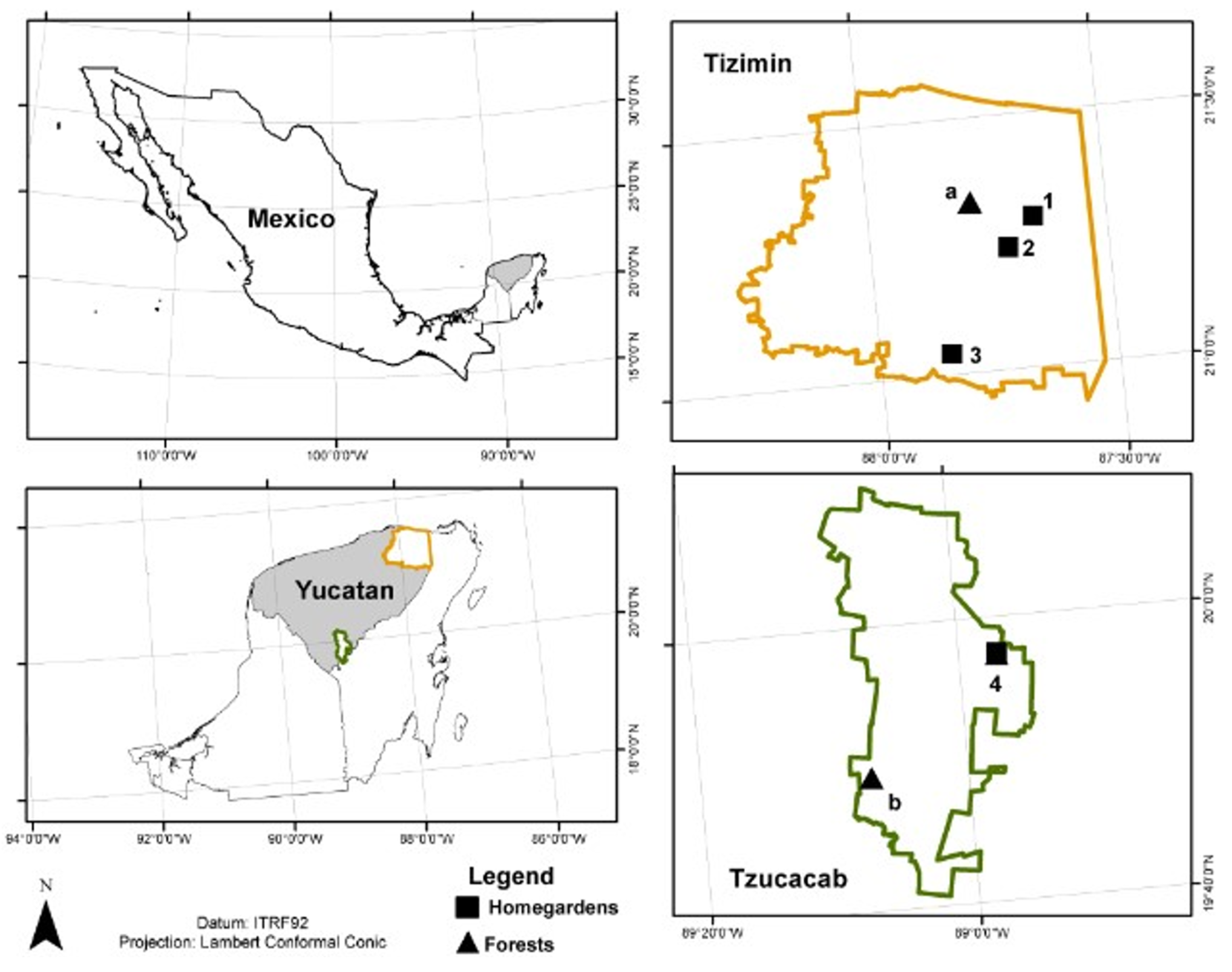
4.1. Study Area
4.2. Sample Collection and Storage
4.3. DNA Extraction
4.4. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Object ID | Design_Description | Compartment | Population | Region | Link |
|---|---|---|---|---|---|
| 31018490 | 16S_forward_phy_hom_SW | Phyllosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018491 | 16S_reverse_phy_hom_SW | Phyllosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018492 | 16S_forward_phy_for_NE | Phyllosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018493 | 16S_reverse_phy_for_NE | Phyllosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018494 | 16S_forward_phy_hom_NE | Phyllosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018495 | 16S_reverse_phy_hom_NE | Phyllosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018496 | 16S_forward_phy_for_SW | Phyllosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018497 | 16S_reverse_phy_for_SW | Phyllosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018502 | 16S_forward_rhi_hom_NE | Rhizosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018503 | 16S_reverse_rhi_hom_NE | Rhizosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018504 | 16S_forward_rhi_for_NE | Rhizosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018505 | 16S_reverse_rhi_for_NE | Rhizosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018506 | 16S_forward_rhi_hom_SW | Rhizosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018507 | 16S_reverse_rhi_hom_SW | Rhizosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018508 | 16S_forward_rhi_for_SW | Rhizosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018509 | 16S_reverse_rhi_for_SW | Rhizosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018510 | ITS_forward_phy_hom_SW | Phyllosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018511 | ITS_reverse_phy_hom_SW | Phyllosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018512 | ITS_forward_phy_for_NE | Phyllosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018513 | ITS_reverse_phy_for_NE | Phyllosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018514 | ITS_forward_phy_hom_NE | Phyllosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018515 | ITS_reverse_phy_hom_NE | Phyllosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018516 | ITS_forward_phy_for_SW | Phyllosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018517 | ITS_reverse_phy_for_SW | Phyllosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018522 | ITS_forward_rhi_hom_NE | Rhizosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018523 | ITS_reverse_rhi_hom_NE | Rhizosphere | Homegarden | Northeast | BioProject ID: PRJNA884306 |
| 31018524 | ITS_forward_rhi_for_NE | Rhizosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018525 | ITS_reverse_rhi_for_NE | Rhizosphere | Forest | Northeast | BioProject ID: PRJNA884306 |
| 31018526 | ITS_forward_rhi_hom_SW | Rhizosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018527 | ITS_reverse_rhi_hom_SW | Rhizosphere | Homegarden | Southwest | BioProject ID: PRJNA884306 |
| 31018528 | ITS_forward_rhi_for_SW | Rhizosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
| 31018529 | ITS_reverse_rhi_for_SW | Rhizosphere | Forest | Southwest | BioProject ID: PRJNA884306 |
References
- Cordovez, V.; Dini, F.A.; Carrión, V.J.; Raaijmakers, J. Ecology and Evolution of Plant Microbiomes. Ann. Rev. Microbiol. 2019, 73, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.L.; Orozco, M.d.C.; Loeza, P.D.; Parra, F.I.; De los Santos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 1–12. [Google Scholar] [CrossRef]
- Griffin, E.A.; Carson, W.P. Tree Endophytes: Cryptic Drivers of Tropical Forest Diversity. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 63–103. [Google Scholar]
- Redford, J.A.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Whipps, M.J.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere microbiology with special reference to diversity and plant genotype. J. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef]
- Stone, G.; Weingarten, E.A.; Jackson, C.R. The Role of the Phyllosphere Microbiome in Plant Health and Function. Annu. Plant Rev. Online 2018, 1, 533–556. [Google Scholar]
- Zhang, Y.; Trivedi, P.; Xu, J.; Roper, M.C.; Wang, N. The Citrus Microbiome: From Structure and Function to Microbiome Engineering and Beyond. Phytobiomes J. 2021, 5, 249–262. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liang, W.; Liu, S. Rhizosphere Microbiome: The Emerging Barrier in Plant-Pathogen Interactions. Front. Microbiol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Xiong, C.; Zhu, Y.-G.; Wang, J.-T.; Shing, B.; Han, L.; Shen, J.; Li, P.; Wang, G.; Wu, C.; Ge, A.I.; et al. Host selection shapes crop microbiome assembly and network complexity. New Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, D. Diversity of Endophytes in Tropical Forests. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–62. [Google Scholar]
- Sivakumar, N.; Sathishkumar, R.; Selvakumar, G.; Shyamkumar, R.; Arjunekumar, K. Phyllospheric Microbiomes: Diversity, Ecological Significance, and Biotechnological Applications. In Plant Microbiomes for Sustainable Agriculture; Yadav, A.N., Singh, J., Rastegari, A.A., Rastegari, A.A., Yadav, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 113–172. [Google Scholar]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Knief, C. Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front. Plant Sci. 2014, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Riesenfeld, S.C.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic Analysis of Microbial Communities. Annu. Rev. Genet. 2004, 38, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Shapiro, L.R.; Benrey, B.; Benrey, B.; Cibrián, J.A. Back to the Origin: In Situ Studies Are Needed to Understand Selection during Crop Diversification. Front. Ecol. Evol. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeenl, S.; Woyke, T.; North, G.; Visel, A.; Partida, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Blancas, J.; Lira, R. Mexican Ethnobotany: Interactions of People and Plants in Mesoamerica. In Ethnobotany of Mexico: Interactions of People and Plants in Mesoamerica; Lira, R., Casas, A., Blancas, J., Eds.; Springer: New York, NY, USA, 2016; pp. 1–19. [Google Scholar]
- Casas, A.; Otero-Arnaiz, A.; Pérez-Negrón, E.; Valiente-Banuet, A. In situ Management and Domestication of Plants in Mesoamerica. Ann. Bot. 2007, 100, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Montañez, P.I.; Ruenes, M.d.R.; Ferrer, M.M.; Estrada, H. Los Huertos familiares Maya-Yucatecos: Situación actual y perspectiva en México. Ambienta 2014, 107, 100–109. [Google Scholar]
- Abdollahzadeh, J.; Groenewald, J.Z.; Coetzee, M.P.A. Evolution of lifestyles in Capnodiales. Stud. Mycol. 2020, 95, 381–414. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, M.C.; Montañez, P.I.; Ruenes, M.d.R.; Jiménez, J.; Estrada, H. Assessment of population structure and management of Cordia dodecandra A. DC. in homegardens and tropical forest in Yucatán, Mexico. Rev. Fac. Cienc. Agrar. 2020, 52, 140–152. [Google Scholar]
- Colunga, P.; Zizumbo, D. Domestication of Plants in Maya Lowlands. Econ. Bot. 2004, 58, S101–S110. [Google Scholar] [CrossRef]
- Zizumbo, D.; Colunga, P. Origin of agriculture and plant domestication in West Mesoamerica. Genet. Resour. Crop. Evol. 2010, 57, 813–825. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Montañez, P.I.; Ruenes, M.R.; Estrada, H.; Jiménez, J. Growing out of the tropical forests: Domestication syndrome of native Mesoamerican trees in Mayan homegardens. Genet. Resour. Crop. Evol. 2020, 67, 587–604. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Tapia, C.A.; Estrada, H.; Ruenes, M.; Montañez, P.; Jiménez, J. Growing Out of the Tropical Forests: Gene Flow of Native Mesoamerican Trees Among Forest and Mayan Homegardens. Front. Ecol. Evol. 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Tuyub-Chan, J.A. Anatomía De La Epidermis Foliar Del Ciricote (Cordia dodecandra A. DC., Cordiaceae) en Poblaciones Silvestres Y Huertos Familiares de Tizimín Y Tzucacab, Yucatán; Universidad Autónoma de Yucatán: Mérida, México, 2021. [Google Scholar]
- Pech-Puch, G.J. Carbono Y Nitrógeno en Suelos Y Tejido Foliar De Individuos Silvestres Y Cultivados de Cordia dodecandra DC. Brosimum alicastrum Sw. Y Spondias purpurea L.; Universidad Autónoma de Yucatán: Mérida, México, 2019. [Google Scholar]
- Bazzicalupo, A.L.; Bálint, M.; Schmitt, I. Comparison of ITS1 and ITS2 rDNA in 454 sequencing of hyperdiverse fungal communities. Fungal Ecol. 2013, 6, 102–109. [Google Scholar] [CrossRef]
- Wemheuer, F.; Berkelmann, D.; Wemheuer, B.; Daniel, R.; Vidal, S.; Bisseleua, H.D. Agroforestry Management Systems Drive the Composition, Diversity, and Function of Fungal and Bacterial Endophyte Communities in Theobroma cacao Leaves. Microorganisms 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Dias, A.C.F.; Homma, S.K.; Cardoso, E.J. Phyllosphere bacterial assembly in citrus crop under conventional and ecological management. PeerJ 2020, 8, e9152. [Google Scholar] [CrossRef]
- Martínez-Romero, E.; Aguirre-Noyola, J.L.; Taco-Taype, N.; Martínez-Romero, J.; Zuñiga-Dávila, D. Plant microbiota modified by plant domestication. Syst. App. Microbiol. 2020, 43, 126106. [Google Scholar] [CrossRef]
- Cowan, J.A.; Gehring, C.A.; Ilstedt, U.; Grady, K.C. Host identity and neighborhood trees affect belowground microbial communities in a tropical rainforest. Trop. Ecol. 2022, 63, 216–228. [Google Scholar] [CrossRef]
- Kembel, S.W.; Mueller, R.C. Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany 2014, 92, 303–311. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef]
- Dong, C.-J.; Wang, L.-L.; Li, Q.; Shang, Q.-M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Lemanceau, P.; Barret, M.; Mazurier, S.; Mondy, S.; Pivato, B.; Fort, T.; Vacher, C. Plant Communication with Associated Microbiota in the Spermosphere, Rhizosphere and Phyllosphere. In Advances in Botanical Research; Becard, G., Ed.; Academic Press: London, UK, 2017; pp. 101–133. [Google Scholar]
- Flores, J.; Durán, R.; Ortiz-Díaz, J. Comunidades vegetales terrestres. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, R., Méndez, M., Eds.; CICY, PPD-FMAM, CONABIO, SEDUMA: Mérida, México, 2010; pp. 125–129. [Google Scholar]
- Zamora Crescencio, P.; Gil, G.G.; Guido, J.S.F.; Ortiz, J.J. Estructura y composición florística de la selva mediana subcaducifolia en el sur del estado de Yucatán, México. Polibotánica 2008, 26, 39–66. [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. MMBR 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; Von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, H.; Yurimoto, H.; Sakai, Y. Interactions of Methylotrophs with Plants and Other Heterotrophic Bacteria. Microorganisms 2015, 3, 137–151. [Google Scholar] [CrossRef]
- Jiang, Y.; Wiese, J.; Tang, S.-K.; Xu, L.-H.; Imhoff, J.F.; Jiang, C. Actinomycetospora chiangmaiensis gen. nov., sp. nov., a new member of the family Pseudonocardiaceae. IJSEM 2008, 58, 408–413. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Hu, C.J.; Roy, J.J.; Kim, S.-J.; Weon, H.-Y.; Kwon, S.-H.; Ji, L. Aureimonas jatrophae sp. nov. and Aureimonas phyllosphaerae sp. nov., leaf-associated bacteria isolated from Jatropha curcas L. Int. J. Syst. Evol. 2013, 63, 1702–1708. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 1–20. [Google Scholar] [CrossRef]
- Xie, J.; Dawwam, G.E.; Sehim, A.E.; Li, X.; Wu, J.; Chen, S.; Zhang, D. Drought Stress Triggers Shifts in the Root Microbial Community and Alters Functional Categories in the Microbial Gene Pool. Front. Microbiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Tremblay, J.; Bainard, L.D.; Cade-Menun, B.; Hamel, C. Long-term effects of nitrogen and phosphorus fertilization on soil microbial community structure and function under continuous wheat production. Environ. Microbiol. 2020, 22, 1066–1088. [Google Scholar] [CrossRef]
- Zhong, C.; Fu, J.; Jiang, T.; Zhang, C.; Cao, G. Polyphosphate metabolic gene expression analyses reveal mechanisms of phosphorus accumulation and release in Microlunatus phosphovorus strain JN459. FEMS Microbiol. Lett. 2018, 365, fny034. [Google Scholar] [CrossRef]
- Baker, C.M.; Chitrakar, R.; Obulareddy, N.; Panchal, S.; Williams, P.; Melotto, M. Molecular battles between plant and pathogenic bacteria in the phyllosphere. Braz. J. Med. Biol. Res. 2010, 43, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal Endophytes and Their Role in Agricultural Plant Protection against Pests and Pathogens. Plants 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Palma-Onetto, V.; Aguilera-Sammaritano, J.; Mithöfer, A. Roles of leaf functional traits in fungal endophyte colonization: Potential implications for host–pathogen interactions. J. Ecol. 2021, 109, 3972–3987. [Google Scholar] [CrossRef]
- Estrada, H.; Canto, B.B.; de los Santos, C.; O’Connor, A. Yucatán in black and red: Linking edaphic analysis and pyrosequencing-based assessment of bacterial and fungal community structures in the two main kinds of soil of Yucatán State. Microbiol. Res. 2016, 188–189, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Oger, P.; Tisserand, E.; Cebrón, A.; Turpault, M.P.; Buée, M.; De Boer, W.; Leveau, J.H.; Frey-Klett, P. Specific impacts of beech and Norway spruce on the structure and diversity of the rhizosphere and soil microbial communities. Sci. Rep. 2016, 6, 27756. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rui, J.; Xiong, J.; He, Z.; Zhou, J.; Yannarell, A.C.; Mackie, R.I. Functional Potential of Soil Microbial Communities in the Maize Rhizosphere. PLoS ONE 2014, 9, e112609. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L.; Hiscox, J. Fungal Ecology: Principles and Mechanisms of Colonization and Competition by Saprotrophic Fungi. Microbiol. Spectr. 2016, 4, 17. [Google Scholar] [CrossRef]
- Hallenberg, N.; Larsson, E.; Mahlapuu, M. Phylogenetic studies in Peniophora. Mycol. Res. 1996, 100, 179–187. [Google Scholar] [CrossRef]
- Liang, J.; Xu, J.; Yang, Z. Divergence, dispersal and recombination in Lepiota cristata from China. Fungal Divers. 2009, 38, 105–124. [Google Scholar]
- Babu, A.G.; Reddy, M.S. Aspergillus tubingensis Improves the Growth and Native Mycorrhizal Colonization of Bermudagrass in Bauxite Residue. Bioremediat J. 2011, 15, 157–164. [Google Scholar] [CrossRef]
- García, E. Modificaciones Al Sistema De Clasificación Climática De Kõeppen (Para Adaptarlo a Las Condiciones De La República Mexicana), 2nd ed.; I.d. Geografía, Instittuo de Geografía, Universidad Nacional Autónoma de México: México City, México, 1973; p. 91. [Google Scholar]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Suzuki, M.T.; Giovannoni, S.J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 1996, 62, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; Mcdonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curre. Protoc. Bioinform. 2020, 70, e100. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Chao, A.; Bunge, J. Estimating the Number of Species in a Stochastic Abundance Model. Biometrics 2002, 58, 531–539. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication Part II. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Cameron, E.S.; Schmidt, P.J.; Tremblay, B.J.M.; Emelko, M.B.; Müller, K.M. Enhancing diversity analysis by repeatedly rarefying next generation sequencing data describing microbial communities. Sci. Rep.-UK 2021, 11, 22302. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
May-Mutul, C.G.; López-Garrido, M.A.; O’Connor-Sánchez, A.; Peña-Ramírez, Y.J.; Labrín-Sotomayor, N.Y.; Estrada-Medina, H.; Ferrer, M.M. Hidden Tenants: Microbiota of the Rhizosphere and Phyllosphere of Cordia dodecandra Trees in Mayan Forests and Homegardens. Plants 2022, 11, 3098. https://doi.org/10.3390/plants11223098
May-Mutul CG, López-Garrido MA, O’Connor-Sánchez A, Peña-Ramírez YJ, Labrín-Sotomayor NY, Estrada-Medina H, Ferrer MM. Hidden Tenants: Microbiota of the Rhizosphere and Phyllosphere of Cordia dodecandra Trees in Mayan Forests and Homegardens. Plants. 2022; 11(22):3098. https://doi.org/10.3390/plants11223098
Chicago/Turabian StyleMay-Mutul, Carla G., Miguel A. López-Garrido, Aileen O’Connor-Sánchez, Yuri J. Peña-Ramírez, Natalia Y. Labrín-Sotomayor, Héctor Estrada-Medina, and Miriam M. Ferrer. 2022. "Hidden Tenants: Microbiota of the Rhizosphere and Phyllosphere of Cordia dodecandra Trees in Mayan Forests and Homegardens" Plants 11, no. 22: 3098. https://doi.org/10.3390/plants11223098
APA StyleMay-Mutul, C. G., López-Garrido, M. A., O’Connor-Sánchez, A., Peña-Ramírez, Y. J., Labrín-Sotomayor, N. Y., Estrada-Medina, H., & Ferrer, M. M. (2022). Hidden Tenants: Microbiota of the Rhizosphere and Phyllosphere of Cordia dodecandra Trees in Mayan Forests and Homegardens. Plants, 11(22), 3098. https://doi.org/10.3390/plants11223098








