Genome-Wide Identification and Expression Profiling of the SRS Gene Family in Melilotus albus Reveals Functions in Various Stress Conditions
Abstract
1. Introduction
2. Results
2.1. Identification and Analysis of SRS Family Members in M. albus
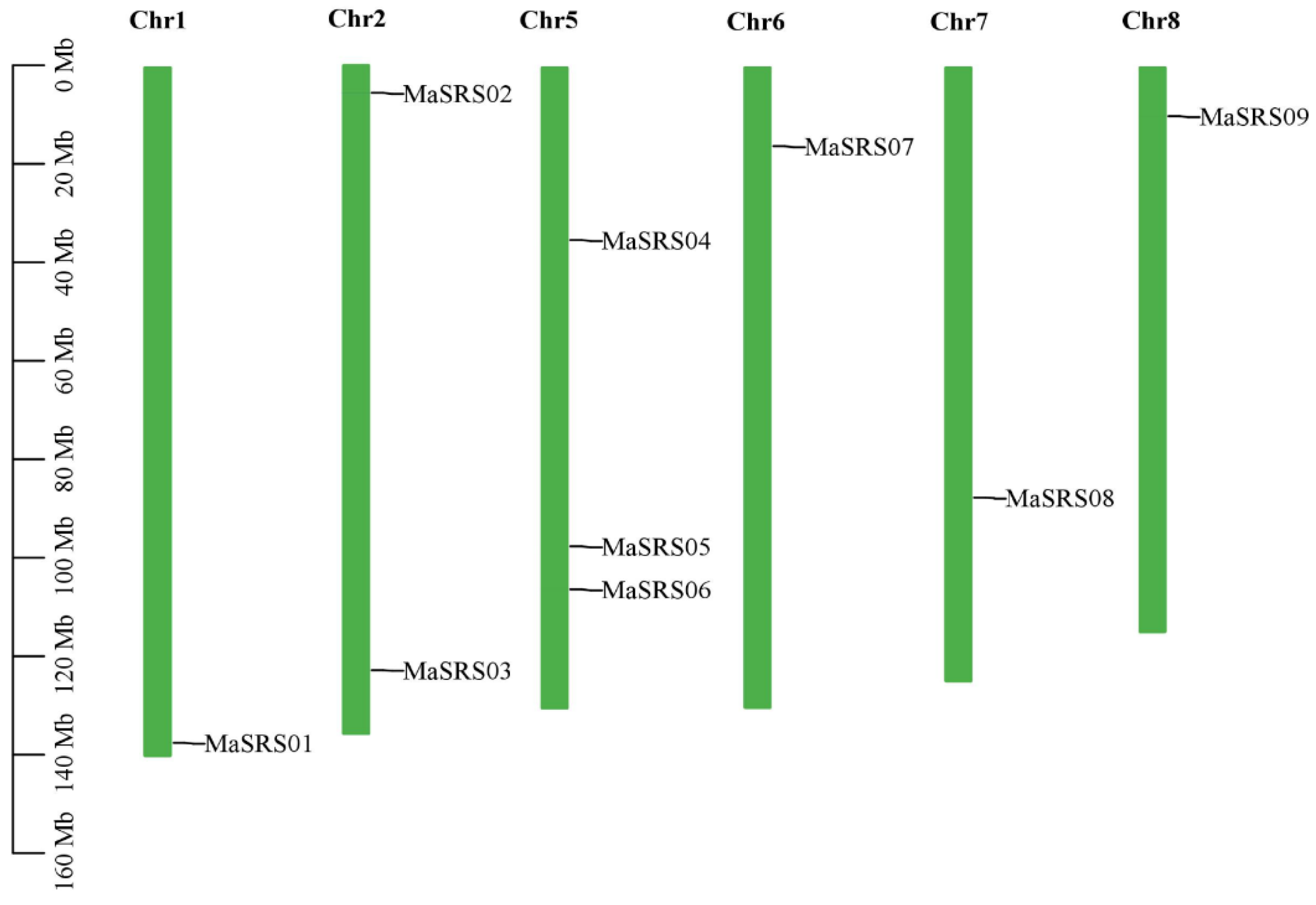
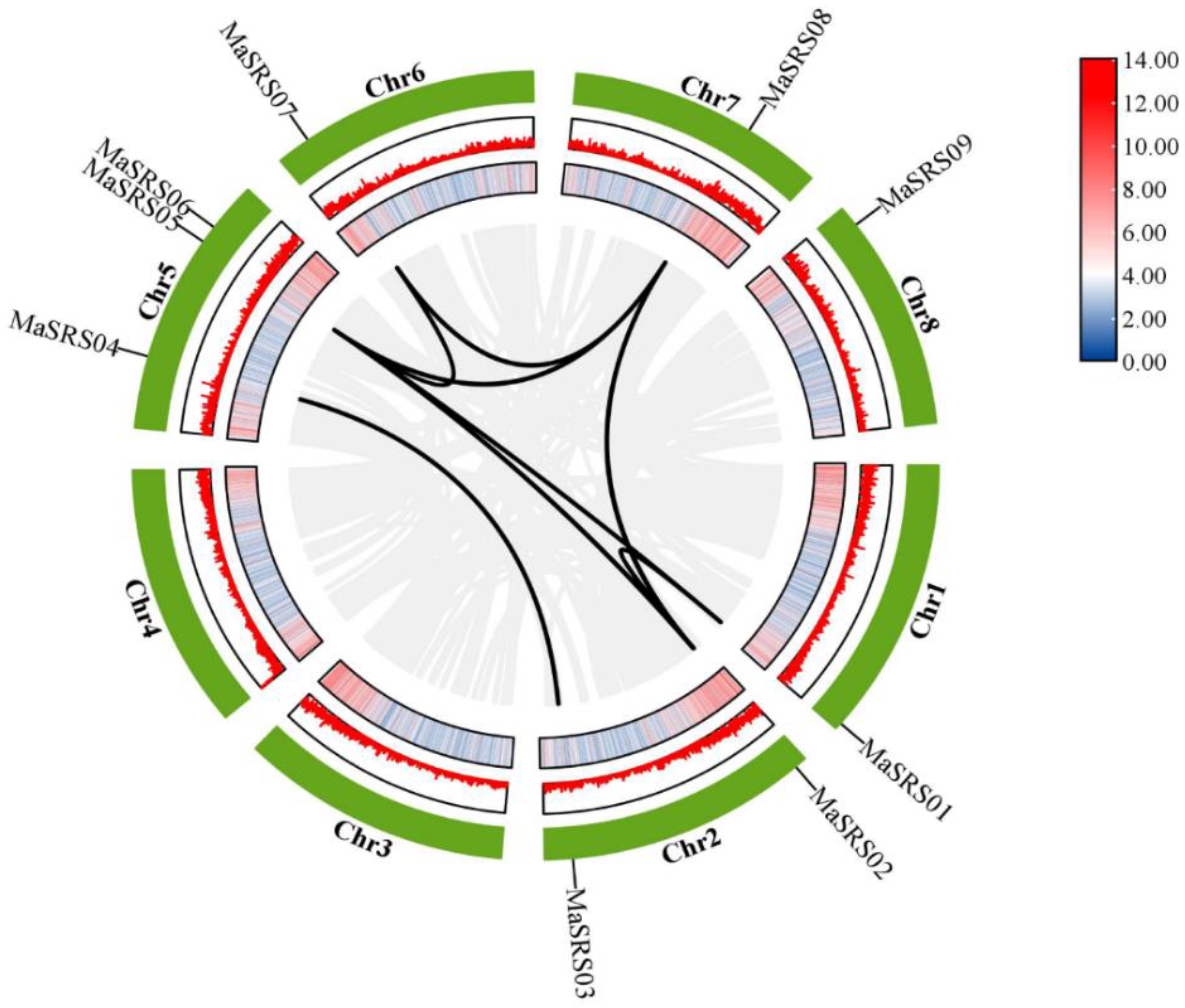
2.2. Chromosomal Locations and Gene Duplication Analysis of MaSRS Genes
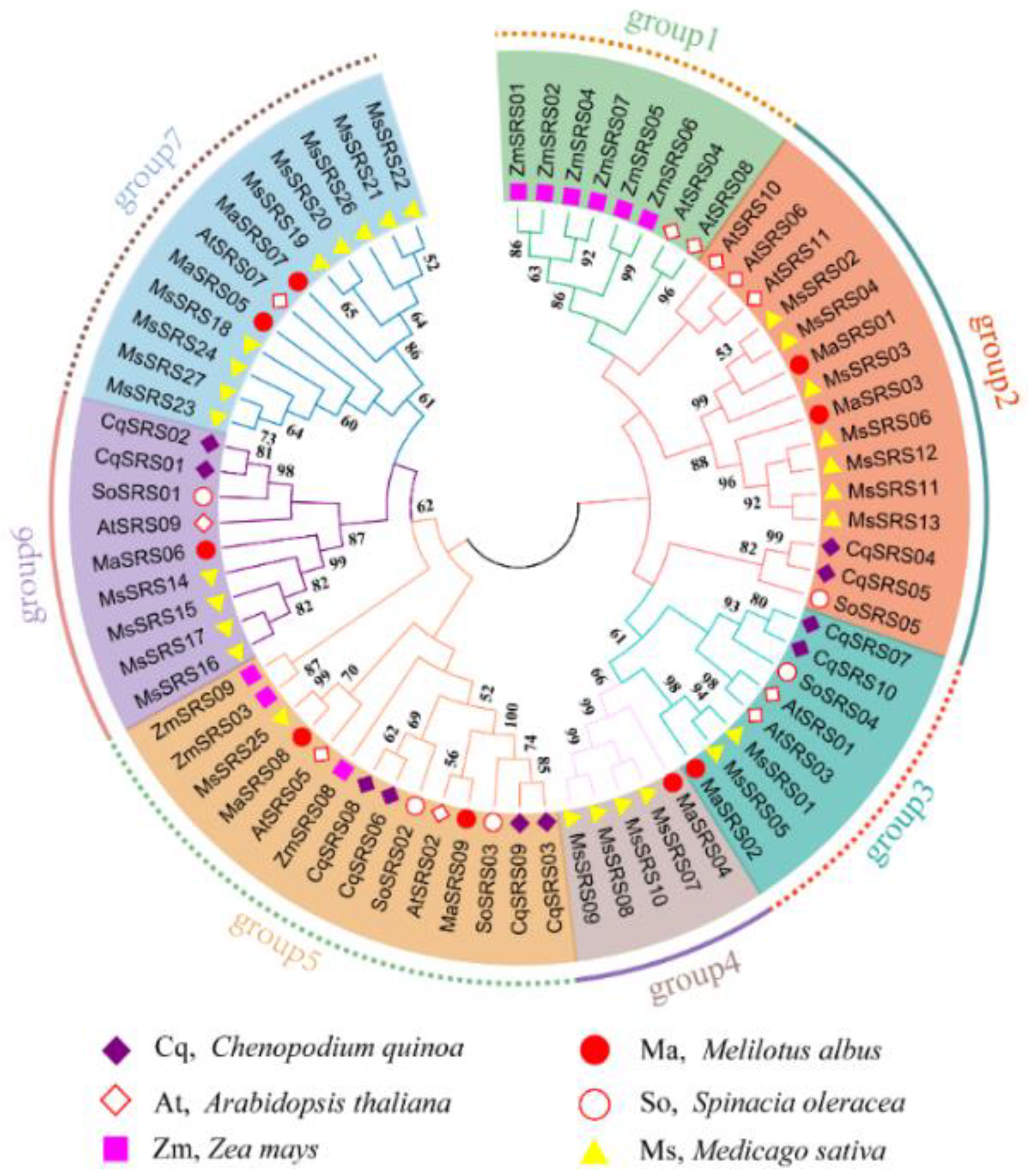
2.3. Phylogenetic Relationship, Gene Structure, Conserved Motif, and Domain Analysis of MaSRS Family
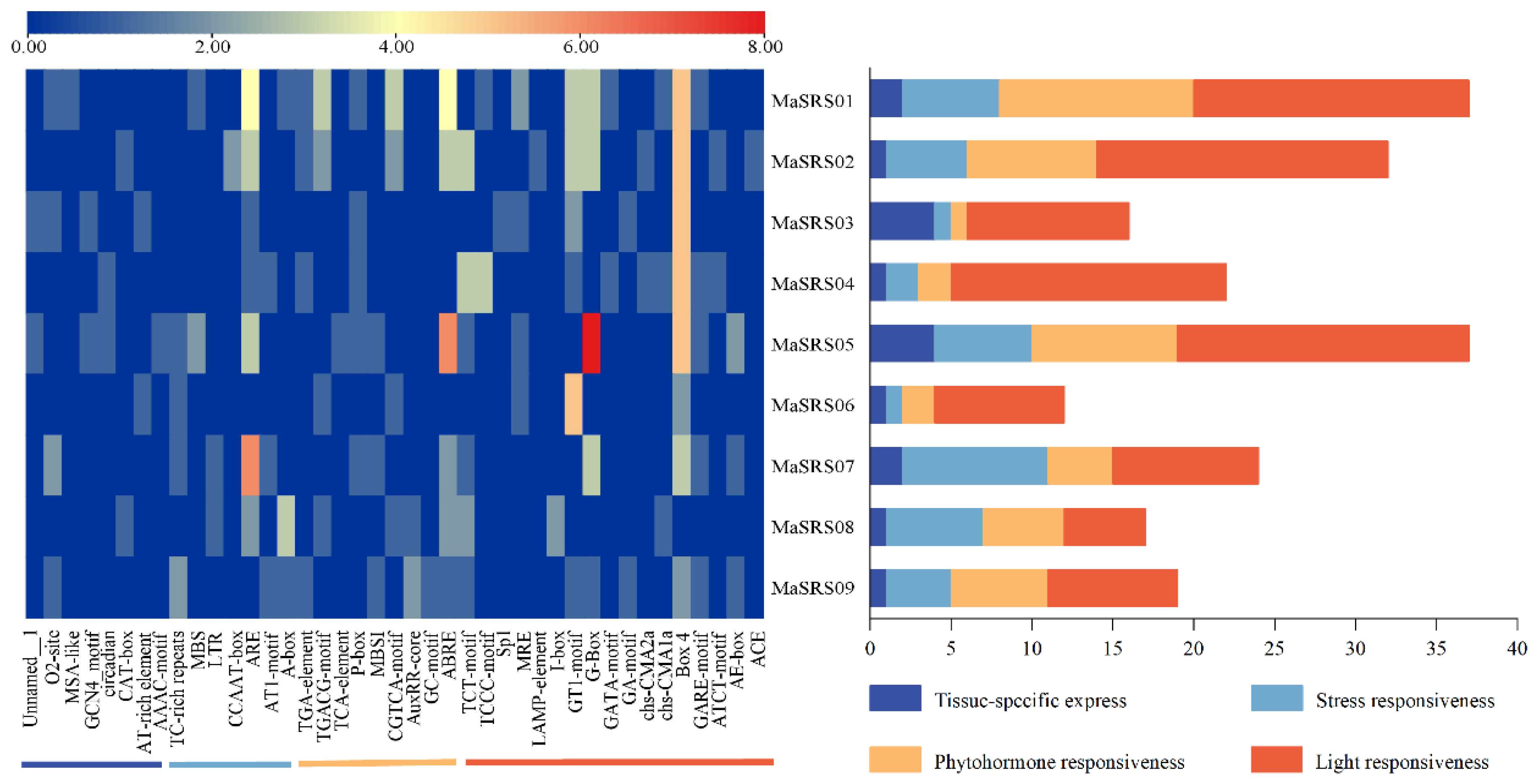
2.4. Identification and Analysis of MaSRS Promoter Cis-Acting Elements
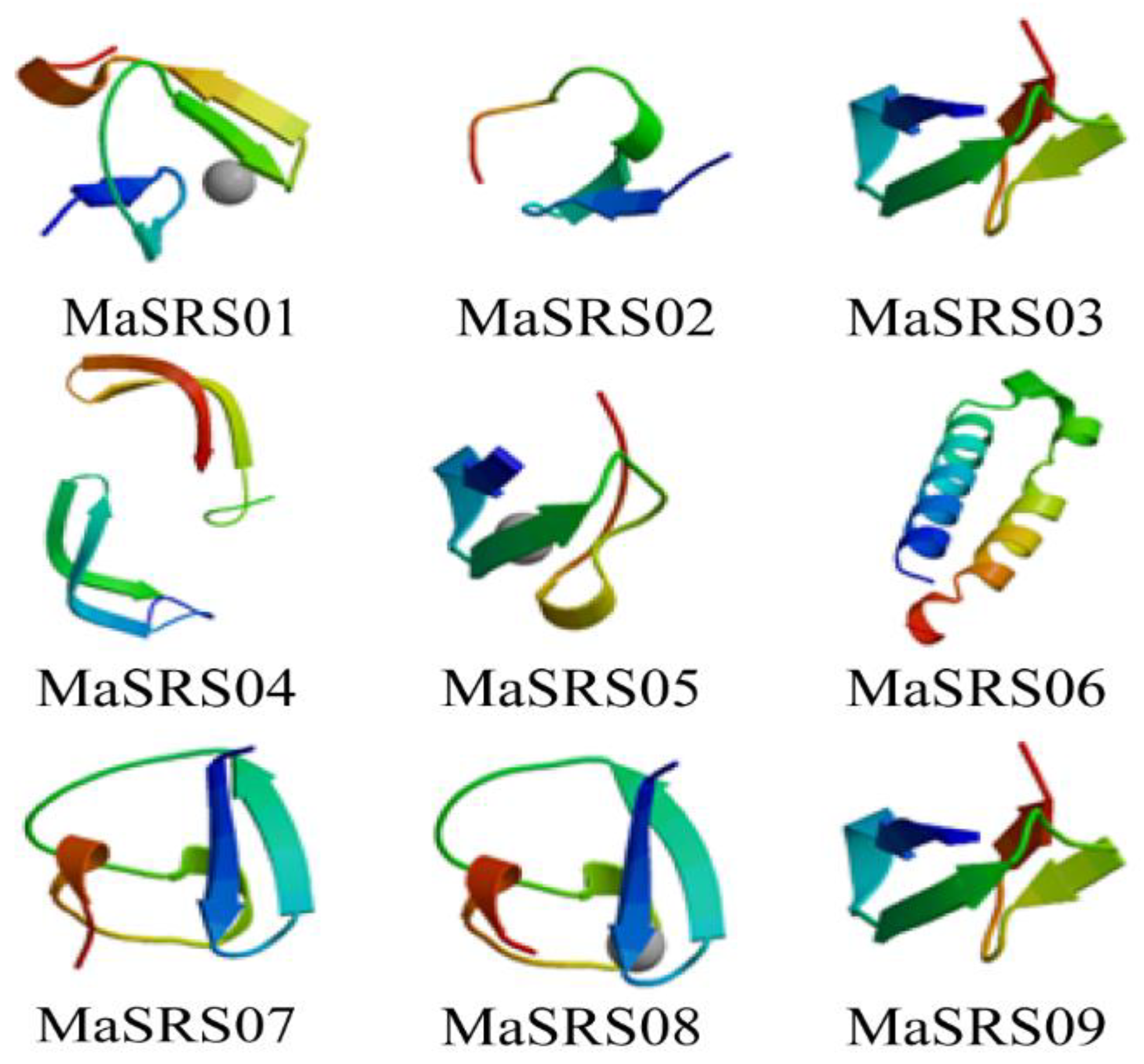
2.5. Protein Interaction Network Construction and Structure Prediction of MaSRS Tfs
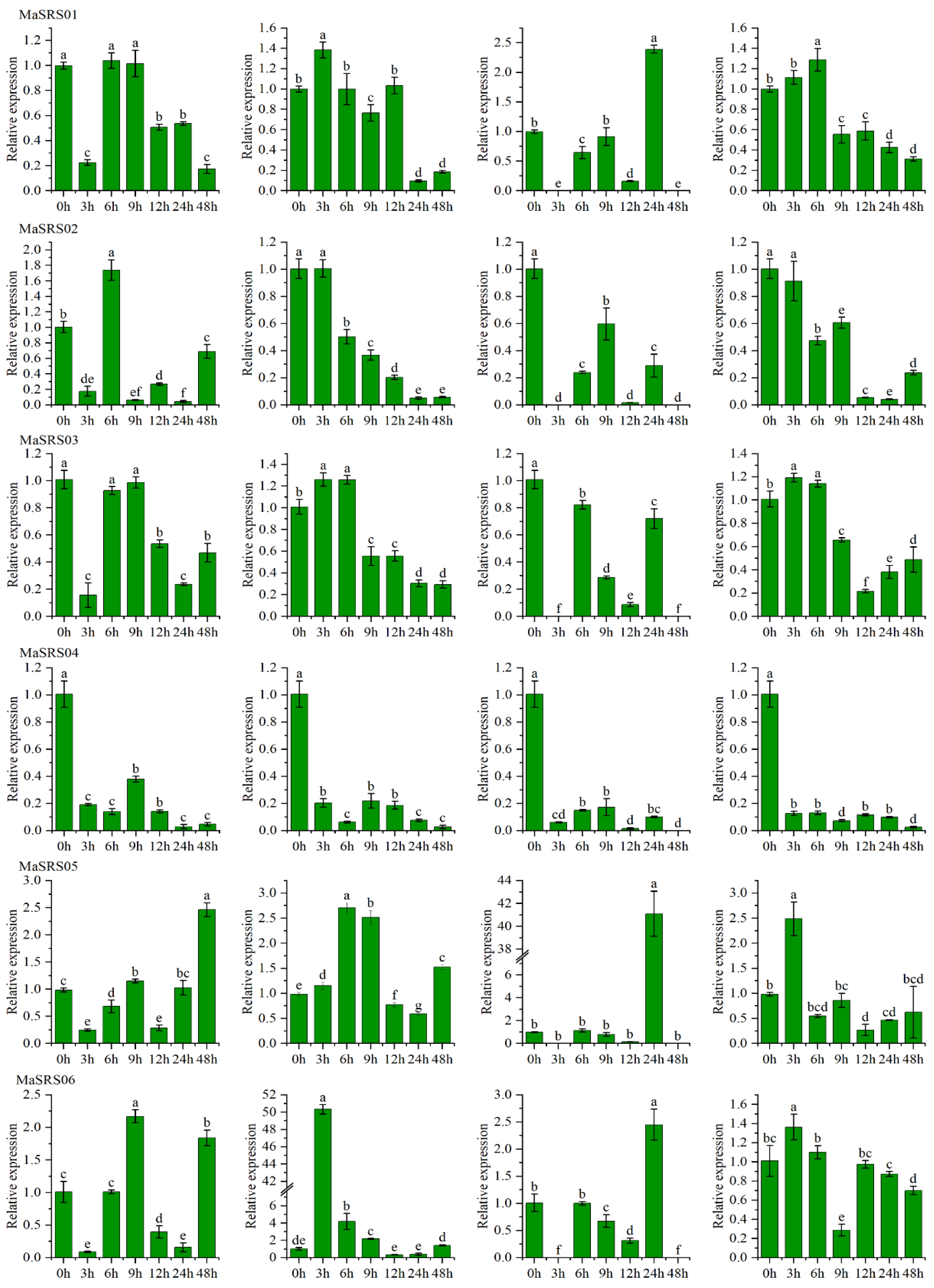
2.6. qRT-PCR Analysis of MaSRSs under Different Biotic and Abiotic Stress
3. Discussion
4. Materials and Methods
4.1. Identification of SRS Genes in Melilotus albus
4.2. Analysis of Synteny, Gene Duplication, and Phylogenetic
4.3. Analysis of the Conserved Motifs and Gene Structures
4.4. Analysis of Cis-Acting Elements of MaSRS Genes, MaSRSs Protein-Protein Interaction Networks, and Prediction of Protein 3D Structure
4.5. Plant Cultivation and Stress Treatments
4.6. RNA Extraction and Real-Time Fluorescence Quantitative PCR Analysis of MaSRS
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Qi, L.; Nian, L.; Zhu, X.; Yi, X.; Zhang, J.; Qiu, J. Genome-Wide Identification and Expression Analysis of the SRS Gene Family in Medicago sativa. DNA Cell Biol. 2021, 40, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Song, X.Y.; Guo, L.L.; Zhang, X.Z.; Zheng, W.J. Genome-Wide Analysis of the Shi-Related Sequence Family and Functional Identification of Gmsrs18 Involving in Drought and Salt Stresses in Soybean. Int. J. Mol. Sci. 2020, 21, 1810. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, P.; Yu, D. Genome-Wide Identification and Characterization of the SHI-Related Sequence Gene Family in Rice. Evol. Bioinforma. 2020, 16. [Google Scholar] [CrossRef]
- Büyük, İ.; Okay, A.; Aras, S. Identification and Characterization of SRS Genes in Phaseolus Vulgaris Genome and Their Responses Under Salt Stress. Biochem. Genet. 2022, 60, 482–503. [Google Scholar] [CrossRef]
- Stone, S.L.; Hauksdóttir, H.; Troy, A.; Herschleb, J.; Kraft, E.; Callis, J. Functional Analysis of the RING-Type Ubiquitin Ligase Family of Arabidopsis. Plant Physiol. 2005, 137, 13–30. [Google Scholar] [CrossRef]
- Elenbaas, B.; Dobbelstein, M.; Roth, J.; Shenk, T.; Levine, A.J. The MDM2 Oncoprotein Binds Specifically to RNA through Its Ring Finger Domain. Mol. Med. 1996, 2, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Fridborg, I.; Kuusk, S.; Robertson, M.; Sundberg, E. The Arabidopsis Protein SHI Represses Gibberellin Responses in Arabidopsis and Barley. Plant Physiol. 2001, 127, 937–948. [Google Scholar] [CrossRef]
- Kuusk, S.; Sohlberg, J.J.; Magnus Eklund, D.; Sundberg, E. Functionally Redundant SHI Family Genes Regulate Arabidopsis Gynoecium Development in a Dose-Dependent Manner. Plant J. 2006, 47, 99–111. [Google Scholar] [CrossRef]
- Eklund, D.M.; Ståldal, V.; Valsecchi, I.; Cierlik, I.; Eriksson, C.; Hiratsu, K.; Takagi, M.O.; Sundström, J.F.; Thelander, M.; Ezcurra, I.; et al. The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. The Plant Cell 2010, 22, 349–363. [Google Scholar] [CrossRef]
- Hossain, M.S.; Shrestha, A.; Zhong, S.; Miri, M.; Austin, R.S.; Sato, S.; Ross, L.; Huebert, T.; Tromas, A.; Torres-Jerez, I.; et al. Lotus Japonicus NF-YA1 Plays an Essential Role during Nodule Differentiation and Targets Members of the SHI/STY Gene Family. Mol. Plant-Microbe Interact. 2016, 29, 950–964. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, S.; Singh, A.; Mahima, M.; Singh, A.; Gautam, V.; Sarkar, A.K. Auxin Signaling Modulates LATERAL ROOT PRIMORDIUM1 (LRP1) Expression during Lateral Root Development in Arabidopsis. Plant J. 2020, 101, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Baylis, T.; Cierlik, I.; Sundberg, E.; Mattsson, J. SHORT INTERNODES/STYLISH Genes, Regulators of Auxin Biosynthesis, Are Involved in Leaf Vein Development in Arabidopsis thaliana. New Phytol. 2013, 197, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Gomariz-Fernández, A.; Sánchez-Gerschon, V.; Fourquin, C.; Ferrándiz, C. The Role of SHI/STY/SRS Genes in Organ Growth and Carpel Development Is Conserved in the Distant Eudicot Species Arabidopsis Thaliana and Nicotiana Benthamiana. Front. Plant Sci. 2017, 8, 814. [Google Scholar] [CrossRef]
- Yuan, T.T.; Xu, H.H.; Zhang, Q.; Zhang, L.Y.; Lu, Y.T. The Cop1 Target Shi-Related Sequence5 Directly Activates Photomorphogenesis-Promoting Genes. Plant Cell 2018, 30, 2368–2382. [Google Scholar] [CrossRef]
- Yuan, T.T.; Xu, H.H.; Li, J.; Lu, Y.T. Auxin Abolishes SHI-RELATED SEQUENCE5-Mediated Inhibition of Lateral Root Development in Arabidopsis. New Phytol. 2020, 225, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.M.; Eggert, K.; Koppolu, R.; Alqudah, A.M.; Poursarebani, N.; Fazeli, A.; Sakuma, S.; Tagiri, A.; Rutten, T.; Govind, G.; et al. VRS2 Regulates Hormone-Mediated Inflorescence Patterning in Barley. Nat. Genet. 2017, 49, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Takahisa, Y.; Yuko, Y.; Hiroyuki, K.; Takashi, M.; Udda, L.; Kazuhiro, S.; Masahiki, I.; Stephen, A.J.; Shin, T. A SHORT INTERNODES (SHI) Family Transcription Factor Gene Regulates Awn Elongation and Pistil Morphology in Barley. J. Exp. Bot. 2012, 63, 5223–5232. [Google Scholar]
- He, B.; Shi, P.; Lv, Y.; Gao, Z.; Chen, G. Gene Coexpression Network Analysis Reveals the Role of SRS Genes in Senescence Leaf of Maize (Zea Mays L.). J. Genet. 2020, 99, 3. [Google Scholar] [CrossRef]
- Wu, F.; Duan, Z.; Xu, P.; Yan, Q.; Meng, M.; Cao, M.; Jones, C.S.; Zong, X.; Zhou, P.; Wang, Y.; et al. Genome and Systems Biology of Melilotus Albus Provides Insights into Coumarins Biosynthesis. Plant Biotechnol. J. 2022, 20, 592–609. [Google Scholar] [CrossRef]
- Zhang, J.; Di, H.; Luo, K.; Jahufer, Z.; Wu, F.; Duan, Z.; Stewart, A.; Yan, Z.; Wang, Y. Coumarin Content, Morphological Variation, and Molecular Phylogenetics of Melilotus. Molecules 2018, 23, 810. [Google Scholar] [CrossRef]
- Wu, F.; Luo, K.; Yan, Z.; Zhang, D.; Yan, Q.; Zhang, Y.; Yi, X.; Zhang, J. Analysis of MiRNAs and Their Target Genes in Five Melilotus Albus NILs with Different Coumarin Content. Sci. Rep. 2018, 8, 14138. [Google Scholar] [CrossRef] [PubMed]
- Zabala, J.M.; Marinoni, L.; Giavedoni, J.A.; Schrauf, G.E. Breeding Strategies in Melilotus Albus Desr., a Salt-Tolerant Forage Legume. Euphytica 2018, 214, 22. [Google Scholar] [CrossRef]
- Hong, J.K.; Kim, J.A.; Kim, J.S.; Lee, S.I.; Koo, B.S.; Lee, Y.H. Overexpression of Brassica Rapa SHI-RELATED SEQUENCE Genes Suppresses Growth and Development in Arabidopsis thaliana. Biotechnol. Lett. 2012, 34, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kaeppler, S.M.; Vogel, K.P.; Casler, M.D. Selection Signatures in Four Lignin Genes from Switchgrass Populations Divergently Selected for In Vitro Dry Matter Digestibility. PLoS ONE 2016, 11, e0167005. [Google Scholar] [CrossRef]
- Trigueros, M.; Navarrete-Gómez, M.; Sato, S.; Christensen, S.K.; Pelaz, S.; Weigel, D.; Yanofsky, M.F.; Ferrándiza, C. The NGATHA Genes Direct Style Development in the Arabidopsis Gynoecium. Plant Cell 2009, 21, 1394–1409. [Google Scholar] [CrossRef]
- Clark, A. Managing Cover Crops Profitably, 3rd ed.; SARE Handbook Series, Book 9; DIANE Publishing: Collingdale, PA, USA, 2007. [Google Scholar]
- Sohlberg, J.J.; Myrenås, M.; Kuusk, S.; Lagercrantz, U.; Kowalczyk, M.; Sandberg, G.; Sundberg, E. STY1 Regulates Auxin Homeostasis and Affects Apical-Basal Patterning of the Arabidopsis Gynoecium. Plant J. 2006, 47, 112–123. [Google Scholar] [CrossRef]
- Ståldal, V.; Cierlik, I.; Landberg, K.; Myrenås, M.; Sundström, J.F.; Eklund, D.M.; Chen, S.; Baylis, T.; Ljung, K.; Sundberg, E. The Arabidopsis Thaliana Transcriptional Activator STYLISH1 Regulates Genes Affecting Stamen Development, Cell Expansion and Timing of Flowering. Plant Mol. Biol. 2012, 78, 545–559. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, S.; Kim, Y.S.; Yun, D.J.; Woo, J.C.; Park, C.M. Activation Tagging of an Arabidopsis SHI-RELATED SEQUENCE Gene Produces Abnormal Anther Dehiscence and Floral Development. Plant Mol. Biol. 2010, 74, 337–351. [Google Scholar] [CrossRef]
- Eklund, D.M.; Thelander, M.; Landberg, K.; Ståldal, V.; Nilsson, A.; Johansson, M.; Valsecchi, I.; Pederson, E.R.A.; Kowalczyk, M.; Ljung, K.; et al. Homologues of the Arabidopsis Thaliana SHI/STY/LRP1 Genes Control Auxin Biosynthesis and Affect Growth and Development in the Moss Physcomitrella Patens. Development 2010, 137, 1275–1284. [Google Scholar] [CrossRef]
- Duan, E.; Wang, Y.; Li, X.; Lin, Q.; Zhang, T.; Wang, Y.; Zhou, C.; Zhang, H.; Jiang, L.; Wang, J.; et al. Osshi1 Regulates Plant Architecture through Modulating the Transcriptional Activity of Ipa1 in Rice. Plant Cell 2019, 31, 1026–1042. [Google Scholar] [CrossRef]
- Nian, L.; Zhang, X.; Yi, X.; Liu, X.; Ain, N.u.; Yang, Y.; Li, X.; Haider, F.U.; Zhu, X. Genome-Wide Identification of ABA Receptor PYL/RCAR Gene Family and Their Response to Cold Stress in Medicago Sativa L. Physiol. Mol. Biol. Plants 2021, 27, 1979–1995. [Google Scholar] [CrossRef] [PubMed]
- Conant, G.C.; Wolfe, K.H. Turning a Hobby into a Job: How Duplicated Genes Find New Functions. Nat. Rev. Genet. 2008, 9, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. How Introns Enhance Gene Expression. Int. J. Biochem. Cell Biol. 2017, 91, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Hooft, R.W.W.; Sander, C.; Vriend, G. Positioning Hydrogen Atoms by Optimizing Hydrogen-bond Networks in Protein Structures. Proteins Struct. Funct. Genet. 1996, 26, 363–376. [Google Scholar] [CrossRef]
- Jin, X.; Yin, X.; Ndayambaza, B.; Zhang, Z.; Min, X.; Lin, X.; Wang, Y.; Liu, W. Genome-Wide Identification and Expression Profiling of the ERF Gene Family in Medicago Sativa L. Under Various Abiotic Stresses. DNA Cell Biol. 2019, 38, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Rajaofera, M.J.N.; Zhu, L.; Liu, W.; Zheng, F.; Miao, W. WY7 Is a Newly Identified Promoter from the Rubber Powdery Mildew Pathogen That Regulates Exogenous Gene Expression in Both Monocots and Dicots. PLoS ONE 2020, 15, e0233911. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, N.; Wang, Q.; Liu, Z.; Liu, W. Genome-Wide Identification and Characterization of the Heavy Metal ATPase (HMA) Gene Family in Medicago Truncatula under Copper Stress. Int. J. Biol. Macromol. 2021, 193, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between Two Cis-Acting Elements, ABRE and DRE, in ABA-Dependent Expression of Arabidopsis Rd29A Gene in Response to Dehydration and High-Salinity Stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hobo, T.; Asada, M.; Kowyama, Y.; Hattori, T. ACGT-Containing Abscisic Acid Response Element (ABRE) and Coupling Element 3 (CE3) Are Functionally Equivalent. Plant J. 1999, 19, 679–689. [Google Scholar] [CrossRef]
- Mukherjee, K.; Choudhury, A.R.; Gupta, B.; Gupta, S.; Sengupta, D.N. An ABRE-Binding Factor, OSBZ8, Is Highly Expressed in Salt Tolerant Cultivars than in Salt Sensitive Cultivars of Indica Rice. Handb. Environ. Chem. Vol. 5 Water Pollut. 2006, 6, 18. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Wang, Y.; Qin, T.; Xu, D.; Sun, C.; Yao, P.; Liu, Y.; Bi, Z.; Bai, J. Identification and Expression Analysis of Calcium-Dependent Protein Kinases Gene Family in Potato Under Drought Stress. Front. Genet. 2022, 13, 874397. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Song, R.T.; Yu, M.Y.; Sui, J.M.; Wang, J.S.; Qiao, L.X. Cloning and Functional Analysis of the Chitinase Gene Promoter in Peanut. Genet. Mol. Res. 2015, 14, 12710–12722. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ren, H.; Luo, Y.; Feng, C.; Li, H. Genome-Wide Identification of Wheat (Triticum Aestivum L.) Expansin Genes and Functional Characterization of TaEXPB1A. Environ. Exp. Bot. 2021, 182, 104307. [Google Scholar] [CrossRef]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Schölkopf, B.; Weigel, D.; Lohmann, J.U. A Gene Expression Map of Arabidopsis Thaliana Development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Zawaski, C.; Kadmiel, M.; Ma, C.; Gai, Y.; Jiang, X.; Strauss, S.H.; Busov, V.B. SHORT INTERNODES-like Genes Regulate Shoot Growth and Xylem Proliferation in Populus. New Phytol. 2011, 191, 678–691. [Google Scholar] [CrossRef]
- Lyu, G.; Li, D.; Li, S. Bioinformatics Analysis of BBX Family Genes and Its Response to UV-B in Arabidopsis thaliana. Plant Signal. Behav. 2020, 15, 1782647. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Gardy, J.L.; Laird, M.R.; Chen, F.; Rey, S.; Walsh, C.J.; Ester, M.; Brinkman, F.S.L. PSORTb v.2.0: Expanded Prediction of Bacterial Protein Subcellular Localization and Insights Gained from Comparative Proteome Analysis. Bioinformatics 2005, 21, 617–623. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Ayaz, A.; Saqib, S.; Huang, H.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant Physiol. Biochem. 2021, 161, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Baxter, L.; Hickman, R.; Beynon, J.; Moore, J.D.; Ott, S. MEME-LaB: Motif Analysis in Clusters. Bioinformatics 2013, 29, 1696–1697. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van De Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein-Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. Sopma: Significant Improvements in Protein Secondary Structure Prediction by Consensus Prediction from Multiple Alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. Peitsch SWISS-MODEL: An Automated Protein Homology-Modeling Server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Hooft, R.W.W.; Sander, C.; Vriend, G. Objectively Judging the Quality of a Protein Structure from a Ramachandran Plot. Bioinformatics 1997, 13, 425–430. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene ID | Gene Name | Protein Length (aa) | Molecular Weight (kDa) | Isoelectric Point (PI) | GRAVY | Instability Index | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| Malbus0105973.1 | MaSRS01 | 339 | 36.69790 | 7.63 | −0.845 | 60.61 | Nucleus |
| Malbus0200518.1 | MaSRS02 | 273 | 29.96145 | 8.53 | −0.562 | 59.56 | Nucleus |
| Malbus0205193.1 | MaSRS03 | 332 | 36.18219 | 7.24 | −0.696 | 48.69 | Nucleus |
| Malbus0501767.1 | MaSRS04 | 324 | 35.59567 | 7.55 | −0.653 | 56.92 | Nucleus |
| Malbus0503375.1 | MaSRS05 | 339 | 36.94744 | 6.70 | −0.750 | 61.44 | Nucleus |
| Malbus0503792.1 | MaSRS06 | 368 | 40.82662 | 5.91 | −0.804 | 48.56 | Nucleus. |
| Malbus0601083.1 | MaSRS07 | 323 | 34.95433 | 5.79 | −0.576 | 55.77 | Nucleus |
| Malbus0702600.1 | MaSRS08 | 197 | 22.52211 | 8.70 | −0.951 | 59.98 | Nucleus |
| Malbus0800677.1 | MaSRS09 | 353 | 37.35217 | 8.79 | −0.604 | 47.07 | Nucleus |
| Gene_1 | Gene_2 | Ka | Ks | Ka_Ks | Duplication Type | T (Mya.) |
|---|---|---|---|---|---|---|
| MaSRS01 | MaSRS02 | 0.28247617 | 0.923572967 | 0.305851492 | Segmental | 102.6192185 |
| MaSRS01 | MaSRS05 | 0.412837564 | 1.299059979 | 0.317797153 | Segmental | 144.3399977 |
| MaSRS02 | MaSRS05 | 0.372374639 | 1.280259294 | 0.290858767 | Segmental | 142.2510326 |
| MaSRS02 | MaSRS08 | 0.499505712 | 1.917012554 | 0.260564654 | Segmental | 213.0013949 |
| MaSRS03 | MaSRS04 | 0.224039169 | 0.718741807 | 0.311710223 | Segmental | 79.8602008 |
| MaSRS05 | MaSRS07 | 0.277413196 | 1.061327635 | 0.261383183 | Segmental | 117.9252928 |
| MaSRS05 | MaSRS08 | 0.580204202 | 2.042528126 | 0.284061793 | Segmental | 226.9475696 |
| MaSRS07 | MaSRS08 | 0.449255747 | 3.245713835 | 0.138415082 | Segmental | 360.6348705 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Nian, L.; Ain, N.u.; Liu, X.; Yang, Y.; Zhu, X.; Haider, F.U.; Lv, Y.; Bai, P.; Zhang, X.; et al. Genome-Wide Identification and Expression Profiling of the SRS Gene Family in Melilotus albus Reveals Functions in Various Stress Conditions. Plants 2022, 11, 3101. https://doi.org/10.3390/plants11223101
Ma B, Nian L, Ain Nu, Liu X, Yang Y, Zhu X, Haider FU, Lv Y, Bai P, Zhang X, et al. Genome-Wide Identification and Expression Profiling of the SRS Gene Family in Melilotus albus Reveals Functions in Various Stress Conditions. Plants. 2022; 11(22):3101. https://doi.org/10.3390/plants11223101
Chicago/Turabian StyleMa, Biao, Lili Nian, Noor ul Ain, Xuelu Liu, Yingbo Yang, Xiaolin Zhu, Fasih Ullah Haider, Ying Lv, Pengpeng Bai, Xiaoning Zhang, and et al. 2022. "Genome-Wide Identification and Expression Profiling of the SRS Gene Family in Melilotus albus Reveals Functions in Various Stress Conditions" Plants 11, no. 22: 3101. https://doi.org/10.3390/plants11223101
APA StyleMa, B., Nian, L., Ain, N. u., Liu, X., Yang, Y., Zhu, X., Haider, F. U., Lv, Y., Bai, P., Zhang, X., Li, Q., Mao, Z., & Xue, Z. (2022). Genome-Wide Identification and Expression Profiling of the SRS Gene Family in Melilotus albus Reveals Functions in Various Stress Conditions. Plants, 11(22), 3101. https://doi.org/10.3390/plants11223101








