Abstract
In recent years, mulberry has acquired a special importance due to its phytochemical composition and its beneficial effects on human health, including antioxidant, anticancer, antidiabetic and immunomodulatory effects. Botanical parts of Morus sp. (fruits, leaves, twigs, roots) are considered a rich source of secondary metabolites. The aim of our study was to highlight the phytochemical profile of each of the botanical parts of Morus tree, their health benefits and applications in food industry with an updated review of literature. Black and white mulberries are characterized in terms of predominant phenolic compounds in correlation with their medical applications. In addition to anthocyanins (mainly cyanidin-3-O-glucoside), black mulberry fruits also contain flavonols and phenolic acids. The leaves are a rich source of flavonols, including quercetin and kaempferol in the glycosylated forms and chlorogenic acid as predominant phenolic acids. Mulberry bark roots and twigs are a source of prenylated flavonoids, predominantly morusin. In this context, the exploitation of mulberry in food industry is reviewed in this paper, in terms of developing novel, functional food with multiple health-promoting effects.
Keywords:
Morus sp.; anthocyanins; flavonols; chlorogenic acid; morusin; health benefits; functional food 1. Introduction
Morus is a perennial, woody, economic, fast-growing deciduous tree, distributed worldwide (India, China, Japan, North Africa, Arabia, South Europe) and used for centuries in agriculture, food, cosmetics and medicine due to its chemical composition and pharmacological activity [1,2,3].
There are 11 main species in the world, but the most common species of mulberry are black (Morus nigra L.), white (Morus alba L.) and red (Morus rubra L., also called American mulberry and native to the eastern United States), followed by other species: Morus australis, Morus bomycis, Morus laevigata, Morus serrata, Morus macroura, Morus cathayana, Morus multicaulis and Morus insignis [4,5,6].
The intensive selection and reproduction of mutations led to the emergence of thousands of varieties, hybrids and polyploids; today, there are 24 species of Morus and one subspecies, with at least 100 known varieties, with androgynous flowers, both male or female. Among the varieties with androgynous flowers, pistillate, staminate or even hermaphroditic types can appear [6,7,8].
In general, the mulberry (Morus nigra L.) is a diploid plant and has 28 chromosomes (2n = 28), but when analyzing its ploidy, many triploid varieties were also found (2n = (3x) = 42), cultivated for their adaptability, vigorous growth and leaf quality. Tetraploids, pentaploid, hexaploidy and dexoploids varieties, the latter having the highest number of chromosomes (2n = 308), represent one of the most valuable adhesions in row of mulberry genetic resources [9,10].
Mulberry tree is the most important plant belonging to the genus Morus of the family Moraceae. It is not a sensitive tree; it is attractive and long lasting and can be planted not only for consumption but also for gardening, edible landscaping, street shade and reducing pollution in the environment and soil. Over thousands of years, it has been domesticated and is now well adapted to grow in almost all types of soil, except swampy, saline or acidic soils [1,11,12].
Mulberry trees can be grown almost throughout the territory, but resistant only where environmental conditions are ensured (light, temperature, water, wind). Light is one of the factors that directly conditions the physiological processes of the mulberry trees, with a preponderant role in its production. The insufficiency of light favors the appearance of mulberry trees pathogens; for this reason, they are planted in sunny areas, and the mulberry rows are placed in an east–west direction. In hilly areas, they are located only on southern, southeastern or southwestern exposures. Being a light-loving tree, for almost all varieties cultivated, a vegetation period of approximately 200 days is required [13,14].
From the point of view of the humidity regime, the mulberry tree is part of the category of mesophilic plants, developing harmoniously on sufficiently moist soils. The content of water from leaf, shoots and roots represents 60–80%, 60–63% and 55–56%, respectively, of the weight of organs. Their water needs are ensured since most of the precipitation is evenly distributed in all the months of the vegetation period, and to a lesser extent, in the groundwater table [4,15,16]. Planting mulberry in nutrient-rich soils with a low acidic or neutral pH will result in the best yield in terms of leaf quality, but also fruit [16,17,18,19].
Asian countries have a long and rich history of large-scale cultivation of white mulberry as a key habitat for silkworm rearing, also known as Sang Shen in China and Oddi in Korea [20]. It is believed that about 5000 years ago, around 2700 B.C., Chinese Empress Si Ling-Shi accidentally discovered that silkworms could be obtained from the cocoon of mulberry leaf worms, and the secret of this art has been kept in China for about 2000 years [4]. On the other hand, in Turkey and Greece, mulberries are mainly grown for fruit production than for foliage [21].
Morus species gained interest in the food industry due to their health benefits, being used in the treatment of anemia, tonsillitis and other sore throats, and today, it is supplied in markets in the form of paste called sangshengao, used in the preparation of teas with benefits for sight, hearing, kidneys and the liver [22,23].
Herbal preparations are the oldest and richest remedies in human health care, and today, the human being studies, tests and regains confidence in natural, effective remedies that have survived for several generations. According to the World Health Organization, almost eighty percent of the world’s population continues to use complementary therapies, many of which are derived from plants such as mulberry.
Based on the recent literature on mulberry, this study aims to review the effectiveness of mulberry as a whole plant, by highlighting the phytochemical profiles of each botanical part of Morus tree and attributing each part to various functional properties, exploring its nutraceuticals applications in medicine and food industries. A systematic update of the existing literature was conducted.
2. Research Methodology
Recent studies regarding the phytochemical composition of fruits, leaves, twigs and roots and their beneficial effects for curing different diseases were selected using a PRISMA 2020 flow diagram based on the suggestion of Page et al., 2021 [24]. The steps and selection criteria, followed by the number of the studies used for our review, are shown in the Figure 1. Databases such as PubMed, Scopus, Science Direct, Elsevier, Google Scholar, Google Patents were accessed to search the literature. The Medical Subject Headings keywords included in the search were: “Morus” “Morus nigra”, “Morus alba”, “nutrients Morus”, “bioactive compounds Morus”, “phytochemicals Morus”, “antioxidant capacity Morus”, “Morus diabetes”, “anticancer Morus”, “immunomodulatory Morus”, “food industry Morus” etc. All information systematized in the tables was obtained from research articles (in vivo/ex vivo/in vitro studies) between 2016 to 2021. Studies published in languages other than English were excluded, except one patent which was written in Romanian. A total of 199 studies were selected and included in this review.
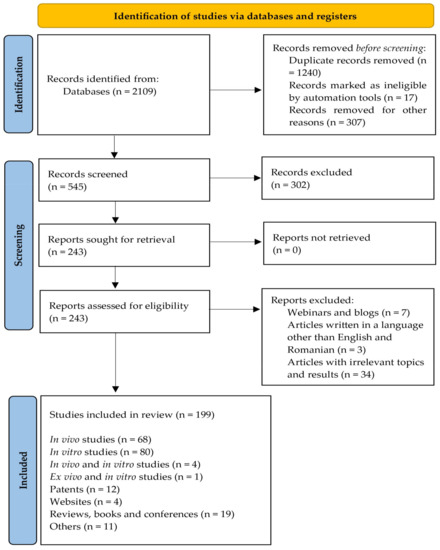
Figure 1.
PRISMA 2020 flow diagram for the present review.
3. Nutritional Values of the Botanical Parts of Morus sp.
Morus tree is considered a medicinal plant. All of its botanical parts (fruits, leaves, twigs, root bark) have their own special effects on the human body, which is why they are used in traditional Chinese and Indian medicine [1,2,23].
3.1. Mulberry Fruits
There are multiple Morus sp. fruits (about 2 to 3 cm long); they form together and are arranged longitudinally around the central axis, similar to blackberries, and are low in calories, but rich in nutrients and antioxidants, so they can ensure good overall health [25]. The fruits contain a high-water content (over 70%), and the pH values differ between species: M. alba presents the highest value (5.6), while the M. rubra and M. nigra have values of 4.04 and 3.52, respectively [21]. Based on values of pH, total soluble solid and total dry weight, the M. alba could be recommended for processing, while M nigra may be recommended for fresh fruit production [21,26].
The taste is better in the case of black fruits, which is due to the lower pH value compared to white fruits, which are sometimes characterized as tasteless. All these own connections, together with the nutritional and medicinal ones, make the black mulberry fruits increasingly sought after and studied [2,21,23,27,28,29,30,31].
The mineral content of mulberry fruits depends on the species, fruit maturity and composition of soil and environmental conditions (light, humidity, temperature, altitude) [21,32,33,34]. In the study of Ercisli & Orhan, 2007 [21], ten elements were determined from mulberry fruits collected from Turkey, where potassium was predominant.
Iron, an essential mineral and very rare in berry fruits, has a high value of 4.2 mg/100 g in M. alba and M. nigra. In another study [32] of the macroelements, N, K and p are found in large levels, while sodium is present in a very low concentration (0.01 mg) in M. alba and M. nigra grown in Spain. The levels of iron varied between 28.20 to 46.74 mg/kg and 23.92 to 37.09 mg/kg in M. alba and M. nigra, respectively, demonstrating good sources of non-heme iron. In the black mulberry, grown in Western Serbia at different altitudes, the highest amount of minerals was determined for phosphorus and potassium, with a significant difference depending on altitude. Additionally, the authors showed that black mulberries are a good source of iron, with the highest content (1.95 mg/100 g) found at 187 m altitude [33]. The form of iron in foods is ferric iron (Fe3+), which is less bioavailable than ferrous iron (Fe2+). In vivo, the increase in iron bioavailability was attributed to ascorbic acid’s chelating and reducing properties [35]. The high content of vitamin C and iron from mulberry resulted in the better bioavailability of iron and can be used to treat anemia [36].
In the mulberries fruits grown in Xinjiang region, China, potassium was the dominant macronutrient, followed by calcium and magnesium. Due to the high Fe content of Russian mulberry and black mulberry from China (11.4–11.9 mg/100 g fw), they can be used as dietary supplements to treat the iron deficiency, anemia [36].
In terms of the fatty acids profile of mulberry fruits, linoleic acid is dominant, followed by palmitic acid and oleic acid, the latter being detected only in M. alba and M. nigra [21,26,37]. Additionally, the high level of linoleic acid (52.3%) was found in white mulberry cultivated from Xinjiang region, China [36].
Regarding protein content, the mulberry fruits grown in southeastern Spain are a good source of protein; M. alba had a higher protein content than M. nigra [32]. In the mulberries cultivated in China, the ratio between essential amino acids (EAA) and total amino acid (TAA) was 44%, 42% and 29% for Russian, white and black mulberries, respectively. Foods with EAA/TAA ratio 40% are an ideal protein source, suggesting that the Russian and white mulberries cultivated in China could be used as an important quality protein source [36].
Ascorbic acid (vitamin C), a powerful water-soluble antioxidant compound, was quantified with the reflectometer in the mulberry fruits (n = 30); the highest value was recorded in M. alba (22.4 mg/100 mL), followed by M. nigra and M. rubra with 21.8 and 19.4 mg/100 mL, respectively [21]. Black mulberries had the highest content in ascorbic acid (48.4 mg/100 g fw), compared with Russian and white mulberry fruits (5.64 mg/100 g fw and 6.01 mg/100 g fw, respectively) grown in China [36].
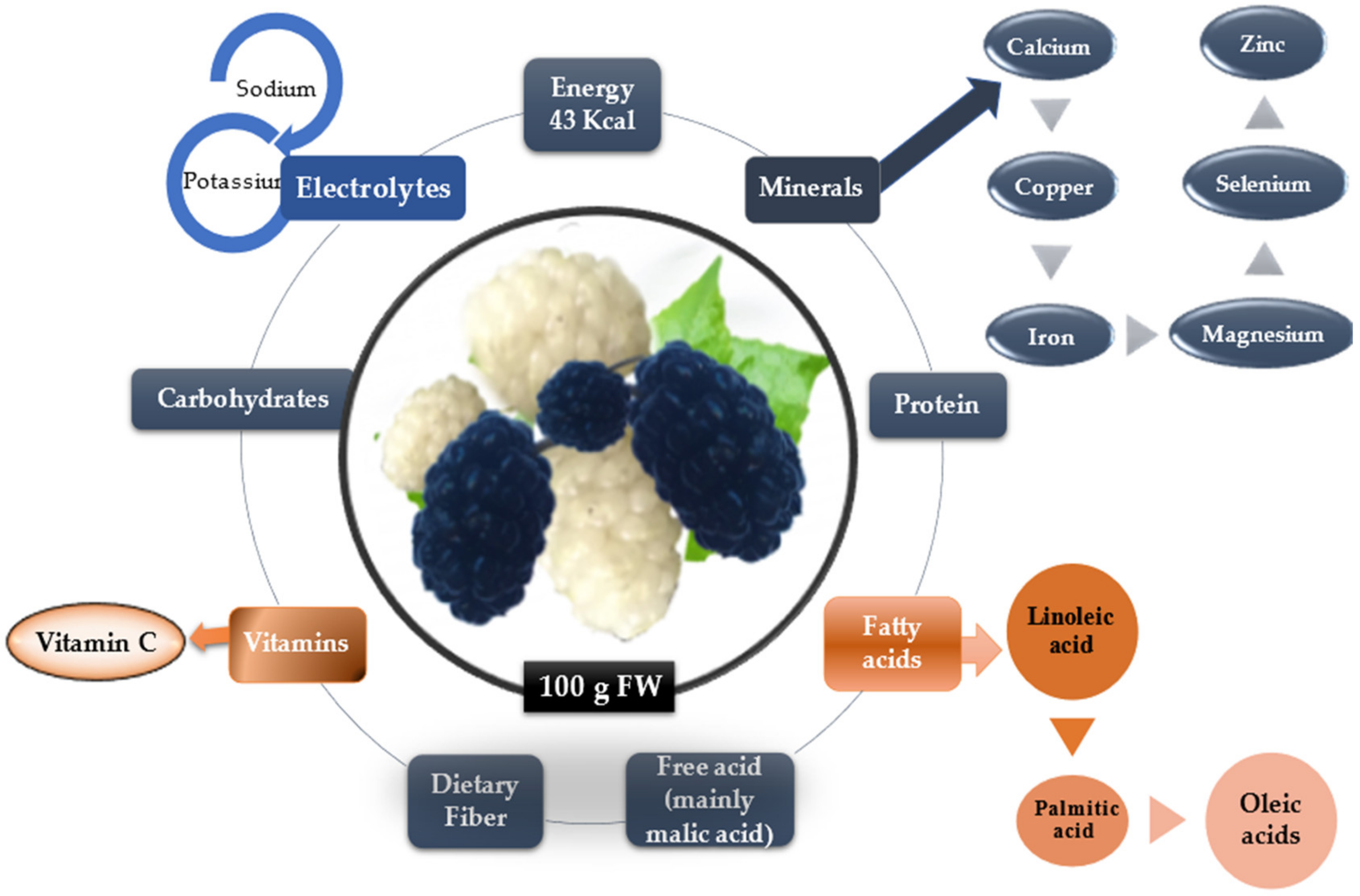
Figure 2 summarizes the nutrients in mulberry fruits with huge importance in human metabolism.
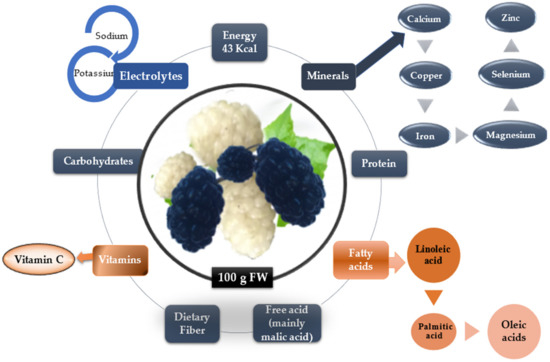
Figure 2.
The nutrients components of mulberry fruits.
Nguyen et al., 2008, mentioned that unripe mulberry, i.e., the green parts of fruits, contain a white sap that may be toxic, stimulating, or mildly hallucinogenic [23].
3.2. Morus Leaves
The leaves of mulberry are just as valuable as the fruits, not only because they are the only known source of food for the development of silkworms (Bombyx mori), but also because they contain, in addition to bioactive compounds, vitamins (C, B1, D), organic acids, minerals and proteins [16,27,38,39].
The proximate compositions of three different varieties of mulberry leaves were investigated by Iqubal, 2012 [40]. The high ash content was found in M. rubra, followed by M. nigra and M. alba, indicates the presence of considerable amounts of inorganic nutrients in leaves. Among the three varieties of mulberry, M. alba had the highest lipid content, the M. rubra had the highest protein level, while M. nigra contained high amounts of fiber. Based on their chemical composition, mulberry leaves can present dietary sources with promising nutritional values [40].
The leaves of M. alba and M. nigra possess a high iron content (119.3–241.8 mg/kg), while the other minerals are found in low concentrations. Among the macronutrients, Ca was found to be predominant, followed by N, K and Mg [26,32].
The protein content in mulberry leaves varied between mulberry species grown in Spain. The leaves of M. alba had a higher protein content (ranging from 14.1 ± 0.4 to 19.4 ± 0.7 % dw) than the leaves of M. nigra (from 13.4 ± 0.3 to 18.7 ± 0.7 % dw) [41].
Mulberry foliage is especially used to feed silkworms, but also other animals such as cattle, goats, and pigs [26,42,43]. New studies showed that M. nigra leaves can be introduced into pig feed in controlled concentrations as an alternative source of protein, without adversely affecting the animals. Mulberry leaves, reporting beneficial effects on the quality of the meat and the chemical composition of the muscles, the growth and finishing performance of their carcass, reduce the thickness of back fat (longissimus dorsi muscle) and increase the fat deposition in the muscles, crude protein levels and amino acids in muscle tissue [42,43].
3.3. Mulberry Twig and Root Bark
Mulberry twigs contains arabinosis, glucose, fructose, maltose, stachyose, tannin, and are also used in medicine, with a series of beneficial effects on serious diseases that affect the human body [4,44]. So far, no data are available on the content of minerals, carbohydrates, lipids, or root bark proteins, but bioactive compounds were identified, which are detailed in the next chapter.
4. Phytochemical Composition of the Botanical Parts of Morus sp.
Polyphenols are secondary metabolites with an important and varied role, widely found in the plant kingdom and constituting a large class of phytochemicals. Among the phytochemical compounds present in Morus sp., flavonoids predominate. Table 1 presents recent data from the literature (the last years) on the screening of phytochemical content found in both the fruits and leaves of Morus sp. Flavonoids are present in high concentrations in the epidermis of leaves and fruit skin, but also in the bark, and have a remarkable spectrum of biological activities [45,46,47,48].

Table 1.
The content of phytochemicals (polyphenols, anthocyanins and flavonoids) from mulberry fruits and leaves from data of literature of the last years (2016–2021).
4.1. Mulberry Fruits
The total phenols and flavonoids of M. nigra fruits varied between 485.5 to 1580 mg GAE/100 g fw, and 129.2 to 219.12 mg QE/100 g fw, respectively. In the case of M. alba, the total phenols and flavonoids varied between 60.4 to 663 mg GAE/100 g fw and 217 to 370 mg QE/100 g fw, respectively (Table 1). Anthocyanins, water-soluble pigments, are found in M. nigra fruits [34,60]. Many studies showed that anthocyanins are powerful antioxidants belonging to a group of flavonoids, and their color depends on the structure and presence of copigments and the acidity of the environment [9,59,61,62,63,64]. The color of black mulberries is due to the presence of anthocyanins and their content varies between 81.36 to 206.1 mg cyanidin-3-O-glucoside equivalent/100 g fw (Table 1). In generally, the contents of bioactive compounds in M. nigra L. are higher compared to M. alba L. due to the presence of anthocyanins [30,65]. Although, the presence of anthocyanins was detected in the case of some varieties of white mulberry [50,56,57], because the ripening of the fruit is accompanied by the change of color from green (unripe) to white and light purple (at full maturity) due to the accumulation of anthocyanins.
There are several studies that reported polyphenolic compounds and their functionality in mulberry fruits, and the amount varies considerably depending on the variety, as well as the climate, soil, agricultural and processing conditions [4,6,7,32,34,45,66].
The effects of ethanol concentration (20, 50 and 80%), the extraction method and time on the total yield of phenols, flavonoids and anthocyanins from black, red and white mulberry from southeast Serbia were investigated [67]. The content of total phenols of M. nigra depends on the extraction method and ranges from 60.04 to 150.13 mg GAE/100 g fw and 69.11 to 142.18 mg GAE/100 g fw for maceration and ultrasonic extractions, respectively. In terms of flavonoid content, M. rubra and M. white have a lower content of flavonoids than M. nigra. The content of the monomeric anthocyanins ranges from 64.08 to 137.06 mg cyanidin-3-O-glucoside/100 g fw for ultrasonic extraction. Ultrasonic extraction was the most efficient in all samples due to the intensification of mass transfer, and thus the penetration of the solvent into the mulberry cells was easier [67].
In recent years, due to the development of analytical techniques for the separation and identification of compounds, it was possible to identify the composition of mulberry metabolites [8,44,68,69,70,71,72].
In M. nigra, there are four main anthocyanins, where cyanidin-3-O-glucoside is the main pigment (Figure S1A) [38,65,73,74].
Thirty-five metabolites were identified in white and black mulberry fruits from Italy (Campania region) [72]. Four anthocyanins from M. nigra were identified, these being pelargonidin and cyanidin conjugated with one, two or three hexose sugars (Figure S1A). The flavonol compounds, quercetin and kampferol, esterified with one to three sugars or one or two sugars with one malonyl group, were identified in both white and black mulberries (Table 2). Two flavanonols were detected in black mulberries, dihydroquercetin (taxifolin) hexoside and dihydrokaempferol hexoside, which are precursors of the biosynthesis of anthocyanidins, such as cyaniding and pelargonidin, respectively [72,75].

Table 2.
Summary of phenolic components isolated from different parts of mulberry tree across-studies (2017–2021).
The total anthocyanin contents of 12 mulberry cultivars in Korea depended on the variety and ranged from 0.51 to 28.61 mg/g dw. Cyanidin-3-O-glucoside was the major anthocyanin compound, followed by cyanidin-3-O-rutinoside, and the lowest content was recorded in the case of pelargonidin-3-O-glucoside [56]. Using UHPLC-(ESI)-qTOF, 16 anthocyanin compounds were identified, including six that were identified for the first time. These are malvidin hexoside, cyanidin malonyl hexose hexoside, cyaniding pentoside, cyanidin malonyl hexoside, petunidindeoxyhexose hexoside, and cyanidin deoxyhexoside [56].
A complex study investigated and compared different medicinal parts of M. alba (root barks, twigs, fruits, leaves) in terms of chemical composition [59]. In the white mulberries, chlorogenic acid, rutin, isoquercitrin were identified, but in lower levels than in the leaves.
In M. nigra fruits, the main flavonoids identified were quercetin 3-O-glucoside and rutin (quercetin 3-rutinoside) (Figure S1A) [38]. Another study showed that the main compounds present in mulberry fruit come in the various glycosylated forms of quercetin and kaempferol, and the content of quercetin glycoside was found to be higher than the amount of kaempferol-O-rutinoside [32].
The major phenolic group profile found in M. nigra, according to the amount recorded, was phenolic acids (benzoic acid and cinnamic acid derivatives), followed by flavonols and anthocyanins. Protocatechuic acid is the main derivative of hydroxybenzoic acid, while chlorogenic acid is the predominant hydroxycinnamic acid. In other studies, a different pattern was found, where anthocyanins were higher than the amount of flavonols [2,79,80,81].
Miljkovic et al., 2015, identified the presence of anthocyanins in black and red mulberries, other than those mentioned above: cyanidin-3-O-arabinoside, peonidin-3-O-arabinoside, delfinidin-3-O-arabinoside, peonidin-3-O-glucoside or galactoside or malvidin-3-O-arabinoside, delfinidin-3-O-glucoside or galactoside, petunidin-3-O-glucoside or galactoside, malvidin-3-O-glucoside or galactoside [82].
Özgen et al., 2009, investigated the phytochemical profile and antioxidant properties of the anthocyanin-rich mulberry species, M. nigra and M. rubra, harvested from Turkey. Their study showed that M. nigra had the richest amount of anthocyanin, with an average of 571 μg cyanidin-3-glucoside equivalent/g FW (fresh weight) compared to M. rubra [83].
4.2. Mulberry Leaves
Flavonols are the main group of flavonoids in mulberry leaves, whose concentrations differ depending on the geographical area. In Spanish mulberry leaves, flavonols range from 3.7 to 9.8 mg/g dw [32], the Polish variety of M. alba leaves contain 17.6 mg/g dw [84], while Tunisian M. rubra leaves reach up to 85.45 mg/g dw [77].
In terms of caffeoylquinic acid derivatives, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeoylquinic acid isomer and caffeoylquinic acid glucoside were identified in the leaves of Morus [32]. In general, chlorogenic acid is predominant in mulberry leaves (Figure S1B), for example in M. alba leaves reaching 51.04% from total phenolic acids [84]. In contrast, syringic acid was the major phenolic acid identified in M. nigra leaves, and is considered the key compound for the biological effects of mulberry leaves [71,85,86].
The phytochemical composition of mulberry leaves belonging to eight clones from Spain (white and black) was investigated [81]. The flavonol derivatives, mainly quercetin and kaempferol in glycosylated forms were identified in mulberry leaves. Among the quercetin derivatives detected in Morus leaves, the compounds quercetin malonyl-dihexoside, quercetin-rhamnose-hexose-rhamnose and quercetin-malonyl-rutinoside were identified for the first time. Concerning kaempferol derivatives, kaempferol rutinoside hexoside, kaempferol-malonyl-dihexoside and kaempferol-malonyl-rutinoside were identified for the first time.
In the leaf samples of M. alba, rutin, isoquercitrin, astragalin, skimmin, and chlorogenic acid were predominant [87].
In the leaves of mulberry, it was also found that the predominant glycosides of flavonol were rutin and quercetin 3 (6-malonylglucoside), which are flavonol glycosides mainly responsible for the antioxidant capacity of the leaves, and have properties that reduce oxidative stress in the liver and improve hyperglycemia [88,89,90,91].
Other flavonoids were identified in mulberry leaves, such as mornigrol E, mornigrol F and morusin, the latter being a prenylated phenolic present in all parts of the mulberry plant, the largest amount being in the bark of the root, while its content decreased in the following order: stem, branches and leaves [40,63].
4.3. Mulberry Twig and Root Bark
The root barks of mulberry, especially of M. alba L., were widely used in Chinese herbal medicine for treating various diseases. The main constituents of root barks are prenylated flavonoids such as morusin, kuwanon C (known as mulberrin) and kuwanon G [92,93]. Another kind of polyphenols found in mulberry are Diels–Alder-type adducts, most of which contain flavonoid groups, and the C-2 and C-3 of the flavonoid unit could be replaced by prenyls and their correspondents [94].
Morusalbanol A, a neuro-protective DielseAlder adduct was isolated from the bark of M. alba grown in China [78]. Ha et al., 2018, investigated phytochemicals from the root bark of M. alba collected from Ulsan province, Korea, and identified four Diels–Alder-type adducts (morusalbins A-D), one isoprenylated flavonoid (albanin T), among which 21 other known phenolic compounds were found [95]. A phytochemical investigation of the root bark of M. alba (Korea), and their anti-Alzheimer’s disease activity, was evaluated by Kuk et al., 2017 [96]. The authors identified two Diels–Alder-type adducts (mulberrofuran G and albanol B) and one prenylated flavonoid (kuwanon G).
Morusin, a prenylated flavonoid, from the perspective of chemical structure has hydroxy groups at 5 (from A ring), 2′ and 4′ (from B ring), a prenyl unit at position 3, and dimethyl pyran group across positions 7 and 8 (Figure S1C) [88].
The major flavonoid constituents, isolated from root barks of M. alba and collected in Korea, were kuwanon E, (3′-prenylated flavanones), kuwanon G, (tetrahydroxyflavone), and norartocarpanone, a member of flavanones [92].
The position and number of hydroxy and prenyl groups on the flavone backbone influences their bioactivities [94,97].
The phenolic phytochemicals in mulberry bark were found to be rich in oxyresveratrol, resveratrol p-coumaric acid, chrysin, catechin, vanillic acid, ferulic acid, chlorogenic acid, mulberroside A, maclurin, and moracins [98,99].
The stilbene, mulberroside A and prenylated flavonoids, kuwanon G, and morusin identified in the root barks were higher than in twigs. Instead, oxyresveratrol content in twigs was higher than in root barks [87]. Oxyresveratrol (2,3′,4,5′-tetrahydroxystilbene) is a natural stilbene, a phytoalexin that has recently received attention due to its therapeutic potentials [100].
Mulberry root bark also contains other flavonoids, such as mulberrin, mulberrochromene and cyclomulberrin [4,76,101].
5. Health Benefits and Effects
The presence of polyphenolic compounds of the genus Morus, have recently received increased attention and interest due to its biological effects, such as anticancer, anti-inflammatory, antidiabetic, antihypertensive, antinociceptive, antiaging, antianemic, antibacterial and antioxidant activities [26,102,103,104,105].
M. nigra is recognized as an appreciable source of flavonoids, especially anthocyanins, which are considered to possess certain protective effects on human health. M. nigra fruits are of huge importance for medicine and pharmaceuticals production, being recommended for the prevention and treatment of serious diseases such as diabetes, cancer, bacterial infections, arthrosclerosis, hyperlipidemia, neurological diseases, inflammation, hypertension, or to improve drugs with their natural flavor and color [1,21,23,27,45,102]. In addition, due to the anthocyanin pigments responsible for the black/purple color, mulberry fruits are also used as color or flavor additives in the food industry [104].
5.1. Antioxidant Potential
Oxidative stress is an imbalance between free radicals and antioxidants in favor of radicals. The high level of free radicals induces many biochemical changes in organisms, with an important contributing factor to a vast number of diseases such as diabetes, atherosclerosis, cardiovascular and neurodegenerative diseases, and cancer [4,6,84,106,107]. Some free radicals are naturally produced in the body and others come from different sources, mainly from polluted environments [108]. Free radicals can alter important classes of biological molecules, thus altering the body’s normal redox state, resulting in increased oxidative stress [109,110,111]. Increasing the level of exogenous antioxidants in the body after their intake can help maintain a good antioxidant status. Antioxidants can counteract the effects of oxidative damage caused by free radicals by preventing and repairing damage caused by reactive oxygen species and reactive nitrogen species; therefore, they can increase immune defense and reduce the risk of cancer, cardiovascular disease, cataracts, diabetes and other degenerative diseases [55,63,72,110,112,113]. A variety of methods were developed to evaluate in vitro the antioxidant capacity of food components [109]. The most commonly used methods are based on the single electron transfer (SET) mechanism, including the Folin–Ciocalteu assay, ABTS, DPPH, FRAP etc. [109]. The methods are based on the ability of samples to scavenge a synthetic-colored radical or to reduce the redox-active compound, which is spectrophotometrically monitored. Assessing the antioxidant capacity of a sample by a single method is not relevant; therefore, several procedures should be carried out to investigate the antioxidant capacity of foods [109].
The in vitro antioxidant capacity of fruits and leaves from different mulberries species, evaluated through various methods, are presented in Table 3.

Table 3.
The in vitro SET assays that investigated antioxidant capacity of mulberry fruits and leaves from data from the literature (2016–2021).
The main compounds that contribute to the antioxidant capacity, identified in M. nigra fruits, are phenolic acids, flavonoids and anthocyanins that promote beneficial effects on human health [26,117].
A comparative study between black and white mulberry methanol and acetone extracts revealed that M. nigra exhibited the highest antioxidant capacity compared with M. alba using the ABTS, DPPH and reducing power assays [118].
Sánchez-Salcedo et al. (2015) determined the antioxidant activity of white and black mulberry fruits, and results showed a significant difference between the clones from 3.84 to 20.73 mg Trolox/g dw and 3.62 to 12.91 mg Trolox/g dw for ABTS and DPPH methods, respectively. In general, the antioxidant activity was significantly higher in M. nigra fruits than in M. alba fruits, and an intra-species variability was observed. The MN1 black mulberry clone showed a significant antioxidant potential (p < 0.05) higher than the rest of the clones [32].
Other studies showed that M. nigra has a high antioxidant activity in the method of bleaching β-carotene (>70% inhibition). In addition, the anthocyanin mulberries (cyaniding derivates) showed the highest liposome antioxidant activity [119].
Mulberry leaves also have a strong antioxidant capacity, and the predominant antioxidants are kaempherol and quercetin, which effectively protect red blood cells against oxidative damage caused by free radicals [5,40,103].
The methanol extract of M. nigra leaves enhanced antioxidant enzymes such as SOD, GSH-Px, CAT in serum, brain and liver tissues, and reduced MDA level, effects that are related to the improved cognitive impairment in mice D-galactose group. Vanilic and chlorogenic acids are the major compounds in the extract and are responsible for antioxidant actions [120,121].
Different parts of the Morus species—the leaves, root and fruits of M. nigra—have a high content of antioxidants. Morusin is a powerful antioxidant of the root bark and was reported to be a promising candidate in the treatment of cancer, including lung cancer, but also attracted scientific interest for its strong antinociceptive and antitumor properties [88,101,117].
Mulberry leaves and fruits contain resveratrol and oxyiresverrol, both of which are natural dietary phenolic compounds, considered powerful antioxidants. M. rubra fruits contain the highest content of resveratrol (50.61 μg/g dw) compared to jamun fruits (Syzygium cumini L.) and jackfruit. The scavenging effect of the DPPH radical is dependent on the concentration dose, and IC50 was found to be 0.40 mg/mL [122]. Resveratrol was studied for its cardioprotective, neuroprotective and antiviral properties. Due to the fact that its antiviral activity was demonstrated in the fight against several viruses, it was recently considered to be an anti-inflammatory agent against coronavirus, a severe acute respiratory syndrome type 2 [123,124].
Mulberry leaves are recognized for their beneficial effects on health, and various studies have shown that they protect red blood cells against oxidative damage, due to their content in flavonoids, such as quercetin and kaempferol [31,103].
In a study performed on rats, the phytochemical profile of M. nigra leaves and their hypolipidemic effect were evaluated. The results of this study demonstrated the potential therapeutic effects of M. nigra leaf infusion, due to large amounts of phenols, flavonoids and antioxidant activity. The high content of polyphenols, mainly chlorogenic acid, normalized the hyperlipidemic disturbance of rats. The antioxidant capacity of mulberry leaves depends on the extraction methods, the highest DPPH-scavenging activity was recorded in the infusion and hydrometanolic extracts (83.85± 0.99% and 81.71 ± 0.05%, respectively) [103].
The antioxidant capacity of M. nigra leaves was evaluated by DPPH and β-carotene/linoleic acid co-oxidation system assays depending on the seasonal variations. A high antioxidant level highlighted that black mulberry leaves were rich in phenolic compounds (syringic acid, quercetin and rutin) that strongly contributed to antioxidant capacity [71].
Morusin, a prenylated flavonoid identified in the root bark of Morus is a powerful antioxidant agent due to its chemical features, reducing ROS formation and inflammation [88].
Interest in natural antioxidants from mulberries has grown considerably in recent years and new approaches are being explored for their most effective extraction. [112,125,126]. Different extraction techniques and modern separation technologies were used to separate and isolate the bioactive components from mulberry [125].
5.2. Anticancer Activity
The International Agency for Research on Cancer (IARC), a specialized agency of the World Health Organization (WHO), reported that by 2020, about 10 million deaths worldwide were caused by cancer, with Europe ranking second (19.6%) after Asia (58.3%). Due to drug resistance and side effects, there is an urgent need to study and develop new therapies for cancer treatment [127].
The anticarcinogenic mechanisms of mulberry phenols demonstrated in several cell cancer lines or in animal tumor models include antioxidant activity, antiproliferation, the induction of apoptosis and antiangiogenic activity [2,128,129,130].
The antioxidant compounds in M. nigra fruit, leaves and bark, were shown to have an anticancer effect in addition to other properties [47,48,131]. The bioactive components in M. nigra leaves were proven to be a good candidate in the genetic protection and treatment of organotoxicity induced by oxidative stress. The methanolic extract of mulberry leaves had an effective cytotoxic behavior against cancer cells [1,132].
The anthocyanins found in M. nigra fruits, such as cyanidin and pelarginidine, had the ability to reduce the viability of cancer cells and inhibit tumor progression. Hydroxynamic acid derivatives of mulberry fruit were shown to increase reactive oxygen species production by acting as prooxidants, and thus destroying cancer cells [2,133,134].
Cho et al., 2017, isolated cyanidin-3-glucoside from mulberry fruit, showing that it had the ability to inhibit tumor growth in nude female mice inoculated with MDA-MB-453 cells, showing active apoptosis by caspase-3 cleavage and DNA fragmentation by proteins of the Bcl-2 family and Bax [133].
Shu et al., 2021, isolated guangsangon E compound from white mulberry leaves, with the ability to inhibit cell proliferation, induce autophagy and apoptosis in lung and nasopharyngeal cancer cells, and increase the flow of autophagy by increasing lysosomal activity and the fusion of autophagosomes and lysosomes. Guangsangon E activates the stress of the endoplasmic reticulum, which is involved in inducing autophagy, by accumulating reactive oxygen species, eventually attenuating autophagy-mediated cell death [135].
Mulberry bark has, in addition to anti-inflammatory and antioxidant activities, antitumor and anticancer effects due to the presence of morusin, which was reported to be a promising candidate for the treatment of certain types of cancer, including lung cancer [45,88,91,136].
The potential of morusin to inhibit the growth of breast cancer cells was confirmed by some in vitro and in vivo studies through C/EBPβ and PPARγ-mediated lipoapoptosis, and induced the adipogenic differentiation, apoptosis and lipoapoptosis of cancer cells [137]. Morusin inhibited the growth and migration of human cervical cancer stem cells by decreasing the expression levels of NF-κBp65 and Bcl-2 and Bax, and increased caspase-3 in a dose-dependent manner [138]. Morusin has a strong antitumor activity for human hepatocellular carcinoma in vitro and in vivo, by inducing apoptosis and inhibiting angiogenesis; moreover, it inhibited the proliferation, migration and tube formation of human umbilical vein endothelial cells [139].
5.3. Antidiabetic Therapy
Diabetes is associated with increased oxidative stress. Hyperglycemia, excess fatty acids and insulin resistance lead to an increase in oxidative stress, followed by other metabolic disorders such as obesity, diabetes, high blood pressure and cardiovascular disease [113,140].
Many research studies reported the phytochemical and pharmacological aspects of M. nigra as an antidiabetic drug [103,113,141] and found that all botanical parts (leaves, root, branches and fruits) have special effects on antidiabetic activity [26,72,113,142].
Leaf and leaf tea is considered a healthy and suitable food in the fight against diabetes, regulating energy metabolism, improving the blood lipid profile, improving insulin sensitivity and lowering blood glucose [143,144]. The main compounds in mulberry leaf tea that were reported to have an antidiabetic effect are phenolic acids, flavonoids, polysaccharides, and 1-deoxynojirimycin, the latter has the effect of lowering high levels of sugar blood [103,145].
Some components identified in mulberry leaves, such as tricetin, gallic and chlorogenic acid, possess the ability to regulate the insulin receptor substrate 1, glycogen synthase kinase-3 beta, interleukin 6, and other proteins responsible for insulin signaling pathway, glucose metabolism, nephropathy diabetes, obesity and other diabetes-dependent factors [146].
In traditional Egyptian culture, black mulberry leaves were also used as a remedy for the treatment of diabetes, improving the redox profile and glycemic response in the liver [113,147]. In an in vivo study of streptozotocin-induced diabetic male albino rats by Hago et al., 2021, the leaf extract caused a significant decrease in blood glucose. In vitro model confirms the anti-diabetic effect of mulberry leaf extract, which has the ability to reverse hyperglycemia, strongly inhibit the α-glucosidase enzyme and prevent the liver and kidney damage associated with diabetes [147].
M. nigra fruits and seeds are also used to treat diabetes, especially type II diabetes and its associated complications [4,140].
M. alba fruit polysaccharides repaired damaged pancreatic tissue, significantly lowered fasting blood glucose levels, curved the oral glucose tolerance zone, and significantly increased high-density lipoprotein cholesterol and total cholesterol of a high-fat diet in streptozotocin-induced type 2 diabetes in rats. Additionally, in the same model, there were significant decreases in fasting serum insulin levels, the homeostasis model for assessing insulin resistance, serum alanine transaminase levels and triglyceride levels [148].
Mulberry twigs and bark are also known to contain polyphenols and polysaccharides. Thus, M. nigra twig methanol extract is a tyrosinase inhibitor and has the ability to lower blood sugar [105,148].
Ha et al., 2020, isolated the farnesylated 2-arylbenzofuran compound from M. alba extract which exerts an anti-obesity effect and is a potent inhibitor of the intracellular protein tyrosine phosphatase 1B (PTP1B), which plays a critical role in the insulin receptor signaling pathway [149].
5.4. Immunomodulatory Effect
Immune stimulation in defense against disease in humans is currently receiving much attention, and there is a growing interest in the discovery of natural products with the effect of strengthening immunity. The immunomodulatory effect of plants has gained great interest, especially in recent years due to a human awareness to modulate the immune system [150].
The bioactive compounds present in the botanical parts of the M. nigra plant have immunomodulatory properties on infectious diseases of bacterial, parasitic and viral etiology. Thus, the mulberry could be exploited to find new avenues for the development of food supplements with a strong immunostimulatory and immunosuppressive effect to enhance quality of life [132,151].
For example, resveratrol is one of the compounds present in M. nigra as a powerful antioxidant with antiviral activity, and recent studies suggested that it has a therapeutic effect in homeostatic disorders associated with COVID-19 and is a potential adjunct to COVID treatment [124,152,153,154].
Mulberry root bark has been used for medicinal purposes since ancient times [155,156,157]. It has an acidic taste, as well as anthelmintic and cathartic, anti-inflammatory, vermifuge and vermicide, diuretic, anti-inflammatory, antitussive and antipyretic properties in oriental medicine, but is also used to treat high blood pressure [42,45,102,158]. The juice extracted from the mulberry root fluidified the blood, and it is very useful for fighting tapeworms and other parasites in the digestive tract [159,160,161].
The immunomodulatory effect generated by both the leaves and fruits of M. alba was proven. Orally administered methanol M. alba leaves extract increased serum immunoglobulin levels and prevented mortality induced by bovine Pasteurella multocida in Swiss albino mice. Additionally, it significantly increased the phagocytic index in the carbon clearance test, and the circulating antibody titer in the indirect haemagulation test was recorded as having positive effects on both humoral and cell-mediated immunity [132].
Recently, it was reported that M. alba fruits induce the phenotypic maturation of dendritic cells due to the polysaccharides in the composition, and can thus be used as an adjunct in dendritic-cell cancer immunotherapy [128].
Numerous studies confirmed that various parts of mulberry have various benefits for human health, such as anti-inflammatory, neuroprotective, antiatherosclerosis, hepato- and gastro-protective, antibacterial, anti-melanogenesis properties, which are presented in Table 4.

Table 4.
The health-promoting properties of different parts of mulberry fruits, as determined in various experimental designs (animal and cell models) (2017–2021).
6. Applications of Mulberry in the Food Industry
Epidemiological studies suggest the role of oxidative stress in the generation and propagation of many chronic diseases. Therefore, to counteract the unwanted effects of oxidative stress exogenous antioxidants in the form of dietary supplements or even functional foods are necessary for the human body [40].
Black mulberry fruits are considered functional foods that, when ripe, have a black–purple color and can be eaten fresh or dehydrated [165,172,173]. Baked mulberries are very perishable fruits, mainly due to their smooth texture, high softening and breathing rate, and susceptibility to fungal attacks. After harvesting, their aroma and appearance change, decomposition processes increase, and synthesis processes are reduced in intensity, which is why an optimal method of preservation is recommended [172,174]. In order to satisfy the requirements of consumers by ensuring that products are both healthy and delicious, and since mulberries are famous all over the world, they have started to be processed in different forms, so that they can be stored and consumed in the long term [172,175,176]. Due to the anthocyanin pigments responsible for their dark color, mulberry fruits are also used as color or flavor additives in the food industry for the production of healthy foods without synthetic food additives [165,176].
In recent years, the Morus plant species began to occupy an important position in the food industry, due to its health benefits (Table 4). Recently, various mulberry-based foods were developed and marketed in Asian regions [41]. As a food, in addition to being consumed fresh, but also dried, mulberry is suitable for the preparation of several foods, such as juices, syrups, wine, vinegar, brandy, jellies, marmalades or jams [27,42].
Mulberry fruits can be eaten ripe or can be found in the market in various forms as nutritional supplements, as a tonic and sedative. The only official medicinal product in the British Pharmacopoeia is Siropus mori, used mainly as an adjunct to its slightly laxative and expectorant qualities [5,102,158].
The water-soluble anthocyanin pigments responsible for the color of mulberry fruits are also used as color or flavoring additives in the food industry, a practice increasingly used to create foods that contain natural additives [165,176].
Taking into account the requirements of consumers, the nutritional value and bioactive compounds that provide a number of beneficial effects on the human body, the mulberry plant is becoming increasingly studied or even exploited for the production of food or food supplements. It is also used as a main ingredient in the production of foods, such as jams [177], lollipops and jellies [178], wines [179,180], syrups [181] and other functional beverages and foods [145,182,183,184,185,186] (Table 5).

Table 5.
Product foodstuffs, their compositions and health benefits (2016–2021).
The mulberry plant is also used in the form of food supplements. In recent years, a number of patents (Table 6) were developed based on mulberry with various functional and therapeutic applications.
Mulberry fruits possess a lower pectin content than other fruits used in the manufacture of jams. There is a patent (RO 135033 A2) that implements different processes for obtaining jams from black, white or red mulberry fruit by gelling with the addition of pectin [189]. An ethnobotanical study conducted by Li et al., 2017, on white mulberry leaf tea, showed that teas could be included in the category of functional foods, having a detoxifying effect, treating coughs and sore throats, colds, etc. [190]. Lin et al., 2020 [187], confirmed that drinks obtained from mulberry leaves are functional products that prevent diseases related to aging and Meng et al., 2020, mention that white mulberry leaf tea is used in Asian countries in order to control diabetes [104].

Table 6.
List of patents based on the therapeutic and functional applications of Morus (2017–2021).
Table 6.
List of patents based on the therapeutic and functional applications of Morus (2017–2021).
| Application No. | Species/Part | Sample Type | Results/Mechanism | Ref. |
|---|---|---|---|---|
| US 11,090,349 B2 | Morus alba L.; Morus alba var. multicaulis L.; Morus nigra; Morus australis Poir. | Raw material, dry leaves | Inhibits α-glucosidase. It has the ability to control blood glucose levels and reduce melanin production for the treatment of conditions caused by pigmentation, such as freckles, chloasma, striae gravidarum, sensitive plaque and melanoma. | [191] |
| AU 2019201188 B2 | M. alba root bark; acacia barks; Uncaria gambir, leaves; Curcuma longa L. | Mixture extract | The compound mixture, demonstrated beneficial synergistic effects with improved anti-inflammatory and anti-nociceptive efficacy, but also the attenuation of joint stiffness. | [192] |
| US 10,588,927 B2 | Mulberry (M. alba) and poria cocos peel | Mixed extract | Used either as a food product or as a pharmaceutical composition with the aim of preventing or treating degenerative neurological diseases, having the ability to improve memory and protection on neurons. | [97] |
| US 2020/0360457 A1 | M. alba and M. nigra root | Macerate extract | As an active ingredient, at least one extract from the root of the plant is used, according to the invention. It is rich in moracenine A, moracenin B, kuwanon C, wittiorumin F and mulberrofuran T, also used in cosmetic composition and a pharmaceutical or nutraceutical composition. | [193] |
| US 2020/0178585 A1 | Morus sp. fruits | Savory concentrate/seasoning, with vegetable fat. | Used as a cooking aid in the preparation of starch-rich food. | [194] |
| US 2020/0197429 A1 | Astragalus root; phlorizin; M. alba root bark; olive leaf and bitter melon. | Standardized extracts | Dietary supplement with the aim of controlling postprandial blood sugar. | [181] |
A patented formula containing M. alba leaf extract (200 mg), along with berberine and red yeast rice powder has benefits on cardiovascular prevention in patients with dyslipidemia and reduces insulin resistance by protecting subjects from the side effects of smoking [195].
Yimam et al., 2017, studied the effect of UP1306, a composition based on a patented mixture of heartwood of Acacia catechu and the root bark of Morus alba, with beneficial effect on joints, evidenced by the attenuation of symptoms associated with osteoarthritis in monosodium–iodoacetate-induced rats, reducing pain sensitivity, significantly improving the integrity of the articular cartilage matrix and causing minimal subchondral bone damage [196].
An aqueous and ethanolic extract of M. alba was developed (US7815949 B2) with estrogenic effects, and used for the treatment of climatic symptoms, osteoporosis and breast or uterine cancer [197]. Another product (US2010/0166898 A1) from the bark of Morus australis Poir, containing kiwanon H, was used as an antimicrobial agent [198]. An ethanolic extract of white mulberry branches, which has a whitening effect, is the basis of another patent (US2006/0216253 A1) [199].
7. Conclusions
All botanical parts of mulberry trees (fruits, leaves, twigs, root bark) are rich in nutrients and phytochemical compounds, exhibiting various pharmacological properties which may help to treat various chronic diseases. This review aimed to highlight the huge potential of the Morus plant and its application in the food, pharmaceutical and cosmetic industries. Among the phytochemical compounds present in Morus sp., flavonoids are the predominant compounds. In M. nigra fruits, anthocyanins are the main compounds, along with the flavonols, quercetin and kaempferol, present in various glycosylated forms. Flavonols are the main group of flavonoids in mulberry leaves, and among the phenolic acids, chlorogenic acid was predominant. Mulberry root bark is an important source of bioactive compounds, in which prenylated flavonoids such as morusin, mulberrin and kuwanon G, have various health-promoting effects. According to the bioactive phytochemical composition, Morus species display a variety of biomedical applications including antioxidants, anticancer, antidiabetic, anti-inflammatory properties, etc. Based on these findings, white and black mulberries can be used as a promising and rich source of phytochemicals, in order to develop food supplements or functional foods to prevent or ameliorate various chronic diseases. Since mulberries are perishable, various mulberry-based products were developed and patented in the food industry as functional foods. Further studies are needed to develop fruit preservation strategies, such as the development and application of edible films or coatings to increase the safety, quality and shelf life of mulberry fruits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11020152/s1, Figure S1: A. The chemical structures of the four main anthocyanins identified in black mulberry fruit. B. Chemical structure of the main flavonols and phenolic acids identified in mulberry’s fruit and leaves. C. The chemical structure of morusin. The chemical structure of phenols was generated using the molview.org program.
Author Contributions
Conceptualization, S.I.V. and A.R.M.; investigation, A.R.M., F.M., A.C.V. and A.N.V.; resources, A.V.T. and A.N.V.; writing—review and editing, A.R.M., F.M. and S.I.V.; visualization, A.V.T. and A.C.V.; supervision, S.I.V.; project administration, A.R.M. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union, through the Horizon 2020 project “NextFood”, Grant agreement No. 771738.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
S.I. Vicas, A.R. Memete, F. Miere (Groza) acknowledge the support provided by University of Oradea through the grant competition “Excellent scientific research related to the priority fields with the goal of technology transfer: INO-TRANSFER-UO”, Project number 309/21.12.2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gr, A.; Yankanchi, G.M.; Gowda, M. Chemical Composition and Pharmacological Functions and Principles of Mulberry: A Review. Int. J. Appl. Res. 2017, 3, 251–254. [Google Scholar]
- Khalifa, I.; Zhu, W.; Li, K. Polyphenols of Mulberry Fruits as Multifaceted Compounds: Compositions, Metabolism, Health Benefits, and Stability—A Structural Review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Investigation of Saccharomyces Cerevisiae in Fermented Mulberry Juice|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Investigation-Of-Saccharomyces-Cerevisiae-In-Juice-Minh-Thi/68008c51f29a627450a7785dfdc4a9e1c09bdcf2 (accessed on 16 September 2021).
- Wei, H.; Liu, S.; Liao, Y.; Ma, C.; Wang, D.; Tong, J.; Feng, J.; Yi, T.; Zhu, L. A Systematic Review of the Medicinal Potential of Mulberry in Treating Diabetes Mellitus. Am. J. Chin. Med. 2018, 46, 1743–1770. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, H.; Venkataramegowda, S. Murthy Antioxidant and Medicinal Properties of Mulberry (Morus sp.): A Review. World J. Pharm. Res. 2014, 3, 320–343. [Google Scholar]
- Turan, I.; Demir, S.; Kilinc, K.; Burnaz, N.A.; Yaman, S.O.; Akbulut, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Antiproliferative and Apoptotic Effect of Morus nigra Extract on Human Prostate Cancer Cells. Saudi Pharm. J. 2017, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Moise, A.; Marghitas, L.; Bobis, O.; Copaciu, F.; Dezmirean, D. Morus spp. Material Conservation and Characterization and Its Importance for Romanian Sericulture and GCEARS-PSP Development—A Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2018, 75, 57. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry Fruit Extract Affords Protection against Ethyl Carbamate-Induced Cytotoxicity and Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef]
- Bukhari, R.; Kour, H. Polyploidy in Agriculture: With Special Reference to Mulberry. J. Pharmacogn. Phytochem. 2019, 8, 1795–1808. [Google Scholar]
- Shafiei, D.; Prof, B. Cytogenetic Characterization of a Triploid Mulberry (Morus spp.) Cultivar Suvarna-2. Ann. Plant Sci. 2018, 7, 2156–2159. [Google Scholar] [CrossRef][Green Version]
- Lucia, K.; Grygorieva, O.; Eva, I.; Terentjeva, M.; Brindza, J. Biological Properties of Black Mulberry-Derived Food Products (Morus nigra L.). J. Berry Res. 2016, 6, 333–343. [Google Scholar] [CrossRef]
- Rafati, M.; Khorasani, N.; Moattar, F.; Shirvany, A.; Moraghebi, F.; Hosseinzadeh, S. Phytoremediation Potential of Populus Alba and Morus alba for Cadmium, Chromuim and Nickel Absorption from Polluted Soil. Int. J. Environ. Res. 2011, 5, 961–970. [Google Scholar] [CrossRef]
- Rohela, G.K.; Muttanna, P.S.; Kumar, R.; Chowdhury, S.R. Mulberry (Morus spp.): An Ideal Plant for Sustainable Development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Begum, N.; Kiran, B.R.; Purushothama, R. Mulberry Cultivation Practices and Diseases: An Overview. Int. J. Curr. Eng. Sci. Res. 2018, 5, 61–68. [Google Scholar]
- Hutasoit, R.; Ginting, S.; Sirait, J.; Tarigan, A. Productivity and Chemical Composition of Several Mulberry Species (Morus spp.) Agains Spacing Plant, and Cutting Age. Int. J. Trop. Vet. Biomed. Res. 2016, 1, 50–56. [Google Scholar] [CrossRef]
- Sudhakar, P.; Velayudhan, S.; Gowda, M.S.; Kumar, J.; Sivaprasad, V. Soil Fertility Status of Mulberry (Morus alba L.) Soils under Bivoltine Sericultural Areas of North, South and Eastern Regions of Karnataka. Int. J. Adv. Res. 2018, 6, 132–140. [Google Scholar] [CrossRef]
- CABI. Morus nigra. Available online: https://www.cabi.org/isc/datasheet/34830 (accessed on 23 June 2021).
- Heuzé, V.; Tran, G.; Lebas, F. Dud Negru (Morus nigra). Available online: https://www.feedipedia.org/node/122 (accessed on 23 June 2021).
- Hutasoit, R.; Ginting, S.P.; Tarigan, A. Effect of Cutting Interval on Yield, Nutrient Composition and Digestibility Several Species of Mulberry. In Proceedings of the International Seminar on Livestock Production and Veterinary Technology, Bali, Indonesia, 10 August 2016; Indonesian Center for Animal Research and Development (ICARD): Kota Bogor, Indonesia, 2016; pp. 476–485. [Google Scholar]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit-A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E. Chemical Composition of White (Morus Alba), Red (Morus Rubra) and Black (Morus Nigra) Mulberry Fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Mohiuddin, E.; Usmanghani, K.; Akram, M.; Asif, M.; Naveed, A.; Shah, P.; Uzair, M. Morus nigra-LA. J. Med. Plant Res. 2011, 5, 5197–5199. [Google Scholar]
- Nguyen, P.M.; Niemeyer, E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Minh, N.P.; Dao, D.T.A. Investigation of Saccharomyces Cerevisiae in Fermented Mulberry Juice. Middle East J. Sci. Res. 2013, 17, 814–820. [Google Scholar]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.d.C.A.; Freitas, K.d.C. Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef]
- Okatan, V. Phenolic Compounds and Phytochemicals in Fruits of Black Mulberry (L.) Genotypes from the Aegean Region in Turkey. Folia Hortic. 2018, 30, 93–101. [Google Scholar] [CrossRef]
- Aboulfadl, M.; Hashem, H.; Assous, M.T.M. Improvement the Nutritional Value of Especial Biscuits (Children School Meal) by Using Some Fruits and Vegetables. J. Appl. Sci. Res. 2013, 9, 5679–5691. [Google Scholar]
- Kobus-Cisowska, J.; Szczepaniak, O.; Szymanowska-Powałowska, D.; Piechocka, J.; Szulc, P.; Dziedziński, M. Antioxidant Potential of Various Solvent Extract from Morus alba Fruits and Its Major Polyphenols Composition. Ciênc. Rural 2019, 50. [Google Scholar] [CrossRef]
- Skender, A.; Kurtovic, M.; Becirspahic, D. Some Physicochemical Characteristics of Black and White Mulberry Genotypes from Bosnia and Herzegovina. Genetika 2019, 51, 1089–1101. [Google Scholar] [CrossRef]
- Bobis, O.; Dezmirean, D.S.; Mărghitaș, L.A.; Bonta, V.; Urcan, A.; Pașca, C.; Moise, A.R. Morus sp. for Revigorating Silkworm Breeding in Romania and Promoting Health Benefits of Leaves and Fruits. Sci. Pap.—Ser. B Hortic. 2018, LXI, 211–215. [Google Scholar]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Hernández, F.; Martínez, J.J. (Poly)Phenolic Compounds and Antioxidant Activity of White (Morus alba) and Black (Morus nigra) Mulberry Leaves: Their Potential for New Products Rich in Phytochemicals. J. Funct. Foods 2015, 18, 1039–1046. [Google Scholar] [CrossRef]
- Paunović, S.M.; Mašković, P.; Milinković, M. Determination of Primary Metabolites, Vitamins and Minerals in Black Mulberry (Morus nigra) Berries Depending on Altitude. Erwerbs-Obstbau 2020, 62, 355–360. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Duralija, B.; Voća, S.; Sengul, M.; Turan, M. Phytochemical Content of Some Black (Morus nigra L.) and Purple (Morus rubra L.) Mulberry Genotypes. Food Technol. Biotechnol. 2010, 48, 102–106. [Google Scholar]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of Iron Absorption: Ascorbic Acid and Other Organic Acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.-J. Chemical Properties in Fruits of Mulberry Species from the Xinjiang Province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Elmacı, Y.; Altuğ, T. Flavour Evaluation of Three Black Mulberry (Morus nigra) Cultivars Using GC/MS, Chemical and Sensory Data. J. Sci. Food Agric. 2002, 82, 632–635. [Google Scholar] [CrossRef]
- Perez, R.; Regueiro, J.; Alonso, E.; Pastrana, L.; Simal-Gandara, J. Influence of Alcoholic Fermentation Process on Antioxidant Activity and Phenolic Levels from Mulberries (Morus nigra L.). LWT—Food Sci. Technol. 2011, 44, 1793–1801. [Google Scholar] [CrossRef]
- Kadam, R.; Dhumal, N.; Khyade, V. The Mulberry, Morus alba (L.): The Medicinal Herbal Source for Human Health. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2941–2964. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Sarfraz, R.A.; Uddin, M.K. Proximate Composition and Antioxidant Potential of Leaves from Three Varieties of Mulberry (Morus sp.): A Comparative Study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, E.M.; Amorós, A.; Hernández, F.; Martínez, J.J. Physicochemical Properties of White (Morus alba) and Black (Morus nigra) Mulberry Leaves, a New Food Supplement. J. Food Nutr. Res. 2017, 5, 253–261. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Peng, Y.; He, J.; Xiao, D.; Chen, C.; Li, F.; Huang, R.; Yin, Y. Dietary Mulberry Leaf Powder Affects Growth Performance, Carcass Traits and Meat Quality in Finishing Pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1934–1945. [Google Scholar] [CrossRef]
- Fan, L.; Peng, Y.; Wu, D.; Hu, J.; Shi, X.; Yang, G.; Li, X. Morus nigra L. Leaves Improve the Meat Quality in Finishing Pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.-J.; Wang, B.; Jiang, C.-S.; Xie, B.-G.; Liu, A.-H.; Li, S.-W.; Guo, Y.-W.; Li, J.; Mao, S.-C. Rearranged Diels-Alder Adducts and Prenylated Flavonoids as Potential PTP1B Inhibitors from Morus nigra. J. Nat. Prod. 2021, 84, 2303–2311. [Google Scholar] [CrossRef]
- Zoofishan, Z.; Hohmann, J.; Hunyadi, A. Phenolic Antioxidants of Morus nigra Roots, and Antitumor Potential of Morusin. Phytochem. Rev. 2018, 17, 1031–1045. [Google Scholar] [CrossRef]
- Kay, C.; Cassidy, A. Encyclopedia of Human Nutrition: Phytochemicals: Classification and Occurrence; Academic Press: Cambridge, MA, USA, 2013; pp. 490–497. ISBN 978-0-12-375083-9. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- De Pádua Lúcio, K.; Rabelo, A.C.S.; Araújo, C.M.; Brandão, G.C.; de Souza, G.H.B.; da Silva, R.G.; de Souza, D.M.S.; Talvani, A.; Bezerra, F.S.; Cruz Calsavara, A.J.; et al. Anti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, e5048031. [Google Scholar] [CrossRef] [PubMed]
- Suttisansanee, U.; Charoenkiatkul, S.; Jongruaysup, B.; Tabtimsri, S.; Siriwan, D.; Temviriyanukul, P. Mulberry Fruit Cultivar “Chiang Mai” Prevents Beta-Amyloid Toxicity in PC12 Neuronal Cells and in a Drosophila Model of Alzheimer’s Disease. Molecules 2020, 25, 1837. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; De Bellis, L.; Miceli, A. Nutraceutical Properties of Mulberries Grown in Southern Italy (Apulia). Antioxidants 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pu, J.; Liu, D.; Yu, W.; Shao, Y.; Yang, G.; Xiang, Z.; He, N. Anti-Inflammatory and Antinociceptive Properties of Flavonoids from the Fruits of Black Mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef]
- Hooshmand, S.; Mahdinezhad, M.R.; Taraz Jamshidi, S.; Soukhtanloo, M.; Mirzavi, F.; Iranshahi, M.; Hasanpour, M.; Ghorbani, A. Morus nigra L. Extract Prolongs Survival of Rats with Hepatocellular Carcinoma. Phytother. Res. PTR 2021, 35, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bao, T.; Chen, W. Comparison of the Protective Effect of Black and White Mulberry against Ethyl Carbamate-Induced Cytotoxicity and Oxidative Damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, W.; Wang, C.; Yang, F.; Chen, X.; Zhang, Q. Effect of Cellulase and Lactobacillus Casei on Ensiling Characteristics, Chemical Composition, Antioxidant Activity, and Digestibility of Mulberry Leaf Silage. J. Dairy Sci. 2019, 102, 9919–9931. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Zhang, X.; Jin, Q.; Li, J. Phenolic Profiles, Antioxidant Activities, and Neuroprotective Properties of Mulberry (Morus Atropurpurea Roxb.) Fruit Extracts from Different Ripening Stages. J. Food Sci. 2016, 81, C2439–C2446. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef]
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of Anthocyanins from Four Kinds of Berries and Their Inhibition Activity to α-Glycosidase and Protein Tyrosine Phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221. [Google Scholar] [CrossRef]
- Przeor, M.; Flaczyk, E.; Kmiecik, D.; Buchowski, M.S.; Staniek, H.; Tomczak-Graczyk, A.; Kobus-Cisowska, J.; Gramza-Michałowska, A.; Foksowicz-Flaczyk, J. Functional Properties and Antioxidant Activity of Morus alba L. Leaves Var. Zolwinska Wielkolistna (WML-P)-The Effect of Controlled Conditioning Process. Antioxidants 2020, 9, 668. [Google Scholar] [CrossRef]
- Kim, M.; Nam, D.-G.; Ju, W.-T.; Choe, J.-S.; Choi, A.-J. Response Surface Methodology for Optimization of Process Parameters and Antioxidant Properties of Mulberry (Morus alba L.) Leaves by Extrusion. Molecules 2020, 25, 5231. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ayvaz, H.; Ruginǎ, D.; Leopold, L.; Stǎnilǎ, A.; Socaciu, C.; Tăbăran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma Inhibition by Anthocyanins Is Associated with the Reduction of Oxidative Stress Biomarkers and Changes in Mitochondrial Membrane Potential. Plant Foods Hum. Nutr. Dordr. Neth. 2017, 72, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.-I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Shebis, Y.; Iluz, D.; Kinel-Tahan, Y.; Dubinsky, Z.; Yehoshua, Y. Natural Antioxidants: Function and Sources. Food Nutr. Sci. 2013, 4, 643–649. [Google Scholar] [CrossRef]
- Ahlawat, T.; Patel, N.L.; Agnihotri, R.; Patel, C.R.; Tandel, Y. Black Mulberry (Morus nigra); Narendra Publishing House: Delhi, India, 2016; pp. 195–212. ISBN 978-93-86110-09-1. [Google Scholar]
- Liu, P.; Li, W.; Hu, Z.; Qin, X.; Liu, G. Isolation, Purification, Identification, and Stability of Anthocyanins from Lycium Ruthenicum Murr. LWT 2020, 126, 109334. [Google Scholar] [CrossRef]
- Zeng, Q.-W.; Zhang, C.; Chen, H.-Y.; Ding, T.-L.; Zhao, A.-C.; Xiang, Z.-H.; He, N.-J. Introduction trial of medicine mulberry (Morus nigra) in Chongqing. China J. Chin. Mater. Med. 2016, 41, 1450–1455. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Kostić, E.; Arsić, B.; Mitić, M.; Dimitrijević, D.; Marinkovic, E.P. Optimization of the Solid-Liquid Extraction Process of Phenolic Compounds from Mulberry Fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 629–633. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Peiretti, P.G. Identification of Polyphenolic Compounds in Edible Wild Fruits Grown in the North-West of Italy by Means of HPLC-DAD-ESI HRMS. Plant Foods Hum. Nutr. Dordr. Neth. 2020, 75, 420–426. [Google Scholar] [CrossRef]
- Mascarello, A.; Orbem Menegatti, A.C.; Calcaterra, A.; Martins, P.G.A.; Chiaradia-Delatorre, L.D.; D’Acquarica, I.; Ferrari, F.; Pau, V.; Sanna, A.; De Logu, A.; et al. Naturally Occurring Diels-Alder-Type Adducts from Morus nigra as Potent Inhibitors of Mycobacterium Tuberculosis Protein Tyrosine Phosphatase B. Eur. J. Med. Chem. 2018, 144, 277–288. [Google Scholar] [CrossRef]
- Figueredo, K.C.; Guex, C.G.; Reginato, F.Z.; da Silva, A.R.H.; Cassanego, G.B.; Lhamas, C.L.; Boligon, A.A.; Lopes, G.H.H.; de Freitas Bauermann, L. Safety Assessment of Morus nigra L. Leaves: Acute and Subacute Oral Toxicity Studies in Wistar Rats. J. Ethnopharmacol. 2018, 224, 290–296. [Google Scholar] [CrossRef]
- Dalmagro, A.; Camargo, A.; Filho, H.; Valcanaia, M.; Jesus, P.; Zeni, A. Seasonal Variation in the Antioxidant Phytocompounds Production from the Morus nigra Leaves. Ind. Crops Prod. 2018, 123, 323–330. [Google Scholar] [CrossRef]
- D’Urso, G.; Mes, J.J.; Montoro, P.; Hall, R.D.; de Vos, R.C.H. Identification of Bioactive Phytochemicals in Mulberries. Metabolites 2019, 10, 7. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Oleszek, W.; Braca, A. Quali-Quantitative Analyses of Flavonoids of Morus nigra L. and Morus alba L. (Moraceae) Fruits. J. Agric. Food Chem. 2008, 56, 3377–3380. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Niu, W.; Ding, Y.; Zhang, R.; Shang, X.; Shang, Z. Analysis and Characterisation of Anthocyanins in Mulberry Fruit. Czech J. Food Sci. 2009, 28, 117–126. [Google Scholar] [CrossRef]
- Qi, X.; Shuai, Q.; Chen, H.; Fan, L.; Zeng, Q.; He, N. Cloning and Expression Analyses of the Anthocyanin Biosynthetic Genes in Mulberry Plants. Mol. Genet. Genom. MGG 2014, 289, 783–793. [Google Scholar] [CrossRef]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ Acqua, S.; Babula, P.; Cvačka, J.; Hošek, J. Evaluation of Anti-Inflammatory Activity of Prenylated Substances Isolated from Morus alba and Morus nigra. J. Nat. Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef]
- Thabti, I.; Elfalleh, W.; Hannachi, H.; Ferchichi, A.; Campos, M.D.G. Identification and Quantification of Phenolic Acids and Flavonol Glycosides in Tunisian Morus Species by HPLC-DAD and HPLC–MS. J. Funct. Foods 2012, 1, 367–374. [Google Scholar] [CrossRef]
- Chen, H.-D.; Ding, Y.; Yang, S.-P.; Li, X.-C.; Wang, X.-J.; Zhang, H.-Y.; Ferreira, D.; Yue, J.-M. ChemInform Abstract: Morusalbanol A, a Neuro-Protective Diels-Alder Adduct with an Unprecedented Architecture from Morus alba. Tetrahedron 2012, 68, 6054–6058. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, J.; Ma, L.; Wen, D.; Chen, F.; Li, J. Identification of Polyphenols in Mulberry (Genus Morus) Cultivars by Liquid Chromatography with Time-of-Flight Mass Spectrometer. J. Food Compos. Anal. 2017, 63, 55–64. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin Composition of Different Wild and Cultivated Berry Species. LWT—Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Tassotti, M.; Del Rio, D.; Hernández, F.; Martínez, J.J.; Mena, P. (Poly)Phenolic Fingerprint and Chemometric Analysis of White (Morus alba L.) and Black (Morus nigra L.) Mulberry Leaves by Using a Non-Targeted UHPLC–MS Approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef]
- Miljkovic, V.; Nikolic, L.; Radulović, N.; Arsic, B.; Nikolić, G.; Kostic, D.; Bojanic, Z.; Zvezdanović, J. Flavonoids in Mulberry Fruit. Identification of Nonanthocyanin Phenolics in Some Mulberry Fruit Species (Morus alba L., Morus rubra L. and Morus nigra L.). Agro Food Ind. Hi-Tech 2015, 26, 38–42. [Google Scholar]
- Özgen, M.; Serce, S.; Kaya, C. Phytochemical and Antioxidant Properties of Anthocyanin-Rich Morus nigra and Morus rubra Fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical Characterization and Antioxidative Properties of Polish Variety of Morus alba L. Leaf Aqueous Extracts from the Laboratory and Pilot-Scale Processes. Agric. Sci. 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Dalmagro, A.P.; Camargo, A.; Zeni, A.L.B. Morus nigra and Its Major Phenolic, Syringic Acid, Have Antidepressant-like and Neuroprotective Effects in Mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef]
- Dalmagro, A.P.; Camargo, A.; Severo Rodrigues, A.L.; Zeni, A.L.B. Involvement of PI3K/Akt/GSK-3β Signaling Pathway in the Antidepressant-like and Neuroprotective Effects of Morus nigra and Its Major Phenolic, Syringic Acid. Chem. Biol. Interact. 2019, 314, 108843. [Google Scholar] [CrossRef]
- Kim, J.-H.; Doh, E.-J.; Lee, G. Quantitative Comparison of the Marker Compounds in Different Medicinal Parts of Morus alba L. Using High-Performance Liquid Chromatography-Diode Array Detector with Chemometric Analysis. Molecules 2020, 25, 5592. [Google Scholar] [CrossRef]
- Choi, D.W.; Cho, S.W.; Lee, S.-G.; Choi, C.Y. The Beneficial Effects of Morusin, an Isoprene Flavonoid Isolated from the Root Bark of Morus. Int. J. Mol. Sci. 2020, 21, 6541. [Google Scholar] [CrossRef]
- Chung, H.; Kim, J.; Kim, J.; Kwon, O. Acute Intake of Mulberry Leaf Aqueous Extract Affects Postprandial Glucose Response after Maltose Loading: Randomized Double-Blind Placebo-Controlled Pilot Study. J. Funct. Foods 2013, 5, 1502–1506. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Shiwaku, K.; Katsube, T.; Kitajima, K.; Anuurad, E.; Yamasaki, M.; Yamane, Y. Mulberry (Morus alba L.) Leaves and Their Major Flavonol Quercetin 3-(6-Malonylglucoside) Attenuate Atherosclerotic Lesion Development in LDL Receptor-Deficient Mice. J. Nutr. 2005, 135, 729–734. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zheng, H.; Liu, Q.; Zhang, H.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Morusin Induces Apoptosis and Autophagy via JNK, ERK and PI3K/Akt Signaling in Human Lung Carcinoma Cells. Chem. Biol. Interact. 2020, 331, 109279. [Google Scholar] [CrossRef]
- Jung, J.-W.; Park, J.-H.; Lee, Y.-G.; Seo, K.-H.; Oh, E.-J.; Lee, D.-Y.; Lim, D.-W.; Han, D.; Baek, N.-I. Three New Isoprenylated Flavonoids from the Root Bark of Morus alba. Molecules 2016, 21, 1112. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Katayanagi, M. Studies on the Constituents of the Cultivated Mulberry Tree. III. Isolation of Four New Flavones, Kuwanon A, B, C and Oxydihydromorusin from the Root Bark of Morus alba L. Chem. Pharm. Bull. 1978, 26, 1453–1458. [Google Scholar] [CrossRef]
- Yan, J.; Ruan, J.; Huang, P.; Sun, F.; Zheng, D.; Zhang, Y.; Wang, T. The Structure–Activity Relationship Review of the Main Bioactive Constituents of Morus Genus Plants. J. Nat. Med. 2020, 74, 331–340. [Google Scholar] [CrossRef]
- Ha, M.T.; Seong, S.H.; Nguyen, T.D.; Cho, W.-K.; Ah, K.J.; Ma, J.Y.; Woo, M.H.; Choi, J.S.; Min, B.S. Chalcone Derivatives from the Root Bark of Morus alba L. Act as Inhibitors of PTP1B and α-Glucosidase. Phytochemistry 2018, 155, 114–125. [Google Scholar] [CrossRef]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s Disease Activity of Compounds from the Root Bark of Morus alba L. Arch. Pharm. Res. 2017, 40, 338–349. [Google Scholar] [CrossRef]
- Choi, S.Z.; Sohn, M.W.; Go, H.S.; Ryu, J.Y.; Jeong, J.S.; Choi, S.H.; Kim, E.J.; Cho, Y.W.; Kim, S.Y. Composition Containing Mixed Extract of Mulberry and Poria Cocos Peel. U.S. Patent 10,588,927, 2020. [Google Scholar]
- Inyai, C.; Komaikul, J.; Kitisripanya, T.; Tanaka, H.; Sritularak, B.; Putalun, W. Development of a Rapid Immunochromatographic Strip Test for the Detection of Mulberroside A. Phytochem. Anal. 2015, 26, 423–427. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.H.; Jo, Y.-Y.; Kim, S.-W.; Kim, H.-B.; Kweon, H.; Ju, W.-T. Characterization of Mulberry Root Bark Extracts (Morus alba L.) Based on the Extraction Temperature and Solvent. Int. J. Ind. Entomol. 2020, 41, 36–44. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef]
- Zoofishan, Z.; Kúsz, N.; Csorba, A.; Tóth, G.; Hajagos-Tóth, J.; Kothencz, A.; Gáspár, R.; Hunyadi, A. Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules 2019, 24, 2497. [Google Scholar] [CrossRef]
- Khalid, N.; Fawad, S.A.; Ahmed, I. Antimicrobial Activity, Phytochemical Profile and Trace Minerals of Black Mulberry (Morus nigra L.) Fresh juice. Pak. J. Bot. 2011, 43, 91–96. [Google Scholar]
- Zeni, A.L.B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of Phenolic Compounds and Lipid-Lowering Effect of Morus nigra Leaves Extract. An. Acad. Bras. Ciênc. 2017, 89, 2805–2815. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Fu, Y.; Chen, Q.; Cheng, P.; Yu, X.; Sun, X.; Wu, J.; Li, W.; Zhang, Q.; et al. Flavonoids Extracted from Mulberry (Morus alba L.) Leaf Improve Skeletal Muscle Mitochondrial Function by Activating AMPK in Type 2 Diabetes. J. Ethnopharmacol. 2020, 248, 112326. [Google Scholar] [CrossRef]
- Zheng, Z.-P.; Cheng, K.-W.; Zhu, Q.; Wang, X.-C.; Lin, Z.-X.; Wang, M. Tyrosinase Inhibitory Constituents from the Roots of Morus nigra: A Structure-Activity Relationship Study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, M.; Nie, W.-J.; Yang, X.-R.; Zeng, X.-C. Effects of the Ethanol Extract of Black Mulberry (Morus nigra L.) Fruit on Experimental Atherosclerosis in Rats. J. Ethnopharmacol. 2017, 200, 228–235. [Google Scholar] [CrossRef]
- Fahimi, Z.; Jahromy, M.H. Effects of Blackberry (Morus nigra) Fruit Juice on Levodopa-Induced Dyskinesia in a Mice Model of Parkinson’s Disease. J. Exp. Pharmacol. 2018, 10, 29–35. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. IJCB 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar]
- Cavalu, S.; Damian, G. Rotational Correlation Times of 3-Carbamoyl-2,2,5,5-Tetramethyl-3-Pyrrolin-1-Yloxy Spin Label with Respect to Heme and Nonheme Proteins. Biomacromolecules 2003, 4, 1630–1635. [Google Scholar] [CrossRef]
- Seal, T.; Chaudhuri, K. Effect of Solvent Extraction System on the Antioxidant Activity of Some Selected Wild Leafy Vegetables of Meghalaya State in India. Int. Res. J. Nat. Appl. Sci. 2015, 2, 69–83. [Google Scholar]
- Araujo, C.M.; Lúcio, K.d.P.; Silva, M.E.; Isoldi, M.C.; de Souza, G.H.B.; Brandão, G.C.; Schulz, R.; Costa, D.C. Morus nigra Leaf Extract Improves Glycemic Response and Redox Profile in the Liver of Diabetic Rats. Food Funct. 2015, 6, 3490–3499. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Liu, X. Insight into the Conformational and Functional Properties of Myofibrillar Protein Modified by Mulberry Polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar] [CrossRef]
- Li, E.; Yang, S.; Zou, Y.; Cheng, W.; Li, B.; Hu, T.; Li, Q.; Wang, W.; Liao, S.; Pang, D. Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries. Molecules 2019, 24, 2329. [Google Scholar] [CrossRef]
- Pannakal, S.T.; Eilstein, J.; Prasad, A.; Ekhar, P.; Shetty, S.; Peng, Z.; Bordier, E.; Boudah, S.; Paillat, L.; Marrot, L.; et al. Comprehensive Characterization of Naturally Occurring Antioxidants from the Twigs of Mulberry (Morus alba) Using on-Line High-Performance Liquid Chromatography Coupled with Chemical Detection and High-Resolution Mass Spectrometry. Phytochem. Anal. PCA 2021. [Google Scholar] [CrossRef]
- Radojković, M.M.; Zeković, Z.P.; Dojčinović, B.P.; Stojanović, Z.S.; Cvetanović, A.D.; Manojlović, D.D. Characterization of Morus Species in Respect to Micro, Macro, and Toxic Elements. Acta Period. Technol. 2014, 45, 229–237. [Google Scholar] [CrossRef]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant Activity of Mulberry Fruit Extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Hassimotto, N.; Genovese, M.; Lajolo, F. Antioxidant Activity of Dietary Fruits, Vegetables, and Commercial Frozen Fruit Pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef]
- Turgut, N.H.; Mert, D.G.; Kara, H.; Egilmez, H.R.; Arslanbas, E.; Tepe, B.; Gungor, H.; Yilmaz, N.; Tuncel, N.B. Effect of Black Mulberry (Morus nigra) Extract Treatment on Cognitive Impairment and Oxidative Stress Status of d-Galactose-Induced Aging Mice. Pharm. Biol. 2016, 54, 1052–1064. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-Cancer Potential of Flavonoids: Recent Trends and Future Perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol Content and Antioxidant Properties of Underutilized Fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of Resveratrol on Cognitive and Memory Performance and Mood: A Meta-Analysis of 225 Patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef]
- Marinella, M.A. Indomethacin and Resveratrol as Potential Treatment Adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020, 74, e13535. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.; Linhardt, R.; Liao, S.; Wu, H.; Zou, Y. Mulberry: A Review of Bioactive Compounds and Advanced Processing Technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin Extraction from Plant Tissues: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. Available online: http://publications.iarc.fr/586 (accessed on 30 December 2021). Licence: CC BY-NC-ND3.0 IGO.
- Chang, B.-Y.; Koo, B.-S.; Kim, S.-Y. Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods 2021, 10, 1966. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional Constituents of Mulberry and Their Potential Applications in Food and Pharmaceuticals: A Review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.-I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.F.; Masih, A.P.; Alqasmi, I.; Alsulimani, A.; Khan, F.H.; Haque, S. Oxidative-Stress-Induced Cellular Toxicity and Glycoxidation of Biomolecules by Cosmetic Products under Sunlight Exposure. Antioxidants 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Bharani, S.E.R.; Asad, M.; Dhamanigi, S.S.; Chandrakala, G.K. Immunomodulatory Activity of Methanolic Extract of Morus alba Linn. (Mulberry) Leaves. Pak. J. Pharm. Sci. 2010, 23, 63–68. [Google Scholar]
- Cho, E.; Chung, E.Y.; Jang, H.-Y.; Hong, O.-Y.; Chae, H.S.; Jeong, Y.-J.; Kim, S.-Y.; Kim, B.-S.; Yoo, D.J.; Kim, J.-S.; et al. Anti-Cancer Effect of Cyanidin-3-Glucoside from Mulberry via Caspase-3 Cleavage and DNA Fragmentation in Vitro and in Vivo. Anticancer Agents Med. Chem. 2017, 17, 1519–1525. [Google Scholar] [CrossRef]
- Chon, S.-U.; Kim, Y.-M.; Park, Y.-J.; Heo, B.-G.; Park, Y.-S.; Gorinstein, S. Antioxidant and Antiproliferative Effects of Methanol Extracts from Raw and Fermented Parts of Mulberry Plant (Morus alba L.). Eur. Food Res. Technol. 2009, 230, 231–237. [Google Scholar] [CrossRef]
- Shu, Y.-H.; Yuan, H.-H.; Xu, M.-T.; Hong, Y.-T.; Gao, C.-C.; Wu, Z.-P.; Han, H.-T.; Sun, X.; Gao, R.-L.; Yang, S.-F.; et al. A Novel Diels-Alder Adduct of Mulberry Leaves Exerts Anticancer Effect through Autophagy-Mediated Cell Death. Acta Pharmacol. Sin. 2021, 42, 780–790. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Dong, L.; Liu, C.; Sun, Z.; Gao, L.; Wang, X. Morusin Suppresses Breast Cancer Cell Growth in Vitro and in Vivo through C/EBPβ and PPARγ Mediated Lipoapoptosis. J. Exp. Clin. Cancer Res. CR 2015, 34, 137. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Yang, L.; Dong, L.; Lin, C.; Zhang, J.; Lin, P.; Wang, X. Morusin Inhibits Human Cervical Cancer Stem Cell Growth and Migration through Attenuation of NF-ΚB Activity and Apoptosis Induction. Mol. Cell. Biochem. 2013, 379, 7–18. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Sun, Z.; Li, H.; Wang, Q.; Yi, C.; Wang, X. Morusin Shows Potent Antitumor Activity for Human Hepatocellular Carcinoma in Vitro and in Vivo through Apoptosis Induction and Angiogenesis Inhibition. Drug Des. Dev. Ther. 2017, 11, 1789–1802. [Google Scholar] [CrossRef]
- Chahwala, V.; Arora, R. Cardiovascular Manifestations of Insulin Resistance. Am. J. Ther. 2009, 16, e14–e28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, W.; Zhang, X.; Zhao, H.; Wang, Y. Antioxidant Activity and the Potential for Cholesterol-Lowering of Phenolic Extract of Morus alba, Morus Multicaulis, and Morus Laevigata Leaves from Yunnan (China). J. Food Biochem. 2017, 41, e12339. [Google Scholar] [CrossRef]
- Ghorbani, A.; Hooshmand, S. Protective Effects of Morus nigra and Its Phytochemicals against Hepatotoxicity: A Review of Preclinical Studies. Pharmacology 2021, 106, 233–243. [Google Scholar] [CrossRef]
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry Leaf Alleviates Streptozotocin-Induced Diabetic Rats by Attenuating NEFA Signaling and Modulating Intestinal Microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.T.; Calderon, I.M.P.; Sinzato, S.; Campos, K.E.; Rudge, M.V.C.; Damasceno, D.C. Effect of Morus nigra Aqueous Extract Treatment on the Maternal-Fetal Outcome, Oxidative Stress Status and Lipid Profile of Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2011, 138, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zheng, S.; Zhang, C.; Zhao, C.; He, X.; Xu, W.; Huang, K. Mulberry Leaf Tea Alleviates Diabetic Nephropathy by Inhibiting PKC Signaling and Modulating Intestinal Flora. J. Funct. Foods 2018, 46, 118–127. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Tang, M.; Zhang, S.; Liu, L.; Gao, L.; Ma, S.; Kong, M.; Yao, Q.; Feng, F.; et al. Analysis of Mulberry Leaf Components in the Treatment of Diabetes Using Network Pharmacology. Eur. J. Pharmacol. 2018, 833, 50–62. [Google Scholar] [CrossRef]
- Hago, S.; Mahrous, E.A.; Moawad, M.; Abdel-Wahab, S.; Abdel-Sattar, E. Evaluation of Antidiabetic Activity of Morus nigra L. and Bauhinia variegata L. Leaves as Egyptian Remedies Used for the Treatment of Diabetes. Nat. Prod. Res. 2021, 35, 829–835. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic Effects of Morus alba Fruit Polysaccharides on High-Fat Diet- and Streptozotocin-Induced Type 2 Diabetes in Rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef]
- Ha, M.T.; Shrestha, S.; Tran, T.H.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Min, B.S. Inhibition of PTP1B by Farnesylated 2-Arylbenzofurans Isolated from Morus alba Root Bark: Unraveling the Mechanism of Inhibition Based on in Vitro and in Silico Studies. Arch. Pharm. Res. 2020, 43, 961–975. [Google Scholar] [CrossRef]
- Dhillon, N.K.; Ahuja, S. Plants as Immunomodulators: A Review. J. Pharmacogn. Phytochem. 2019, 8, 364–368. [Google Scholar]
- Labro, M.-T. Immunomodulatory Effects of Antimicrobial Agents. Part I: Antibacterial and Antiviral Agents. Expert Rev. Anti Infect. Ther. 2012, 10, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Rao, A. Probiotics: A Potential Immunomodulator in COVID-19 Infection Management. Nutr. Res. 2021, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Zinellu, A.; Eid, A.H.; Pintus, G. Therapeutic Potential of Resveratrol in COVID-19-Associated Hemostatic Disorders. Molecules 2021, 26, 856. [Google Scholar] [CrossRef] [PubMed]
- Gyebi, G.A.; Adegunloye, A.P.; Ibrahim, I.M.; Ogunyemi, O.M.; Afolabi, S.O.; Ogunro, O.B. Prevention of SARS-CoV-2 Cell Entry: Insight from in Silico Interaction of Drug-like Alkaloids with Spike Glycoprotein, Human ACE2, and TMPRSS2. J. Biomol. Struct. Dyn. 2020, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Poiroux, G.; Barre, A.; Simplicien, M.; Pelofy, S.; Segui, B.; Van Damme, E.J.M.; Rougé, P.; Benoist, H. Morniga-G, a T/Tn-Specific Lectin, Induces Leukemic Cell Death via Caspase and DR5 Receptor-Dependent Pathways. Int. J. Mol. Sci. 2019, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Hause, B.; Hu, J.; Barre, A.; Rougé, P.; Proost, P.; Peumans, W.J. Two Distinct Jacalin-Related Lectins with a Different Specificity and Subcellular Location Are Major Vegetative Storage Proteins in the Bark of the Black Mulberry Tree. Plant Physiol. 2002, 130, 757–769. [Google Scholar] [CrossRef]
- Youssef, F.S.; Labib, R.M.; Eldahshan, O.A.; Singab, A.N.B. Synergistic Hepatoprotective and Antioxidant Effect of Artichoke, Fig, Blackberry Herbal Mixture on HepG2 Cells and Their Metabolic Profiling Using NMR Coupled with Chemometrics. Chem. Biodivers. 2017, 14, e1700206. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated Alkaloids Isolated from Mulberry Trees (Morusalba L.) and Silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef]
- Vochyánová, Z.; Pokorná, M.; Rotrekl, D.; Smékal, V.; Fictum, P.; Suchý, P.; Gajdziok, J.; Šmejkal, K.; Hošek, J. Prenylated Flavonoid Morusin Protects against TNBS-Induced Colitis in Rats. PLoS ONE 2017, 12, e0182464. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-Z.; Li, N.; Tuo, Z.-D.; Li, J.-L.; Xing, S.-S.; Li, B.-B.; Zhang, L.; Lee, H.-S.; Chen, J.-G.; Cui, L. Effects of Morus Root Bark Extract and Active Constituents on Blood Lipids in Hyperlipidemia Rats. J. Ethnopharmacol. 2016, 180, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Chen, J.; Yuan, Y.; Chen, J.; Sayed, M.D.M.; Luo, L.; Zhu, Y.; Li, S.; Bu, S. Cortex Mori Radicis Extract Attenuates Myocardial Damages in Diabetic Rats by Regulating ERS. Biomed. Pharmacother. 2017, 90, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.T.; Ozan, G.; Dundar, S.; Bozoglan, A.; Karaman, T.; Dildes, N.; Kaya, C.A.; Kaya, N.; Erdem, E. The Effects of Morus nigra on the Alveolar Bone Loss in Experimentally-Induced Periodontitis. Eur. Oral Res. 2019, 53, 99–105. [Google Scholar] [CrossRef]
- Gu, P.S.; Moon, M.; Choi, J.G.; Oh, M.S. Mulberry Fruit Ameliorates Parkinson’s-Disease-Related Pathology by Reducing α-Synuclein and Ubiquitin Levels in a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine/Probenecid Model. J. Nutr. Biochem. 2017, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Nesello, L.A.N.; Beleza, M.L.M.L.; Mariot, M.; Mariano, L.N.B.; de Souza, P.; Campos, A.; Cechinel-Filho, V.; Andrade, S.F.; da Silva, L.M. Gastroprotective Value of Berries: Evidences from Methanolic Extracts of Morus nigra and Rubus Niveus Fruits. Gastroenterol. Res. Pract. 2017, 2017, 7089697. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2017, 23, 4. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of Black Mulberry and Grape Seeds: Chemical Characterization and Bioactive Potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef]
- Costa, J.P.L.; Brito, H.O.; Galvão-Moreira, L.V.; Brito, L.G.O.; Costa-Paiva, L.; Brito, L.M.O. Randomized Double-Blind Placebo-Controlled Trial of the Effect of Morus nigra L. (Black Mulberry) Leaf Powder on Symptoms and Quality of Life among Climacteric Women. Int. J. Gynaecol. Obstet. 2020, 148, 243–252. [Google Scholar] [CrossRef]
- Li, H.X.; Park, J.U.; Su, X.D.; Kim, K.T.; Kang, J.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Identification of Anti-Melanogenesis Constituents from Morus alba L. Leaves. Molecules 2018, 23, 2559. [Google Scholar] [CrossRef]
- Xu, L.; Huang, T.; Huang, C.; Wu, C.; Jia, A.; Hu, X. Chiral Separation, Absolute Configuration, and Bioactivity of Two Pairs of Flavonoid Enantiomers from Morus nigra. Phytochemistry 2019, 163, 33–37. [Google Scholar] [CrossRef]
- Yao, J.; He, H.; Xue, J.; Wang, J.; Jin, H.; Wu, J.; Hu, J.; Wang, R.; Kuchta, K. Mori Ramulus (Chin.Ph.)-the Dried Twigs of Morus alba L./Part 1: Discovery of Two Novel Coumarin Glycosides from the Anti-Hyperuricemic Ethanol Extract. Molecules 2019, 24, 629. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Paudel, K.R.; Gong, D.-S.; Oak, M.-H. Vascular Protection by Ethanol Extract of Morus alba Root Bark: Endothelium-Dependent Relaxation of Rat Aorta and Decrease of Smooth Muscle Cell Migration and Proliferation. Evid.-Based Complement. Altern. Med. ECAM 2018, 2018, 7905763. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and Ozone Treatments Affects Postharvest Quality of Black Mulberry (Morus nigra) Fruits. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Jiang, Q.; Xu, Y.; Zhou, X.; Zhang, N.; Sun, D.; Li, H.; Chen, L. Chemical Components of the Fruits of Morus nigra Linn.: Methyl Caffeate as a Potential Anticancer Agent by Targeting 3-Phosphoglycerate Dehydrogenase. J. Agric. Food Chem. 2021, 69, 12433–12444. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, J.; Ma, L.; Cai, J.; Li, J. Comparison of Polyphenol Profile and Inhibitory Activities Against Oxidation and α-Glucosidase in Mulberry (Genus Morus) Cultivars from China. J. Food Sci. 2015, 80, C2440–C2451. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Demir, S.; Haznedaroglu, M.Z.; Yesil-Celiktas, O. Optimization of Microwave Assisted Extraction of Morus nigra L. Fruits Maximizing Tyrosinase Inhibitory Activity with Isolation of Bioactive Constituents. Food Chem. 2018, 248, 183–191. [Google Scholar] [CrossRef]
- Vega, E.N.; Molina, A.K.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Stojković, D.; Soković, M.; et al. Anthocyanins from Rubus fruticosus L. and Morus nigra L. Applied as Food Colorants: A Natural Alternative. Plants 2021, 10, 1181. [Google Scholar] [CrossRef]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.D.; Beekwilder, J.; Capanoglu, E. Processing Black Mulberry into Jam: Effects on Antioxidant Potential and in Vitro Bioaccessibility. J. Sci. Food Agric. 2017, 97, 3106–3113. [Google Scholar] [CrossRef]
- Kurt, A.; Bursa, K.; Toker, O.S. Gummy Candies Production with Natural Sugar Source: Effect of Molasses Types and Gelatin Ratios. Food Sci. Technol. Int. Cienc. Tecnol. Los Aliment. Int. 2021, 1082013221993566. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Xiao, L.; Apaliya, M.T. Statistical Interpretation of Chromatic Indicators in Correlation to Phytochemical Profile of a Sulfur Dioxide-Free Mulberry (Morus nigra) Wine Submitted to Non-Thermal Maturation Processes. Food Chem. 2018, 239, 470–477. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Xiao, L.; Tahir, H.E. Aroma Profile and Sensory Characteristics of a Sulfur Dioxide-Free Mulberry (Morus nigra) Wine Subjected to Non-Thermal Accelerating Aging Techniques. Food Chem. 2017, 232, 89–97. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ergün, S.; Yigit, M.; Yilmaz, E.; Ahmadifar, E. Dietary Supplementation of Black Mulberry (Morus nigra) Syrup Improves the Growth Performance, Innate Immune Response, Antioxidant Status, Gene Expression Responses, and Disease Resistance of Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 107, 211–217. [Google Scholar] [CrossRef]
- Kuwahara, M.; Kim, H.-K.; Ozaki, M.; Nanba, T.; Chijiki, H.; Fukazawa, M.; Okubo, J.; Mineshita, Y.; Takahashi, M.; Shibata, S. Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults. Nutrients 2020, 12, 1580. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Chao, Y.; Chen, Q.; Cheng, P.; Yu, X.; Kuai, M.; Wu, J.; Li, W.; Zhang, Q.; et al. IRS1/PI3K/AKT Pathway Signal Involved in the Regulation of Glycolipid Metabolic Abnormalities by Mulberry (Morus alba L.) Leaf Extracts in 3T3-L1 Adipocytes. Chin. Med. 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Miłek, M.; Sidor, E.; Kapusta, I.; Litwińczuk, W.; Puchalski, C.; Dżugan, M. The Effect of Adding the Leaves and Fruits of Morus alba to Rape Honey on Its Antioxidant Properties, Polyphenolic Profile, and Amylase Activity. Molecules 2019, 25, 84. [Google Scholar] [CrossRef]
- Tubaş, F.; Per, S.; Taşdemir, A.; Bayram, A.K.; Yıldırım, M.; Uzun, A.; Saraymen, R.; Gümüş, H.; Elmalı, F.; Per, H. Effects of Cornus mas L. and Morus rubra L. Extracts on Penicillin-Induced Epileptiform Activity: An Electrophysiological and Biochemical Study. Acta Neurobiol. Exp. 2017, 77, 45–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, Ş.; Özkal, B.; Özçelik, B. Fortification of Dark Chocolate with Spray Dried Black Mulberry (Morus nigra) Waste Extract Encapsulated in Chitosan-Coated Liposomes and Bioaccessability Studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Wu, C.-J.; Kuo, P.-C.; Chen, W.-Y.; Tzen, J.T.C. Quercetin 3-O-Malonylglucoside in the Leaves of Mulberry (Morus alba) Is a Functional Analog of Ghrelin. J. Food Biochem. 2020, 44, e13379. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Pannangpetch, P.; Kukongviriyapan, V.; Prawan, A.; Kukongviriyapan, U.; Itharat, A. Mulberry Leaf Extract Stimulates Glucose Uptake and GLUT4 Translocation in Rat Adipocytes. Am. J. Chin. Med. 2012, 40, 163–175. [Google Scholar] [CrossRef]
- Baston, O.; Pricop, E.M. Gem Din Dude (Morus) Şi Procedeu de Fabricaţie. 2021. Available online: https://inovaliment.ro/gem-din-dude-morus-si-procedeu-de-fabricatie/ (accessed on 3 December 2021).
- Li, D.; Zheng, X.; Duan, L.; Deng, S.; Ye, W.; Wang, A.; Xing, F.-W. Ethnobotanical Survey of Herbal Tea Plants from the Traditional Markets in Chaoshan, China. J. Ethnopharmacol. 2017, 205, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zou, Y. Plant Extract Obtained from Morus Plant Leaves, Compositions Containing Same, Method of Extraction and Uses Thereof. U.S. Patent 11,090,349, 17 August 2021. [Google Scholar]
- Brownell, L.A.; Chu, M.; Hong, M.-F.; Hyun, E.-J.; Jia, Q.; Jiao, P.; Kim, H.-J.; Kim, M.-R.; Kim, T.-W.; Lee, B.-S.; et al. Compositions and Methods for Joint Health. U.S. Patent 11,123,393, 21 September 2020. [Google Scholar]
- Duriot, L.C.; Salwinski, A.B.; Rangoni, L.I.H. Root Extracts from Plants of the Morus Genus and Uses of Same. U.S. Patent Application 16/753,912, 19 November 2020. [Google Scholar]
- Mellema, M.; Seuen-Ten-Hoorn, J.W.; Verhoef, P. Savoury Concentrate with Mulberry Fruit Extract. U.S. Patent Application 16/309,273, 11 June 2020. [Google Scholar]
- Grassi, D.; Necozione, S.; Desideri, G.; Abballe, S.; Mai, F.; De Feo, M.; Carducci, A.; Ferri, C. Acute and Long Term Effects of a Nutraceutical Combination on Lipid Profile, Glucose Metabolism and Vascular Function in Patients with Dyslipidaemia with and Without Cigarette Smoking. High Blood Press. Cardiovasc. Prev. 2021, 28, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Lee, Y.-C.; Wright, L.; Jiao, P.; Horm, T.; Hong, M.; Brownell, L.; Jia, Q. A Botanical Composition Mitigates Cartilage Degradations and Pain Sensitivity in Osteoarthritis Disease Model. J. Med. Food 2017, 20, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I. Estrogenic Extracts of Morus alba and Uses Thereof. U.S. Patent 7,815,949, 19 October 2010. [Google Scholar]
- Ku, Y.-L.; Liang, S.-T.; Lin, Y.-H.; Chen, L.-H.; Kuo, Y.-Y. Anti-Bacterial Use of Extract from Morus Australis Poir. and Compound Kuwanon h. U.S. Patent 8,071,142, 6 December 2010. [Google Scholar]
- Kim, J.-H.; Wang, M.; Simon, J.; Kim, A. Whitening Cosmetics Containing Morus alba Extracts. U.S. Patent Application 11/347,884, 28 September 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).