Phytochemical Profile, Safety and Efficacy of a Herbal Mixture Used for Contraception by Traditional Health Practitioners in Ngaka Modiri Molema District Municipality, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Voucher Specimens
2.2. Preparation of the Herbal Mixture
2.3. Phytochemical Profile of the Herbal Mixture
2.4. In Vitro Cytotoxicity Test Based on 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assay
2.5. Acute Oral Toxicity of Herbal Mixture
2.6. In Vivo Contraceptive Efficacy of Herbal Mixture
2.7. Ethical Considerations
2.8. Data Analysis
- d = 0.2 represented small effect and no practically significant difference;
- d = 0.5 represented medium effect and practically visible difference;
- d = 0.8 represented large effect and practically significant difference.
3. Results
3.1. Phytochemical Profile of the Herbal Mixture
3.2. Cytotoxicity of the Herbal Mixture
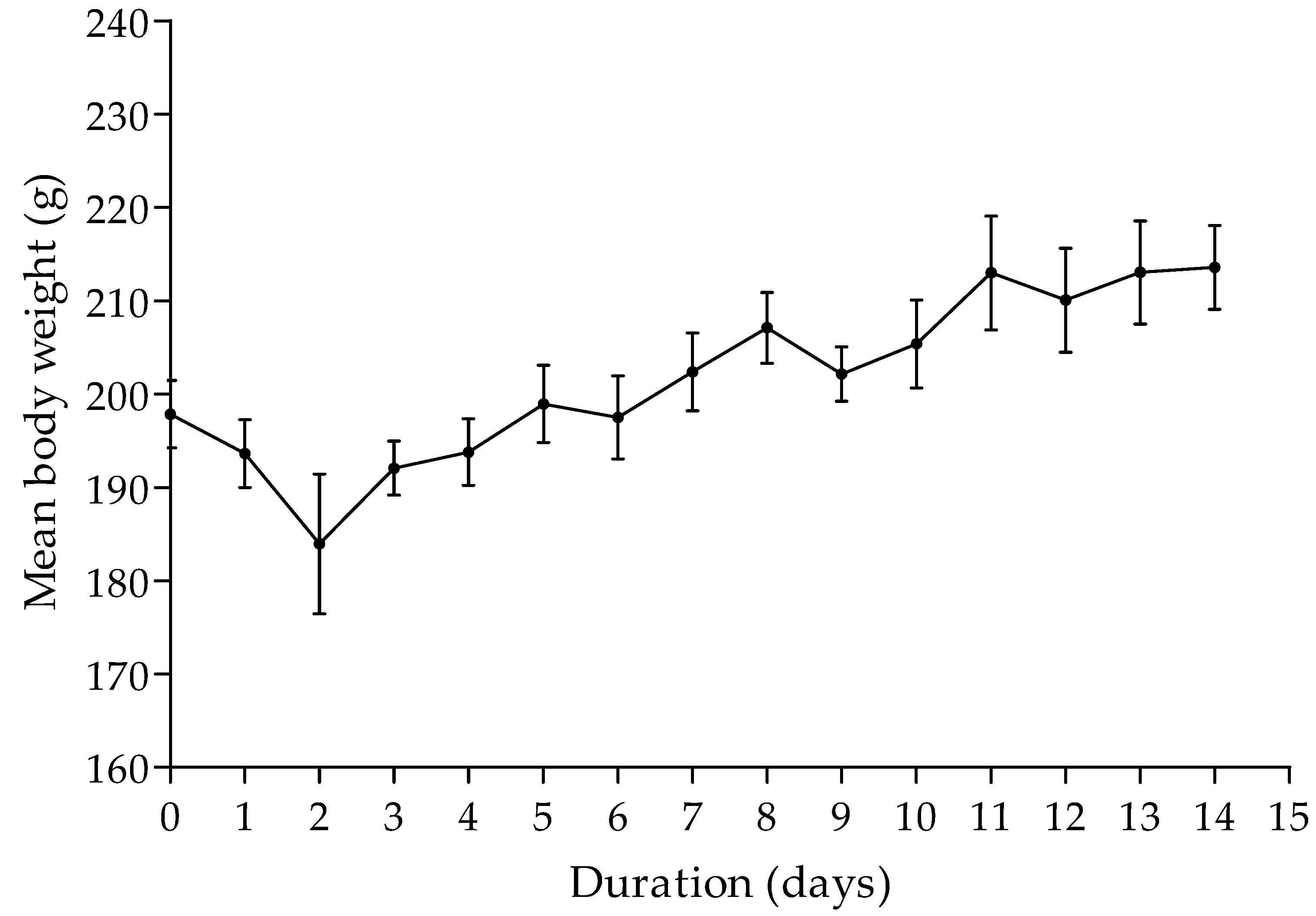
3.3. Acute Oral Toxicity of the Herbal Mixture
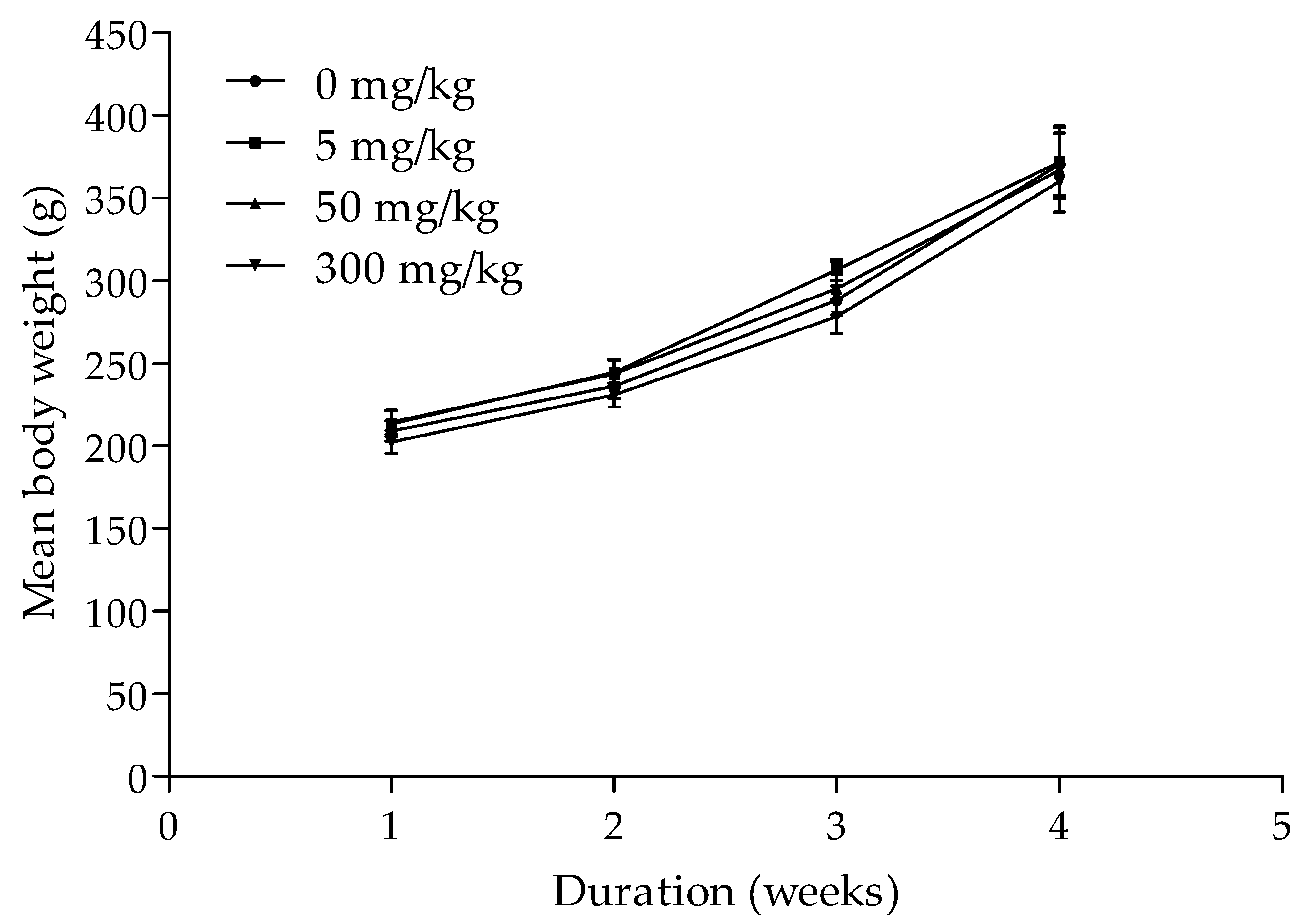
3.4. Contraceptive Efficacy of the Herbal Mixture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keshri, G.; Lakshmi, V.; Singh, M.M. Postcoital contraceptive activity of some indigenous plants in rats. Contraception 1998, 57, 357–360. [Google Scholar] [CrossRef]
- Abdillahi, H.S.; Van Staden, J. South African plants and male reproductive healthcare: Conception and contraception. J. Ethnopharmacol. 2012, 143, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.K.; Mishra, M.R.; Mishra, A.; Panda, A.K.; Behera, R.K.; Jha, S.; Choudhury, S. A comprehensive review of plants used as contraceptives. Int. J. Pharm. Sci. Res. 2013, 4, 148–155. [Google Scholar]
- Verma, S.; Yadav, A. Rising trends towards the development of oral herbal male contraceptive: An insight review. Future J. Pharm. Sci. 2021, 7, 23. [Google Scholar] [CrossRef]
- Zaman, W.; Ahmad, M.; Zafar, M.; Amina, H.; Ullah, F.; Bahadur, S.; Ayaz, A.; Saqib, S.; Begum, N.; Jahan, S. The quest for some novel antifertility herbals used as male contraceptives in district Shangla, Pakistan. Acta Ecol. Sin. 2020, 40, 102–112. [Google Scholar] [CrossRef]
- Jain, S.; Choudhary, G.P.; Jain, D.K. Medicinal plants with potential anti-fertility activity: A review. Int. J. Green Pharm. 2015, 9, 223–228. [Google Scholar]
- Moroole, M.A.; Materechera, S.A.; Otang-Mbeng, W.; Aremu, A.O. Medicinal plants used for contraception in South Africa: A review. J. Ethnopharmacol. 2019, 235, 19–27. [Google Scholar] [CrossRef]
- Shehab, N.G.; Abu-Gharbieh, E. Phenolic profiling and evaluation of contraceptive effect of the ethanolic extract of Salsola imbricata Forssk. in male albino rats. Evid.-Based Complementary Altern. Med. 2014, 2014, 695291. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Shirahatti, P.S.; Ramu, R.; Prasad, N. Azadirachta indica A. Juss (neem) as a contraceptive: An evidence-based review on its pharmacological efficiency. Phytomedicine 2021, 88, 153596. [Google Scholar] [CrossRef]
- Moroole, M.A.; Materechera, S.A.; Otang-Mbeng, W.; Aremu, A.O. African indigenous contraception: A review. Afr. J. Reprod. Health 2020, 24, 173–184. [Google Scholar]
- Keele, J.J.; Forste, R.; Flake, D.F. Hearing native voices: Contraceptive use in Matemwe village, East Africa. Afr. J. Reprod. Health 2005, 9, 32–41. [Google Scholar] [CrossRef]
- Bledsoe, C.H.; Hill, A.G.; d’Alessandro, U.; Langerock, P. Constructing natural fertility: The use of Western contraceptive technologies in rural Gambia. Popul. Dev. Rev. 1994, 20, 81–113. [Google Scholar] [CrossRef]
- Jinadu, M.K.; Olusi, S.; Ajuwon, B. Traditional fertility regulation among the Yoruba of Southwestern Nigeria: I. A study of prevalence, attitudes, practice and methods. Afr. J. Reprod. Health 1997, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jaravaza, D.C. Traditional contraceptives and indigenous knowledge systems in Mutasa District of Manicaland Province, Zimbabwe. Int. J. Acad. Res. Progress. Educ. Dev. 2013, 2, 29–35. [Google Scholar]
- Mothiba, T.; Lebese, R.; Davhana-Maselesele, M. Indigenous family planning practices in Capricorn district of Limpopo province, South Africa. Afr. J. Phys. Health Educ. Recreat. Danc. 2012, 18, 228–239. [Google Scholar]
- Sewani-Rusike, C.R. Antifertility effects of Pouzolzia mixta in female wistar rats. Afr. J. Tradit. Complementary Altern. Med. 2013, 10, 526–532. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Van Staden, J. Smokescreens and mirrors in safety and quality of herbal medicines: A case of commercialized herbal preparations. S. Afr. J. Bot. 2012, 82, 4–10. [Google Scholar] [CrossRef][Green Version]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Moyo, M.; Aremu, A.O.; van Staden, J. Ethnopharmacology in Sub-Sahara Africa: Current Trends and Future Perspectives. In Ethnopharmacology; Heinrich, M., Jäger, A.K., Eds.; Wiley Blackwell: West Sussex, UK, 2015; pp. 263–278. [Google Scholar]
- Heinrich, M.; Appendino, G.; Efferth, T.; Fürst, R.; Izzo, A.A.; Kayser, O.; Pezzuto, J.M.; Viljoen, A. Best practice in research—Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 2020, 246, 112230. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Cordell, G.A. Phytochemistry and traditional medicine—The revolution continues. Phytochem. Lett. 2014, 10, xxviii–xl. [Google Scholar] [CrossRef]
- Moroole, M.A.; Materechera, S.A.; Otang-Mbeng, W.; Aremu, A.O. Practices, taboos and techniques of indigenous contraception among Batswana traditional healers in Ngaka Modiri Molema district, South Africa. Afr. J. Phys. Act. Health Sci. 2020, 26, 427–437. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Harmonized integrated hazard classification system for human health and environmental effects of chemical substances as endorsed by the 28th Joint Meeting of the Chemicals Committee and the Working Party on Chemicals in November 1998, Part 2, p.11. Available online: http://webnet1.oecd.org/oecd/pages/home/displaygeneral/0,3380,EN-documents-521-14-no-24-no-0,FF.html (accessed on 21 January 2020).
- Patnaik, R.; Padhy, R.N. Probit analysis of comparative assays on toxicities of lead chloride and lead acetate to in vitro cultured human umbilical cord blood lymphocytes. Interdiscip. Toxicol. 2015, 8, 35–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive substances of plant origin. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 967, pp. 967–1008. [Google Scholar]
- Botelho, G.; Canas, S.; Lameiras, J. Development of phenolic compounds encapsulation techniques as a major challenge for food industry and for health and nutrition fields. In Nutrient Delivery; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 535–586. [Google Scholar]
- Zaneveld, L.J.D.; Kaminski, J.M.; Waller, D.P.; Quigg, J.; Anderson, R.A.; Mack, S.R.; Bauer, L. Aryl 4-guanidinobenzoates: Potential vaginal contraceptives. In Female Contraception; Springer: Berlin/Heidelberg, Germany, 1988; pp. 286–292. [Google Scholar]
- Zatuchni, G.; Osborn, C. Gossypol: A possible male antifertility agent. Report of a workshop. Res. Front. Fertil. Regul. 1981, 1, 1–15. [Google Scholar]
- Mamabolo, M.P.; Muganza, F.M.; Olivier, M.T.; Olaokun, O.O.; Nemutavhanani, L.D. Evaluation of antigonorrhea activity and cytotoxicity of Helichrysum caespititium (DC) Harv. whole plant extracts. Biol. Med. 2018, 10, 422. [Google Scholar] [CrossRef]
- Ngulde, S.I.; Sandabe, U.K.; Abounader, R.; Dawson, T.K.; Zhang, Y.; Iliya, I.; Hussaini, I.M. Ethanol extract of Securidaca longipedunculata induces apoptosis in brain tumor (U87) cells. BioMed Res. Int. 2019, 2019, 9826590. [Google Scholar] [CrossRef]
- Halder, B.; Singh, S.; Thakur, S.S. Withania somnifera root extract has potent cytotoxic effect against human malignant melanoma cells. PLoS ONE 2015, 10, e0137498. [Google Scholar]
- Etuk, E.U.; Adebiyi, R.A.; Elsa, A.T.; Agaie, B.M. Acute and subchronic (28-day) oral toxicity studies of the aqueous root extract of Securidaca longepedunculata Fresen (Polygalaceae) in mice. Int. J. Pharmacol. 2006, 2, 421–425. [Google Scholar]
- Houghton, P.J.; Howes, M.J.; Lee, C.C.; Steventon, G. Uses and abuses of in vitro tests in ethnopharmacology: Visualizing an elephant. J. Ethnopharmacol. 2007, 110, 391–400. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complementary Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Vambe, M.; Aremu, A.O.; Chukwujekwu, J.C.; Finnie, J.F.; Van Staden, J. Antibacterial screening, synergy studies and phenolic content of seven South African medicinal plants against drug-sensitive and -resistant microbial strains. S. Afr. J. Bot. 2018, 114, 250–259. [Google Scholar] [CrossRef]
| Plant (Family) | Voucher Specimen | Local Name | Parts Used | Preparation, Dosage and Administration of Herbal Mixture |
|---|---|---|---|---|
| Bulbine frutescens (L). Willd. (Xanthorrhoeaceae) | Moroole M.A 2 | Makgabenyane | Roots | Dried plant parts of B. frutescens, H. caespititium and T. trifidum were cut into small pieces, mixed and crushed into powder. Thereafter, 100 g of the herbal mixture was added to 2 l of water, heated until boiling point and allowed to cool. For women, aliquot of 250 mL daily drank once a day, three days before or after sex. |
| Helichrysum caespititium (DC.) Harv. (Compositae) | Moroole M.A 1 | Phate ya Ngaka | Leaves | |
| Teucrium trifidum Retz. (Lamiaceae) | Moroole M.A 4 | Setlhokotlhoko | Leaves |
| Peak | Compound | Molecular Formula * | Molar Weight (G/Mol) * | Retention Time (S) | Area (%) |
|---|---|---|---|---|---|
| 1 | 2,3-Butanedione | C4H6O2 | 86.09 | 130.9 | 0.0904 |
| 2 | Butanoic acid, methyl ester | C5H10O2 | 102.131 | 136.5 | 0.0184 |
| 3 | Acetic acid | C2H4O2 | 60.052 | 314.7 | 5.9440 |
| 4 | Unknown 1 | - | - | 316.3 | 2.8014 |
| 5 | 2-Propanone, 1-hydroxy- | C3H6O2 | 74.078 | 631.9 | 0.8804 |
| 6 | Unknown 2 | - | - | 669.9 | 7.4327 |
| 7 | Unknown 3 | - | - | 674.4 | 19.942 |
| 8 | 2,3 Butanediol | C4H10O2 | 90.122 | 679.6 | 44.660 |
| 9 | Unknown 4 | - | - | 680.2 | 1.7356 |
| 10 | Pyrrrolidin-1-acetic acid | - | - | 695.1 | 0.5558 |
| 11 | Unknown 5 | - | - | 849.3 | 0.8718 |
| 12 | 2,5-Dimethyl-4-hydroxy-3(2H)-Furanone | C6H8O3 | 128.13 | 1003.6 | 1.5595 |
| 13 | Unknown 6 | - | - | 1079.4 | 2.3000 |
| 14 | Phenol, 4-ethyl- | C8H10O | 122.164 | 1163.4 | 0.2300 |
| 15 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.17 | 1219.1 | 2.6723 |
| 16 | Glycerin | C3H8O3 | 92.09 | 1232.9 | 4.8424 |
| 17 | Unknown 7 | - | - | 1298.1 | 0,4270 |
| 18 | Benzaldehyde, 3-methyl- | C8H8O | 120.15 | 1308.2 | 1.1407 |
| 19 | Unknown 8 | - | - | 1549.8 | 0.3190 |
| 20 | Unknown 9 | - | - | 1584 | 0.97792 |
| 21 | Benzeneethanol, 4-hydroxy- | C8H10O2 | 138.163 | 1725.2 | 0.0030054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroole, M.A.; Materechera, S.A.; Otang-Mbeng, W.; Hayeshi, R.; Bester, C.; Aremu, A.O. Phytochemical Profile, Safety and Efficacy of a Herbal Mixture Used for Contraception by Traditional Health Practitioners in Ngaka Modiri Molema District Municipality, South Africa. Plants 2022, 11, 193. https://doi.org/10.3390/plants11020193
Moroole MA, Materechera SA, Otang-Mbeng W, Hayeshi R, Bester C, Aremu AO. Phytochemical Profile, Safety and Efficacy of a Herbal Mixture Used for Contraception by Traditional Health Practitioners in Ngaka Modiri Molema District Municipality, South Africa. Plants. 2022; 11(2):193. https://doi.org/10.3390/plants11020193
Chicago/Turabian StyleMoroole, Molelekwa Arthur, Simeon Albert Materechera, Wilfred Otang-Mbeng, Rose Hayeshi, Cor Bester, and Adeyemi Oladapo Aremu. 2022. "Phytochemical Profile, Safety and Efficacy of a Herbal Mixture Used for Contraception by Traditional Health Practitioners in Ngaka Modiri Molema District Municipality, South Africa" Plants 11, no. 2: 193. https://doi.org/10.3390/plants11020193
APA StyleMoroole, M. A., Materechera, S. A., Otang-Mbeng, W., Hayeshi, R., Bester, C., & Aremu, A. O. (2022). Phytochemical Profile, Safety and Efficacy of a Herbal Mixture Used for Contraception by Traditional Health Practitioners in Ngaka Modiri Molema District Municipality, South Africa. Plants, 11(2), 193. https://doi.org/10.3390/plants11020193







