Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Content
2.2. HPLC-ESI-MS n Qualitative Analysis
2.3. Distribution of Compounds between the Tested Extracts
2.4. Quantification of Phytochemicals by HPLC-DAD
2.5. Antioxidant Properties
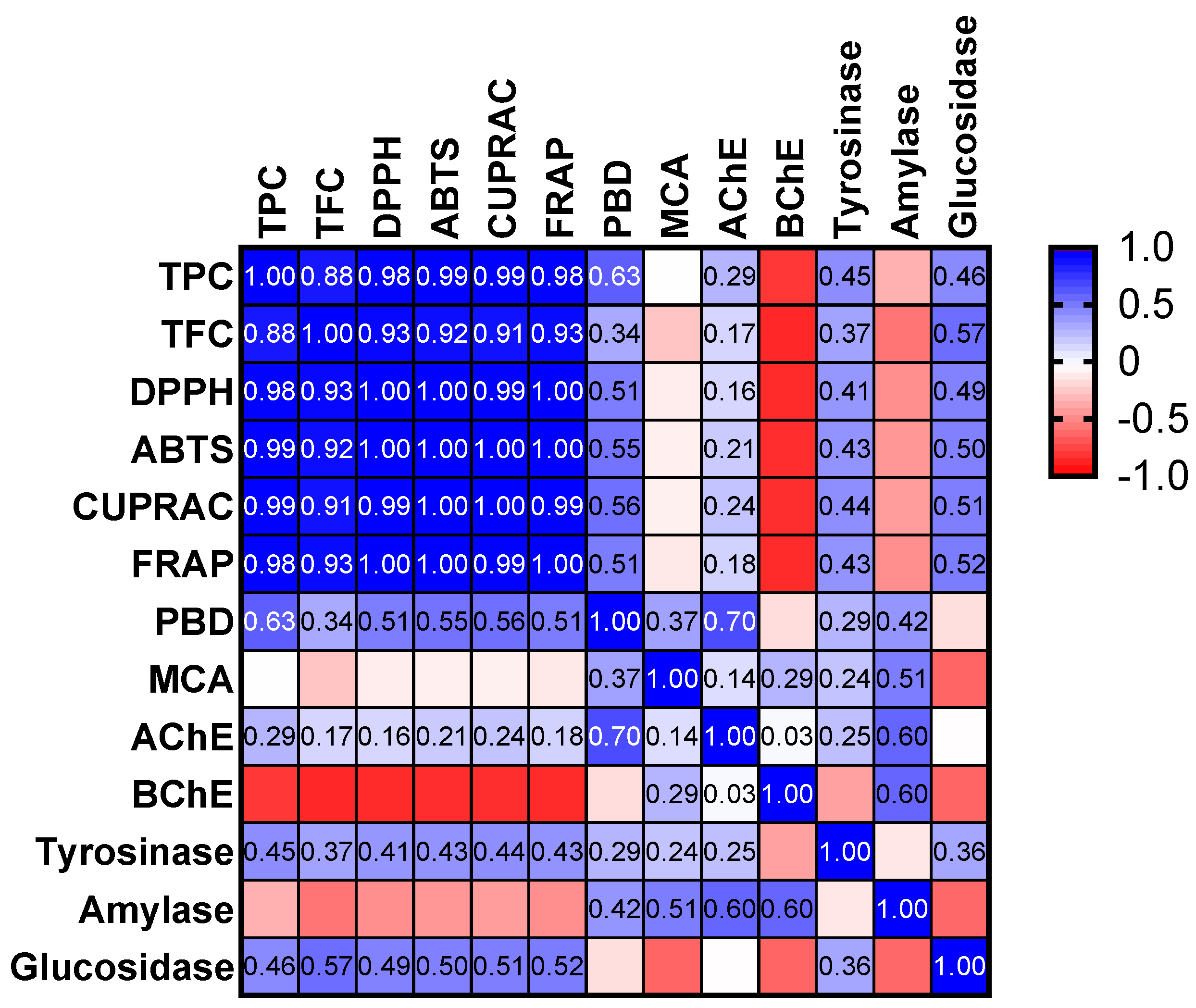
2.6. Enzyme Inhibitory Properties
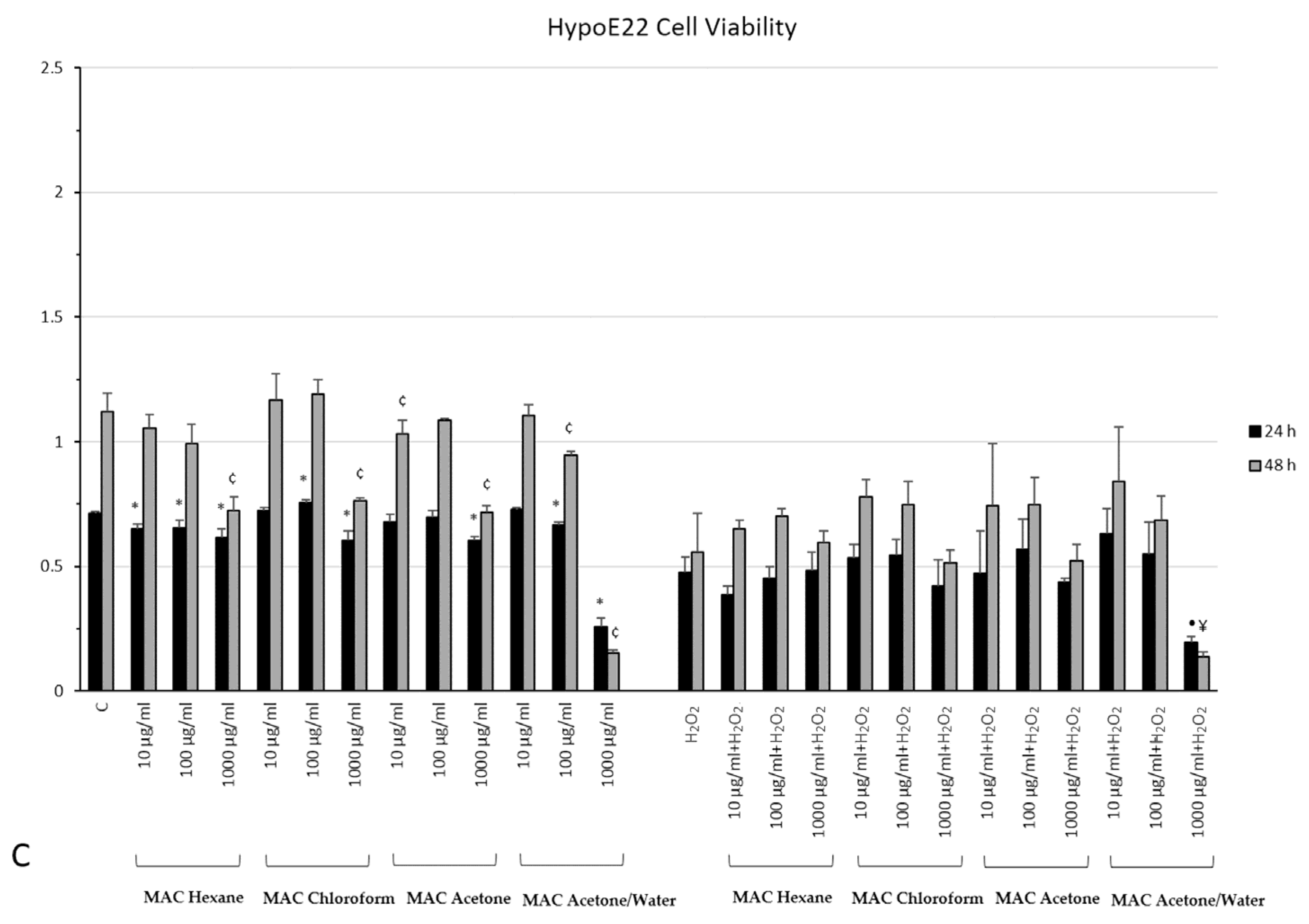
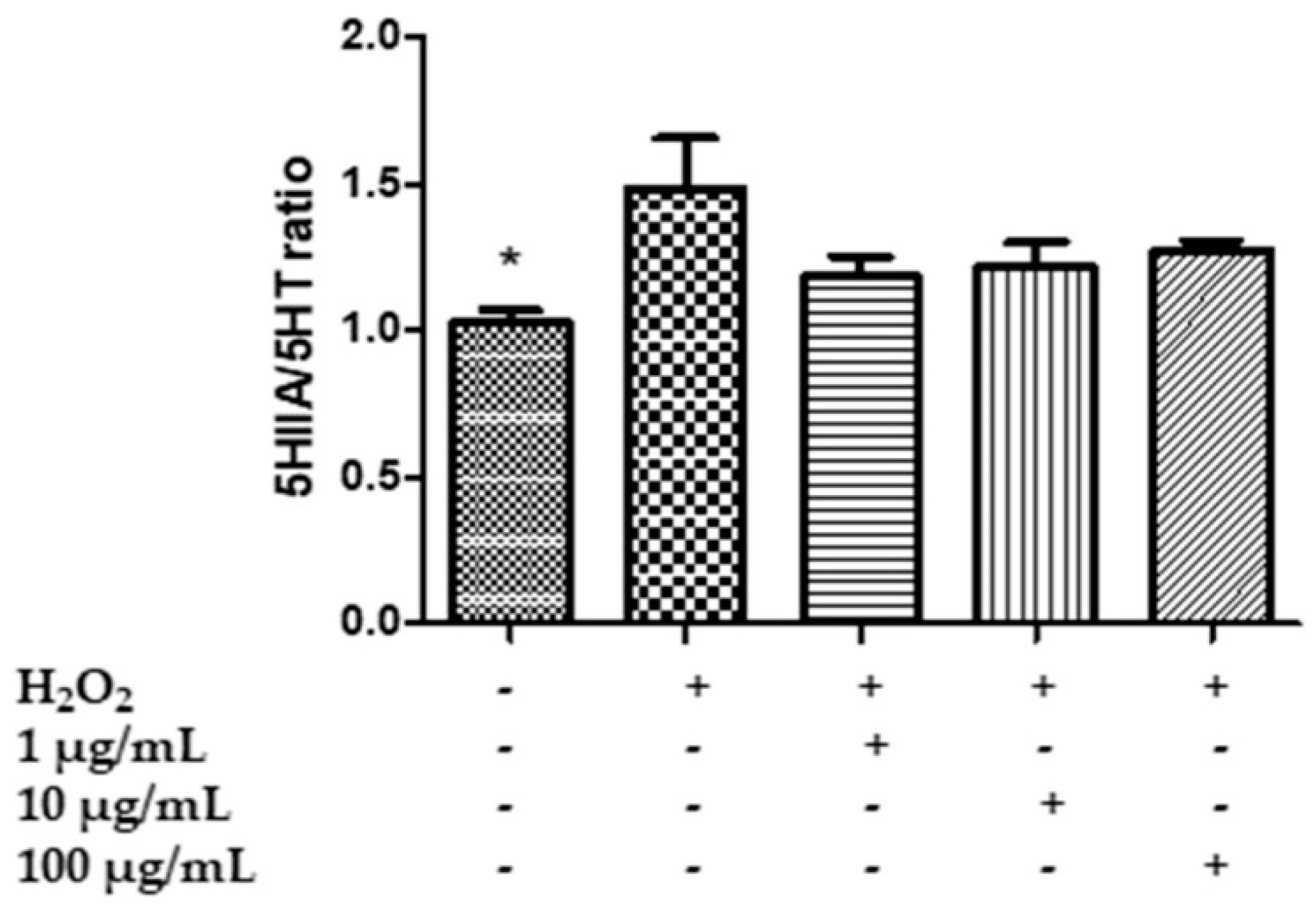
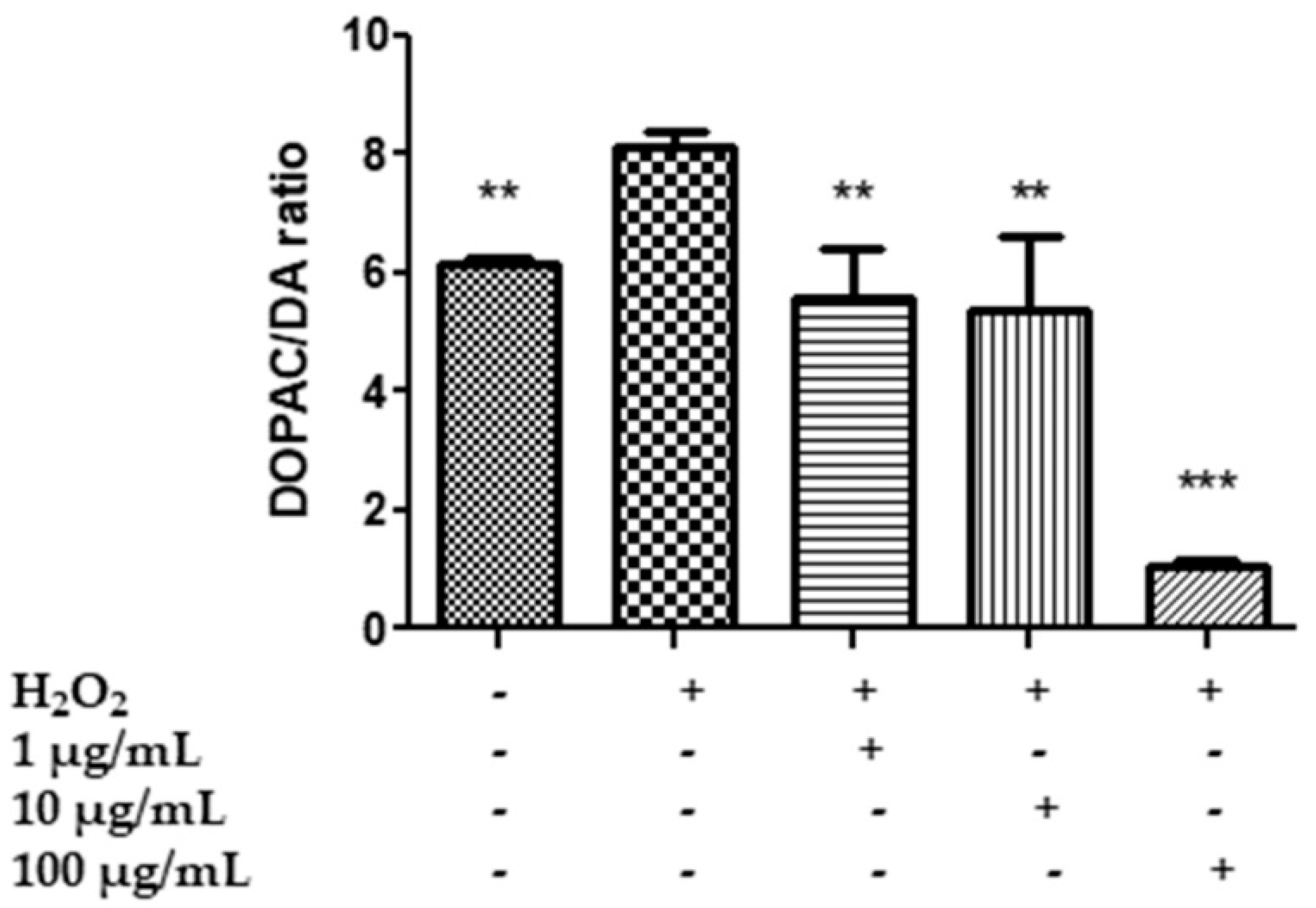
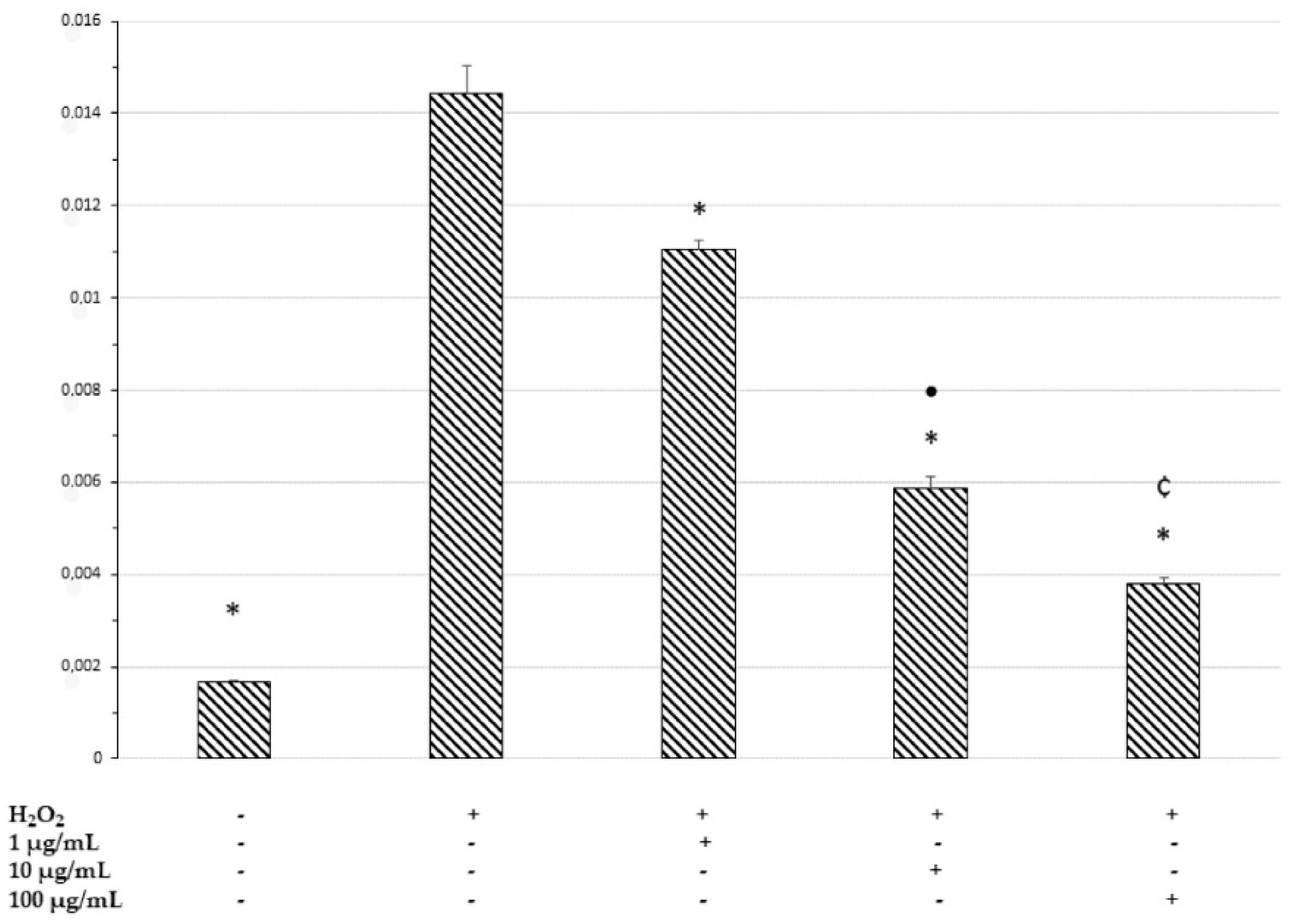
2.7. In Vitro Neuromodulatory and Neuroprotective Effects of Mentha spicata Extracts
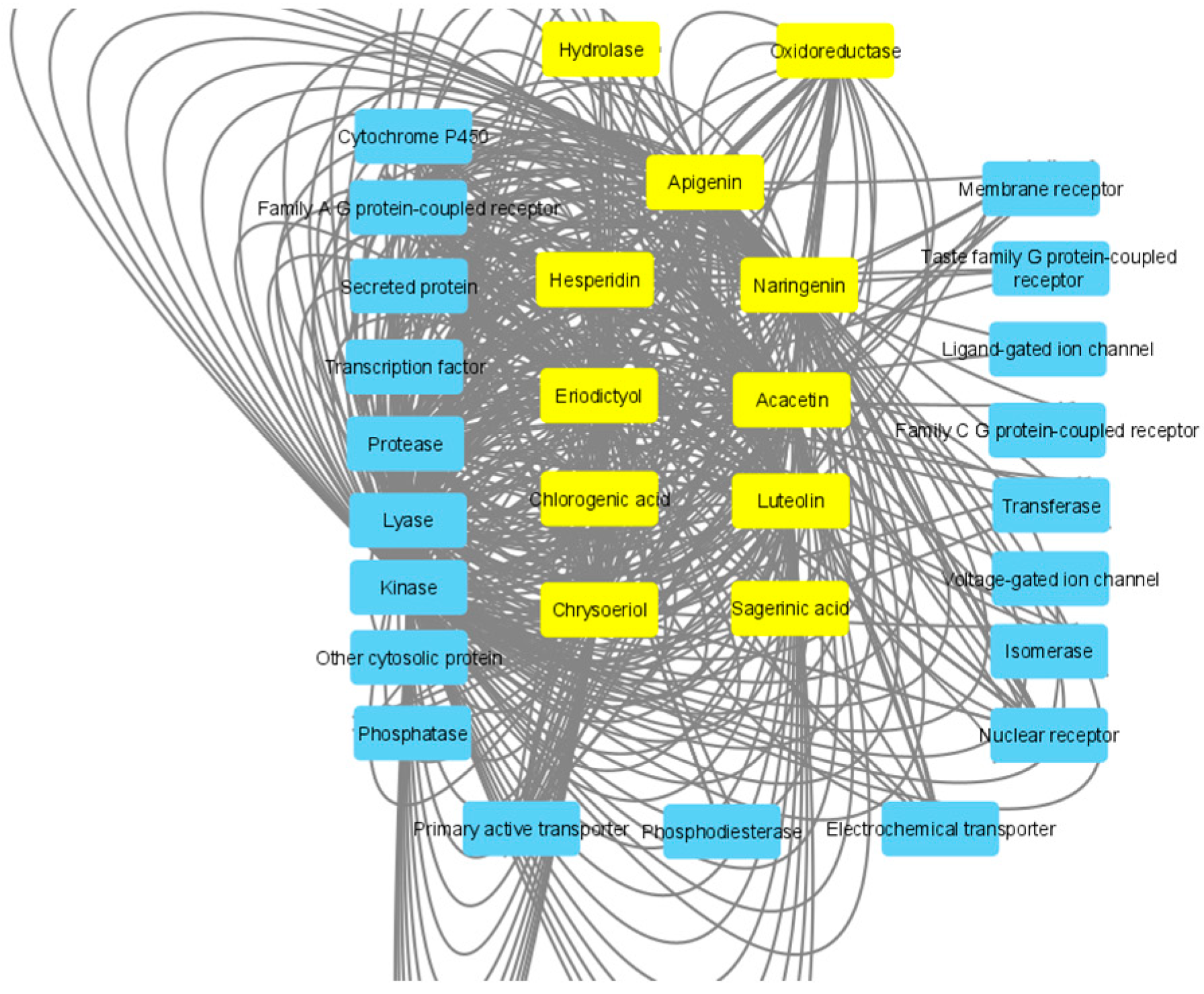
2.8. In Silico Study
3. Materials and Methods
3.1. Plant Materials
3.2. Total Phenolic and Flavonoid Content
3.3. Chromatographic Analysis of the Extracts
3.4. Antioxidant and Enzyme Inhibitory Assays
3.5. Cell Culture and Treatment
3.6. MTT Assay
3.7. PGE2 ELISA Assay
3.8. Quantitative Determination of Dopamine (DA), Dihydroxyphenilacetic Acid (DOPAC), Levodopa (L-Dopa), Serotonin (5-HT) and 5-Hydroxyindolacetic Acid (5HIIA)
3.9. Gene Expression Analysis
3.10. In Silico Studies
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahendran, G.; Verma, S.K.; Rahman, L.-U. The traditional uses, Phytochemistry and pharmacology of Spearmint (Mentha spicata L.): A review. J. Ethnopharmacol. 2021, 114266. [Google Scholar] [CrossRef] [PubMed]
- Güner, A.; Aslan, S. Türkiye Bitkileri Listesi: (Damarlı Bitkiler); Nezahat Gökyiǧit Botanik Bahçesi Yayınları: Ataşehir, Turkey, 2012. [Google Scholar]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata essential oil: Chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of Vibrio spp. strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef] [PubMed]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid Screening of Mentha spicata Essential Oil and L-Menthol in Mentha piperita Essential Oil by ATR-FTIR Spectroscopy Coupled with Multivariate Analyses. Foods 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Mahboubi, M. Mentha spicata L. essential oil, phytochemistry and its effectiveness in flatulence. J. Tradit. Complement. Med. 2021, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, T.; Akter, R.; Ahmed, J.; Mazumdar, S.; Talukder, D.; Nandi, N.C.; Nurulamin, M. Evaluation of acute oral toxicity, cytotoxicity, antidepressant and antioxidant activities of Japanese mint (Mentha arvensis L.) oil. Phytomedicine Plus 2021, 1, 100140. [Google Scholar] [CrossRef]
- Brandão, F.R.; Farias, C.F.S.; de Melo Souza, D.C.; de Oliveira, M.I.B.; de Matos, L.V.; Majolo, C.; de Oliveira, M.R.; Chaves, F.C.M.; de Almeida O’Sullivan, F.L.; Chagas, E.C. Anesthetic potential of the essential oils of Aloysia triphylla, Lippia sidoides and Mentha piperita for Colossoma macropomum. Aquaculture 2021, 534, 736275. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Özgüven, M.; Hassanpouraghdam, M.B. Planting-date and cutting-time affect the growth and essential oil composition of Mentha × piperita and Mentha arvensis. Ind. Crops Prod. 2021, 170, 113790. [Google Scholar] [CrossRef]
- Yakoubi, R.; Megateli, S.; Hadj Sadok, T.; Gali, L. Photoprotective, antioxidant, anticholinesterase activities and phenolic contents of different Algerian Mentha pulegium extracts. Biocatal. Agric. Biotechnol. 2021, 34, 102038. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef] [PubMed]
- Sebai, E.; Abidi, A.; Serairi, R.; Marzouki, M.; Saratsi, K.; Darghouth, M.A.; Sotiraki, S.; Akkari, H. Essential oil of Mentha pulegium induces anthelmintic effects and reduces parasite-associated oxidative stress in rodent model. Exp. Parasitol. 2021, 225, 108105. [Google Scholar] [CrossRef] [PubMed]
- Chraibi, M.; Fadil, M.; Farah, A.; Lebrazi, S.; Fikri-Benbrahim, K. Antimicrobial combined action of Mentha pulegium, Ormenis mixta and Mentha piperita essential oils against S. aureus, E. coli and C. tropicalis: Application of mixture design methodology. LWT 2021, 145, 111352. [Google Scholar] [CrossRef]
- Gabetti, E.; Sgorbini, B.; Stilo, F.; Bicchi, C.; Rubiolo, P.; Chialva, F.; Reichenbach, S.E.; Bongiovanni, V.; Cordero, C.; Cavallero, A. Chemical fingerprinting strategies based on comprehensive two-dimensional gas chromatography combined with gas chromatography-olfactometry to capture the unique signature of Piemonte peppermint essential oil (Mentha x piperita var Italo-Mitcham). J. Chromatogr. A 2021, 1645, 462101. [Google Scholar] [CrossRef] [PubMed]
- Spadaccino, G.; Frabboni, L.; Petruzzi, F.; Disciglio, G.; Mentana, A.; Nardiello, D.; Quinto, M. Essential oil characterization of Prunus spinosa L., Salvia officinalis L., Eucalyptus globulus L., Melissa officinalis L. and Mentha x piperita L. by a volatolomic approach. J. Pharm. Biomed. Anal. 2021, 202, 114167. [Google Scholar] [CrossRef] [PubMed]
- Djamila, B.; Fatima Zohra, K.; Lahcene, K.; Zohra, R.F. Drying methods affect the extracts and essential oil of Mentha aquatica L. Food Biosci. 2021, 41, 101007. [Google Scholar] [CrossRef]
- Eghbalian, M.; Shavisi, N.; Shahbazi, Y.; Dabirian, F. Active packaging based on sodium caseinate-gelatin nanofiber mats encapsulated with Mentha spicata L. essential oil and MgO nanoparticles: Preparation, properties, and food application. Food Packag. Shelf Life 2021, 29, 100737. [Google Scholar] [CrossRef]
- Alsaraf, S.; Hadi, Z.; Akhtar, M.J.; Khan, S.A. Chemical profiling, cytotoxic and antioxidant activity of volatile oil isolated from the mint (Mentha spicata L.) grown in Oman. Biocatal. Agric. Biotechnol. 2021, 34, 102034. [Google Scholar] [CrossRef]
- Yang, X.; Han, H.; Li, B.; Zhang, D.; Zhang, Z.; Xie, Y. Fumigant toxicity and physiological effects of spearmint (Mentha spicata, Lamiaceae) essential oil and its major constituents against Reticulitermes dabieshanensis. Ind. Crops Prod. 2021, 171, 113894. [Google Scholar] [CrossRef]
- Abootalebian, M.; Keramat, J.; Kadivar, M.; Ahmadi, F.; Abdinian, M. Comparison of total phenolic and antioxidant activity of different Mentha spicata and M. longifolia accessions. Ann. Agric. Sci. 2016, 61, 175–179. [Google Scholar] [CrossRef]
- Begaa, S.; Messaoudi, M.; Ouanezar, A.; Hamidatou, L.; Malki, A. Chemical elements of Algerian Mentha spicata L. used in the treatment of digestive system disorders by employing instrumental neutron activation analysis technique. J. Radioanal. Nucl. Chem. 2018, 317, 1107–1112. [Google Scholar] [CrossRef]
- Elmastas, M.; Telci, I.; Aksit, H.; Erenler, R. Comparison of total phenolic contents and antioxidant capacities in mint genotypes used as spices. Turk. J. Biochem. 2015, 40, 456–462. [Google Scholar]
- Brahmi, F.; Nury, T.; Debbabi, M.; Hadj-Ahmed, S.; Zarrouk, A.; Prost, M.; Madani, K.; Boulekbache-Makhlouf, L.; Lizard, G. Evaluation of antioxidant, anti-inflammatory and cytoprotective properties of ethanolic mint extracts from algeria on 7-ketocholesterol-treated murine RAW 264.7 macrophages. Antioxidants 2018, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Parveen, T.; Amin, N.; Saleem, D.; Razi, F.; Haider, S.; Haleem, D.J. Antistress effect of Mentha piperita in rats and the role of brain serotonin and dopamine. Asian J. Pharm. Biol. Res. (AJPBR) 2012, 2, 73–78. [Google Scholar]
- Ahmad, M.; Khan, M.P.Z.; Mukhtar, A.; Zafar, M.; Sultana, S.; Jahan, S. Ethnopharmacological survey on medicinal plants used in herbal drinks among the traditional communities of Pakistan. J. Ethnopharmacol. 2016, 184, 154–186. [Google Scholar] [CrossRef]
- Ali, L.; Khan, S.; Nazir, M.; Raiz, N.; Naz, S.; Zengin, G.; Mukhtar, M.; Parveen, S.; Shazmeen, N.; Saleem, M.; et al. Chemical profiling, in vitro biological activities and Pearson correlation between phenolic contents and antioxidant activities of Caragana brachyantha Rech.f. S. Afr. J. Bot. 2021, 140, 189–193. [Google Scholar] [CrossRef]
- Ikram, S.; Bhatti, K.H.; Parvaiz, M. Ethnobotanical studies of aquatic plants of district Sialkot, Punjab (Pakistan). J. Med. Plants 2014, 2, 58–63. [Google Scholar]
- Asowata-Ayodele, A.M.; Afolayan, A.J.; Otunola, G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 104, 69–75. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Moradi, M.-T.; Mahmoodnia, L.; Alaei, S.; Asadi-Samani, F.; Luther, T. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J. Nephropathol. 2017, 6, 118. [Google Scholar] [CrossRef] [Green Version]
- Bardaweel, S.K.; Bakchiche, B.; ALSalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complementary Altern. Med. 2018, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Karaca, N.; Demirci, B.; Demirci, F. Evaluation of Lavandula stoechas L. subsp. stoechas L., Mentha spicata L. subsp. spicata L. essential oils and their main components against sinusitis pathogens. Z. Für Nat. C 2018, 73, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Laojun, S.; Chaiphongpachara, T. Comparative study of larvicidal activity of commercial essential oils from aromatic rosemary, vanilla, and spearmint against the mosquito Aedes aegypti. Biodiversitas J. Biol. Divers. 2020, 21, 2383–2389. [Google Scholar]
- Nardoni, S.; Giovanelli, S.; Pistelli, L.; Mugnaini, L.; Profili, G.; Pisseri, F.; Mancianti, F. In vitro activity of twenty commercially available, plant-derived essential oils against selected dermatophyte species. Nat. Prod. Commun. 2015, 10, 1934578X1501000840. [Google Scholar] [CrossRef] [Green Version]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia Island (Italy). Nat. Prod. Res. 2021, 35, 993–999. [Google Scholar] [CrossRef]
- Ben Saad, A.; Rjeibi, I.; Alimi, H.; Ncib, S.; Bouhamda, T.; Zouari, N. Protective effects of Mentha spicata against nicotine-induced toxicity in liver and erythrocytes of Wistar rats. Appl. Physiol. Nutr. Metab. 2018, 43, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Farr, S.A.; Niehoff, M.L.; Ceddia, M.A.; Herrlinger, K.A.; Lewis, B.J.; Feng, S.; Welleford, A.; Butterfield, D.A.; Morley, J.E. Effect of botanical extracts containing carnosic acid or rosmarinic acid on learning and memory in SAMP8 mice. Physiol. Behav. 2016, 165, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, P.M.H.; Noba, N.Y.; Shohel, M.; Bhattacherjee, R.; Das, B.K. Analgesic, anti-inflammatory and antipyretic effect of Mentha spicata (Spearmint). J. Pharm. Res. Int. 2013, 3, 854–864. [Google Scholar] [CrossRef]
- Kumar, V.; Kural, M.R.; Pereira, B.; Roy, P. Spearmint induced hypothalamic oxidative stress and testicular anti-androgenicity in male rats–Altered levels of gene expression, enzymes and hormones. Food Chem. Toxicol. 2008, 46, 3563–3570. [Google Scholar] [CrossRef]
- Erenler, R.; Telci, I.; Elmastas, M.; Aksit, H.; Gul, F.; Tufekci, A.R.; Demirtas, I.; Kayir, O. Quantification of flavonoids isolated from Mentha spicata in selected clones of Turkish mint landraces. Turk. J. Chem. 2018, 42, 1695–1705. [Google Scholar] [CrossRef]
- Najda, A.; Klimek, K.; Balant, S.; Wrzesinska-Jedrusiak, E.; Piekarski, W. Optimization of the process of polyphenol extraction from Mentha spicata with various solvents. Przem. Chem. 2019, 98, 1286–1289. [Google Scholar]
- Zekri, N.; Zerkani, H.; Elazzouzi, H.; Zair, T.; El Belghiti, M.A. Extracts of M. pulegium (L.) and M. spicata (L.): Effect of Extraction Conditions on Phenolics and Flavonoids Contents and Their Antioxidant Power. Egypt. J. Chem. 2021, 64, 1447–1459. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Sun, P.; Feng, J.; Yuan, J.; Wang, Y.; Shang, Y.-F.; Niu, X.-L.; Yang, S.-H.; Wei, Z.-J. Solvent effect on phenolics and antioxidant activity of Huangshan Gongju (Dendranthema morifolium (Ramat) Tzvel. cv. Gongju) extract. Food Chem. Toxicol. 2021, 147, 111875. [Google Scholar] [CrossRef]
- Rudke, A.R.; Andrade, K.S.; Mazzutti, S.; Zielinski, A.A.F.; Alves, V.R.; Vitali, L.; Ferreira, S.R.S. A comparative study of phenolic compounds profile and in vitro antioxidant activity from buriti (Mauritia flexuosa) by-products extracts. LWT 2021, 150, 111941. [Google Scholar] [CrossRef]
- Wang, R.; He, R.; Li, Z.; Lin, X.; Wang, L. HPLC-Q-Orbitrap-MS/MS phenolic profiles and biological activities of extracts from roxburgh rose (Rosa roxburghii Tratt.) leaves. Arab. J. Chem. 2021, 14, 103257. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Verardo, G.; Duse, I.; Callea, A. Analysis of underivatized oligosaccharides by liquid chromatography/electrospray ionization tandem mass spectrometry with post-column addition of formic acid. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2009, 23, 1607–1618. [Google Scholar] [CrossRef]
- Justesen, U. Negative atmospheric pressure chemical ionisation low-energy collision activation mass spectrometry for the characterisation of flavonoids in extracts of fresh herbs. J. Chromatogr. A 2000, 902, 369–379. [Google Scholar] [CrossRef]
- Hu, P.; Liang, Q.-L.; Luo, G.-A.; Zhao, Z.-Z.; Jiang, Z.-H. Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem. Pharm. Bull. 2005, 53, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Rita, I.; Pereira, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: Emerging reserve lots with special harvest requirements. Food Funct. 2016, 7, 4188–4192. [Google Scholar] [CrossRef]
- Nagy, T.O.; Solar, S.; Sontag, G.; Koenig, J. Identification of phenolic components in dried spices and influence of irradiation. Food Chem. 2011, 128, 530–534. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Stafilov, T. LC/DAD/MS n and ICP-AES Assay and Correlations between Phenolic Compounds and Toxic Metals in Endemic Thymus alsarensis from the Thallium Enriched Allchar Locality. Nat. Prod. Commun. 2017, 12, 1934578X1701200206. [Google Scholar]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In vitro inhibition of the transcription factor NF-κB and cyclooxygenase by Bamboo extracts. Phytother. Res. 2014, 28, 224–230. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Wairata, J.; Fadlan, A.; Setyo Purnomo, A.; Taher, M.; Ersam, T. Total phenolic and flavonoid contents, antioxidant, antidiabetic and antiplasmodial activities of Garcinia forbesii King: A correlation study. Arab. J. Chem. 2022, 15, 103541. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Scherer, R.; Lemos, M.F.; Lemos, M.F.; Martinelli, G.C.; Martins, J.D.L.; da Silva, A.G. Antioxidant and antibacterial activities and composition of Brazilian spearmint (Mentha spicata L.). Ind. Crops Prod. 2013, 50, 408–413. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahiti, K.S.; Rohan, B.; Raji, P. Evaluation of bioactivity of Annona muricata, Piper betle and Mentha spicata. Int. J. Pharm. Phytopharm. Res. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Qadir, M.A.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of Phenolic Compounds and Antioxidant and Antimicrobial Activities of Some Common Herbs. Int. J. Anal. Chem. 2017, 2017, 6. [Google Scholar]
- Tsimogiannis, D.I.; Oreopoulou, V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′, 4′-hydroxy substituted members. Innov. Food Sci. Emerg. Technol. 2006, 7, 140–146. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Lucero-Prisno, D.E.; Abdullah, A.S.; Huang, J.; Laurence, C.; Liang, X.; Ma, Z.; Mao, Z.; Ren, R.; et al. What is global health? Key concepts and clarification of misperceptions. Glob. Health Res. Policy 2020, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Ferruzzi, M.G.; Hamaker, B.R. Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem. 2022, 370, 130981. [Google Scholar] [CrossRef]
- Ozten, O.; Zengin Kurt, B.; Sonmez, F.; Dogan, B.; Durdagi, S. Synthesis, molecular docking and molecular dynamics studies of novel tacrine-carbamate derivatives as potent cholinesterase inhibitors. Bioorg. Chem. 2021, 115, 105225. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s disease and its treatment by different approaches: A review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- The Lancet Global Health. Global health 2021: Who tells the story? Lancet Glob. Health 2021, 9, e99. [Google Scholar]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzyme Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Şöhretoğlu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Habasi, M.; Xie, L.Z.; Aisa, H.A. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef] [Green Version]
- Roseiro, L.B.; Rauter, A.P.; Serralheiro, M.L.M. Polyphenols as acetylcholinesterase inhibitors: Structural specificity and impact on human disease. Nutr. Aging 2012, 1, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Abbou, F.; Azzi, R.; Ouffai, K.; El Haci, I.A.; Belyagoubi-Benhammou, N.; Bensouici, C.; Benamar, H. Phenolic profile, antioxidant and enzyme inhibitory properties of phenolic-rich fractions from the aerial parts of Mentha pulegium L. S. Afr. J. Bot. 2022, 146, 196–204. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Cam, M.; Basyigit, B.; Alasalvar, H.; Yilmaztekin, M.; Ahhmed, A.; Sagdic, O.; Konca, Y.; Telci, I. Bioactive properties of powdered peppermint and spearmint extracts: Inhibition of key enzymes linked to hypertension and type 2 diabetes. Food Biosci. 2020, 35, 100577. [Google Scholar] [CrossRef]
- Benabdallah, A.; Boumendjel, M.; Aissi, O.; Rahmoune, C.; Boussaid, M.; Messaoud, C. Chemical composition, antioxidant activity and acetylcholinesterase inhibitory of wild Mentha species from northeastern Algeria. S. Afr. J. Bot. 2018, 116, 131–139. [Google Scholar] [CrossRef]
- Lee, J.; Chang, C.; Liu, I.; Chi, T.; Yu, H.; Cheng, J. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Stephenson, G.; Shachar, D.B. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: A lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann. N. Y. Acad. Sci. 2004, 1012, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.-N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential nutraceutical properties of leaves from several commonly cultivated plants. Biomolecules 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Priya, N.G.; Subathra, M.; Ramesh, A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ. Toxicol. Pharmacol. 2008, 26, 92–95. [Google Scholar] [CrossRef]
- Cavar Zeljkovic, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, H.; Magudeeswaran, S.; Kandasamy, S.; Poomani, K. Binding mechanism of naringenin with monoamine oxidase–B enzyme: QM/MM and molecular dynamics perspective. Heliyon 2021, 7, e06684. [Google Scholar] [CrossRef]
- Kiraly, A.J.; Soliman, E.; Jenkins, A.; Van Dross, R.T. Apigenin inhibits COX-2, PGE2, and EP1 and also initiates terminal differentiation in the epidermis of tumor bearing mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 44–53. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ojo, A.B.; Okolie, C.; Nwakama, M.-A.C.; Iyobhebhe, M.; Evbuomwan, I.O.; Nwonuma, C.O.; Maimako, R.F.; Adegboyega, A.E.; Taiwo, O.A. Deciphering the Interactions of Bioactive Compounds in Selected Traditional Medicinal Plants against Alzheimer’s Diseases via Pharmacophore Modeling, Auto-QSAR, and Molecular Docking Approaches. Molecules 2021, 26, 1996. [Google Scholar] [CrossRef]
- Prasopthum, A.; Pouyfung, P.; Sarapusit, S.; Srisook, E.; Rongnoparut, P. Inhibition effects of Vernonia cinerea active compounds against cytochrome P450 2A6 and human monoamine oxidases, possible targets for reduction of tobacco dependence. Drug Metab. Pharmacokinet. 2015, 30, 174–181. [Google Scholar] [CrossRef]
- Sharif Siam, M.K.; Sarker, A.; Sayeem, M.M.S. In silico drug design and molecular docking studies targeting Akt1 (RAC-alpha serine/threonine-protein kinase) and Akt2 (RAC-beta serine/threonine-protein kinase) proteins and investigation of CYP (cytochrome P450) inhibitors against MAOB (monoamine oxidase B) for OSCC (oral squamous cell carcinoma) treatment. J. Biomol. Struct. Dyn. 2020, 39, 6467–6479. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Park, C.M. Chrysoeriol ameliorates COX-2 expression through NF-κB, AP-1 and MAPK regulation via the TLR4/MyD88 signaling pathway in LPS-stimulated murine macrophages. Exp. Ther. Med. 2021, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.d.P.; Ruiz-Medina, A.; Salazar-Mendías, C.; Llorent-Martínez, E.J. Spectrophotometric determination of the antioxidant properties and characterization of the phenolic content by high-performance liquid chromatography–diode array detection–tandem mass spectrometry (HPLC–DAD–MS/MS) of Berberis hispanica Boiss. & Reut. leaves. Anal. Lett. 2021, 54, 646–657. [Google Scholar]
- Rapino, M.; Di Valerio, V.; Zara, S.; Gallorini, M.; Marconi, G.D.; Sancilio, S.; Marsich, E.; Ghinassi, B.; di Giacomo, V.; Cataldi, A. Chitlac-coated thermosets enhance osteogenesis and angiogenesis in a co-culture of dental pulp stem cells and endothelial cells. Nanomaterials 2019, 9, 928. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M. Central apelin-13 administration modulates hypothalamic control of feeding. J. Biol. Regul. Homeost. Agents 2016, 30, 883–888. [Google Scholar]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Gu, L.; Lu, J.; Li, Q.; Wu, N.; Zhang, L.; Li, H.; Xing, W.; Zhang, X. A network-based analysis of key pharmacological pathways of Andrographis paniculata acting on Alzheimer’s disease and experimental validation. J. Ethnopharmacol. 2020, 251, 112488. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int. J. Mol. Sci. 2020, 21, 3575. [Google Scholar] [CrossRef]





| Extraction Method | Solvent | Total Phenolic Content | Total Flavonoid Content |
|---|---|---|---|
| (mg GAE/g) | (mg RE/g) | ||
| HAE | Hexane | 32.75 ± 0.42 c,* | nd |
| Chloroform | 27.39 ± 0.95 d | 16.44 ± 0.30 b | |
| Acetone | 52.19 ± 0.67 b | 14.43 ± 0.70 c | |
| Acetone/Water | 129.68 ± 1.43 a | 92.20 ± 0.69 a | |
| UAE | Hexane | 11.88 ± 0.29 d | nd |
| Chloroform | 28.71 ± 1.15 c | 18.16 ± 0.66 b | |
| Acetone | 55.95 ± 1.12 b | nd | |
| Acetone/Water | 142.62 ± 3.52 a | 84.95 ± 0.76 a | |
| MAC | Hexane | 13.15 ± 0.05 d | 1.02 ± 0.13 b |
| Chloroform | 30.99 ± 0.25 c | nd | |
| Acetone | 58.62 ± 1.12 b | nd | |
| Acetone/Water | 87.69 ± 0.38 a | 84.42 ± 1.56 a |
| No. | tR | [M-H]− | m/z (% Base Peak) | Assigned Identification | HAE-Hexane | HAE-Chloroform | HAE-Acetone | HAE-Aceton/Water | UAE-Hexane | UAE-Chloroform | UAE-Acetone | UAE-Aceton/Water | MAC-Hexane | MAC-Chloroform | MAC-Acetone | MAC-Aceton/Water |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (min) | m/z | |||||||||||||||
| 1 | 1.8 | 341 | MS2 [341]: 179 (100), 161 (37), 149 (14), 143 (12), 131 (10), 119 (9), 113 (15), 101 (8) | Disaccharide | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | 2.7 | 191 | MS2 [191]: 173 (29), 129 (6), 111 (100) | Citric acid * | + | + | + | − | + | + | + | − | + | + | + | + |
| 3 | 3.8 | 315 | MS2 [315]: 153 (100) | Dihydroxybenzoic acid-O-hexoside | - | − | + | + | − | − | − | + | − | − | − | + |
| MS3 [315→153]: 123 (100) | ||||||||||||||||
| 4 | 9.0 | 353 | MS2 [353]: 191 (25), 179 (60), 173 (100) | Chlorogenic acid * | − | − | + | + | − | − | + | + | − | − | + | + |
| 5 | 9.8 | 305 | MS2 [305]: 225 (100) | Unknown | − | − | − | + | − | − | − | + | − | − | − | + |
| MS3 [305→225]: 163 (100) | ||||||||||||||||
| 6 | 11.2 | 179 | MS2 [179]: 135 (100) | Caffeic acid * | - | − | + | − | − | − | − | − | − | − | − | + |
| 7 | 17.9 | 595 | MS2 [595]: 287 (100) | Eriodictyol-O-rutinoside | + | + | + | + | + | + | + | + | + | + | + | + |
| MS3 [595→287]: 151 (100), 135 (8) | ||||||||||||||||
| 8 | 19.1 | 449 | MS2 [449]: 287 (100), 151 (7) | Eriodictyol-O-hexoside | − | − | + | + | − | − | + | + | − | − | + | + |
| MS3 [449→287]: 151 (100), 135 (12) | ||||||||||||||||
| 9 | 19.8 | 497 | MS2 [497]: 451 (100) | Unknown | + | + | + | − | + | + | + | − | + | + | + | − |
| MS3 [497→451]: 225 (100) | ||||||||||||||||
| 10 | 20.1 | 609 | MS2 [609]: 301 (100) | Rutin * | − | − | + | + | − | − | − | + | − | − | − | + |
| MS3 [609→301]: 179 (100), 151 (85) | ||||||||||||||||
| 11 | 20.4 | 593 | MS2 [593]: 285 (100) | Luteolin-O-rutinoside | + | + | + | + | + | − | + | + | + | + | + | + |
| MS3 [593→285]: 285 (100), 243(17), 241 (38) | ||||||||||||||||
| 12 | 21.5 | 447 | MS2 [447]: 285 (100) | Luteolin-O-hexoside | + | + | + | + | − | − | + | + | + | + | + | + |
| MS3 [447→285]: 285 (100), 243 (24), 241 (18) | ||||||||||||||||
| 13 | 21.5 | 461 | MS2 [461]: 285 (100) | Luteolin-O-glucuronide | + | − | + | + | − | − | − | + | + | + | − | + |
| MS3 [461→285]: 285 (100), 241 (15) | ||||||||||||||||
| 14 | 22.1 | 579 | MS2 [579]: 271 (100) | Naringenin-O-rutinoside | + | − | + | + | − | − | + | + | − | − | + | + |
| MS3 [579→271]: 177 (25), 151 (100) | ||||||||||||||||
| 15 | 24.0 | 577 | MS2 [577]: 269 (100) | Apigenin-O-rutinoside | + | − | + | + | − | + | + | + | + | + | + | + |
| MS3 [577→269]: 225 (100) | ||||||||||||||||
| 16 | 24.3 | 609 | MS2 [609]: 301 (100) | Hesperidin * | + | + | + | + | − | + | + | + | − | + | + | + |
| MS3 [609→301]: 286 (34), 283 (57), 241 (100), 227 (58), 125 (58) | ||||||||||||||||
| 17 | 25.1 | 717 | MS2 [717]: 537 (38), 519 (100) | Salvianolic acid B/E/L | − | − | − | + | − | − | − | + | − | − | − | + |
| MS3 [717→519]: 339 (100), 321 (14), 295 (7), 277 (7) | ||||||||||||||||
| 18 | 25.4 | 431 | MS2 [431]: 269 (100)MS3 [431→269]: 225 (100), 151 (49) | Apigenin-O-hexoside | + | − | − | + | − | + | + | + | + | + | + | + |
| 19 | 25.5 | 607 | MS2 [607]: 299 (100), 284 (43) | Chrysoeriol-O-rutinoside | + | − | + | + | − | − | + | + | − | − | + | + |
| MS3 [607→299]: 284 (100) | ||||||||||||||||
| 20 | 26.1 | 719 | MS2 [719]: 359 (100) | Sagerinic acid | + | − | + | + | − | − | + | + | − | − | + | + |
| MS3 [719→359]: 197 (18), 179 (25), 161 (100), 135 (2) | ||||||||||||||||
| 21 | 29.6 | 537 | MS2 [537]: 493 (100), 359 (25) | Salvianolic acid I | − | − | − | + | − | + | + | + | − | + | + | + |
| MS3 [537→493]: 359 (100), 179 (12), 161 (10) | ||||||||||||||||
| 22 | 33.5 | 591 | MS2 [591]: 283 (100), 268 (16) | Acacetin-O-rutinoside | − | − | + | + | − | − | + | + | − | − | + | + |
| 23 | 36.0 | 285 | MS2 [285]: 285 (100), 243 (28), 241 (8) | Luteolin * | − | − | + | + | − | − | + | − | − | − | + | + |
| 24 | 37.3 | 299 | MS2 [299]: 284 (100) | Chrysoeriol | − | + | + | − | − | + | + | − | − | + | + | + |
| 25 | 38.2 | 551 | MS2 [551]: 519 (100), 359 (60) | Monomethyl lithospermate | − | − | + | + | − | − | + | + | − | − | + | + |
| MS3 [551→519]: 339 (100), 179 (28), 161 (23) | ||||||||||||||||
| 26 | 39.1 | 327 | MS2 [327]: 291 (31), 229 (100), 221 (16), 211 (46), 171 (52) | Oxo-dihydroxy-octadecenoic acid | − | + | + | + | − | + | + | + | + | + | + | − |
| 27 | 40.5 | 329 | MS2 [329]: 311 (23), 293 (35), 229 (100), 211 (58) | Trihydroxy-octadecenoic acid | − | + | + | + | − | + | + | + | + | + | + | − |
| N° | Assigned Identification | HAE-Acet | HAE-Acet: H2O | UAE-Acet | UAE-Acet: H2O | MAC-Acet | MAC-Acet: H2O |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| 4 | Chlorogenic acid | 0.19 ± 0.01 | 3.4 ± 0.2 | 0.15 ± 0.01 | 2.9 ± 0.2 | 0.14 ± 0.01 | 2.4 ± 0.2 |
| 20 | Sagerinic acid | 4.2 ± 0.3 | 30 ± 2 | 2.4 ± 0.2 | 29 ± 2 | 8.7 ± 0.5 | 17 ± 1 |
| Total | 4.4 ± 0.3 | 33 ± 2 | 2.6 ± 0.2 | 32 ± 2 | 8.8 ± 0.5 | 19 ± 1 | |
| Flavonoids | |||||||
| 7 | Eriodictyol-O-rutinoside | 26 ± 2 | 74 ± 5 | 14.8 ± 0.9 | 75 ± 4 | 62 ± 3 | 69 ± 4 |
| 8 | Eriodictyol-O-hexoside | 0.35 ± 0.03 | 12.6 ± 0.9 | 0.42 ± 0.03 | 15 ± 1 | 5.9 ± 0.4 | 15 ± 1 |
| 11 | Luteolin-O-rutinoside | 1.3 ± 0.1 | 7.4 ± 0.5 | 0.47 ± 0.03 | 7.5 ± 0.5 | 2.4 ± 0.2 | 6.8 ± 0.5 |
| 12+13 | Luteolin glycosides | 2.1 ± 0.1 | 25 ± 2 | 0.65 ± 0.04 | 25 ± 2 | 3.1 ± 0.2 | 26 ± 1 |
| 14 | Naringenin-O-rutinoside | 6.4 ± 0.4 | 6.8 ± 0.5 | 0.22 ± 0.02 | 9.4 ± 0.6 | 14 ± 1 | 8.2 ± 0.5 |
| 15 | Apigenin-O-rutinoside | 1.5 ± 0.1 | 4.3 ± 0.3 | 0.84 ± 0.05 | 4.2 ± 0.3 | 3.0 ± 0.2 | 4.6 ± 0.3 |
| 16 | Hesperidin | 1.7 ± 0.1 | 9.8 ± 0.7 | 0.21 ± 0.01 | 11.8 ± 0.7 | 5.4 ± 0.3 | 10.1 ± 0.7 |
| 18+19 | Apigenin+ chrysoeriol glycosides | 1.2 ± 0.1 | 3.1 ± 0.2 | 0.57 ± 0.04 | 3.3 ± 0.2 | 2.2 ± 0.1 | 3.4 ± 0.2 |
| 22 | Acacetin-O-rutinoside | 0.54 ± 0.04 | 0.97 ± 0.07 | 0.36 ± 0.02 | 0.91 ± 0.06 | 0.91 ± 0.06 | 0.89 ± 0.06 |
| 23 | Luteolin | 0.72 ± 0.05 | 0.88 ± 0.06 | 0.36 ± 0.02 | 0.82 ± 0.05 | 0.71 ± 0.05 | 1.2 ± 0.1 |
| 24 | Chrysoeriol | 0.68 ± 0.05 | 0.33 ± 0.02 | 0.45 ± 0.03 | 0.30 ± 0.02 | 0.61 ± 0.04 | 0.28 ± 0.02 |
| Total | 43 ± 2 | 145 ± 6 | 19 ± 1 | 153 ± 5 | 100 ± 3 | 145 ± 4 | |
| TIPC | 47 ± 2 | 178 ± 6 | 22 ± 1 | 185 ± 5 | 109 ± 3 | 164 ± 4 |
| Extraction Methods | Solvent | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | PBD (mmol TE/g) | Metal Chelating (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| HAE | Hexane | 33.15 ± 0.69 c | 38.16 ± 1.13 c | 82.87 ± 1.67 c | 41.71 ± 0.57 c | 3.23 ± 0.15 a | 24.09 ± 3.37 a |
| Chloroform | 11.84 ± 0.70 d | 29.63 ± 1.12 c | 94.12 ± 0.76 c | 37.34 ± 1.25 c | 2.14 ± 0.11 c | 3.30 ± 0.29 b | |
| Acetone | 84.88 ± 0.62 b | 84.43 ± 1.62 b | 200.27 ± 3.38 b | 100.15 ± 1.45 b | 2.67 ± 0.14 b | 21.80 ± 1.00 a | |
| Acetone/Water | 419.18 ± 1.52 a | 348.78 ± 6.48 a | 678.48 ± 17.75 a | 458.51 ± 8.09 a | 2.91 ± 0.02 b | 7.98 ± 1.19 b | |
| UAE | Hexane | 7.33 ± 0.25 d | 9.11 ± 0.83 d | 37.72 ± 0.81 d | 22.69 ± 0.50 d | 0.76 ± 0.07 d | na |
| Chloroform | 17.46 ± 0.67 c | 32.71 ± 2.30 c | 102.70 ± 0.53 c | 42.45 ± 0.63 c | 1.97 ± 0.05 c | 11.32 ± 0.96 b | |
| Acetone | 90.29 ± 0.12 b | 100.54 ± 2.11 b | 248.13 ± 4.83 b | 123.43 ± 2.55 b | 2.64 ± 0.11 b | 21.54 ± 2.58 a | |
| Acetone/Water | 427.29 ± 2.35 a | 392.44 ± 14.91 a | 782.24 ± 9.99 a | 492.27 ± 14.05 a | 3.48 ± 0.36 a | 8.86 ± 3.84 b | |
| MAC | Hexane | 7.53 ± 0.40 d | 8.38 ± 1.34 d | 42.32 ± 1.38 d | 24.16 ± 0.84 d | 1.03 ± 0.03 c | 15.49 ± 1.04 b |
| Chloroform | 12.99 ± 0.76 c | 32.03 ± 1.92 c | 101.13 ± 1.11 c | 41.59 ± 0.83 c | 2.38 ± 0.17 a | na | |
| Acetone | 89.71 ± 0.22 b | 99.70 ± 1.41 b | 239.91 ± 2.05 b | 119.14 ± 3.25 b | 2.54 ± 0.19 a | 20.18 ± 0.92 a | |
| Acetone/Water | 266.60 ± 3.09 a | 228.82 ± 8.90 a | 456.73 ± 3.56 a | 302.10 ± 5.33 a | 1.90 ± 0.04 b | 9.53 ± 1.80 c |
| Extraction Methods | Solvent | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| HAE | Hexane | 3.77 ± 0.27 ab | 8.70 ± 1.29 a | 95.99 ± 4.83 ab | 0.83 ± 0.01 a | 0.62 ± 0.06 d |
| Chloroform | 4.18 ± 0.39 a | 6.50 ± 0.24 b | 91.82 ± 3.81 ab | 0.71 ± 0.03 b | 1.40 ± 0.03 b | |
| Acetone | 4.30 ± 0.36 a | 5.61 ± 0.77 b | 87.41 ± 7.58 b | 0.86 ± 0.03 a | 0.90 ± 0.03 c | |
| Acetone/Water | 3.11 ± 0.28 b | 2.12 ± 0.13 c | 101.08 ± 1.78 a | 0.61 ± 0.04 c | 1.66 ± 0.01 a | |
| UAE | Hexane | na | 4.36 ± 0.80 b | 85.33 ± 9.38 b | 0.57 ± 0.01 b | 1.27 ± 0.03 bc |
| Chloroform | 4.84 ± 0.20 a | 7.62 ± 0.97 a | 98.81 ± 3.10 ab | 0.80 ± 0.01 a | 1.39 ± 0.03 ab | |
| Acetone | 4.30 ± 0.20 b | 5.24 ± 0.18 b | 107.67 ± 4.34 a | 0.83 ± 0.01 a | 1.20 ± 0.01 c | |
| Acetone/Water | 4.21 ± 0.03 b | 1.33 ± 0.11 c | 100.75 ± 1.06 a | 0.57 ± 0.02 b | 1.52 ± 0.12 a | |
| MAC | Hexane | na | 7.49 ± 0.44 a | 96.03 ± 5.33 bc | 0.54 ± 0.01 b | 1.29 ± 0.03 c |
| Chloroform | 4.82 ± 0.36 a | 6.69 ± 0.61 ab | 95.03 ± 1.93 c | 0.75 ± 0.01 a | 1.49 ± 0.02 b | |
| Acetone | 2.85 ± 0.16 b | 5.46 ± 0.76 b | 103.61 ± 2.81 ab | 0.74 ± 0.01 a | 1.45 ± 0.03 b | |
| Acetone/Water | 3.37 ± 0.20 b | na | 108.38 ± 1.52 a | 0.52 ± 0.01 b | 1.62 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Ak, G.; Ceylan, R.; Uysal, S.; Llorent-Martínez, E.; Di Simone, S.C.; Rapino, M.; Acquaviva, A.; Libero, M.L.; Chiavaroli, A.; et al. Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle. Plants 2022, 11, 233. https://doi.org/10.3390/plants11020233
Zengin G, Ak G, Ceylan R, Uysal S, Llorent-Martínez E, Di Simone SC, Rapino M, Acquaviva A, Libero ML, Chiavaroli A, et al. Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle. Plants. 2022; 11(2):233. https://doi.org/10.3390/plants11020233
Chicago/Turabian StyleZengin, Gokhan, Gunes Ak, Ramazan Ceylan, Sengul Uysal, Eulogio Llorent-Martínez, Simonetta Cristina Di Simone, Monica Rapino, Alessandra Acquaviva, Maria Loreta Libero, Annalisa Chiavaroli, and et al. 2022. "Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle" Plants 11, no. 2: 233. https://doi.org/10.3390/plants11020233
APA StyleZengin, G., Ak, G., Ceylan, R., Uysal, S., Llorent-Martínez, E., Di Simone, S. C., Rapino, M., Acquaviva, A., Libero, M. L., Chiavaroli, A., Recinella, L., Leone, S., Brunetti, L., Cataldi, A., Orlando, G., Menghini, L., Ferrante, C., Balaha, M., & di Giacomo, V. (2022). Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle. Plants, 11(2), 233. https://doi.org/10.3390/plants11020233



















