Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress
Abstract
:1. Introduction
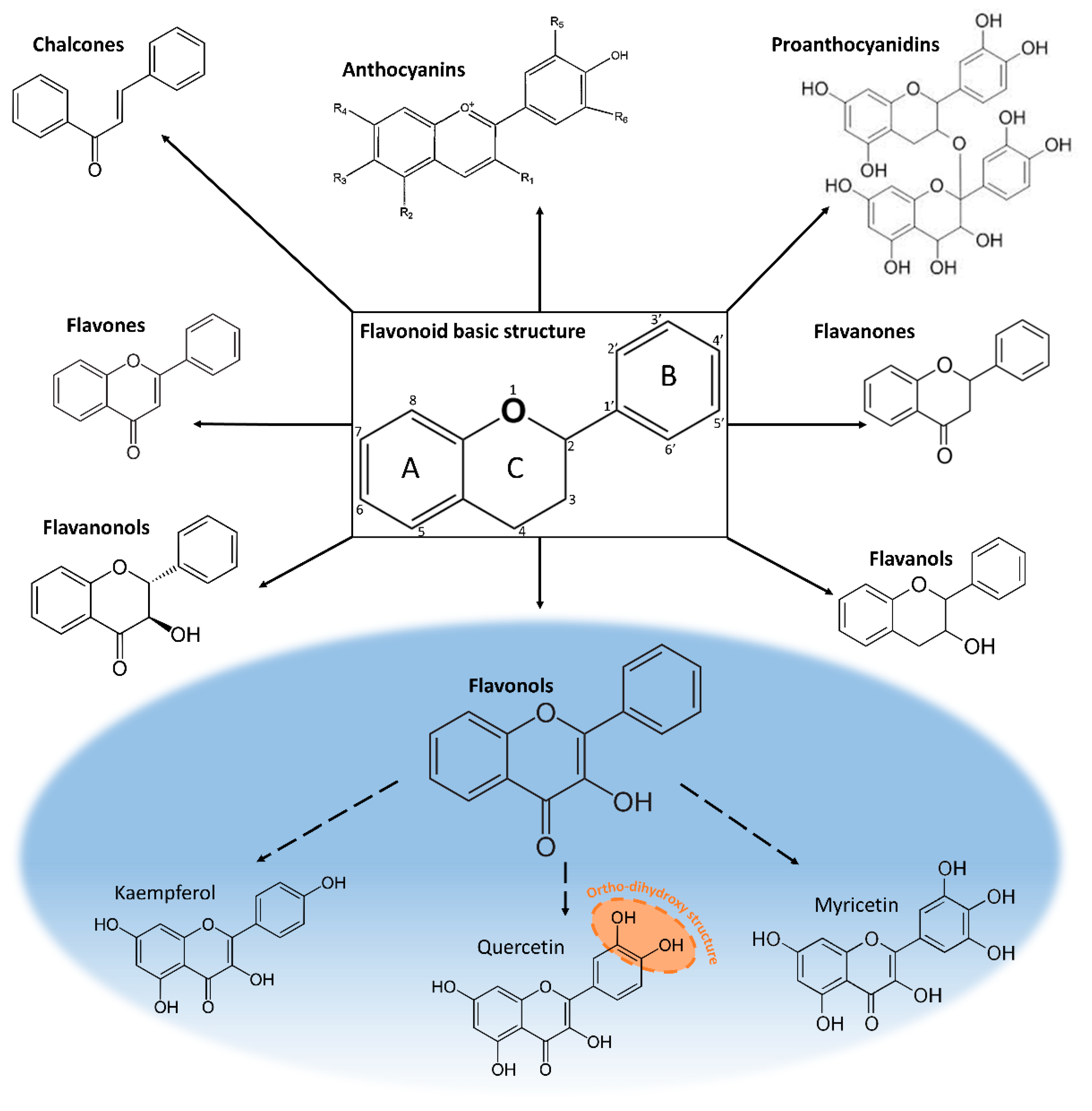
2. Chemical and Physical Properties of Flavonols: Relationship with Their Function
3. Biosynthesis and Storage of Flavonols: Relationship with Their Function
4. Flavonols in Plants: An Important Polyphenol to Cope with Rapid Climate Change
4.1. Drought
4.2. Warming
4.3. UV Radiation
4.4. Salinity
5. Flavonols as Antioxidants: A Unifying Mode of Action against Climate Stresses
6. Flavonols as Indirect Growth Regulators
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publising: Oxford, UK, 2006; pp. 1–22. [Google Scholar]
- Treutter, D. Significance of Flavonoids in Plant Resistance: A Review. Environ. Chem. Lett. 2006, 4, 147. [Google Scholar] [CrossRef]
- Gori, A.; Tattini, M.; Centritto, M.; Ferrini, F.; Marino, G.; Mori, J.; Guidi, L.; Brunetti, C. Seasonal and Daily Variations in Primary and Secondary Metabolism of Three Maquis Shrubs Unveil Different Adaptive Responses to Mediterranean Climate. Conserv. Physiol. 2019, 7, coz070. [Google Scholar] [CrossRef]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and Diurnal Variation in Leaf Phenolics of Three Medicinal Mediterranean Wild Species: What Is the Best Harvesting Moment to Obtain the Richest and the Most Antioxidant Extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef] [Green Version]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Berny-Mier y Teran, J.C.; Timmermans, B.G.H.; Bruun, H.H.; Covelo, F.; Glauser, G.; Rasmann, S.; Tack, A.J.M. Latitudinal Variation in Plant Chemical Defences Drives Latitudinal Patterns of Leaf Herbivory. Ecography 2018, 41, 1124–1134. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A.; Rasmann, S.; Petry, W.K.; Carrillo-Gavilán, A.; Zas, R.; Sampedro, L. Trade-Offs between Constitutive and Induced Defences Drive Geographical and Climatic Clines in Pine Chemical Defences. Ecol. Lett. 2014, 17, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential Accumulation of Flavonoids and Hydroxycinnamates in Leaves of Ligustrum vulgare under Excess Light and Drought Stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids Accumulate in Leaves and Glandular Trichomes of Phillyrea latifolia Exposed to Excess Solar Radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, Physiological and Biochemical Adjustments in Response to Root Zone Salinity Stress and High Solar Radiation in Two Mediterranean Evergreen Shrubs, Myrtus cmmunis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef]
- Tattini, M.; Matteini, P.; Saracini, E.; Traversi, M.L.; Giordano, C.; Agati, G. Morphology and Biochemistry of Non-Glandular Trichomes in Cistus salvifolius L. Leaves Growing in Extreme Habitats of the Mediterranean Basin. Plant Biol. 2007, 9, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple Functions of Polyphenols in Plants Inhabiting Unfavorable Mediterranean Areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Munné-Bosch, S. Drought-Induced Changes in Flavonoids and Other Low Molecular Weight Antioxidants in Cistus Clusii Grown under Mediterranean Field Conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-Ubach, A.; Gargallo-Garriga, A.; Sardans, J.; Oravec, M.; Mateu-Castell, L.; Pérez-Trujillo, M.; Parella, T.; Ogaya, R.; Urban, O.; Peñuelas, J. Drought Enhances Folivory by Shifting Foliar Metabolomes in Quercus ilex Trees. New Phytol. 2014, 202, 874–885. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, M.; Guevara, M.Á.; Sánchez-Gómez, D.; de María, N.; Díaz, L.M.; Mancha, J.A.; Fernández de Simón, B.; Cadahía, E.; Desai, N.; Aranda, I.; et al. Organ-Specific Metabolic Responses to Drought in Pinus pinaster Ait. Plant Physiol. Biochem. 2016, 102, 17–26. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids from Biosynthesis to Human Health; Justino, C.G., Ed.; InTech: London, UK, 2017; Volume 6, pp. 3–17. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, L.P.; Grotewold, E. Flavonoids as Developmental Regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Murphy, A.S. Flavonoids and Auxin Transport: Modulators or Regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Morita, Y.; Takagi, K.; Fukuchi-Mizutani, M.; Ishiguro, K.; Tanaka, Y.; Nitasaka, E.; Nakayama, M.; Saito, N.; Kagami, T.; Hoshino, A.; et al. A Chalcone Isomerase-like Protein Enhances Flavonoid Production and Flower Pigmentation. Plant J. 2014, 78, 294–304. [Google Scholar] [CrossRef]
- Gayomba, S.R.; Watkins, J.M.; Muday, G.K. Flavonols Regulate Plant Growth and Development through Regulation of Auxin Transport and Cellular Redox Status. Recent Adv. Polyphen. Res. 2016, 5, 143–170. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzio, V.; Lattanzino, V.M.T.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Chapter 9—Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Southston, UK, 2019; pp. 157–168. [Google Scholar]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.W.; Tobler, M.A.; Keefover-Ring, K.; Flint, S.D.; Barkley, A.E.; Ryel, R.J.; Lindroth, R.L. Rapid Modulation of Ultraviolet Shielding in Plants Is Influenced by Solar Ultraviolet Radiation and Linked to Alterations in Flavonoids. Plant Cell Environ. 2016, 39, 222–230. [Google Scholar] [CrossRef]
- Davies, K.M.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Functions of Flavonoid and Betalain Pigments in Abiotic Stress Tolerance in Plants. Annu. Plant Rev. Online 2018, 1, 21–62. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant Properties of Phenolic Compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Brown, E.J.; Khodr, H.; Hider, C.R.; Rice-Evans, C.A. Structural Dependence of Flavonoid Interactions with Cu2+ Ions: Implications for Their Antioxidant Properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Agrawal, A.D. Pharmacological Activities of Flavonoids: A Review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant–Environment Interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, J.-L.; Austin, M.B.; Stewart, C.; Noel, J.P. Structure and Function of Enzymes Involved in the Biosynthesis of Phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [Green Version]
- Pollastri, S.; Tattini, M. Flavonols: Old Compounds for Old Roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought Stress Has Contrasting Effects on Antioxidant Enzymes Activity and Phenylpropanoid Biosynthesis in Fraxinus ornus Leaves: An Excess Light Stress Affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.M.; Robberecht, R.; Flint, S.D. Internal Filters: Prospects for UV-Acclimation in Higher Plants. Physiol. Plant. 1983, 58, 445–450. [Google Scholar] [CrossRef]
- Smith, G.J.; Markham, K.R. Tautomerism of Flavonol Glucosides: Relevance to Plant UV Protection and Flower Colour. J. Photochem. Photobiol. A Chem. 1998, 118, 99–105. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.R.; Krishna, D.R. Bioflavonoids Classification, Pharmacological, Biochemical Effects and Therapeutic Potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Aherne, S.A.; O’Brien, N.M. Dietary Flavonols: Chemistry, Food Content, and Metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Soufi, O.; Romero, C.; Louaileche, H. Ortho-Diphenol Profile and Antioxidant Activity of Algerian Black Olive Cultivars: Effect of Dry Salting Process. Food Chem. 2014, 157, 504–510. [Google Scholar] [CrossRef]
- Melidou, M.; Riganakos, K.; Galaris, D. Protection against Nuclear DNA Damage Offered by Flavonoids in Cells Exposed to Hydrogen Peroxide: The Role of Iron Chelation. Free Radic. Biol. Med. 2005, 39, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation Is a Major Regulator of Phenylpropanoid Availability and Biological Activity in Plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Smith, D.L. Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Markham, K.R.; Porter, L.J. Flavonoids of the Primitive Liverwort Takakia and Their Taxonomic and Phylogenetic Significance. Phytochemistry 1979, 18, 611–615. [Google Scholar] [CrossRef]
- Iwashina, T. The Structure and Distribution of the Flavonoids in Plants. J. Plant Res. 2000, 113, 287. [Google Scholar] [CrossRef]
- Williamson, G.; Plumb, G.W.; Garcia-Conesa, M.T. Glycosylation, Esterification, and Polymerization of Flavonoids and Hydroxycinnamates: Effects on Antioxidant Properties. In Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology; Gross, G.G., Hemingway, R.W., Yoshida, T., Branham, S.J., Eds.; Springer: Boston, MA, USA, 1999; pp. 483–494. [Google Scholar]
- Vogt, T.; Jones, P. Glycosyltransferases in Plant Natural Product Synthesis: Characterization of a Supergene Family. Trends Plant Sci. 2000, 5, 380–386. [Google Scholar] [CrossRef]
- Gachon, C.M.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant Secondary Metabolism Glycosyltransferases: The Emerging Functional Analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, M.; Neutelings, G.; El Jaziri, M.; Baucher, M. You Want It Sweeter: How Glycosylation Affects Plant Response to Oxidative Stress. Front. Plant Sci. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Swain, T.; Williams, C.A. The Role of Phenylalanine in Flavonoid Biosynthesis. Phytochemistry 1970, 9, 2115–2122. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Gutensohn, M.; Yoo, H.; Adebesin, F.; Qian, Y.; Guo, L.; Jaini, R.; Lynch, J.H.; McCoy, R.M.; Shreve, J.T.; et al. Identification of a Plastidial Phenylalanine Exporter That Influences Flux Distribution through the Phenylalanine Biosynthetic Network. Nat. Commun. 2015, 6, 8142. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lynch, J.H.; Guo, L.; Rhodes, D.; Morgan, J.A.; Dudareva, N. Completion of the Cytosolic Post-Chorismate Phenylalanine Biosynthetic Pathway in Plants. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.E.; Deroles, S.C.; Manson, D.G.; Lewis, D.H.; Bloor, S.J.; Bradley, J.M. Enhancing Anthocyanin Production by Altering Competition for Substrate between Flavonol Synthase and Dihydroflavonol 4-Reductase. Euphytica 2003, 131, 259–268. [Google Scholar] [CrossRef]
- Owens, D.K.; Alerding, A.B.; Crosby, K.C.; Bandara, A.B.; Westwood, J.H.; Winkel, B.S.J. Functional Analysis of a Predicted Flavonol Synthase Gene Family in Arabidopsis. Plant Physiol. 2008, 147, 1046–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quattrocchio, F.; Baudry, A.; Lepiniec, L.; Grotewold, E. The Regulation of Flavonoid Biosynthesis. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; pp. 97–122. [Google Scholar]
- Abrahams, S.; Tanner, G.J.; Larkin, P.J.; Ashton, A.R. Identification and Biochemical Characterization of Mutants in the Proanthocyanidin Pathway in Arabidopsis. Plant Physiol. 2002, 130, 561–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Y.; Wenger, J.P.; Saathoff, K.; Peel, G.J.; Wen, J.; Huhman, D.; Allen, S.N.; Tang, Y.; Cheng, X.; Tadege, M.; et al. A WD40 Repeat Protein from Medicago Truncatula Is Necessary for Tissue-Specific Anthocyanin and Proanthocyanidin Biosynthesis But Not for Trichome Development. Plant Physiol. 2009, 151, 1114–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional Control of Flavonoid Biosynthesis by MYB–BHLH–WDR Complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Ishihara, H.; Huep, G.; Barsch, A.; Mehrtens, F.; Niehaus, K.; Weisshaar, B. Differential Regulation of Closely Related R2R3-MYB Transcription Factors Controls Flavonol Accumulation in Different Parts of the Arabidopsis thaliana Seedling. Plant J. 2007, 50, 660–677. [Google Scholar] [CrossRef] [Green Version]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The Grapevine R2R3-MYB Transcription Factor VvMYBF1 Regulates Flavonol Synthesis in Developing Grape Berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravaglia, D.; Espley, R.V.; Henry-Kirk, R.A.; Andreotti, C.; Ziosi, V.; Hellens, R.P.; Costa, G.; Allan, A.C. Transcriptional Regulation of Flavonoid Biosynthesis in Nectarine (Prunus persica) by a Set of R2R3 MYB Transcription Factors. BMC Plant Biol. 2013, 13, 68. [Google Scholar] [CrossRef] [Green Version]
- Tirumalai, V.; Swetha, C.; Nair, A.; Pandit, A.; Shivaprasad, P.V. MiR828 and MiR858 Regulate VvMYB114 to Promote Anthocyanin and Flavonol Accumulation in Grapes. J. Exp. Bot. 2019, 70, 4775–4792. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.J.; Hrazdina, G. Endoplasmic Reticulum as a Site of Phenylpropanoid and Flavonoid Metabolism in Hippeastrum. Plant Physiol. 1984, 74, 901–906. [Google Scholar] [CrossRef] [Green Version]
- Winkel-Shirley, B. Evidence for Enzyme Complexes in the Phenylpropanoid and Flavonoid Pathways. Physiol. Plant. 1999, 107, 142–149. [Google Scholar] [CrossRef]
- Winkel, B.S.J. Metabolic Channeling in Plants. Annu. Rev. Plant Biol. 2004, 55, 85–107. [Google Scholar] [CrossRef]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA Damage by Hydrogen Peroxide Through the Fenton Reaction In Vivo and In Vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Saslowsky, D.E.; Warek, U.; Winkel, B.S.J. Nuclear Localization of Flavonoid Enzymes in Arabidopsis. J. Biol. Chem. 2005, 280, 23735–23740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaprometov, M.N.; Nikolaeva, T.N. Chloroplasts Isolated from Kidney Bean Leaves Are Capable of Phenolic Compound Biosynthesis. Russ. J. Plant Physiol. 2003, 50, 623–626. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How Relevant Are Flavonoids as Antioxidants in Plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Peeters, A.J.M.; Léon-Kloosterziel, K.M.; Koornneef, M. The TRANSPARENT TESTA12 Gene of Arabidopsis Encodes a Multidrug Secondary Transporter-like Protein Required for Flavonoid Sequestration in Vacuoles of the Seed Coat Endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef] [Green Version]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.-M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and Biochemistry of Seed Flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-Peroxidase Reaction as a Detoxification Mechanism of Plant Cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.K.; De Luca, V.; Khouri, H.; Latchinian, L.; Brisson, L.; Charest, P.M. Enzymology and Compartmentation of Polymethylated Flavonol Glucosides in Chrysosplenium americanum. Phytochemistry 1987, 26, 1237–1245. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared Spectroscopy as a Tool to Study Plant Cuticles. Spectrosc. Eur. 2016, 28, 4. [Google Scholar]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Herrmann, K. On the Occurrence of Flavonol and Flavone Glycosides in Vegetables. Z. Lebensm. Unters. 1988, 186, 1–5. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.-P. Tissue Localization of Phenolic Compounds in Plants by Confocal Laser Scanning Microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. The ‘Ins’ and ‘Outs’ of Flavonoid Transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J. Flavonoid Transport Mechanisms: How to Go, and with Whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.-M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE Transporter TT12 Acts as a Vacuolar Flavonoid/H+-Antiporter Active in Proanthocyanidin-Accumulating Cells of the Seed Coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef] [Green Version]
- Rea, P.A. Plant ATP-Binding Cassette Transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K.; Djordjevic, M.A. Flavonoids Are Differentially Taken up and Transported Long Distances in Arabidopsis. Plant Physiol. 2007, 145, 478–490. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, R. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Caldwell, M.M.; Flint, S.D. Stratospheric Ozone Reduction, Solar UV-B Radiation and Terrestrial Ecosystems. Clim. Chang. 1994, 28, 375–394. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Lionello, P.; Abrantes, F.; Gacic, M.; Planton, S.; Trigo, R.; Ulbrich, U. The Climate of the Mediterranean Region: Research Progress and Climate Change Impacts. Reg. Environ. Chang. 2014, 14, 1679–1684. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Chang. 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The Threat of Soil Salinity: A European Scale Review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Davis, M.B. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardans, J.; Peñuelas, J. Plant-Soil Interactions in Mediterranean Forest and Shrublands: Impacts of Climatic Change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Matesanz, S.; Valladares, F. Ecological and Evolutionary Responses of Mediterranean Plants to Global Change. Environ. Exp. Bot. 2014, 103, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Sosa, T.; Alías, J.C.; Escudero, J.C.; Chaves, N. Interpopulational Variation in the Flavonoid Composition of Cistus ladanifer L. Exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Ždero-Pavlović, R.; Tumbas-Šaponjac, V.; Čanadanović-Brunet, J.; Orlović, S. Water Stress Induces Changes in Polyphenol Profile and Antioxidant Capacity in Poplar Plants (Populus spp.). Plant Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological Drought Resistance and Accumulation of Leaf Phenolics in White Clover Interspecific Hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Fischbach, R.J.; Kossmann, B.; Panten, H.; Steinbrecher, R.; Heller, W.; Seidlitz, H.K.; Sandermann, H.; Hertkorn, N.; Schnitzler, J.-P. Seasonal Accumulation of Ultraviolet-B Screening Pigments in Needles of Norway Spruce (Picea abies (L.) Karst.). Plant Cell Environ. 1999, 22, 27–37. [Google Scholar] [CrossRef]
- Csepregi, K.; Coffey, A.; Cunningham, N.; Prinsen, E.; Hideg, É.; Jansen, M.A.K. Developmental Age and UV-B Exposure Co-Determine Antioxidant Capacity and Flavonol Accumulation in Arabidopsis Leaves. Environ. Exp. Bot. 2017, 140, 19–25. [Google Scholar] [CrossRef]
- Hectors, K.; Van Oevelen, S.; Geuns, J.; Guisez, Y.; Jansen, M.A.K.; Prinsen, E. Dynamic Changes in Plant Secondary Metabolites during UV Acclimation in Arabidopsis thaliana. Physiol. Plant. 2014, 152, 219–230. [Google Scholar] [CrossRef]
- Sebastiani, F.; Torre, S.; Gori, A.; Brunetti, C.; Centritto, M.; Ferrini, F.; Tattini, M. Dissecting Adaptation Mechanisms to Contrasting Solar Irradiance in the Mediterranean Shrub Cistus Incanus. Int. J. Mol. Sci. 2019, 20, 3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brossa, R.; Casals, I.; Pintó-Marijuan, M.; Fleck, I. Leaf Flavonoid Content in Quercus ilex L. Resprouts and Its Seasonal Variation. Trees 2009, 23, 401–408. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of Secondary Metabolism in Citrus Plants Is Associated to Sensitivity to Combined Drought and High Temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [Green Version]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The Biosynthesis of Flavonoids Is Enhanced Similarly by UV Radiation and Root Zone Salinity in L. vulgare Leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef]
- Jorge, T.F.; Tohge, T.; Wendenburg, R.; Ramalho, J.C.; Lidon, F.C.; Ribeiro-Barros, A.I.; Fernie, A.R.; António, C. Salt-Stress Secondary Metabolite Signatures Involved in the Ability of Casuarina Glauca to Mitigate Oxidative Stress. Environ. Exp. Bot. 2019, 166, 103808. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, J.; Ren, T.; Du, H.; Liu, H.; Li, Y.; Zhang, C. Salt Stress Decreases Seedling Growth and Development but Increases Quercetin and Kaempferol Content in Apocynum venetum. Plant Biol. 2020, 22, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.D.M.; Ruiz, J.M. Differential Responses of Five Cherry Tomato Varieties to Water Stress: Changes on Phenolic Metabolites and Related Enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of Flavonoid Accumulation under Drought Stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe Drought Stress Is Affecting Selected Primary Metabolites, Polyphenols, and Volatile Metabolites in Grapevine Leaves (Vitis vinifera cv. Pinot Noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crop. Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liu, Y.; Cao, B. Regulation of Flavanone 3-Hydroxylase Gene Involved in the Flavonoid Biosynthesis Pathway in Response to UV-B Radiation and Drought Stress in the Desert Plant, Reaumuria soongorica. Plant Physiol. Biochem. 2013, 73, 161–167. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The Effect of Drought Stress on Polyphenolic Compounds and Expression of Flavonoid Biosynthesis Related Genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A Grape BHLH Transcription Factor Gene, VvbHLH1, Increases the Accumulation of Flavonoids and Enhances Salt and Drought Tolerance in Transgenic Arab. thaliana. Plant Cell Tiss. Organ Cult. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- De Simón, B.F.; Sanz, M.; Cervera, M.T.; Pinto, E.; Aranda, I.; Cadahía, E. Leaf Metabolic Response to Water Deficit in Pinus Pinaster Ait. Relies upon Ontogeny and Genotype. Environ. Exp. Bot. 2017, 140, 41–55. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant Molecular Stress Responses Face Climate Change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagnè, D.; Rowan, D.D.; Troggio, M.; et al. High Temperature Reduces Apple Fruit Colour via Modulation of the Anthocyanin Regulatory Complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The Grapevine VviPrx31 Peroxidase as a Candidate Gene Involved in Anthocyanin Degradation in Ripening Berries under High Temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole Plant Temperature Manipulation Affects Flavonoid Metabolism and the Transcriptome of Grapevine Berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Virjamo, V.; Du, W.; Yin, Y.; Nissinen, K.; Nybakken, L.; Guo, H.; Julkunen-Tiitto, R. Effects of Soil Pyrene Contamination on Growth and Phenolics in Norway Spruce (Picea abies) Are Modified by Elevated Temperature and CO2. Env. Sci. Pollut. Res. 2018, 25, 12788–12799. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, K.; Nybakken, L.; Virjamo, V.; Julkunen-Tiitto, R. Slow-Growing Salix Repens (Salicaceae) Benefits from Changing Climate. Environ. Exp. Bot. 2016, 128, 59–68. [Google Scholar] [CrossRef]
- Nissinen, K.; Virjamo, V.; Randriamanana, T.; Sobuj, N.; Sivadasan, U.; Mehtätalo, L.; Beuker, E.; Julkunen-Tiitto, R.; Nybakken, L. Responses of Growth and Leaf Phenolics in European Aspen (Populus tremula) to Climate Change during Juvenile Phase Change. Can. J. For. Res. 2017, 47, 1350–1363. [Google Scholar] [CrossRef]
- Kellomäki, S.; Wang, K.-Y. Growth and Resource Use of Birch Seedlings Under Elevated Carbon Dioxide and Temperature. Ann. Bot. 2001, 87, 669–682. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Salleo, S. Different Strategies of Drought Resistance in Three Mediterranean Sclerophyllous Trees Growing in the Same Environmental Conditions. New Phytol. 1988, 108, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.; Proksch, P.; Gülz, P.-G. Epicuticular Flavonoid Aglycones in the Genus Cistus, Cistaceae. J. Plant Physiol. 1987, 131, 25–36. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Seasonal Variation of Exudate of Cistus ladanifer. J. Chem. Ecol. 1993, 19, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Hidalgo-Galvez, M.D.; Vázquez-González, C.; Pérez-Ramos, I.M. Micro-Climatic Effects on Plant Phenolics at the Community Level in a Mediterranean Savanna. Sci. Rep. 2020, 10, 14757. [Google Scholar] [CrossRef] [PubMed]
- Bornman, J.F.; Barnes, P.W.; Robson, T.M.; Robinson, S.A.; Jansen, M.A.K.; Ballaré, C.L.; Flint, S.D. Linkages between Stratospheric Ozone, UV Radiation and Climate Change and Their Implications for Terrestrial Ecosystems. Photochem. Photobiol. Sci. 2019, 18, 681–716. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, A. Ultraviolet Radiation and Plants: Burning Questions. Plant Cell 1992, 4, 1353–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhardt, K.E.; Lampi, M.A.; Greenberg, B.M. The Effects of Far-red Light on Plant Growth and Flavonoid Accumulation in Brassica Napus in the Presence of Ultraviolet B Radiation. Photochem. Photobiol. 2008, 84, 1445–1454. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional Roles of Flavonoids in Photoprotection: New Evidence, Lessons from the Past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Ryan, K.G.; Markham, K.R.; Bloor, S.J.; Bradley, J.M.; Mitchell, K.A.; Jordan, B.R. UVB Radiation Induced Increase in Quercetin: Kaempferol Ratio in Wild-Type and Transgenic Lines of Petunia. Photochem. Photobiol. 1998, 68, 323–330. [Google Scholar] [CrossRef]
- Ryan, K.G.; Swinny, E.E.; Markham, K.R.; Winefield, C. Flavonoid Gene Expression and UV Photoprotection in Transgenic and Mutant Petunia Leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Tiitto, R.; Lindfors, A.; Aphalo, P.J. Metabolite Specific Effects of Solar UV-A and UV-B on Alder and Birch Leaf Phenolics. Glob. Change Biol. 2008, 14, 1294–1304. [Google Scholar] [CrossRef]
- Osmond, C.B.; Foyer, C.H.; Bock, G.; Grace, S.C.; Logan, B.A. Energy Dissipation and Radical Scavenging by the Plant Phenylpropanoid Pathway. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [Green Version]
- Hectors, K.; van Oevelen, S.; Guisez, Y.; Prinsen, E.; Jansen, M.A. The Phytohormone Auxin Is a Component of the Regulatory System That Controls UV-mediated Accumulation of Flavonoids and UV-induced Morphogenesis. Physiol. Plant. 2012, 145, 594–603. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Env. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Libutti, A.; Cammerino, A.R.B.; Monteleone, M. Risk Assessment of Soil Salinization Due to Tomato Cultivation in Mediterranean Climate Conditions. Water 2018, 10, 1503. [Google Scholar] [CrossRef] [Green Version]
- Grattan, S.R.; Grieve, C.M. Mineral Nutrient Acquisition and Response by Plants Grown in Saline Environments. Handb. Plant Crop Stress 1999, 2, 203–229. [Google Scholar]
- Fageria, N.K.; Gheyi, H.R.; Moreira, A. Nutrient Bioavailability in Salt Affected Soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Romero-Aranda, R.; Soria, T.; Cuartero, J. Tomato Plant-Water Uptake and Plant-Water Relationships under Saline Growth Conditions. Plant Sci. 2001, 160, 265–272. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Huang, J.; Peng, S.; Xiong, D. Diffusional Conductance to CO2 Is the Key Limitation to Photosynthesis in Salt-stressed Leaves of Rice (Oryza sativa). Physiol. Plant. 2018, 163, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Sairam, R.K.; Tyagi, A. Physiology and Molecular Biology of Salinity Stress Tolerance in Plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachâal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt Stress (NaCl) Affects Plant Growth and Branch Pathways of Carotenoid and Flavonoid Biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- Mrázová, A.; Belay, S.A.; Eliášová, A.; Perez-Delgado, C.; Kaducová, M.; Betti, M.; Vega, J.M.; Paľove-Balang, P. Expression, Activity of Phenylalanine-Ammonia-Lyase and Accumulation of Phenolic Compounds in Lotus japonicus under Salt Stress. Biologia 2017, 72, 36–42. [Google Scholar] [CrossRef]
- Li, X.; Kim, Y.B.; Kim, Y.; Zhao, S.; Kim, H.H.; Chung, E.; Lee, J.-H.; Park, S.U. Differential Stress-Response Expression of Two Flavonol Synthase Genes and Accumulation of Flavonols in Tartary Buckwheat. J. Plant Physiol. 2013, 170, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Traversi, M.L.; Castelli, S.; Biricolti, S.; Guidi, L.; Massai, R.; Tattini, M.; Traversi, M.L.; Castelli, S.; Biricolti, S.; et al. Contrasting Response Mechanisms to Root-Zone Salinity in Three Co-Occurring Mediterranean Woody Evergreens: A Physiological and Biochemical Study. Funct. Plant Biol. 2009, 36, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Cruz de Carvalho, M.H. Drought Stress and Reactive Oxygen Species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet Oxygen Production in Photosystem II and Related Protection Mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Symonowicz, M.; Kolanek, M. Flavonoids and Their Properties to Form Chelate Complexes. Biotechnol. Food Sci. 2012. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Jogawat, A. Crosstalk Among Phytohormone Signaling Pathways During Abiotic Stress. In Molecular Plant Abiotic Stress; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 209–220. [Google Scholar]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.; Rubery, P.H. Naturally Occurring Auxin Transport Regulators. Science 1988, 241, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids Act as Negative Regulators of Auxin Transport In Vivo in Arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buer, C.S.; Muday, G.K. The Transparent Testa4 Mutation Prevents Flavonoid Synthesis and Alters Auxin Transport and the Response of Arabidopsis Roots to Gravity and Light. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, B.M.; Geisler, M.; Bigler, L.; Ringli, C. Flavonols Accumulate Asymmetrically and Affect Auxin Transport in Arabidopsis. Plant Physiol. 2011, 156, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Han, K.; Heller, W.; Albert, A.; Dobrev, P.I.; Zažímalová, E.; Schäffner, A.R. Kaempferol 3-O-Rhamnoside-7-O-Rhamnoside Is an Endogenous Flavonol Inhibitor of Polar Auxin Transport in Arabidopsis Shoots. New Phytol. 2014, 201, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Teale, W.D.; Pasternak, T.; Dal Bosco, C.; Dovzhenko, A.; Kratzat, K.; Bildl, W.; Schwörer, M.; Falk, T.; Ruperti, B.; V Schaefer, J. Flavonol-mediated Stabilization of PIN Efflux Complexes Regulates Polar Auxin Transport. EMBO J. 2021, 40, e104416. [Google Scholar] [CrossRef]
- Fernández-Marcos, M.; Sanz, L.; Lewis, D.R.; Muday, G.K.; Lorenzo, O. Control of Auxin Transport by Reactive Oxygen and Nitrogen Species. In Polar Auxin Transport; Chen, R., Baluška, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–117. [Google Scholar]
- Lewis, D.R.; Ramirez, M.V.; Miller, N.D.; Vallabhaneni, P.; Ray, W.K.; Helm, R.F.; Winkel, B.S.J.; Muday, G.K. Auxin and Ethylene Induce Flavonol Accumulation through Distinct Transcriptional Networks. Plant Physiol. 2011, 156, 144–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, W.; Smet, I.D.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Bossche, R.V.; Karimi, M.; Rybel, B.D.; Vanholme, B.; et al. Transcription Factor WRKY23 Assists Auxin Distribution Patterns during Arabidopsis Root Development through Local Control on Flavonol Biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of Auxin Content in Arabidopsis Confers Improved Drought Stress Resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K. Ultraviolet-B Radiation Effects on Plants: Induction of Morphogenic Responses. Physiol. Plant. 2002, 116, 423–429. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic Acid Accumulation Modulates Auxin Transport in the Root Tip to Enhance Proton Secretion for Maintaining Root Growth under Moderate Water Stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic Acid and Abiotic Stress Signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, C.; Sebastiani, F.; Tattini, M. Review: ABA, Flavonols, and the Evolvability of Land Plants. Plant Sci. 2019, 280, 448–454. [Google Scholar] [CrossRef]
- Heine, G.F.; Hernandez, J.M.; Grotewold, E. Two Cysteines in Plant R2R3 MYB Domains Participate in REDOX-Dependent DNA Binding. J. Biol. Chem. 2004, 279, 37878–37885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Song, C.-P. Guard-Cell Signalling for Hydrogen Peroxide and Abscisic Acid. New Phytol. 2008, 178, 703–718. [Google Scholar] [CrossRef] [PubMed]


| Flavonoid Class | Compound Subclass | Number of Hydroxyl Groups | TEAC (3) Value (mM) | Maximum Absorption Wavelength (nm) (4) | |
|---|---|---|---|---|---|
| (1) | (2) | ||||
| Flavonols | Kaempferol | 4 | 1.34 ± 0.08 | 1.98 ± 0.13 | 367 |
| Flavonols | Quercetin | 5 | 4.7 ± 0.1 | 4.30 ± 0.16 | 371 |
| Flavonols | Myricetin | 6 | 3.1 ± 0.30 | 2.45 ± 0.35 | 374 |
| Flavones | Chrysin | 2 | 1.43 ± 0.07 | 0.98 ± 0.04 | 313 |
| Flavones | Apigenin | 3 | 1.45 ± 0.08 | 1.04 ± 0.06 | 337 |
| Flavanones | Naringenin | 3 | 1.53 ± 0.05 | 0.59 ± 0.08 | 289 |
| Flavanonols | Taxifolin | 5 | 1.9 ± 0.03 | 2.43 ± 0.12 | 290 |
| Abiotic Stress | Flavonol Type | Species | Plant Organ | Tissue Localization | Growth Conditions | Measurement Technique | Conclusion/Function | References |
|---|---|---|---|---|---|---|---|---|
| Drought | Kaempferol, quercetin | Arabidopsis thaliana | Not specified (all plant) | Not studied | Growth chamber | LC-PDA-MS | Scavenging radical activity (Quercetin 3-O-glucoside and kaempferol 3-O-glucoside). Quercetins had a higher antioxidant activity than kaempferols. | [105] |
| Drought | Myricetin, kaempferol | Populus spp. | Leaves and root | Not studied | Growth chamber | HPLC-PDA | Antioxidant capacity. | [106] |
| Drought | Kaempferol, quercetin | Trifolium repens L. | Leaves | Not studied | Field conditions | HPLC | Under drought stress, kaempferol glycosides accumulation was related to reduced senescence and to less pronounced decreases in shoot dry weight. | [107] |
| Drought and UV radiation | Quercetin | Fraxinus ornus | Leaves | Mesophyll (in the vacuoles of cells) | Grown outdoors in an experimental plot | Confocal microscope for flavonol localization. HPLC–MS for quantification. | Increase in quercetin 3-O-glucoside in severe drought and excess light stresses. Potential function as H2O2 scavenger. | [38] |
| UV radiation | Kaempferol | Picea abies | Needles | Not studied | Field cabinet experiments | RP-HPLC | Potentially UV-B screening. | [108] |
| UV radiation | Kaempferol, quercetin | Arabidopsis thaliana | Leaves | Not studied | Growth chamber | UPLC-TQD | Antioxidant activity. | [109] |
| UV radiation | Kaempferol, quercetin | Arabidopsis thaliana | Leaves | Not studied | Growth chamber | UPLC-MS | Accumulation of specific flavonol glycosides, i.e., kaempferol and quercetin di- and triglycosides (rhamnosylated) in response to UV-radiation. | [110] |
| UV radiation | Myricetin and quercetin | Cistus incanus L. | Leaves | Not studied | Field conditions | HPLC–DAD | Major light-induced increases observed for myricetin and quercetin derivatives. | [111] |
| Low temperature | Quercetin, kaempferol and rhamnetin | Quercus ilex L. | Leaves | Not studied | Field conditions (forest) | HPLC–MS/MS | High amount of flavonol-hexosides detected in winter. They could contribute to photoprotection. | [112] |
| Heat and drought | Kaempferol, quercetin | Citrus spp. (Cleopatra and Carrizo) | Leaves | Not studied | Greenhouses | UPLC/ESI-QTOF-MS | Combination of heat and drought favours accumulation of kaempferol and quercetin derivatives in poorly-drought tolerant species. | [113] |
| Heat and salinity | Kaempferol, quercetin | Solanum lycopersicon L. | Leaves | Not studied | In vitro (using aerated hydroponic systems containing a modified Hoagland solution) | UHPLC/QTOF-MS | Accumulation of kaempferol and quercetin derivatives leads to lower oxidative damage when plant grow under concomitant heat and salt stress. | [114] |
| Salinity and UV-radiation | Quercetin | Ligustrum vulgare | Leaves | Epidermal, boundary of epidermal and adaxial palisade, and in the palisade parenchyma cell layers | Greenhouses | Epifluorescence microscope and Confocal Laser Scanning Microscope (CLSM) for flavonoids localization. HPLC for quantification. | Increase in quercetin 3-O-glycoside in response to UV-radiation and salinity stress (NaCl). Potential role as antioxidant and photoprotection. | [115] |
| Salinity | Kaempferol, quercetin | Casuarina glauca | Nodules, roots and branchets | Not studied | In vitro (using Broughton and Dillworth’s medium) | LC-HRMS | Kaempferol and quercetin derivatives accumulate in case of severe salt stress and play a key role in protection against oxidative damage. | [116] |
| Salinity | Kaempferol, quercetin | Apocynum venetum L. | Leaves | Not studied | Plant culture room | HPLC | Kaempferol and quercetin accumulation under salt stress. | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laoué, J.; Fernandez, C.; Ormeño, E. Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress. Plants 2022, 11, 172. https://doi.org/10.3390/plants11020172
Laoué J, Fernandez C, Ormeño E. Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress. Plants. 2022; 11(2):172. https://doi.org/10.3390/plants11020172
Chicago/Turabian StyleLaoué, Justine, Catherine Fernandez, and Elena Ormeño. 2022. "Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress" Plants 11, no. 2: 172. https://doi.org/10.3390/plants11020172
APA StyleLaoué, J., Fernandez, C., & Ormeño, E. (2022). Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress. Plants, 11(2), 172. https://doi.org/10.3390/plants11020172







