HPLC-MS Profiling, Antioxidant, Antimicrobial, Antidiabetic, and Cytotoxicity Activities of Arthrocnemum indicum (Willd.) Moq. Extracts
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis
2.2. Polyphenolic Profile
2.3. Antioxidant Activity
2.4. Antimicrobial Activity
2.5. α-Glucosidase Inhibitory Activity Evaluation
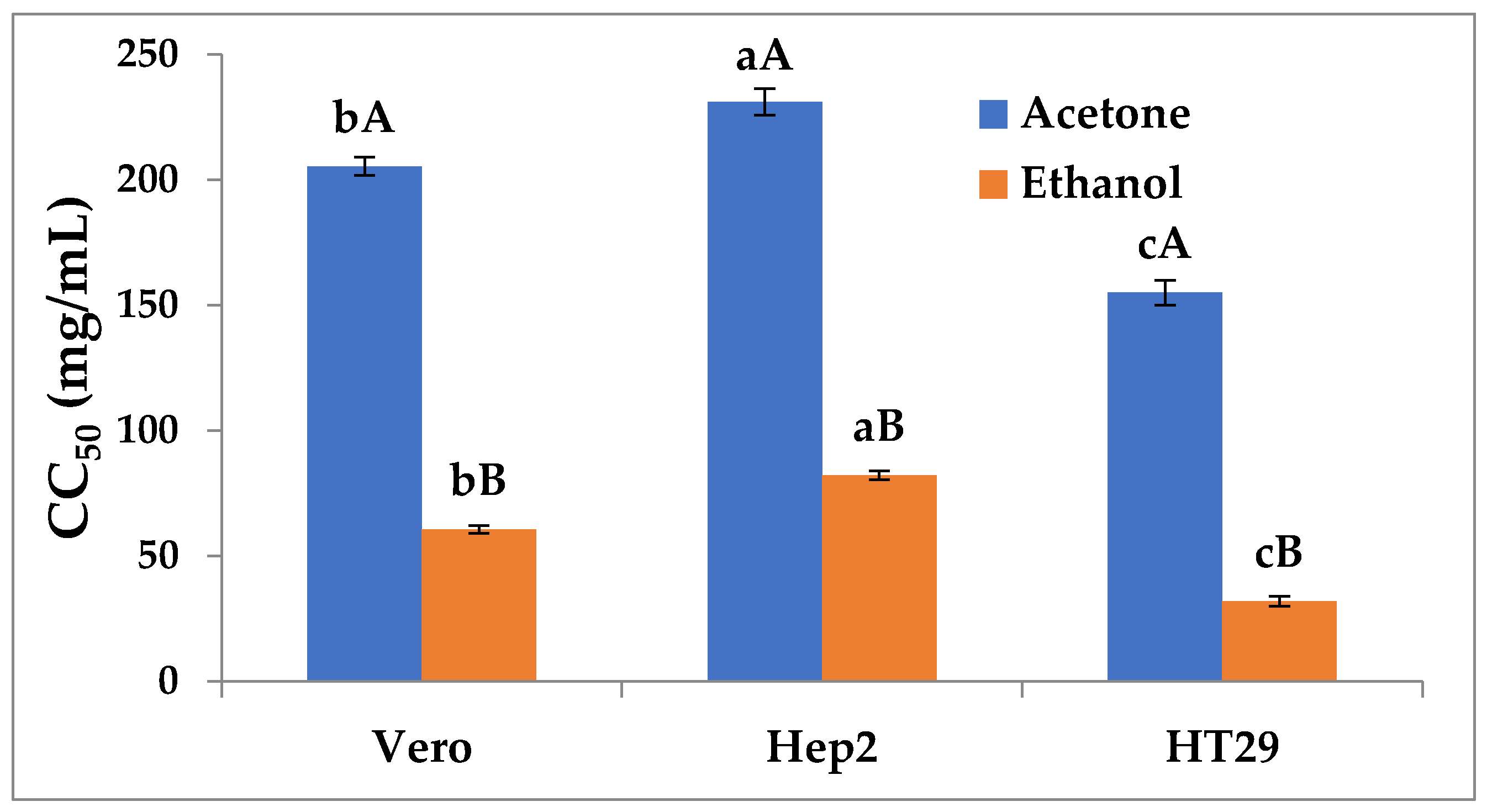
2.6. Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Plant Sampling and Extract Preparation
4.3. Colorimetric Quantification of Antioxidants
4.3.1. TPC Assay
4.3.2. TFC Assay
4.3.3. TCTC Assay
4.4. HPLC-MS Analysis of Phenolic Compounds
4.5. Antioxidant Activity
4.6. Antimicrobial Activity
4.6.1. Microorganisms
4.6.2. Disc-Diffusion Assay
4.6.3. Micro-Well Determination of MIC, MBC, and MFC
4.7. Cytotoxicity Assay
4.8. α-Glucosidase Inhibitory Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Mefteh, F.; Daoud, A.; Bouket, A.C.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date Palm Trees Root-Derived Endophytes as Fungal Cell Factories for Diverse Bioactive Metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef] [Green Version]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Anti-oxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Daoud, A.; Ben Mefteh, F.; Mnafgui, K.; Turki, M.; Jmal, S.; Ben Amar, R.; Ayadi, F.; ElFeki, A.; Abid, L.; Rateb, M.E.; et al. Cardiopreventive effect of ethanolic extract of date palm pollen against isoproterenol induced myocardial infarction in rats through the inhibition of the angiotensin-converting enzyme. Exp. Toxicol. Pathol. 2017, 69, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad-Elkareem, M.A.M.; Abdelgadir, E.H.; Badawy, O.M.; Kadri, A. Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ 2019, 7, e6441. [Google Scholar] [CrossRef] [Green Version]
- Felhi, S.; Hajlaoui, H.; Ncir, M.; Bakari, S.; Ktari, N.; Saoudi, M.; Gharsallah, N.; Kadri, A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze-dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016, 36, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Felhi, S.; Daoud, A.; Hajlaoui, H.; Mnafgui, K.; Gharsallah, N.; Kadri, A. Solvent extraction effects on phytochemical constituents profiles, antioxidant and antimicrobial activities and functional group analysis of Ecballium elaterium seeds and peels fruits. Food Sci. Technol. 2017, 37, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Aouadi, K.; Hajlaoui, H.; Arraouadi, S.; Ghannay, S.; Snoussi, M.; Kadri, A. HPLC/MS Phytochemical Profiling with Antioxidant Activities of Echium humile Desf. Extracts: ADMET Prediction and Computational Study Targeting Human Peroxiredoxin 5 Receptor. Agronomy 2021, 11, 2165. [Google Scholar] [CrossRef]
- Sajkowska-Kozielewicz, J.J.; Kozielewicz, P.; Barnes, N.M.; Wawer, I.; Paradowska, K. Antioxidant, cytotoxic, and antiproliferative activities and total polyphenol contents of the extracts of Geissospermum reticulatum bark. Oxid. Med. Cell. Longev. 2016, 2016, 2573580. [Google Scholar] [CrossRef] [Green Version]
- Hajlaoui, H.; Arraouadi, S.; Mighri, H.; Chaaibia, M.; Gharsallah, N.; Ros, G.; Nieto, G.; Kadri, A. Phytochemical Constituents and Antioxidant Activity of Oudneya Africana L. Leaves Extracts: Evaluation Effects on Fatty Acids and Proteins Oxidation of Beef Burger during Refrigerated Storage. Antioxidants 2019, 8, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felhi, S.; Saoudi, M.; Daoud, A.; Hajlaoui, H.; Ncir, M.; Chaabane, R.; El Feki, A.; Gharsallah, N.; Kadri, A. Investigation of phytochemical contents, in vitro antioxidant and antibacterial behavior and in vivo anti-inflammatory potential of Ecballium elaterium methanol fruits extract. Food Sci. Technol. 2017, 37, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Bakari, S.; Hajlaoui, H.; Daoud, A.; Mighri, H.; Ross-Garcia, J.M.; Gharsallah, N.; Kadri, A. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of hydroalcoholic extracts of leaves and flowers of Erodium glaucophyllum collected from Tunisian Sahara. Food Sci. Biotechnol. 2018, 38, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Bakari, S.; Daoud, A.; Felhi, S.; Smaoui, S.; Gharsallah, N.; Kadri, A. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of cactus cladode. Food Sci. Technol. 2017, 27, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Takomthong, P.; Waiwut, P.; Yenjai, C.; Sombatsri, A.; Reubroycharoen, P.; Lei, L.; Lai, R.; Chaiwiwatrakul, S.; Boonyarat, C. Multi-Target Actions of Acridones from Atalantia monophylla towards Alzheimer’s Pathogenesis and Their Pharmacokinetic Properties. Pharmaceuticals 2021, 14, 888. [Google Scholar] [CrossRef]
- Hurtado-Fernandez, E.; Pacchiarotta, T.; Mayboroda, O.A.; Fernandez-Gutierrez, A.; Carrasco-Pancorbo, A. Quantitative characterization of important metabolites of avocado fruit by gas chromatography coupled to different detectors (APCI-TOF MS and FID). Food Res. Int. 2014, 62, 801–811. [Google Scholar] [CrossRef]
- Liebezeit, G.; Künnemann, T.D.; Gad, G. Biotechnological potential of North sea salt marsh plants-a review of traditional knowledge. Prog. Ind. Microbiol. 1999, 70, 77–84. [Google Scholar]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Figueroa, M.E.; Davy, A.J. Salt stimulation of growth and photosynthesis in an extreme halophyte Arthrocnemum macrostachyum. Plant Biol. 2010, 12, 79–87. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Kumar, A.; Kaushik, P.; Incerpi, S.; Pedersen, J.Z.; Goel, S.; Prasad, A.K.; Rohil, V.; Parmar, V.S.; Saso, L.; Len, C. Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry. Molecules 2021, 26, 4800. [Google Scholar] [CrossRef]
- Majtan, J. Honey: An immunomodulator in wound healing. Wound Repair Regen. 2014, 22, 187–192. [Google Scholar] [CrossRef]
- Kelainy, E.G.; Laila, I.M.I.; Ibrahim, S.R. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ. Sci. Poll. Res. 2019, 26, 31675–31684. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Baby, A.R.; de Almeida, T.S.; Costa, J.G. In vitro cytotoxicity assessment of ferulic, caffeic and p-coumaric acids on human renal cancer cells. Biomed. Biopharm. Res. 2020, 17, 63–74. [Google Scholar] [CrossRef]
- ElKhazendar, M.; Chalak, J.; El-Huneidi, W.; Vinod, A.; Abdel-Rahman, W.M.; Abu-Gharbieh, E. Antiproliferative and proapoptotic activities of ferulic acid in breast and liver cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 2571–2576. [Google Scholar]
- Peng, C.C.; Chyau, C.C.; Wang, H.E.; Chang, C.H.; Chen, K.C.; Chou, K.Y.; Peng, R.Y. Cytotoxicity of ferulic acid on T24 cell line differentiated by different microenvironments. BioMed Res. Int. 2013, 2013, 579859. [Google Scholar] [CrossRef]
- Wang, T.; Gong, X.; Jiang, R.; Li, H.; Du, W.; Kuang, G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am. J. Transl. Res. 2016, 8, 968–980. [Google Scholar]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Karimvand, M.N.; Kalantar, H.; Khodayar, M.J. Cytotoxic and apoptotic effects of ferulic acid on renal carcinoma cell line (ACHN). Jundishapur J. Nat. Pharm. Prod. 2021, 15, e81969. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Kilani Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bami, E.; Ozakpinar, O.B.; Ozdemir-Kumral, Z.N.; Köroglu, K.; Ercan, F.; Cirakli, Z.; Sekerler, T.; Izzettin, F.V.; Sancar, M.; Okuyan, B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 54, 105–111. [Google Scholar] [CrossRef]
- Alam, M.A.; Sernia, C.; Brown, L. Ferulic Acid Improves Cardiovascular and Kidney Structure and Function in Hypertensive Rats. J. Cardiovasc. Pharmacol. 2013, 61, 240–249. [Google Scholar]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Ijabadeniyi, O.A.; Govender, A.; Olagunju, O.F.; Oyedeji, A.B. The antimicrobial activity of two phenolic acids against foodborne Escherichia coli and Listeria monocytogenes and their effectiveness in a meat system. Ital. J. Food Sci. 2021, 33, 39–45. [Google Scholar] [CrossRef]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion–induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Yingbin, S.; Xun, S.; Li, L.; Jian, S.; Yogini, J.; Junqing, H.; Chun, L.; Wenjian, Y.; Leonard, W.; Hui, Z.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar]
- Kilic, I.; Yesiloglu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Roy, N.; Narayanankutty, A.; Nazeem, P.A.; Valsalan, R.; Babu, T.D.; Mathew, D. Plant Phenolics Ferulic Acid and P-Coumaric Acid Inhibit Colorectal Cancer Cell Proliferation through EGFR Down-Regulation. Asian Pac. J. Cancer Prev. 2016, 17, 4019–4023. [Google Scholar] [PubMed]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem. Biol. Interact. 2018, 291, 16–28. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.A.; Novelli, E.L.; Okoshi, K.; Okoshi, M.P.; Di Muzio, B.P.; Guimaraes, J.F.; Fernandes Junior, A. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Yang, J.S.; Lin, J.J.; Lai, K.C.; Lu, H.F.; Ma, C.Y.; Sai-Chuen Wu, R.; Wu, K.C.; Chueh, F.S.; Gibson Wood, W.; et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012, 27, 480–484. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Rutin: A potential antiviral for repurposing as a SARS-CoV-2 main protease (Mpro) inhibitor. Nat. Prod. Commun. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Sun, B.; Richardo-da-Silvia, M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Saini, A.; Pandey, A.; Sharma, S.; Suradkar, U.S.; Ambedkar, Y.R.; Meena, P.; Raman, R.; Gurjar, A.S. Assessment of antioxidant activity of rosemary (Rosmarinus officinalis) leaves extract. J. Pharmacogn. Phytochem. 2020, 9, 14–17. [Google Scholar]
- Kadri, A.; Zarai, Z.; Ben Chobba, I.; Gharsallah, N.; Damak, M.; Bekir, A. Chemical composition and in vitro antioxidant activities of Thymelaea hirsuta L. essential oil from Tunisia. Afri. J. Biotechnol. 2011, 15, 2930–2935. [Google Scholar]
- Hajlaoui, H.; Snoussi, M.; Noumi, E.; Zanetti, S.; Ksouri, R.; Bakhrouf, A. Chemical composition. antioxidant and antibacterial activities of the essential oils of five Tunisian aromatic plants. Ital. J. Food Sci. 2010, 3, 323–332. [Google Scholar]
- Snoussi, M.; Hajlaoui, H.; Noumi, E.; Usai, D.; Sechi, L.A.; Zanetti, S.; Bakhrouf, A. In-vitro anti-Vibrio spp. activity and chemical composition of some Tunisian aromatic plants. World J. Microbiol. Biotechnol. 2008, 24, 3071–3076. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkittawon, P.; Chuenchon, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibito activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Magadula, J.J.; Sulmimani, H.O. Cytotoxic and anti-HIV activities of some Tanzanian Garcinia species. Tanzan. J. Health Res. 2010, 12, 144–1490. [Google Scholar] [CrossRef] [Green Version]
- Asghari, B.; Salehi, P.; Sonboli, A.; Ebrahimi, S.N. Flavonoids from Salvia chloroleuca with alpha-amylsae and alpha-glucosidase inhibitory effect. Iran. J. Pharm. Res. 2015, 14, 609. [Google Scholar]


| Fractions | TPC (mg GAE/g DR) | TFC (mg CE/g DR) | TCTC (mg CE/g DR) |
|---|---|---|---|
| Ethanol | 303.67 ± 4.16 a | 55.33 ± 2.52 a | 11.17 ± 1.26 a |
| Acetone | 207.00 ± 4.00 b | 36.17 ± 1.04 b | 10.33 ± 0.58 a |
| Hexane | 16.00 ± 1.73 c | 6.17 ± 1.26 c | 2.50 ± 0.50 b |
| Peaks | Compounds | MS [M−H]−m/z | Retention Time (min) | Quantity in µg/g Extract | |
|---|---|---|---|---|---|
| Ethanol | Acetone | ||||
| 1 | Quinic acid | 191.00 | 2130 | 305.62 ± 9.62 | 287.90 ± 12.81 |
| 2 | Protocatchuic acid | 153.00 | 7385 | 1598.01 ± 1.73 | 343.01 ± 6.53 |
| 3 | Epicatechin | 289.00 | 13.795 | 54.48 ± 2.22 | - |
| 4 | 4-O-caffeoylquinic acid | 353.00 | 12.562 | 646.71 ± 5.50 | 437.72 ± 0.20 |
| 5 | Caffeic acid | 179.00 | 12.993 | 82.99 ± 2.04 | 115.58 ± 4.88 |
| 6 | 1,3-di-O-caffeoyquinic acid | 515.00 | 14.960 | 198.45 ± 8.14 | 118.46 ± 1.20 |
| 7 | p-Coumaric acid | 163.00 | 17.087 | 5982.57 ± 1.37 | 966.18 ± 32.41 |
| 8 | Trans-Ferulic acid | 193.00 | 18.744 | 7432.51 ± 27.41 | 1469.69 ± 36.27 |
| 9 | Rosmarinic acid | 359.00 | 22.209 | 259.42 ± 2.98 | 58.13 ± 3.27 |
| 10 | Hyperoside (quercetin-3-O-galactoside) | 463.00 | 22.910 | 2067.92 ± 20.65 | 2513.82 ± 69.82 |
| 11 | Rutin | 609.00 | 23.136 | 4108.17 ± 14.31 | 7987.96 ± 18.73 |
| 12 | Salvianolic acid | 717.00 | 23.762 | 174.17 ± 1.91 | 70.40 ± 1.19 |
| 13 | 4,5-di-O-caffeoylquinic acid | 515.00 | 23.902 | 3050.97 ± 8.02 | 2696.01 ± 24.63 |
| 14 | Quercetrin (quercetin-3-O-Rhamonoside) | 447.00 | 25.112 | 212.34 ± 1.677 | 133.02 ± 1.49 |
| 15 | Naringenin | 271.00 | 26.977 | 492.82 ± 7.40 | 94.32 ± 1.00 |
| 16 | Silymarin | 481.00 | 28.852 | 129.87 ± 2.31 | 50.72 ± 1.44 |
| 17 | Apegenin | 269.00 | 32.391 | 49.84 ± 1.15 | 24.40 ± 2.03 |
| 18 | Cirsiliol | 329.00 | 32.451 | 3438.42 ± 19.26 | 791.39 ± 2.25 |
| 19 | Acacetin | 283.00 | 37.061 | 882.42 ± 15.58 | 876.51 ± 26.16 |
| Fractions | DPPH (IC50 μg/mL) | Superoxide Anion (IC50 μg/mL) | Reducing Power (EC50 μg/mL) |
|---|---|---|---|
| Ethanol | 7.17 ± 1.26 c | 31.67 ± 1.53 c | 51.67 ± 1.53 c |
| Acetone | 18.50 ± 1.80 b | 113.67 ± 1.53 b | 75.67 ± 2.08 b |
| Hexane | 321.00 ± 3.61 a | 417.00 ± 2.65 a | 356.67 ± 2.08 a |
| BHT | 10.70 ± 0.61 c | 3.50 ± 0.50 d | 23.33 ± 1.53 d |
| TPC | TFC | CTC | DPPH | Superoxide Anion | FRAP | |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 0.994 ** | 1 | ||||
| TCTC | 0.956 ** | 0.924 ** | 1 | |||
| DPPH | −0.953 ** | −0.932 ** | −0.983 ** | 1 | ||
| Superoxide Anion | −0.991 ** | −0.979 ** | −0.978 ** | 0.985 ** | 1 | |
| FRAP | −0.965 ** | −0.945 ** | −0.984 ** | 0.999 ** | 0.991 ** | 1 |
| Strains | Ethanol | Acetone | Hexane | Antibiotics |
|---|---|---|---|---|
| Gram-positive bacteria | Gent. | |||
| S. epidermidis CIP 106510 | 14.66 ± 1.15 aB | 11.66 ± 0.57 aC | 7.00 ± 1.00 bcD | 22.00 ± 1.00 bcA |
| M. luteus NCIMB 8166 | 14.00 ± 0.00 aB | 12.00 ± 1.00 aC | 8.00 ± 00 bD | 27.50 ± 0.50 aA |
| E. feacalis ATCC 29212 | 14.16 ± 1.25 aB | 12.33 ± 0.57 aB | 8.00 ± 1.00 bC | 26.00 ± 1.00 aA |
| B. cereus ATCC 14579 | 12.33 ± 0.57 bB | 11.00 ± 1.00 abC | 9.66 ± 0.57 aD | 27.66 ± 0.57 aA |
| Gram-negative bacteria | ||||
| E. coli ATCC 35218 | 11.33 ± 0.57 bcB | 11.33 ± 1.15 aB | 7.00 ± 0.00 bcC | 21.66 ± 0.57 bcA |
| L. monocytogenes ATCC19115 | 10.66 ± 0.57 cB | 9.66 ± 0.57 bB | 7.66 ± 0.57 bC | 23.00 ± 1.00 bA |
| P. aeruginosa ATCC 27853 | 8.66 ± 0.57 cdB | 6.66 ± 0.57 cC | 6.00 ± 0.00 cC | 16.00 ± 1.00 dA |
| S. typhimurium LT2 DT104 | 10.00 ± 1.00 dB | 7.66 ± 0.57 cC | 7.66 ± 0.57 bC | 20.66 ± 1.52 cA |
| Fungal strains | Amph. | |||
| Candida albicans ATCC 90028 | 12.33 ± 0.57 bB | 12.33 ± 0.57 abB | 8.00 ± 0.00 abC | 19.00 ± 1.00 aA |
| Candida glabrata ATCC 90030 | 13.66 ± 0.57 abB | 13.33 ± 0.57 aB | 7.00 ± 1.00 bC | 16.66 ± 0.57 bA |
| Candida parapsilosis ATCC 22019 | 14.00 ± 1.00 aB | 11.66 ± 1.52 abC | 7.66 ± 0.57 abD | 18.33 ± 0.57 aA |
| Candida krusei ATCC 6258 | 12.66 ± 0.57 abB | 11.00 ± 1.00 bC | 8.33 ± 0.57 aD | 18.00 ± 1.00 abA |
| Ethanol | Acetone | Antibiotics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| Bacterial strains | Gentamycin | ||||||||
| S. epidermidis CIP 106510 | 0.29 | 1.17 | 4 (Bactericidal) | 1.17 | 4.69 | 4 (Bactericidal) | 0.009 | 0.039 | 4 (Bactericidal) |
| M. luteus NCIMB 8166 | 0.15 | 0.59 | 4 (Bactericidal) | 1.17 | 4.69 | 4 (Bactericidal) | 0.004 | 0.019 | 4 (Bactericidal) |
| E. feacalis ATCC 29212 | 0.29 | 1.17 | 4(Bactericidal) | 0.59 | 2.34 | 2 (Bactericidal) | 0.004 | 0.019 | 4 (Bactericidal) |
| B. cereus ATCC 14579 | 0.29 | 1.17 | 4 (Bactericidal) | 0.59 | 2.34 | 2 (Bactericidal) | 0.004 | 0.039 | 4 (Bactericidal) |
| E. coli ATCC 35218 | 1.17 | 4.69 | 4 (Bactericidal) | 2.34 | 9.38 | 4 (Bactericidal) | 0.004 | 0.039 | 4 (Bactericidal) |
| L. monocytogenes ATCC19115 | 1.17 | 4.69 | 4 (Bactericidal) | 2.34 | 9.38 | 2 (Bactericidal) | 0.019 | 0.078 | 4 (Bactericidal) |
| P. aeruginosa ATCC 27853 | 1.17 | 9.38 | 8(Bacteriostatic) | 1.17 | 9.38 | 8 (Bacteriostatic) | 0.019 | 0.15 | 8 (Bacteriostatic) |
| S. typhimurium LT2 DT104 | 0.59 | 2.34 | 4 (Bactericidal) | 1.17 | 4.69 | 2 (Bactericidal) | 0.019 | 0.039 | 2 (Bactericidal) |
| Fungal strains | MFC | MFC/MIC | MFC | MFC/MIC | Amphotericin B MFC MFC/MIC | ||||
| C. albicans ATCC 90028 | 0.15 | 1.17 | 8 (fungistatic) | 0.59 | 4.69 | 8 (fungistatic) | 0.078 | 0.31 | 4 (Fungicidal) |
| C. glabrata ATCC 90030 | 0.15 | 0.59 | 4 (Fungicidal) | 0.59 | 2.34 | 4 (Fungicidal) | 0.078 | 0.31 | 4 (Fungicidal) |
| C. parapsilosis ATCC 22019 | 0.15 | 0.59 | 4 (Fungicidal) | 0.59 | 2.34 | 4 (Fungicidal) | 0.039 | 0.078 | 2 (Fungicidal) |
| C. krusei ATCC 6258 | 0.15 | 1.17 | 8 (fungistatic) | 0.59 | 4.69 | 8 (fungistatic) | 0.078 | 0.31 | 4 (Fungicidal) |
| α-Glucosidase (IC50 mg/mL) | |
|---|---|
| Ethanol | 13.17 ± 1.04 b |
| Acetone | 111.50 ± 2.78 a |
| Acarbose | 1.12 ± 0.08 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajlaoui, H.; Arraouadi, S.; Mighri, H.; Ghannay, S.; Aouadi, K.; Adnan, M.; Elasbali, A.M.; Noumi, E.; Snoussi, M.; Kadri, A. HPLC-MS Profiling, Antioxidant, Antimicrobial, Antidiabetic, and Cytotoxicity Activities of Arthrocnemum indicum (Willd.) Moq. Extracts. Plants 2022, 11, 232. https://doi.org/10.3390/plants11020232
Hajlaoui H, Arraouadi S, Mighri H, Ghannay S, Aouadi K, Adnan M, Elasbali AM, Noumi E, Snoussi M, Kadri A. HPLC-MS Profiling, Antioxidant, Antimicrobial, Antidiabetic, and Cytotoxicity Activities of Arthrocnemum indicum (Willd.) Moq. Extracts. Plants. 2022; 11(2):232. https://doi.org/10.3390/plants11020232
Chicago/Turabian StyleHajlaoui, Hafedh, Soumaya Arraouadi, Hedi Mighri, Siwar Ghannay, Kaïss Aouadi, Mohd Adnan, Abdelbaset Mohamed Elasbali, Emira Noumi, Mejdi Snoussi, and Adel Kadri. 2022. "HPLC-MS Profiling, Antioxidant, Antimicrobial, Antidiabetic, and Cytotoxicity Activities of Arthrocnemum indicum (Willd.) Moq. Extracts" Plants 11, no. 2: 232. https://doi.org/10.3390/plants11020232
APA StyleHajlaoui, H., Arraouadi, S., Mighri, H., Ghannay, S., Aouadi, K., Adnan, M., Elasbali, A. M., Noumi, E., Snoussi, M., & Kadri, A. (2022). HPLC-MS Profiling, Antioxidant, Antimicrobial, Antidiabetic, and Cytotoxicity Activities of Arthrocnemum indicum (Willd.) Moq. Extracts. Plants, 11(2), 232. https://doi.org/10.3390/plants11020232










