Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]
Abstract
1. Introduction
2. Results
2.1. Carotenoids Evaluation
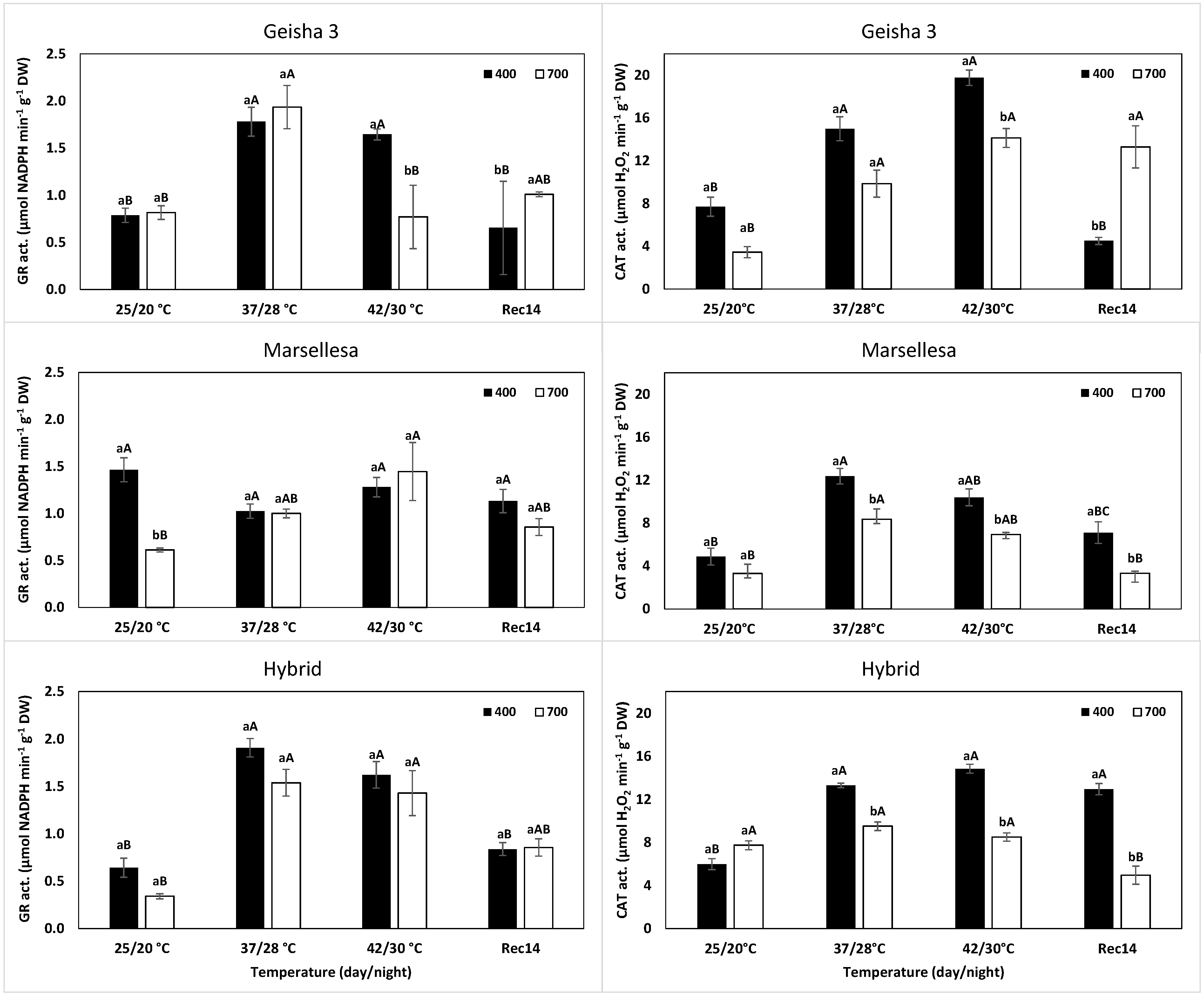
2.2. Antioxidant Enzyme Activity
2.3. Expression of Selected Genes Associated with Protective Roles
3. Materials and Methods
3.1. Plant Material, Growth Conditions and Experimental Design
3.2. Temperature Rise Implementation
3.3. Carotenoids Evaluation
3.4. Activity of Antioxidative Enzymes
3.5. Expression of Genes Associated with Antioxidant and Protective Molecules
3.6. Statistical Analysis
4. Discussion
4.1. Photoprotective Pigments
4.1.1. Xanthophylls
4.1.2. Carotenes
4.2. Antioxidant Enzyme Responses and the Associated Gene Expression
4.3. Expression of Genes Associated with Other Protective Molecules
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: The Physical Science Basis; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- IPCC. Climate Change 2007: The physical science basis. In Summary for Policymakers; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A.S., Connors, L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Pais, I.P.; Reboredo, F.H.; Ramalho, J.C.; Pessoa, M.F.; Lidon, F.C.; Silva, M.M. Potential impacts of climate change on agriculture—A review. Emir. J. Food Agric. 2020, 32, 397–407. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. Synthesis Report. 2009. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 1 October 2021).
- FAO. Looking Ahead in World Food and Agriculture: Perspectives to 2050; Conforti, P., Ed.; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- FAO. The State of Food and Agriculture Climate Change, Agriculture and Food Security; Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Peer, L.A.; Dar, Z.A.; Lone, A.A.; Bhat, M.Y.; Ahamad, N. High temperature triggered plant responses from whole plant to cellular level. Plant Physiol. Rep. 2020, 25, 611–626. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Pais, I.P.; Ribeiro-Barros, A.I.; Martins, L.D.; Tomaz, M.A.; Rodrigues, W.P.; Campostrini, E.; Semedo, J.N.; Fortunato, A.S.; Martins, M.Q.; et al. Lipid profile adjustments may contribute to warming acclimation and to heat impact mitigation by elevated [CO2] in Coffea spp. Environ. Exp. Bot. 2019, 167, 103856. [Google Scholar] [CrossRef]
- Dubberstein, D.; Lidon, F.C.; Rodrigues, A.P.; Semedo, J.N.; Marques, I.; Rodrigues, W.P.; Gouveia, D.; Armengaud, J.; Semedo, M.C.; Martins, S.; et al. Resilient and sensitive key points of the photosynthetic machinery of Coffea spp. to the single and superimposed exposure to severe drought and heat stresses. Front. Plant Sci. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Liu, H.L.; Lee, Z.X.; Chuang, T.W.; Wu, H.C. Effect of heat stress on oxidative damage and antioxidant defense system in white clover (Trifolium repens L.). Planta 2021, 254, 103. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Nash, D.; Miyao, M.; Murata, N. Heat inactivation of oxygen evolution in Photosystem II particles and its acceleration by chloride depletion and exogenous manganese. BBA Bioenerg. 1985, 807, 127–133. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Velitchkova, M.Y.; Allakhverdiev, S.I.; Huner, N.P.A. Heat stress-induced effects of photosystem I: An overview of structural and functional responses. Photosynth. Res. 2017, 133, 17–30. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. USA 2000, 97, 13430–13435. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Silva, J.R.; Ferreira, L.S.; Filho, J.A.M.; Figueiredo, F.A.M.M.A.; Ferraz, T.M.; Bernado, W.P.; Bezerra, B.S.; de Abreu, D.P.; Cespom, L.; et al. Stomatal and photochemical limitations of photosynthesis in coffee (Coffea spp.) plants subjected to elevated temperatures. Crop Pasture Sci. 2018, 69, 317–325. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Fortunato, A.S.; Lidon, F.C.; Batista-Santos, P.; Leitão, A.E.; Pais, I.P.; Ribeiro, A.I.; Ramalho, J.C. Biochemical and molecular characterization of the antioxidative system of Coffea sp. under cold conditions in genotypes with contrasting tolerance. J. Plant Physiol. 2010, 167, 333–342. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Rodrigues, A.P.; Lidon, F.C.; Marques, L.M.C.; Leitão, A.E.; Fortunato, A.S.; Pais, I.P.; Silva, M.J.; Scotti-Campos, P.; Lopes, A.; et al. Stress cross-response of the antioxidative system promoted by superimposed drought and cold conditions in Coffea spp. PLoS ONE 2018, 13, e0198694. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Martins, S.; Semedo, M.C.; Silva, M.J.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. can be strengthened by elevated air CO2. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef]
- Marques, I.; Gouveia, D.; Gaillard, J.-C.; Martins, S.; Semedo, M.C.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; Armengaud Ramalho, J.C. Next-generation proteomics reveals a greater antioxidative response to drought in Coffea arabica than in Coffea canephora. Agronomy 2022, 12, 148. [Google Scholar] [CrossRef]
- Marques, I.; Rodrigues, A.P.; Gouveia, D.; Lidon, F.C.; Martins, S.; Semedo, M.C.; Gaillard, J.-C.; Pais, I.P.; Semedo, J.N.; Scotti-Campos, P.; et al. High-resolution shotgun proteomics reveals that increased air [CO2] amplifies the acclimation response of Coffea species to drought regarding antioxidative, energy, sugar, and lipid dynamics. J. Plant Physiol. 2022, 276, 153788. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.C.; Campos, P.S.; Teixeira, M.; Nunes, M.A. Nitrogen dependent changes in antioxidant system and in fatty acid composition of chloroplast membranes from Coffea arabica L. plants submitted to high irradiance. Plant Sci. 1998, 135, 115–124. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Quartin, V.L.; Leitão, E.; Campos, P.S.; Carelli, M.L.C.; Fahl, J.I.; Nunes, M.A. Cold acclimation ability and photosynthesis among species of the tropical Coffea genus. Plant Biol. 2003, 5, 631–641. [Google Scholar] [CrossRef]
- Batista-Santos, P.; Lidon, F.C.; Fortunato, A.; Leitão, A.E.; Lopes, E.; Partelli, F.; Ribeiro, A.I.; Ramalho, J.C. The impact of cold on photosynthesis in genotypes of Coffea spp.–Photosystem sensitivity, photoprotective mechanisms and gene expression. J. Plant Physiol. 2011, 168, 792–806. [Google Scholar] [CrossRef]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitão, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Reboredo, F.H.; Partelli, F.L.; et al. Protective response mechanisms to heat stress in interaction with high [CO2] conditions in Coffea spp. Front. Plant Sci. 2016, 7, 947. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; David, P.H.C.; Paulo, O.S.; Goulao, L.F.; Fortunato, A.S.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Transcriptomic leaf profiling reveals differential responses of the two most traded coffee species to elevated [CO2]. Int. J. Mol. Sci. 2020, 21, 9211. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; Paulo, O.S.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. A transcriptomic approach to understanding the combined impacts of supra-optimal temperatures and CO2 revealed different responses in the polyploid Coffea arabica and its diploid progenitor C. canephora. Int. J. Mol. Sci. 2021, 22, 3125. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Rahn, E.; Läderach, P.; Ghini, R.; Ramalho, J.C. Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim. Chang. 2019, 152, 167–178. [Google Scholar] [CrossRef]
- Osorio, N. The Global Coffee Crisis: A Threat to Sustainable Development. International Coffee Organization, London. Submission to the World Summit on Sustainable Development, Johannesburg. 2002. Available online: https://www.ico.org/documents/ed1849.pdf (accessed on 2 May 2022).
- Martins, M.Q.; Fortunato, A.S.; Rodrigues, W.P.; Partelli, F.L.; Campostrini, E.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Selection and validation of reference genes for accurate RT-qPCR data normalization in Coffea spp. under a climate changes context of interacting elevated [CO2] and temperature. Front. Plant Sci. 2017, 8, 307. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Rigal, C.; Vaast, P.; Ramalho, J.C.; et al. Shaded-coffee: A nature-based strategy for coffee production under climate change? A review. Front. Sustain. Food Syst. 2022, 6, 877476. [Google Scholar] [CrossRef]
- ICO—International Coffee Organisation. Coffee Price Rise Continues in November Reaching a 10-Year High. 2021. Available online: athttps://www.ico.org/documents/cy2021-22/cmr-1121-e.pdf (accessed on 3 January 2022).
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and agronomic performance of the coffee crop in the context of climate change and global warming: A review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Santos, C.; Leitão, A.; Pais, I.; Lidon, F.; Ramalho, J. Perspectives on the potential impacts of climate changes on coffee plant and bean quality. Emir. J. Food Agric. 2015, 27, 152–163. [Google Scholar] [CrossRef]
- Assad, E.D.; Pinto, H.S.; Zullo, J., Jr.; Ávila, A.M.H. Climatic changes impact in agroclimatic zonning of coffee in Brazil. Pesqui. Agropecu. Bras. 2004, 39, 1057–1064. [Google Scholar] [CrossRef]
- Bunn, C.; Läderach, P.; Ovalle Rivera, O.; Kirschke, D. A bitter cup: Climate change profile of global production of Arabica and Robusta coffee. Clim. Chang. 2015, 129, 89–101. [Google Scholar] [CrossRef]
- Craparo, A.C.W.; Van Asten, P.J.A.; Läderach, P.; Jassogne, L.T.P.; Grab, S.W. Coffea arabica yields decline in Tanzania due to climate change: Global implications. Agric. For. Meteorol. 2015, 207, 1–10. [Google Scholar] [CrossRef]
- Läderach, P.; Ramirez-Villegas, J.; Navarro-Racines, C.; Zelaya, C.; Martinez–Valle, A.; Jarvis, A. Climate change adaptation of coffee production in space and time. Clim. Chang. 2017, 141, 47–62. [Google Scholar] [CrossRef]
- Alégre, C. Climates et caféiers d’Arabie. Agron. Trop. 1959, 14, 23–58. [Google Scholar]
- Camargo, A.P. O clima e a cafeicultura no Brasil. Inf. Agropecuário 1985, 11, 13–26. [Google Scholar]
- Drinnan, J.E.; Menzel, C.M. Temperature affects vegetative growth and flowering of coffee (Coffea arabica L.). J. Hortic. Sci. 1995, 70, 25–34. [Google Scholar] [CrossRef]
- Martins, L.D.; Tomaz, M.A.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C. Combined effects of elevated [CO2] and high temperature on leaf mineral balance in Coffea spp. plants. Clim. Chang. 2014, 126, 365–379. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Ribeiro, T.H.C.; Cardon, C.H.; Fedenia, L.; Maia, V.A.; Barbosa, B.C.F.; Caldeira, C.F.; Klein, P.E.; Chalfun-Junior, A. Elevated temperatures impose transcriptional constraints and elicit intraspecific differences between coffee genotypes. Front. Plant Sci. 2020, 11, 1113. [Google Scholar] [CrossRef]
- Camargo, M.B.P. The impact of climatic variability and climate change on arabic coffee crop in Brazil. Bragantia 2010, 69, 239–247. [Google Scholar] [CrossRef]
- Nunes, M.A.; Bierhuizen, J.F.; Ploegman, C. Studies on productivity of coffee. I. Effect of light, temperature and CO2 concentration on photosynthesis of Coffea arabica. Acta Bot. Neerl. 1968, 17, 93–102. [Google Scholar] [CrossRef]
- Verhage, F.Y.F.; Anten, N.P.R.; Sentelhas, P.C. Carbon dioxide fertilization offsets negative impacts of climate change on arabica coffee yield in Brazil. Clim. Chang. 2017, 144, 671–685. [Google Scholar] [CrossRef]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and hybrid crop breeding: A multidisciplinary review. Front. Genet. 2021, 12, 643761. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.C.; Rodrigues, A.P.; Semedo, J.N.; Pais, I.P.; Martins, L.D.; Simões-Costa, M.C.; Leitão, A.E.; Fortunato, A.S.; Batista-Santos, P.; Palos, I.M.; et al. Sustained photosynthetic performance of Coffea spp. under long-term enhanced [CO2]. PLoS ONE 2013, 8, e82712. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Marques, N.C.; Semedo, J.N.; Matos, M.C.; Quartin, V.L. Photosynthetic performance and pigment composition of leaves from two tropical species is determined by light quality. Plant Biol. 2002, 4, 112–120. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. BBA Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 713–736. [Google Scholar] [CrossRef]
- Pogson, B.J.; Niyogi, K.K.; Björkman, O.; DellaPenna, D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc. Natl. Acad. Sci. USA 1998, 95, 13324–13329. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Björkman, O.; Grossman, A.R. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef]
- Logan, B.A. Reactive oxygen species and photosynthesis. In Antioxidants and Reactive Oxygen in Plants; Smirnoff, N., Ed.; Blackwell Publishing: Oxford, UK, 2005; pp. 250–267. [Google Scholar]
- Kalituho, L.; Rech, J.; Jahns, P. The roles of specific xanthophylls in light utilization. Planta 2007, 225, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Chen, Y.-X.; Caliandro, R.; Govindjee Clegg, R.M. Photosystem II fluorescence life time imaging in avocado leaves: Contributions of the lutein-epoxide and violaxanthin cycles to fluorescence quenching. J. Photochem. Photobiol. B 2011, 104, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Giossi, C.; Cartaxana, P.; Cruz, S. Photoprotective role of neoxanthin in plants and algae. Molecules 2020, 25, 4617. [Google Scholar] [CrossRef]
- Johnson, G.N.; Scholes, J.D.; Horton, P.; Young, A.J. Relationships between carotenoid composition and growth habit in British plant species. Plant Cell Environ. 1993, 16, 681–686. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- De Las Rivas, J.; Telfer, A.; Barber, J. Two coupled β-carotene molecules protect P680 from photodamage in isolated Photosystem II reaction centres. Biochim. Biophys. Acta (BBA) Bioenerg. 1993, 1142, 155–164. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, D.; Cramer, W.A. Stoichiometrically bound β-carotene in the cytochrome b6f complex of oxygenic photosynthesis protects against oxygen damage. J. Biol. Chem. 1999, 274, 1581–1587. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Jorge, T.; Osorio, S.; Pott, D.M.; Lidon, F.C.; DaMatta, F.M.; Marques, I.; Ribeiro-Barros, A.I.; Ramalho, J.C.; António, C. Primary metabolite profile changes in Coffea spp. promoted by single and combined exposure to drought and elevated CO2 concentration. Metabolites 2021, 11, 427. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the impact of drought in Coffea genotypes: Transcriptomic analysis supports a common high resilience to moderate water deficit but a genotype dependent sensitivity to severe water deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdatiand, K., Leslie, C., Eds.; InTech: Milton, QS, Canada, 2013; pp. 169–205. [Google Scholar]
- Feierabend, J. Catalases in plants: Molecular and functional properties and role in stress defense. In Antioxidants and Reactive Oxygen in Plants; Smirnoff, N., Ed.; Blackwell Publishing: Oxford, UK, 2005; pp. 99–140. [Google Scholar]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Abiotic stress, generation of reactive oxygen species, and their consequences: An overview. In Reactive Oxygen Species in Plants: Boon or Bane—Revisiting the Role of ROS; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 23–50. [Google Scholar] [CrossRef]
- Zhang, S.-G.; Han, S.-Y.; Yang, W.-H.; Wei, H.-L.; Zhang, M.; Qi, L.-W. Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tissue Organ Cult. 2010, 100, 21–29. [Google Scholar] [CrossRef]
- Erice, G.; Aranjuelo, I.; Irigoyen, J.J.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and limited water supply on antioxidant status during regrowth of nodulated alfalfa. Physiol. Plant. 2007, 130, 33–45. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Zinta, G.; AbdElgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.G.; Ju, Z.; Van Santen, E.; Qiu, J.; Weaver, D.B.; Prior, S.A.; Rogers, H.H. The influence of elevated CO2 on the activities of antioxidative enzymes in two soybean genotypes. Aust. J. Plant Physiol. 2000, 27, 1061–1068. [Google Scholar] [CrossRef]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Zimmerman, J.L. Introduction of the carrot HSP17.7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant Cell Environ. 2006, 29, 95–104. [Google Scholar] [CrossRef]
- Boston, R.S.; Viitanen, P.V.; Vierling, E. Molecular chaperones and protein folding in plants. Plant Mol. Biol. 1996, 32, 191–222. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Mao, J.; Chia, W.; Ouyang, M.; He, B.; Chen, F.; Zhang, L. PAB is an assembly chaperone that functions downstream of chaperonin 60 in the assembly of chloroplast ATP synthase coupling factor 1. Proc. Natl. Acad. Sci. USA 2015, 112, 4152–4157. [Google Scholar] [CrossRef]
- Montané, M.H.; Dreyer, S.; Triantaphylides, C.; Kloppstech, K. Early light-inducible proteins during long-term acclimation of barley to photooxidative stress caused by light and cold: High level of accumulation by posttranscriptional regulation. Planta 1997, 202, 293–302. [Google Scholar] [CrossRef]
- Hutin, C.; Nussaume, L.; Moise, N.; Moya, I.; Kloppstech, K.; Havaux, M. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl. Acad. Sci. USA 2003, 100, 4921–4926. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yang, H.; Liang, Y.; Li, X.; Oliver, M.J.; Zhang, D. Functional aspects of early light-induced protein (ELIP) genes from the desiccation-tolerant moss Syntrichia caninervis. Int. J. Mol. Sci. 2020, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Anaraki, Z.E.; Tafreshi, S.A.H.; Shariati, M. Transient silencing of heat shock proteins showed remarkable roles or HSP70 during adaptation to stress in plants. Environ. Exp. Bot. 2018, 155, 142–157. [Google Scholar] [CrossRef]
- Panchuk, I.I.; Volkov, R.A.; Schöffl, F. Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002, 129, 838–853. [Google Scholar] [CrossRef] [PubMed]
- Cottee, N.S.; Wilson, I.W.; Tan, D.K.Y.; Bange, M.P. Understanding the molecular events underpinning cultivar differences in the physiological performance and heat tolerance of cotton (Gossypium hirsutum). Funct. Plant Biol. 2014, 41, 56–67. [Google Scholar] [CrossRef]
- Cassamo, C.T.; Draper, D.; Romeiras, M.M.; Marques, I.; Chiulele, R.; Rodrigues, M.; Stalmans, M.; Partelli, F.L.; Ribeiro-Barros, A.; Ramalho, J.C. Impact of climate changes in the suitable areas for Coffea arabica L. production in Mozambique: Agroforestry as an alternative management system to strengthen crop sustainability. Agric. Ecosyst. Environ. 2022. submitted. [Google Scholar]

| Pigment | Genotype | [CO2] (µL L−1) | Temperature (Day/Night) | |||||
|---|---|---|---|---|---|---|---|---|
| 25/20 °C | 31/25 °C | 37/28 °C | 42/30 °C | Rec4 | Rec14 | |||
| Neoxanthin (mg g−1 DW) | Geisha 3 | 400 | 0.195 ± 0.004 aC | 0.293 ± 0.014 aA | 0.269 ± 0.004 aAB | 0.178 ± 0.012 aC | 0.222 ± 0.010 aBC | 0.201 ± 0.028 aC |
| 700 | 0.180 ± 0.019 aA | 0.168 ± 0.020 bA | 0.227 ± 0.025 aA | 0.197 ± 0.006 aA | 0.170 ± 0.014 aA | 0.182 ± 0.020 aA | ||
| Marsellesa | 400 | 0.270 ± 0.039 bA | 0.298 ± 0.041 aA | 0.315 ± 0.015 aA | 0.215 ± 0.042 aA | 0.329 ± 0.019 aA | 0.234 ± 0.022 aA | |
| 700 | 0.404 ± 0.024 aA | 0.363 ± 0.033 aA | 0.368 ± 0.034 aA | 0.321 ± 0.014 aA | 0.304 ± 0.012 aA | 0.295 ± 0.014 aA | ||
| Hybrid | 400 | 0.245 ± 0.003 aB | 0.291 ± 0.025 aAB | 0.346 ± 0.030 aA | 0.219 ± 0.022 aB | 0.219 ± 0.015 aB | 0.236 ± 0.016 aB | |
| 700 | 0.251 ± 0.027 aAB | 0.254 ± 0.019 aAB | 0.236 ± 0.017 bAB | 0.291 ± 0.013 aA | 0.178 ± 0.011 aB | 0.221 ± 0.011 aAB | ||
| Violaxanthin (mg g−1 DW) | Geisha 3 | 400 | 0.203 ± 0.008 aBC | 0.360 ± 0.013 aA | 0.247 ± 0.014 aB | 0.093 ± 0.010 aD | 0.154 ± 0.011 aCD | 0.187 ± 0.023 aBC |
| 700 | 0.178 ± 0.028 aAB | 0.205 ± 0.032 bA | 0.221 ± 0.038 aA | 0.139 ± 0.004 aB | 0.157 ± 0.009 aAB | 0.148 ± 0.009 aB | ||
| Marsellesa | 400 | 0.299 ± 0.050 aA | 0.326 ± 0.050 bA | 0.327 ± 0.016 aA | 0.132 ± 0.031 bB | 0.195 ± 0.008 aAB | 0.182 ± 0.026 aAB | |
| 700 | 0.435 ± 0.032 aA | 0.467 ± 0.031 aA | 0.385 ± 0.032 aAB | 0.276 ± 0.031 aBC | 0.219 ± 0.013 aC | 0.192 ± 0.027 aC | ||
| Hybrid | 400 | 0.270 ± 0.015 aB | 0.351 ± 0.044 aAB | 0.356 ± 0.019 aA | 0.153 ± 0.010 bC | 0.154 ± 0.029 aC | 0.205 ± 0.019 aBC | |
| 700 | 0.246 ± 0.027 aAB | 0.334 ± 0.014 aA | 0.263 ± 0.018 aAB | 0.267 ± 0.022 aAB | 0.120 ± 0.028 aB | 0.169 ± 0.011 aAB | ||
| Antheraxanthin (mg g−1 DW) | Geisha 3 | 400 | 0.058 ± 0.005 aA | 0.043 ± 0.010 aA | 0.062 ± 0.005 aA | 0.048 ± 0.004 aA | 0.045 ± 0.002 aA | 0.021 ± 0.000 aB |
| 700 | 0.059 ± 0.004 aA | 0.059 ± 0.003 aA | 0.066 ± 0.004 aA | 0.040 ± 0.008 aAB | 0.026 ± 0.009 aB | 0.028 ± 0.003 aB | ||
| Marsellesa | 400 | 0.046 ± 0.012 aA | 0.044 ± 0.014 aA | 0.064 ± 0.007 aA | 0.064 ± 0.011 aA | 0.073 ± 0.006 aA | 0.041 ± 0.009 aA | |
| 700 | 0.067 ± 0.008 aA | 0.043 ± 0.010 aA | 0.069 ± 0.016 aA | 0.060 ± 0.007 aA | 0.048 ± 0.009 aA | 0.040 ± 0.008 aA | ||
| Hybrid | 400 | 0.039 ± 0.002 aB | 0.054 ± 0.015 aAB | 0.056 ± 0.012 aAB | 0.077 ± 0.013 aA | 0.069 ± 0.003 aAB | 0.062 ± 0.005 aAB | |
| 700 | 0.047 ± 0.007 aA | 0.038 ± 0.006 aA | 0.059 ± 0.008 aA | 0.040 ± 0.006 aA | 0.042 ± 0.003 aA | 0.046 ± 0.004 aA | ||
| Zeaxanthin (mg g−1 DW) | Geisha 3 | 400 | 0.094 ± 0.005 aA | 0.030 ± 0.014 aB | 0.092 ± 0.014 aA | 0.097 ± 0.015 aA | 0.049 ± 0.019 aAB | 0.008 ± 0.001 aB |
| 700 | 0.057 ± 0.002 aAB | 0.066 ± 0.006 aAB | 0.094 ± 0.010 aAB | 0.056 ± 0.017 aAB | 0.034 ± 0.006 aB | 0.023 ± 0.004 aB | ||
| Marsellesa | 400 | 0.015 ± 0.002 aB | 0.013 ± 0.003 aB | 0.043 ± 0.011 aB | 0.097 ± 0.026 aAB | 0.144 ± 0.035 aA | 0.123 ± 0.058 aA | |
| 700 | 0.089 ± 0.022 aAB | 0.026 ± 0.007 aB | 0.018 ± 0.005 aB | 0.097 ± 0.033 aAB | 0.084 ± 0.036 aAB | 0.182 ± 0.073 aA | ||
| Hybrid | 400 | 0.051 ± 0.007 aB | 0.061 ± 0.011 aB | 0.063 ± 0.016 aB | 0.114 ± 0.034 aB | 0.228 ± 0.040 aA | 0.114 ± 0.037 aB | |
| 700 | 0.066 ± 0.028 aB | 0.022 ± 0.008 aB | 0.037 ± 0.010 aB | 0.029 ± 0.005 aB | 0.179 ± 0.054 aA | 0.106 ± 0.026 aAB | ||
| V + A + Z (mg g−1 DW) | Geisha 3 | 400 | 0.355 ± 0.013 aAB | 0.433 ± 0.031 aA | 0.401 ± 0.030 aA | 0.239 ± 0.025 aB | 0.248 ± 0.020 aB | 0.216 ± 0.022 aB |
| 700 | 0.380 ± 0.039 aAB | 0.343 ± 0.031 aAB | 0.381 ± 0.033 aA | 0.235 ± 0.028 aB | 0.217 ± 0.007 aB | 0.198 ± 0.014 aB | ||
| Marsellesa | 400 | 0.360 ± 0.061 bA | 0.383 ± 0.061 aA | 0.433 ± 0.031 aA | 0.272 ± 0.044 aA | 0.412 ± 0.042 aA | 0.346 ± 0.043 aA | |
| 700 | 0.592 ± 0.051 aA | 0.536 ± 0.035 aAB | 0.473 ± 0.049 aAB | 0.434 ± 0.019 aAB | 0.351 ± 0.033 aB | 0.414 ± 0.059 aAB | ||
| Hybrid | 400 | 0.360 ± 0.020 aA | 0.466 ± 0.062 aA | 0.475 ± 0.031 aA | 0.344 ± 0.050 aA | 0.451 ± 0.025 aA | 0.381 ± 0.023 aA | |
| 700 | 0.387 ± 0.043 aA | 0.394 ± 0.025 aA | 0.360 ± 0.026 aA | 0.336 ± 0.025 aA | 0.341 ± 0.026 aA | 0.321 ± 0.020 aA | ||
| DEPS | Geisha 3 | 400 | 0.345 ± 0.014 aAB | 0.105 ± 0.030 aC | 0.299 ± 0.024 aAB | 0.499 ± 0.034 aA | 0.272 ± 0.055 aB | 0.093 ± 0.013 aC |
| 700 | 0.305 ± 0.030 aA | 0.282 ± 0.026 aA | 0.354 ± 0.049 aA | 0.293 ± 0.053 aA | 0.212 ± 0.032 aA | 0.178 ± 0.012 aA | ||
| Marsellesa | 400 | 0.116 ± 0.008 aB | 0.098 ± 0.015 aB | 0.163 ± 0.021 aB | 0.481 ± 0.072 aA | 0.402 ± 0.051 aAB | 0.320 ± 0.115 aAB | |
| 700 | 0.202 ± 0.024 aAB | 0.088 ± 0.018 aB | 0.105 ± 0.017 aB | 0.280 ± 0.072 aA | 0.257 ± 0.076 aA | 0.383 ± 0.124 aAB | ||
| Hybrid | 400 | 0.193 ± 0.008 aB | 0.189 ± 0.022 aB | 0.187 ± 0.020 aB | 0.383 ± 0.064 aAB | 0.572 ± 0.075 aA | 0.352 ± 0.072 aAB | |
| 700 | 0.203 ± 0.045 aB | 0.097 ± 0.021 aB | 0.178 ± 0.032 aB | 0.145 ± 0.022 bB | 0.524 ± 0.120 aA | 0.367 ± 0.066 aAB | ||
| Lutein (mg g−1 DW) | Geisha 3 | 400 | 0.601 ± 0.007 aC | 0.833 ± 0.027 aB | 0.780 ± 0.033 aB | 0.756 ± 0.043 aB | 1.036 ± 0.058 aA | 0.809 ± 0.104 aB |
| 700 | 0.534 ± 0.042 aBC | 0.503 ± 0.042 bC | 0.650 ± 0.052 aAB | 0.710 ± 0.032 aA | 0.649 ± 0.043 bAB | 0.646 ± 0.071 bAB | ||
| Marsellesa | 400 | 0.779 ± 0.109 bBC | 0.842 ± 0.107 aBC | 0.945 ± 0.051 aB | 0.612 ± 0.067 bC | 1.427 ± 0.045 aA | 0.886 ± 0.076 aBC | |
| 700 | 1.198 ± 0.085 aA | 1.010 ± 0.072 aA | 1.030 ± 0.081 aA | 1.153 ± 0.054 aA | 1.148 ± 0.075 aA | 1.101 ± 0.075 aA | ||
| Hybrid | 400 | 0.731 ± 0.020 aB | 0.913 ± 0.069 aAB | 1.021 ± 0.068 aA | 0.919 ± 0.074 aAB | 1.054 ± 0.068 aA | 1.026 ± 0.035 aA | |
| 700 | 0.742 ± 0.105 aB | 0.706 ± 0.057 aB | 0.683 ± 0.042 bB | 1.042 ± 0.049 aA | 0.807 ± 0.035 bAB | 0.907 ± 0.070 aA | ||
| α-carotene (mg g−1 DW) | Geisha 3 | 400 | 0.129 ± 0.011 aB | 0.265 ± 0.014 aA | 0.256 ± 0.009 aA | 0.074 ± 0.010 aB | 0.071 ± 0.010 aB | 0.046 ± 0.005 aB |
| 700 | 0.120 ± 0.019 aB | 0.113 ± 0.030 bB | 0.224 ± 0.039 aA | 0.094 ± 0.002 aB | 0.056 ± 0.012 aB | 0.062 ± 0.016 aB | ||
| Marsellesa | 400 | 0.225 ± 0.048 aAB | 0.279 ± 0.047 aA | 0.319 ± 0.034 aA | 0.121 ± 0.040 aB | 0.187 ± 0.028 aAB | 0.109 ± 0.026 aB | |
| 700 | 0.364 ± 0.036 aA | 0.306 ± 0.041 aAB | 0.306 ± 0.058 aAB | 0.240 ± 0.027 aAB | 0.190 ± 0.017 aB | 0.117 ± 0.017 aB | ||
| Hybrid | 400 | 0.156 ± 0.017 aBC | 0.278 ± 0.037 aA | 0.255 ± 0.014 aAB | 0.107 ± 0.020 bC | 0.055 ± 0.010 aC | 0.072 ± 0.015 aC | |
| 700 | 0.205 ± 0.038 aA | 0.223 ± 0.041 aA | 0.209 ± 0.025 aAB | 0.223 ± 0.026 aA | 0.060 ± 0.012 aB | 0.080 ± 0.009 aB | ||
| β-carotene (mg g−1 DW) | Geisha 3 | 400 | 0.314 ± 0.016 aC | 0.513 ± 0.023 aA | 0.407 ± 0.009 aB | 0.322 ± 0.025 aBC | 0.277 ± 0.016 aC | 0.291 ± 0.051 aC |
| 700 | 0.286 ± 0.016 aA | 0.298 ± 0.033 bA | 0.374 ± 0.032 aA | 0.317 ± 0.018 aA | 0.251 ± 0.025 aA | 0.254 ± 0.049 aA | ||
| Marsellesa | 400 | 0.353 ± 0.070 aAB | 0.378 ± 0.075 aAB | 0.488 ± 0.027 aA | 0.233 ± 0.048 bB | 0.439 ± 0.030 aAB | 0.367 ± 0.035 aAB | |
| 700 | 0.522 ± 0.042 aA | 0.561 ± 0.046 aA | 0.528 ± 0.079 aA | 0.483 ± 0.030 aA | 0.456 ± 0.015 aA | 0.427 ± 0.028 aA | ||
| Hybrid | 400 | 0.371 ± 0.013 aAB | 0.475 ± 0.047 aA | 0.421 ± 0.016 aAB | 0.381 ± 0.027 aAB | 0.306 ± 0.018 aB | 0.361 ± 0.014 aAB | |
| 700 | 0.289 ± 0.043 aB | 0.392 ± 0.016 aAB | 0.433 ± 0.034 aA | 0.331 ± 0.042 aAB | 0.312 ± 0.024 aAB | 0.360 ± 0.012 aAB | ||
| (α + β) carotene (mg g−1 DW) | Geisha 3 | 400 | 0.443 ± 0.015 aB | 0.778 ± 0.035 aA | 0.662 ± 0.008 aA | 0.396 ± 0.032 aB | 0.348 ± 0.021 aB | 0.336 ± 0.056 aB |
| 700 | 0.406 ± 0.033 aB | 0.411 ± 0.062 bB | 0.598 ± 0.071 aA | 0.411 ± 0.018 aAB | 0.306 ± 0.037 aB | 0.317 ± 0.063 aB | ||
| Marsellesa | 400 | 0.578 ± 0.115 aAB | 0.657 ± 0.121 aAB | 0.807 ± 0.060 aA | 0.355 ± 0.087 bB | 0.626 ± 0.057 aAB | 0.476 ± 0.059 aB | |
| 700 | 0.886 ± 0.057 aA | 0.867 ± 0.081 aA | 0.834 ± 0.132 aA | 0.722 ± 0.033 aA | 0.645 ± 0.028 aA | 0.544 ± 0.043 aA | ||
| Hybrid | 400 | 0.528 ± 0.024 aB | 0.753 ± 0.083 aA | 0.676 ± 0.022 aAB | 0.488 ± 0.046 aBC | 0.362 ± 0.028 aC | 0.433 ± 0.022 aC | |
| 700 | 0.494 ± 0.081 aAB | 0.615 ± 0.051 aAB | 0.642 ± 0.058 aA | 0.554 ± 0.045 aAB | 0.372 ± 0.019 aB | 0.440 ± 0.010 aB | ||
| (α/β) carotene (g g−1 DW) | Geisha 3 | 400 | 0.426 ± 0.052 aB | 0.518 ± 0.018 aAB | 0.633 ± 0.035 aA | 0.228 ± 0.025 aC | 0.259 ± 0.037 aC | 0.175 ± 0.024 aC |
| 700 | 0.404 ± 0.052 aAB | 0.346 ± 0.054 bB | 0.576 ± 0.056 aA | 0.301 ± 0.022 aBC | 0.207 ± 0.034 aC | 0.255 ± 0.031 aBC | ||
| Marsellesa | 400 | 0.658 ± 0.079 aAB | 0.764 ± 0.058 aA | 0.642 ± 0.040 aAB | 0.424 ± 0.074 aBC | 0.411 ± 0.038 aBC | 0.271 ± 0.049 aC | |
| 700 | 0.718 ± 0.077 aA | 0.545 ± 0.070 aAB | 0.597 ± 0.069 aAB | 0.522 ± 0.086 aB | 0.415 ± 0.035 aBC | 0.267 ± 0.026 aC | ||
| Hybrid | 400 | 0.422 ± 0.044 aAB | 0.570 ± 0.027 aA | 0.611 ± 0.034 aA | 0.266 ± 0.033 bB | 0.172 ± 0.025 aB | 0.199 ± 0.041 aB | |
| 700 | 0.696 ± 0.033 aAB | 0.561 ± 0.086 aAB | 0.475 ± 0.034 aB | 0.770 ± 0.135 aA | 0.210 ± 0.048 aC | 0.230 ± 0.030 aC | ||
| Total carotenoids (mg g−1 DW) | Geisha 3 | 400 | 1.594 ± 0.032 aC | 2.338 ± 0.099 aA | 2.113 ± 0.009 aAB | 1.569 ± 0.106 aB | 1.854 ± 0.103 aB | 1.562 ± 0.203 aB |
| 700 | 1.500 ± 0.131 aA | 1.425 ± 0.154 bA | 1.855 ± 0.180 aA | 1.553 ± 0.070 aA | 1.343 ± 0.088 bA | 1.343 ± 0.159 aB | ||
| Marsellesa | 400 | 1.987 ± 0.317 bAB | 2.180 ± 0.320 aAB | 2.500 ± 0.151 aAB | 1.688 ± 0.332 aB | 2.795 ± 0.138 aA | 1.941 ± 0.165 aAB | |
| 700 | 3.079 ± 0.196 aA | 2.777 ± 0.215 aA | 2.705 ± 0.290 aA | 2.630 ± 0.114 aA | 2.448 ± 0.139 aA | 2.354 ± 0.168 aA | ||
| Hybrid | 400 | 1.864 ± 0.042 aB | 2.423 ± 0.227 aAB | 2.519 ± 0.123 aA | 1.970 ± 0.190 aAB | 2.085 ± 0.101 aAB | 2.076 ± 0.059 aAB | |
| 700 | 1.875 ± 0.238 aA | 1.969 ± 0.133 aA | 1.921 ± 0.138 bA | 2.223 ± 0.121 aA | 1.698 ± 0.049 aA | 1.890 ± 0.088 aA | ||
| Genotype | Temperature (Day/Night) | [CO2] (µL L−1) | HSP70 | ELIP | Chape20 | Chape60 | CAT | CuSOD1 | CuSOD2 | APXCyt | APXChl | APXt+s | VDE2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geisha 3 | 25/20 °C | 400 | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aC | 1.00 | aD | 1.00 | aC | 1.00 | aD | 1.00 | aA |
| 700 | 0.98 | aD | 0.96 | aC | 1.02 | aC | 1.21 | aC | 0.98 | aB | 0.98 | aC | 0.95 | aC | 0.88 | aD | 0.98 | aC | 1.02 | aC | 0.99 | aA | ||
| 37/28 °C | 400 | 1.78 | aC | 2.23 | aB | 8.22 | aB | 6.65 | aB | 3.42 | aC | 4.55 | aB | 25.23 | aA | 48.23 | aB | 12.31 | aB | 15.34 | aB | 0.56 | aB | |
| 700 | 1.76 | aB | 2.21 | aA | 4.55 | bA | 5.67 | bB | 2.22 | bA | 3.23 | bA | 12.24 | bA | 14.55 | bB | 10.25 | aA | 14.24 | aA | 0.54 | aC | ||
| 42/30 °C | 400 | 4.22 | aA | 3.45 | aA | 11.21 | aA | 9.21 | aA | 6.56 | aA | 6.66 | aA | 28.91 | aA | 69.89 | aA | 24.55 | aA | 26.77 | aA | 0.98 | aA | |
| 700 | 2.25 | bA | 2.44 | bA | 4.55 | bA | 6.23 | bA | 2.27 | bA | 3.56 | bA | 11.55 | bA | 17.67 | bA | 12.34 | bA | 15.61 | bA | 0.72 | bB | ||
| Rec14 | 400 | 2.21 | aB | 1.25 | aC | 7.72 | aC | 2.23 | aC | 4.46 | aB | 2.23 | aC | 2.66 | aB | 26.55 | aC | 14.55 | aB | 8.6 | aC | 0.98 | aA | |
| 700 | 1.24 | bC | 1.22 | aB | 2.33 | bB | 1.27 | bC | 2.26 | bA | 1.67 | bB | 2.21 | aB | 12.21 | bC | 8.90 | bB | 2.24 | bB | 0.88 | aB | ||
| Marsellesa | 25/20 °C | 400 | 1.00 | aD | 1.00 | aC | 1.00 | aC | 1.00 | aD | 1.00 | aB | 1.00 | aC | 1.00 | aB | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aA |
| 700 | 1.02 | aC | 0.98 | aC | 0.98 | aB | 1.02 | aC | 1.04 | aC | 0.99 | aC | 0.92 | aB | 0.99 | aD | 1.03 | aD | 1.05 | aD | 0.96 | aA | ||
| 37/28 °C | 400 | 2.24 | aB | 3.33 | aA | 3.46 | aB | 3.34 | aB | 2.61 | aA | 4.23 | aB | 14.55 | aA | 48.91 | aB | 18.21 | aB | 22.34 | aB | 0.97 | aA | |
| 700 | 1.88 | bB | 2.24 | bA | 2.23 | bA | 1.67 | bB | 2.18 | bA | 2.29 | bB | 0.98 | bB | 10.98 | bC | 8.23 | bB | 11.36 | bB | 0.76 | bB | ||
| 42/30 °C | 400 | 4.33 | aA | 3.37 | aA | 6.78 | aA | 4.54 | aA | 2.67 | aA | 8.99 | aA | 14.67 | aA | 104.22 | aA | 24.56 | aA | 39.31 | aA | 0.55 | aB | |
| 700 | 2.21 | bA | 2.27 | bA | 2.21 | bA | 2.21 | bA | 2.17 | bA | 4.55 | bA | 1.02 | bB | 55.66 | bA | 19.18 | bA | 22.8 | bA | 0.43 | bC | ||
| Rec14 | 400 | 1.25 | bC | 2.21 | aB | 3.23 | aB | 1.99 | aC | 2.41 | aA | 4.65 | aB | 14.22 | aA | 26.71 | aC | 5.44 | aC | 6.33 | aC | 0.98 | aA | |
| 700 | 2.22 | aA | 1.98 | bB | 1.12 | bB | 1.03 | bC | 1.98 | bB | 2.33 | bB | 2.21 | bA | 14.33 | bB | 2.23 | bC | 3.35 | bC | 0.78 | bB | ||
| Hybrid | 25/20 °C | 400 | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aD | 1.00 | aA |
| 700 | 0.97 | aC | 0.96 | bC | 0.98 | aD | 0.98 | aD | 0.99 | aC | 0.99 | aC | 1.13 | aC | 1.22 | aC | 1.02 | aC | 0.98 | aD | 0.97 | bA | ||
| 37/28 °C | 400 | 6.22 | aB | 5.66 | aB | 11.25 | aB | 8.91 | aB | 3.44 | aB | 8.88 | aB | 35.61 | aB | 23.56 | aB | 26.77 | aB | 32.44 | aB | 0.94 | aA | |
| 700 | 2.43 | bA | 2.23 | bA | 6.78 | bB | 4.56 | bB | 2.23 | bA | 1.44 | bBC | 4.57 | bB | 9.21 | bB | 22.11 | aA | 14.55 | bB | 0.96 | aA | ||
| 42/30 °C | 400 | 13.98 | aA | 8.66 | aA | 26.79 | aA | 11.23 | aA | 5.21 | aA | 12.34 | aA | 55.34 | aA | 36.21 | aA | 33.72 | aA | 43.77 | aA | 0.56 | bB | |
| 700 | 2.49 | bA | 2.21 | bA | 9.87 | bA | 8.86 | bA | 2.21 | bA | 5.57 | bA | 8.87 | bA | 11.23 | bA | 21.40 | bA | 24.78 | bA | 0.67 | aC | ||
| Rec14 | 400 | 3.44 | aC | 2.82 | aC | 5.44 | aC | 2.45 | aC | 2.49 | aC | 4.56 | aC | 13.44 | aC | 17.22 | aC | 14.23 | aC | 24.21 | aC | 0.98 | aA | |
| 700 | 1.22 | bB | 1.45 | bB | 3.56 | bC | 1.23 | bC | 1.89 | bB | 2.26 | bB | 1.22 | bC | 8.54 | bB | 5.51 | bB | 11.20 | bC | 0.77 | bB | ||
| Gene Symbol | Gene Description | Primer Sequence (5′–3′) | Amplicon Size (bp) |
|---|---|---|---|
| HSP70 | Stromal 70 kDa heat shock-related protein, chloroplastic | F: GGGAAGCAATTGACACCAAG | 150 |
| R: AGCCACCAGATACTGCATCC | |||
| ELIP | Chloroplast early light-induced protein | F: GCCATGATAGGGTTTGTTGC | 101 |
| R: GTCCCAATGAACCATTGCAG | |||
| Chape20 | Chloroplast 20 kDa chaperonin | F: GTTAAAGCTGCCGCTGTTG | 150 |
| R: CTCACCTCCTTGAGGTTTCG | |||
| Chape60 | Mitochondria chaperonin CPN60 | F: GGATAGTGAAGCCCTTGC | 80 |
| R: CCCAGGAGCTTTTATTGCAC | |||
| CAT | Catalase isozyme 1 | F: CTACTTCCCCTCGCGGTAT | 150 |
| R: CTGTCTGGTGCAAATGAACG | |||
| CuSOD1 | Superoxide dismutase [Cu-Zn] | F: CCCTTGGAGACACAACGAAT | 141 |
| R: GGCAGTACCATCTTGACCA | |||
| CuSOD2 | Superoxide dismutase [Cu-Zn] | F: GGGGCTCTATCCAATTCCTC | 150 |
| R: GGTTAAAATGAGGCCCAGTG | |||
| APXCyt | Cytosol ascorbate peroxidase | F: TCTGGATTTGAGGGACCTTG | 108 |
| R: GTCAGATGGAAGCCGGATAA | |||
| APXChl | Chloroplast ascorbate peroxidase | F: CACCTGCTGCTCATTTACG | 100 |
| R: GACCTTCCCAATGTGTGTG | |||
| APXt+s | Stromatic ascorbate peroxidase (sAPX) mRNA | F: AGGGCAGAATATGAAGGATTGG | 112 |
| R: CCAAGCAAGGATGTCAAAATAGCC | |||
| VDE2 | Violaxanthin de-epoxidase | F: GGGTTCAAAATGCACAAGACTG | 86 |
| R: CCCTCTTTTACCTCAGGCATTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinci, G.; Marques, I.; Rodrigues, A.P.; Martins, S.; Leitão, A.E.; Semedo, M.C.; Silva, M.J.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; et al. Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]. Plants 2022, 11, 2702. https://doi.org/10.3390/plants11202702
Vinci G, Marques I, Rodrigues AP, Martins S, Leitão AE, Semedo MC, Silva MJ, Lidon FC, DaMatta FM, Ribeiro-Barros AI, et al. Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]. Plants. 2022; 11(20):2702. https://doi.org/10.3390/plants11202702
Chicago/Turabian StyleVinci, Gabriella, Isabel Marques, Ana P. Rodrigues, Sónia Martins, António E. Leitão, Magda C. Semedo, Maria J. Silva, Fernando C. Lidon, Fábio M. DaMatta, Ana I. Ribeiro-Barros, and et al. 2022. "Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]" Plants 11, no. 20: 2702. https://doi.org/10.3390/plants11202702
APA StyleVinci, G., Marques, I., Rodrigues, A. P., Martins, S., Leitão, A. E., Semedo, M. C., Silva, M. J., Lidon, F. C., DaMatta, F. M., Ribeiro-Barros, A. I., & Ramalho, J. C. (2022). Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]. Plants, 11(20), 2702. https://doi.org/10.3390/plants11202702














