Amplified Drought Alters Leaf Litter Metabolome, Slows Down Litter Decomposition, and Modifies Home Field (Dis)Advantage in Three Mediterranean Forests
Abstract
:1. Introduction
2. Results
2.1. Initial Litter Quality
2.2. Initial Metabolomic Litter Signature
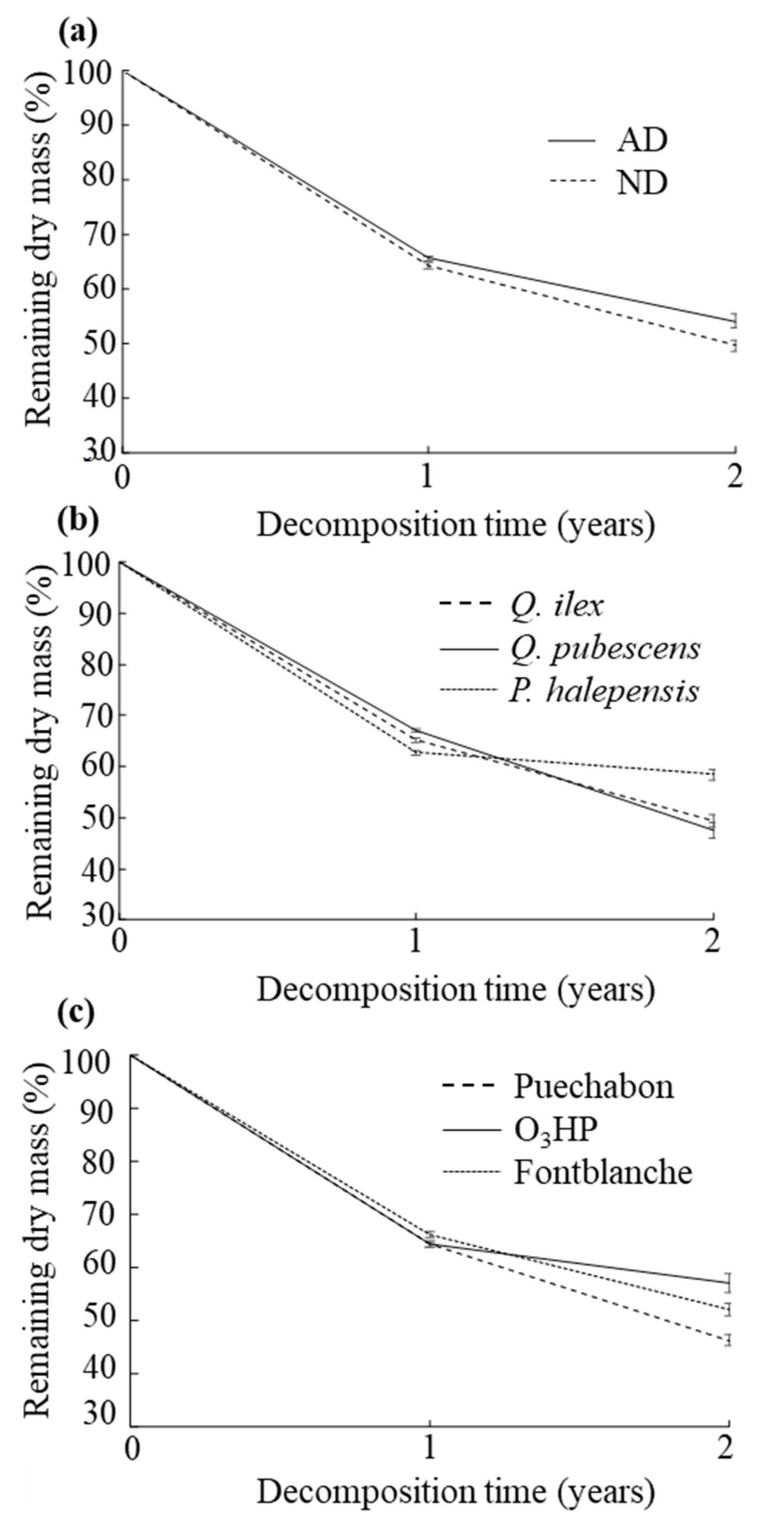
2.3. Leaf Litter Decomposition
2.4. Home Field Advantage (HFA)
3. Discussion
3.1. Amplified Drought Alters Initial Litter Metabolomes and Initial Litter Quality
3.2. Decomposition Dynamics in the Three Mediterranean Forests
3.3. Home Field Advantage in the Three Mediterranean Study Forests
4. Material and Methods
4.1. Study Sites
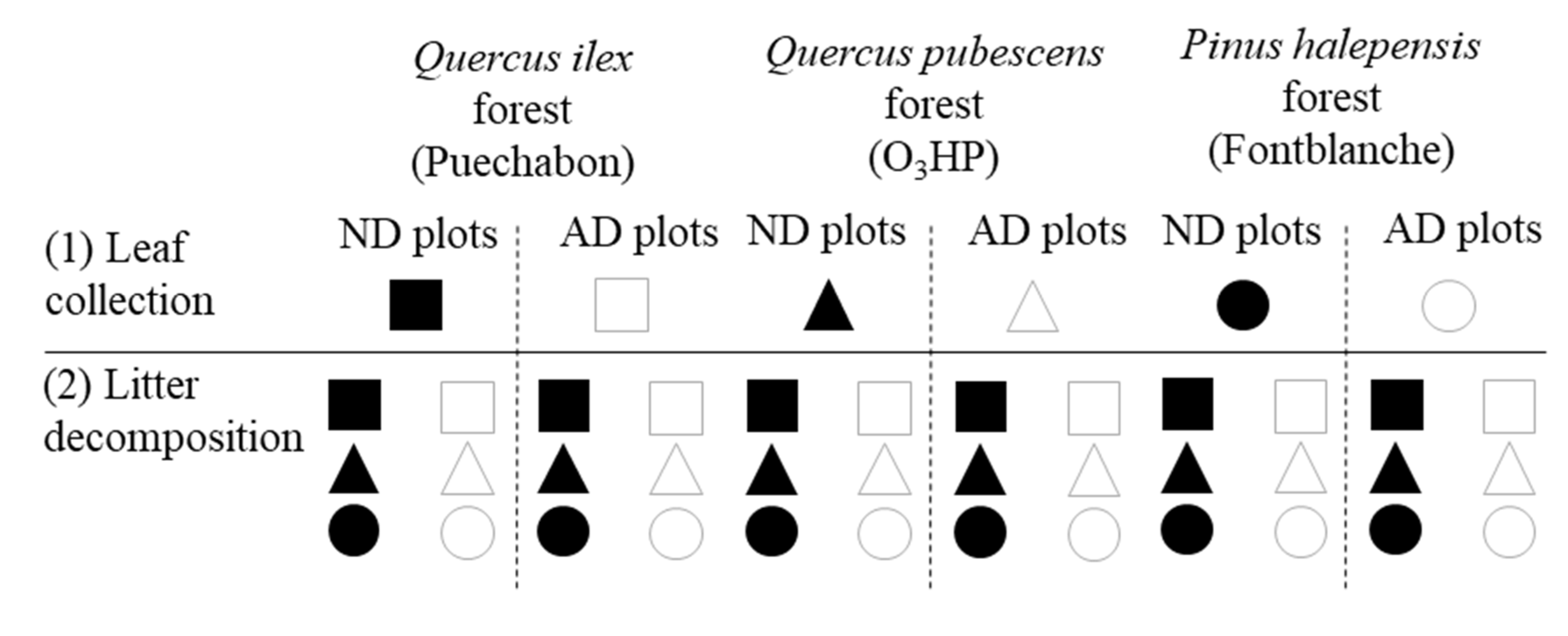
4.2. Experimental Setup and Litter Bag Processing
4.3. Initial Litter Quality
4.4. Initial Metabolomic Litter Signature
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, J. Patterns in the fate of production in plant communities. Am. Nat. 1999, 154, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.H.; Behan-Pelletier, V.; Ritz, K.; Herrick, J.E.; Jones, T.H.; Six, J.; Strong, D.R.; van der Putten, W.H. Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2012; p. 424. [Google Scholar]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439. [Google Scholar] [CrossRef]
- (Ciska) Veen, G.F.; Sundqvist, M.K.; Wardle, D.A. Environmental factors and traits that drive plant litter decomposition do not determine home-field advantage effects. Funct. Ecol. 2015, 29, 981–991. [Google Scholar] [CrossRef]
- Vivanco, L.; Austin, A.T. Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J. Ecol. 2008, 96, 727–736. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; González-Arzac, A.; Pérez, L.I. There’s no place like home? An exploration of the mechanisms behind plant litter-decomposer affinity in terrestrial ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2001, 6, 751–765. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. Multiple mechanisms for trait effects on litter decomposition: Moving beyond home-field advantage with a new hypothesis: Substrate-matrix quality interactions in decay. J. Ecol. 2012, 100, 619–630. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Barker, G.M. Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Funct. Ecol. 2002, 16, 585–595. [Google Scholar] [CrossRef]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, M.; He, T. Home-field advantage of litter decomposition and nitrogen release in forest ecosystems. Biol. Fertil. Soils 2012, 49, 427–434. [Google Scholar] [CrossRef]
- St. John, M.G.; Orwin, K.H.; Dickie, I.A. No ‘home’ versus ‘away’ effects of decomposition found in a grassland–forest reciprocal litter transplant study. Soil Biol. Biochem. 2011, 43, 1482–1489. [Google Scholar] [CrossRef]
- Veen, G.F.C.; Freschet, G.T.; Ordonez, A.; Wardle, D.A. Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 2015, 124, 187–195. [Google Scholar] [CrossRef]
- Liski, J.; Nissinen, A.; Erhard, M.; Taskinen, O. Climatic effects on litter decomposition from arctic tundra to tropical rainforest. Glob. Chang. Biol. 2003, 9, 575–584. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Aupic-Samain, A.; Santonja, M.; Chomel, M.; Pereira, S.; Quer, E.; Lecareux, C.; Limousin, J.-M.; Ourcival, J.-M.; Simioni, G.; Gauquelin, T.; et al. Soil biota response to experimental rainfall reduction depends on the dominant tree species in mature northern mediterranean forests. Soil Biol. Biochem. 2021, 154, 108122. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Santonja, M.; Rancon, A.; Fromin, N.; Baldy, V.; Hättenschwiler, S.; Fernandez, C.; Montès, N.; Mirleau, P. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a mediterranean shrubland. Soil Biol. Biochem. 2017, 111, 124–134. [Google Scholar] [CrossRef]
- Criquet, S.; Tagger, S.; Vogt, G.; Le Petit, J. Endoglucanase and β-Glycosidase activities in an evergreen oak litter: Annual variation and regulating factors. Soil Biol. Biochem. 2002, 34, 1111–1120. [Google Scholar] [CrossRef]
- Reichstein, M.; Tenhunen, J.D.; Roupsard, O.; Ourcival, J.-M.; Rambal, S.; Dore, S.; Valentini, R. Ecosystem respiration in two mediterranean evergreen holm oak forests: Drought effects and decomposition dynamics. Funct. Ecol. 2002, 16, 27–39. [Google Scholar] [CrossRef]
- Rodriguez-Ramirez, N.; Santonja, M.; Baldy, V.; Ballini, C.; Montès, N. Shrub species richness decreases negative impacts of drought in a mediterranean ecosystem. J. Veg. Sci. 2017, 28, 985–996. [Google Scholar] [CrossRef]
- Chapman, S.K.; Koch, G.W. What type of diversity yields synergy during mixed litter decomposition in a natural forest ecosystem? Plant Soil 2007, 299, 153–162. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought changes the dynamics of trace element accumulation in a mediterranean Quercus ilex forest. Environ. Pollut. 2007, 147, 567–583. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J.; et al. Modulation of leaf economic traits and trait relationships by climate: Modulation of leaf traits by climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Saunier, A.; Greff, S.; Blande, J.D.; Lecareux, C.; Baldy, V.; Fernandez, C.; Ormeño, E. Amplified drought and seasonal cycle modulate Quercus pubescens leaf metabolome. Metabolites 2022, 12, 307. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; et al. Opposite metabolic responses of shoots and roots to drought. Sci. Rep. 2015, 4, 6829. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate change effects on secondary compounds of forest trees in the northern hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Saunier, A.; Ormeño, E.; Havaux, M.; Wortham, H.; Ksas, B.; Temime-Roussel, B.; Blande, J.D.; Lecareux, C.; Mévy, J.-P.; Bousquet-Mélou, A.; et al. Resistance of native oak to recurrent drought conditions simulating predicted climatic changes in the mediterranean region: Oak forest under several years of drought. Plant Cell Environ. 2018, 41, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Viros, J.; Mévy, J.-P.; Tonetto, A.; Saunier, A.; Bousquet-Mélou, A.; Fernandez, C. Exogenous isoprene confers physiological benefits in a negligible isoprene emitter (Acer monspessulanum L.) under water deficit. Plants 2020, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Asplund, J.; Kauserud, H.; Bokhorst, S.; Lie, M.H.; Ohlson, M.; Nybakken, L. Fungal communities influence decomposition rates of plant litter from two dominant tree species. Fungal Ecol. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Chomel, M.; Fernandez, C.; Bousquet-Mélou, A.; Gers, C.; Monnier, Y.; Santonja, M.; Gauquelin, T.; Gros, R.; Lecareux, C.; Baldy, V. Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. J. Ecol. 2014, 102, 411–424. [Google Scholar] [CrossRef]
- Fernandez, C.; Santonja, M.; Gros, R.; Monnier, Y.; Chomel, M.; Baldy, V.; Bousquet-Mélou, A. Allelochemicals of Pinus halepensis as drivers of biodiversity in mediterranean open mosaic habitats during the colonization stage of secondary succession. J. Chem. Ecol. 2013, 39, 298–311. [Google Scholar] [CrossRef]
- Kainulainen, P.; Holopainen, J.K. Concentrations of secondary compounds in scots pine needles at different stages of decomposition. Soil Biol. Biochem. 2002, 34, 37–42. [Google Scholar] [CrossRef]
- Kazakou, E.; Violle, C.; Roumet, C.; Pintor, C.; Gimenez, O.; Garnier, E. Litter quality and decomposability of species from a mediterranean succession depend on leaf traits but not on nitrogen supply. Ann. Bot. 2009, 104, 1151–1161. [Google Scholar] [CrossRef]
- Santonja, M.; Fernandez, C.; Gauquelin, T.; Baldy, V. Climate change effects on litter decomposition: Intensive drought leads to a strong decrease of litter mixture interactions. Plant Soil 2015, 393, 69–82. [Google Scholar] [CrossRef]
- Barba, J.; Lloret, F.; Yuste, J.C. Effects of drought-induced forest die-off on litter decomposition. Plant Soil 2016, 402, 91–101. [Google Scholar] [CrossRef] [Green Version]
- de Dios, R.S.; Benito-Garzón, M.; Sainz-Ollero, H. Present and future extension of the iberian submediterranean territories as determined from the distribution of marcescent oaks. Plant Ecol. 2009, 204, 189–205. [Google Scholar] [CrossRef]
- De Rigo, D.; Caudullo, G. Quercus ilex in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., De Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; European Commission: Luxembourg, 2016; pp. 152–153. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The polyphenolic profile of oak (Quercus) species: A phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Michel, T.; Khlif, I.; Kanakis, P.; Termentzi, A.; Allouche, N.; Halabalaki, M.; Skaltsounis, A.-L. UHPLC-DAD-FLD and UHPLC-HRMS/MS based metabolic profiling and characterization of different Olea europaea organs of koroneiki and chetoui varieties. Phytochem. Lett. 2015, 11, 424–439. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Bursal, E.; Boğa, R. Polyphenols analysed by UHPLC-ESI-MS/MS and antioxidant activities of molasses, acorn and leaves of oak (Quercus robur Subsp. pedunculiflora). Prog. Nutr. 2018, 20, 167–175. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Gargallo-Garriga, A.; Sardans, J.; Oravec, M.; Mateu-Castell, L.; Pérez-Trujillo, M.; Parella, T.; Ogaya, R.; Urban, O.; Peñuelas, J. Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol. 2014, 202, 874–885. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Li, C.; Wang, X.; He, X. Anti-inflammatory oleanolic triterpenes from chinese acorns. Molecules 2016, 21, 669. [Google Scholar] [CrossRef]
- Gammacurta, M.; Waffo-Teguo, P.; Winstel, D.; Cretin, B.N.; Sindt, L.; Dubourdieu, D.; Marchal, A. Triterpenoids from Quercus petraea: Identification in wines and spirits and sensory assessment. J. Nat. Prod. 2019, 82, 265–275. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, Z.; Wang, Y.; Xu, J.; He, X. Anti-neuroinflammatory triterpenoids from the seeds of Quercus serrata Thunb. Fitoterapia 2020, 142, 104523. [Google Scholar] [CrossRef]
- Bowers, J.J.; Gunawardena, H.P.; Cornu, A.; Narvekar, A.S.; Richieu, A.; Deffieux, D.; Quideau, S.; Tharayil, N. Rapid screening of ellagitannins in natural sources via targeted reporter ion triggered tandem mass spectrometry. Sci. Rep. 2018, 8, 10399. [Google Scholar] [CrossRef]
- Frost, S.; Lerno, L.; Zweigenbaum, J.; Heymann, H.; Ebeler, S. Characterization of red wine proanthocyanidins using a putative proanthocyanidin database, amide hydrophilic interaction liquid chromatography (HILIC), and time-of-flight mass spectrometry. Molecules 2018, 23, 2687. [Google Scholar] [CrossRef] [PubMed]
- Yuzuak, S.; Ballington, J.; Xie, D.-Y. HPLC-QTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, A.; Papa, S.; Pellegrino, A.; Fuggi, A. Decomposition dynamics of Myrtus communis and Quercus ilex leaf litter: Mass loss, microbial activity and quality change. Appl. Soil Ecol. 2007, 36, 32–40. [Google Scholar] [CrossRef]
- Tu, L.; Hu, H.; Chen, G.; Peng, Y.; Xiao, Y.; Hu, T.; Zhang, J.; Li, X.; Liu, L.; Tang, Y. Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS ONE 2014, 9, e88752. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Prieto, P.; Estiarte, M. Drought and warming induced changes in P and K concentration and accumulation in plant biomass and soil in a mediterranean shrubland. Plant Soil 2008, 306, 261–271. [Google Scholar] [CrossRef]
- Uscola, M.; Villar-Salvador, P.; Oliet, J.; Warren, C.R. Foliar absorption and root translocation of nitrogen from different chemical forms in seedlings of two mediterranean trees. Environ. Exp. Bot. 2014, 104, 34–43. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Ottow, E.A.; Brinker, M.; Teichmann, T.; Fritz, E.; Kaiser, W.; Brosché, M.; Kangasjärvi, J.; Jiang, X.; Polle, A. Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol. 2005, 139, 1762–1772. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant flavonoids in mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef]
- Cárdenas, P.D.; Almeida, A.; Bak, S. Evolution of structural diversity of triterpenoids. Front. Plant Sci. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Chen, H.-T.; Li, T.-C.; Weng, J.-H.; Jhan, Y.-L.; Lin, S.-X.; Chou, C.-H. The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J. Chem. Ecol. 2014, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Simioni, G.; Cailleret, M.; Ruffault, J.; Badel, E.; Carrière, S.; Davi, H.; Gavinet, J.; Huc, R.; Limousin, J.-M.; et al. Consistently Lower Sap Velocity and Growth over Nine Years of Rainfall Exclusion in a Mediterranean Mixed Pine-Oak Forest. Agric. For. Meteorol. 2021, 108472, 308–309. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Franco, A.C.; Moreira, M.Z.; Haridasan, M. Specific Leaf Area explains differences in leaf traits between congeneric savanna and forest trees. Funct. Ecol. 2005, 19, 932–940. [Google Scholar] [CrossRef]
- McDonald, P.G.; Fonseca, C.R.; Overton, J.M.; Westoby, M. Leaf-size divergence along rainfall and soil-nutrient gradients: Is the method of size reduction common among clades? Funct. Ecol. 2003, 17, 50–57. [Google Scholar] [CrossRef]
- Fernandez, C.; Monnier, Y.; Santonja, M.; Gallet, C.; Weston, L.A.; Prévosto, B.; Saunier, A.; Baldy, V.; Bousquet-Mélou, A. The impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front. Plant. Sci. 2016, 27, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gobat, J.-M.; Aragno, M.; Matthey, W. Le sol Vivant: Bases de Pédologie, Biologie des Sols, 3rd ed.; Presses polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2013; p. 848. [Google Scholar]
- Santonja, M.; Pereira, S.; Gauquelin, T.; Quer, E.; Simioni, G.; Limousin, J.-M.; Ourcival, J.-M.; Reiter, I.M.; Fernandez, C.; Baldy, V. Experimental precipitation reduction slows down litter decomposition but exhibits weak to no effect on soil organic carbon and nitrogen stocks in three mediterranean forests of southern france. Forests 2022, 13, 1485. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Raschi, A.; Lanini, M.; Ineson, P. Decomposition and nutrient dynamics of Quercus pubescens leaf litter in a naturally enriched CO2 mediterranean ecosystem. Funct. Ecol. 1999, 13, 343–351. [Google Scholar] [CrossRef]
- Garcia-Pausas, J.; Casals, P.; Romanyà, J. Litter decomposition and faunal activity in mediterranean forest soils: Effects of N content and the moss layer. Soil Biol. Biochem. 2004, 36, 989–997. [Google Scholar] [CrossRef]
- Kaushal, R.; Verma, K.S.; Chaturvedi, O.P.; Alam, N.M. Leaf litter decomposition and nutrient dynamics in four multipurpose tree species. Range Manag. Agrofor. 2012, 33, 20–27. [Google Scholar]
- Pereira, S.; Burešová, A.; Kopecky, J.; Mádrová, P.; Aupic-Samain, A.; Fernandez, C.; Baldy, V.; Sagova-Mareckova, M. Litter traits and rainfall reduction alter microbial litter decomposers: The evidence from three mediterranean forests. FEMS Microbiol. Ecol. 2019, 95, fiz168. [Google Scholar] [CrossRef] [PubMed]
- Aponte, C.; García, L.V.; Marañón, T. Tree species effect on litter decomposition and nutrient release in mediterranean oak forests changes over time. Ecosystems 2012, 15, 1204–1218. [Google Scholar] [CrossRef]
- Santonja, M.; Rodríguez-Pérez, H.; Le Bris, N.; Piscart, C. Leaf nutrients and macroinvertebrates control litter mixing effects on decomposition in temperate streams. Ecosystems 2020, 23, 400–416. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; van Logtestijn, R.S.P.; van Hal, J.R.; Aerts, R. Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 2013, 122, 987–997. [Google Scholar] [CrossRef]
- Almagro, M.; Maestre, F.T.; Martínez-López, J.; Valencia, E.; Rey, A. Climate change may reduce litter decomposition while enhancing the contribution of photodegradation in dry perennial mediterranean grasslands. Soil Biol. Biochem. 2015, 90, 214–223. [Google Scholar] [CrossRef]
- Thakur, M.P.; Reich, P.B.; Hobbie, S.E.; Stefanski, A.; Rich, R.; Rice, K.E.; Eddy, W.C.; Eisenhauer, N. Reduced feeding activity of soil detritivores under warmer and drier conditions. Nat. Clim. Chang. 2018, 8, 75–78. [Google Scholar] [CrossRef]
- Larcher, W. Temperature stress and survival ability of mediterranean sclerophyllous plants. Plant Biosyst. 2000, 134, 279–295. [Google Scholar] [CrossRef]
- Yuste, J.C.; Peñuelas, J.; Estiarte, M.; Garcia-Mas, J.; Mattana, S.; Ogaya, R.; Pujol, M.; Sardans, J. Drought-resistant fungi control soil organic matter decomposition and its response to temperature. Glob. Chang. Biol. 2011, 17, 1475–1486. [Google Scholar] [CrossRef]
- Zheng, Z.; Mamuti, M.; Liu, H.; Shu, Y.; Hu, S.; Wang, X.; Li, B.; Lin, L.; Li, X. Effects of nutrient additions on litter decomposition regulated by phosphorus-induced changes in litter chemistry in a subtropical forest, China. For. Ecol. Manag. 2017, 400, 123–128. [Google Scholar] [CrossRef]
- Strickland, M.S.; Osburn, E.; Lauber, C.; Fierer, N.; Bradford, M.A. Litter quality is in the eye of the beholder: Initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009, 23, 627–636. [Google Scholar] [CrossRef]
- Foudyl-Bey, S.; Brais, S.; Drouin, P. Litter heterogeneity modulates fungal activity, C mineralization and N retention in the Boreal forest floor. Soil Biol. Biochem. 2016, 100, 264–275. [Google Scholar] [CrossRef]
- Isidorov, V.; Tyszkiewicz, Z.; Pirożnikow, E. Fungal succession in relation to volatile organic compounds emissions from scots pine and Norway spruce leaf litter-decomposing fungi. Atmos. Environ. 2016, 131, 301–306. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Coûteaux, M.-M.; Bottner, P.; Berg, B. Litter decomposition, climate and liter quality. Trends Eco. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Voříšková, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B.H. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979; Volume 5, p. 372. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Determination of lignin and cellulose in acid-detergent fiber with permanganate. J. Assoc. Off. Anal. Chem. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1989; p. 521. [Google Scholar]
- Peñuelas, J.; Estiarte, M.; Kimball, B.A.; Idso, S.B.; Pinter, P.J.; Wall, G.M.; Garcia, R.L.; Hansaker, D.J.; LaMorte, R.L.; Hensrik, D.L. Variety of responses of plant phenolic concentration to CO2 enrichment. J. Exp. Bot. 1996, 47, 1463–1467. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL. 2019. Available online: http://www.R-project.org/ (accessed on 8 August 2022).
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Brakni, R.; Ali Ahmed, M.; Burger, P.; Schwing, A.; Michel, G.; Pomares, C.; Hasseine, L.; Boyer, L.; Fernandez, X.; Landreau, A.; et al. UHPLC-HRMS/MS based profiling of algerian lichens and their antimicrobial activities. Chem. Biodivers. 2018, 15, e1800031. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F.; Package ’factoextra’. The Comprehensive R Archive Network (CRAN). 2017. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 8 August 2022).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 504. [Google Scholar]

| Leave/Needles Quality | Litter Types (T) | Litter Species (S) | T × S | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Values | p-Values | Q. ilex | Q. pubescens | P. halepensis | F-Values | p-Values | F-Values | p-Values | |||||
| C (mg·g−1) | ND | 0.01 | ns | 478.09 ± 1.35 | b | 462.61 ± 2.65 | c | 516.09 ± 1.60 | a | 172.61 | *** | 0.16 | ns |
| AD | 477.26 ± 1.29 | b | 462.11 ± 5.49 | c | 518.47 ± 2.90 | a | |||||||
| N (mg·g−1) | ND | 8.67 | ** | 9.62 ± 0.20 | a | 6.39 ± 0.20 | b | 5.36 ± 0.13 | c | 244.41 | *** | 8.24 | ** |
| AD | 8.22 ± 0.18 | a | 6.17 ± 0.12 | b | 5.49 ± 0.09 | c | |||||||
| C/N (mg·g−1) | ND | 9.97 | ** | 49.8 ± 1.00 | c | 72.64 ± 2.31 | b | 96.41 ± 2.19 | a | 348.96 | *** | 8.59 | ** |
| AD | 58.17 ± 1.24 | c | 74.92 ± 0.96 | b | 94.44 ± 1.39 | a | |||||||
| P (mg·g−1) | ND | 0.561 | ns | 3.48 ± 0.21 | a | 1.90 ± 0.05 | b | 1.56 ± 0.05 | c | 198.85 | *** | 7.58 | ** |
| AD | 3.23 ± 0.09 | a | 1.80 ± 0.08 | b | 1.87 ± 0.02 | b | |||||||
| Ca (mg·g−1) | ND | 80.56 | *** | 25.09 ± 0.56 | b | 32.90 ± 0.77 | a | 18.41 ± 0.18 | c | 487.43 | *** | 0.104 | ns |
| AD | 21.68 ± 0.42 | b | 28.89 ± 0.58 | a | 16.10 ± 0.19 | c | |||||||
| K (mg·g−1) | ND | 12.12 | ** | 1.81 ± 0.06 | a | 0.84 ± 0.01 | b | 0.95 ± 0.01 | b | 157.47 | *** | 7.10 | ** |
| AD | 2.77 ± 0.05 | a | 0.91 ± 0.12 | b | 0.97 ± 0.02 | b | |||||||
| Mg (mg·g−1) | ND | 82.74 | *** | 1.36 ± 0.01 | b | 2.83 ± 0.19 | a | 1.48 ± 0.02 | b | 428.45 | *** | 14.87 | *** |
| AD | 1.87 ± 0.03 | b | 3.81 ± 0.08 | a | 1.53 ± 0.01 | c | |||||||
| Na (mg·g−1) | ND | 27.6 | *** | 0.11 ± 0.00 | a | 0.04 ± 0.00 | b | 0.11 ± 0.00 | a | 249.11 | *** | 55.05 | *** |
| AD | 0.16 ± 0.01 | a | 0.07 ± 0.00 | b | 0.08 ± 0.00 | b | |||||||
| Phenols (mg·g−1) | ND | 34.88 | *** | 32.87 ± 1.58 | b | 40.77 ± 2.59 | a | 38.59 ± 1.17 | ab | 2.63 | ns | 19.46 | *** |
| AD | 58.48 ± 2.89 | a | 44.18 ± 2.17 | b | 39.96 ± 1.49 | b | |||||||
| Lignin (mg·g−1) | ND | 0.25 | ns | 337.38 ± 5.49 | a | 273.33 ± 9.00 | b | 302.57 ± 10.61 | ab | 22.11 | *** | 0.992 | ns |
| AD | 326.77 ± 6.24 | a | 283.64 ± 9.01 | b | 310.57 ± 5.66 | ab | |||||||
| Cellulose (mg·g−1) | ND | 1.89 | ns | 204.99 ± 4.18 | a | 159.07 ± 8.01 | b | 150.81 ± 12.92 | b | 9.62 | *** | 2.22 | ns |
| AD | 198.16 ± 7.32 | 163.53 ± 5.93 | 182.75 ± 13.28 | ||||||||||
| Hemicellulose (mg·g−1) | ND | 0.96 | ns | 270.02 ± 16.22 | ab | 274.22 ± 22.47 | a | 206.59 ± 10.42 | b | 18.40 | *** | 0.812 | ns |
| AD | 273.75 ± 11.97 | a | 309.58± 10.35 | a | 205.27 ± 12.22 | b | |||||||
| WSC (mg·g−1) | ND | 5.38 | * | 187.61 ± 10.19 | c | 293.38 ± 12.08 | b | 340.02 ± 7.13 | ab | 74.53 | *** | 4.89 | * |
| AD | 201.32 ± 13.36 | c | 243.24 ± 6.00 | b | 301.41 ± 6.07 | a | |||||||
| WHC (%) | ND | 18.12 | *** | 137.24 ± 0.98 | b | 146.90 ± 1.01 | a | 113.89 ± 1.03 | c | 307.10 | *** | 6.00 | ** |
| AD | 129.89 ± 1.70 | b | 140.06 ± 0.75 | a | 114.43 ± 1.38 | c | |||||||
| SLA (cm2·g−1) | ND | 3.79 | ns | 51.50 ± 0.88 | c | 133.13 ± 0.41 | a | 83.50 ± 2.19 | b | 183.76 | *** | 2.73 | ns |
| AD | 47.80 ± 0.87 | c | 128.16 ± 1.36 | a | 85.06 ± 2.64 | b | |||||||
| Quercus ilex | Quercus pubescens | Pinus halepensis | |||||
|---|---|---|---|---|---|---|---|
| df | F-Values | p-Values | F-Values | p-Values | F-Values | p-Values | |
| Litter types | 1 | 9.23 | 0.013 * | 2.44 | 0.034 * | 0.97 | 0.464 ns |
| Litter Types | VIP | RT (Min) | m/z [M + H]+ | m/z [M − H]− | Molecular Formula [M − H]− | Error (PPM) | mSigma | Putative Identification | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AD | M615T468 | 7.8 | 617.114 | 615.0987 | C28H23O16 | 0.9 | 9.1 | Quercetin-3-O-(6″-O-galloyl)-b-D-glucopyranoside | [44] |
| AD | M629T496 | 8.26 | 631.129 | 629.1144 | C29H25O16 | 1.1 | 28 | Isorhamnetin 3-(6″-galloylglucoside) | [44] |
| AD | M340T388 (isotope) | 6.47 | 341.086 | 339.0717 | C15H15O9 | 1.5 | 2.3 | Aesculin | [45] |
| ND | M387T49 | 0.81 | / | 387.1143 [M + HCOO]− | C13H23O13 | 0.5 | 11 | Disaccharide | |
| 341.1086 | C12H21O11 | 0.9 | 13.8 | ||||||
| AD | M330T66 (isotope) | 1.09 | / | 331.0671 | C13H15O10 | −0.5 | 21.6 | galloyl-β-D-glucose | [44] |
| AD | M399T407 | 6.78 | 355.101 | 399.0934 | C16H17O9 | −3.9 | NA | Chlorogenic acid | [46] [47] [48] |
| 353.0875 | C17H19O1 | −3.3 | 23.0 |
| Litter Types | VIP | RT (min) | m/z [M + H]+ | m/z [M − H]− | Molecular Formula [M − H]− | Error (PPM) | mSigma | Putative Identification | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AD | M711T533 | 8.88 | / | 711.3964 [M + HCOO]− | C37H59O13 | −0.5 | 25.8 | Arjungenin glycoside isomer 1 | [49] |
| 665.3906 | C36H57O11 | 4.6 | na | ||||||
| AD | M711T496 | 8.26 | / | 711.3965 [M + HCOO]− | C37H59013 | −0.9 | 14.9 | Arjungenin glycoside isomer 2 | [49] |
| 665.3906 | C36H57O11 | −5.5 | 425.9 | ||||||
| AD | M503T633 | 10.55 | / | 503.3382 | C30H47O6 | −0.5 | 3.9 | Oleane triterpene | [50] |
| AD | M695T600 | 10.00 | / | 695.4018 [M + HCOO]− | C37H59O12 | −1.4 | 6.9 | Arjunglucoside II or arjunetin | [49] [51] |
| 649.3957 | C36H57O10 | −2.7 | NA | ||||||
| ND | M387T49 | 0.81 | / | 387.1144 | C13H23O13 | 0.5 | 11 | Disaccharide | [48] |
| ND | M702T383 (isotope) | 6.38 | / | 701.5749 [M − H]2− | C57H46O42 | 0.9 | 220.4 | Ellagitanin | [52] |
| AD | M593T520 | 8.66 | / | 593.1591 [M − H]2− | C60H50O26 | −1.6 | 24.7 | Proanthocyanidin (Cat-Cat-GalCat-GalCat) | [53] |
| AD | M343T637 | 10.61 | / | 343.0460 | C17H11O8 | 0.3 | 4.8 | Galloylquinic acid isomer | |
| AD | M289T405 | 6.74 | 291.086 | 289.0716 | C15H14O6 | 1.6 | 11.4 | (epi)catechin | [54] |
| d.f. | %SS | F-Value | p-Value | |
|---|---|---|---|---|
| Litter species | 2 | 1.9 | 9.5 | <0.0001 |
| Litter types | 1 | 0.0 | 0.4 | 0.518 |
| Precipitation treatments | 1 | 1.6 | 16.4 | <0.0001 |
| Forests | 2 | 4.2 | 21.6 | <0.0001 |
| Time | 1 | 34.0 | 348.9 | <0.0001 |
| Litter species × Litter types | 2 | 0.1 | 0.7 | 0.484 |
| Litter species × Precipitation treatments | 2 | 0.0 | 0.2 | 0.803 |
| Litter species × Forests | 4 | 2.7 | 6.8 | <0.0001 |
| Litter types × Forests | 2 | 0.6 | 3 | 0.053 |
| Precipitation Treatments × Forests | 2 | 0.5 | 2.5 | 0.083 |
| Litter species × Time | 2 | 8.3 | 42.4 | <0.0001 |
| Precipitation treatments × Time | 1 | 0.4 | 4.3 | 0.039 |
| Forests × Time | 2 | 3.8 | 19.6 | <0.0001 |
| Litter species × Litter types × Forests | 4 | 0.8 | 2.2 | 0.073 |
| Litter species × Precipitation treatments × Forest | 4 | 1.0 | 2.6 | 0.039 |
| Litter species × Forests × Time | 4 | 1.0 | 2.6 | 0.036 |
| Precipitation treatment × Forests × Time | 2 | 0.6 | 3.2 | 0.040 |
| Residuals | 393 | 38.3 | - | - |
| Decomposition Time | Precipitation Treatments | ND | AD | ||||
|---|---|---|---|---|---|---|---|
| Litter Species | Q. ilex | Q. pubescens | P. halepensis | Q. ilex | Q. pubescens | P. halepensis | |
| First year | ADH | −3.55 | −3.43 | −4.92 | −0.06 | −4.35 | −6.67 |
| T-value | 1.24 | 2.41 ** | 1.84 | 0.03 | 3.36 *** | 2.63 ** | |
| Second year | ADH | −0.30 | −10.10 | 18.48 | −13.15 | −4.03 | −1.59 |
| T-value | −0.04 | −3.38 ** | −3.46 ** | 0.14 | −1.01 | 0.20 | |
| Forest | Quercus ilex L. | Quercus pubescens Willd. | Pinus halepensisMill. |
|---|---|---|---|
| Site | Puechabon | Oak Observatory at the Observatoire de Haute Provence (O3HP) | Font-Blanche |
| Location | 43°44′29″ N, 3035′45″ E | 43°56′115″ N, 05°42′642″ E | 43°14′27″ N 5°40′45″ E |
| Altitude a.s.l. (m) | 270 | 650 | 425 |
| Mediterranean bioclimatic zone | mesomediterranean | supramediterranean | thermos-mesomediterranean |
| Soil type | rhodo-chromic luvisol | pierric calcosol | leptosol |
| Soil texture | clay loam | clay | clay |
| Soil pH | 6.60 | 6.76 | 6.80 |
| Forest types | evergreen broadleaf | deciduous broadleaf | mixed coniferous/broadleaf |
| Other vegetation | Buxus sempervirens L., Phyllirea latifolial L., Pistacia terebinthus L. Juniperus oxycedrus L. | Acer monspessulanum L., Cotinus coggygria Scop. | Quercus ilex L., Quercus coccifera L. And Phyllirea latifolia L. |
| Tree density (stems/ha) | 6070 | 5706 | 6000 |
| Tree height (m) | 5.5 | 5.5 | 13 |
| Stand age (years) | 74 | 70 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quer, E.; Pereira, S.; Michel, T.; Santonja, M.; Gauquelin, T.; Simioni, G.; Ourcival, J.-M.; Joffre, R.; Limousin, J.-M.; Aupic-Samain, A.; et al. Amplified Drought Alters Leaf Litter Metabolome, Slows Down Litter Decomposition, and Modifies Home Field (Dis)Advantage in Three Mediterranean Forests. Plants 2022, 11, 2582. https://doi.org/10.3390/plants11192582
Quer E, Pereira S, Michel T, Santonja M, Gauquelin T, Simioni G, Ourcival J-M, Joffre R, Limousin J-M, Aupic-Samain A, et al. Amplified Drought Alters Leaf Litter Metabolome, Slows Down Litter Decomposition, and Modifies Home Field (Dis)Advantage in Three Mediterranean Forests. Plants. 2022; 11(19):2582. https://doi.org/10.3390/plants11192582
Chicago/Turabian StyleQuer, Elodie, Susana Pereira, Thomas Michel, Mathieu Santonja, Thierry Gauquelin, Guillaume Simioni, Jean-Marc Ourcival, Richard Joffre, Jean-Marc Limousin, Adriane Aupic-Samain, and et al. 2022. "Amplified Drought Alters Leaf Litter Metabolome, Slows Down Litter Decomposition, and Modifies Home Field (Dis)Advantage in Three Mediterranean Forests" Plants 11, no. 19: 2582. https://doi.org/10.3390/plants11192582
APA StyleQuer, E., Pereira, S., Michel, T., Santonja, M., Gauquelin, T., Simioni, G., Ourcival, J.-M., Joffre, R., Limousin, J.-M., Aupic-Samain, A., Lecareux, C., Dupouyet, S., Orts, J.-P., Bousquet-Mélou, A., Gros, R., Sagova-Mareckova, M., Kopecky, J., Fernandez, C., & Baldy, V. (2022). Amplified Drought Alters Leaf Litter Metabolome, Slows Down Litter Decomposition, and Modifies Home Field (Dis)Advantage in Three Mediterranean Forests. Plants, 11(19), 2582. https://doi.org/10.3390/plants11192582







