Pulsed Vacuum Drying of Persimmon Slices: Drying Kinetics, Physicochemical Properties, Microstructure and Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drying Methods
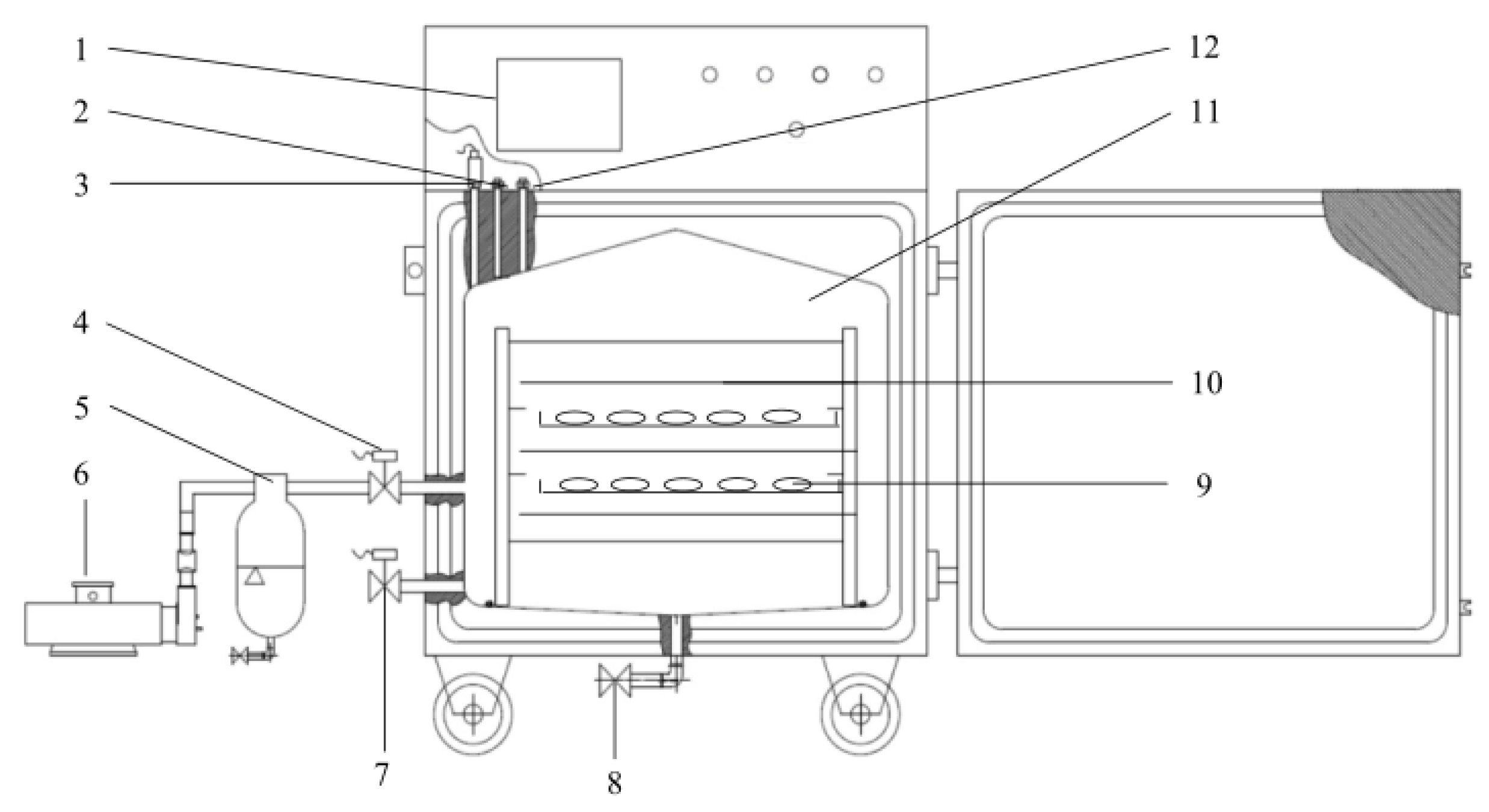
2.1.1. Pulsed Vacuum Drying (PVD)
2.1.2. Hot Air Drying (HAD)
2.2. Drying Kinetics
2.2.1. Drying Characteristics
2.2.2. Kinetic Modeling of Drying Process
2.3. Color Measurements
2.4. Rehydration
2.5. Scanning Electron Microscopy (SEM) Observations
2.6. Methods of Persimmon Extraction
2.7. Soluble Sannin Content
2.8. Total Phenolic Content (TPC)
2.9. Antioxidant Activity
2.10. Statistical Analysis
3. Results and Discussion
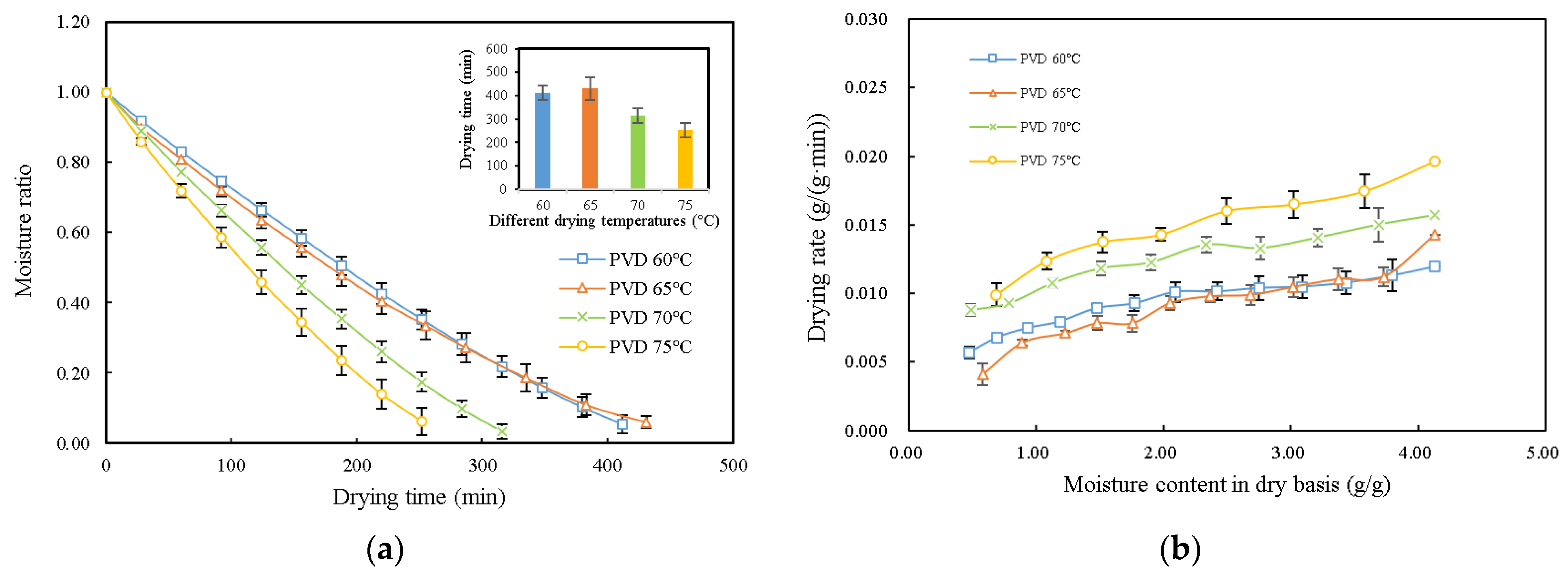
3.1. Drying Characteristics and Kinetic Modeling
3.2. Color Evaluation
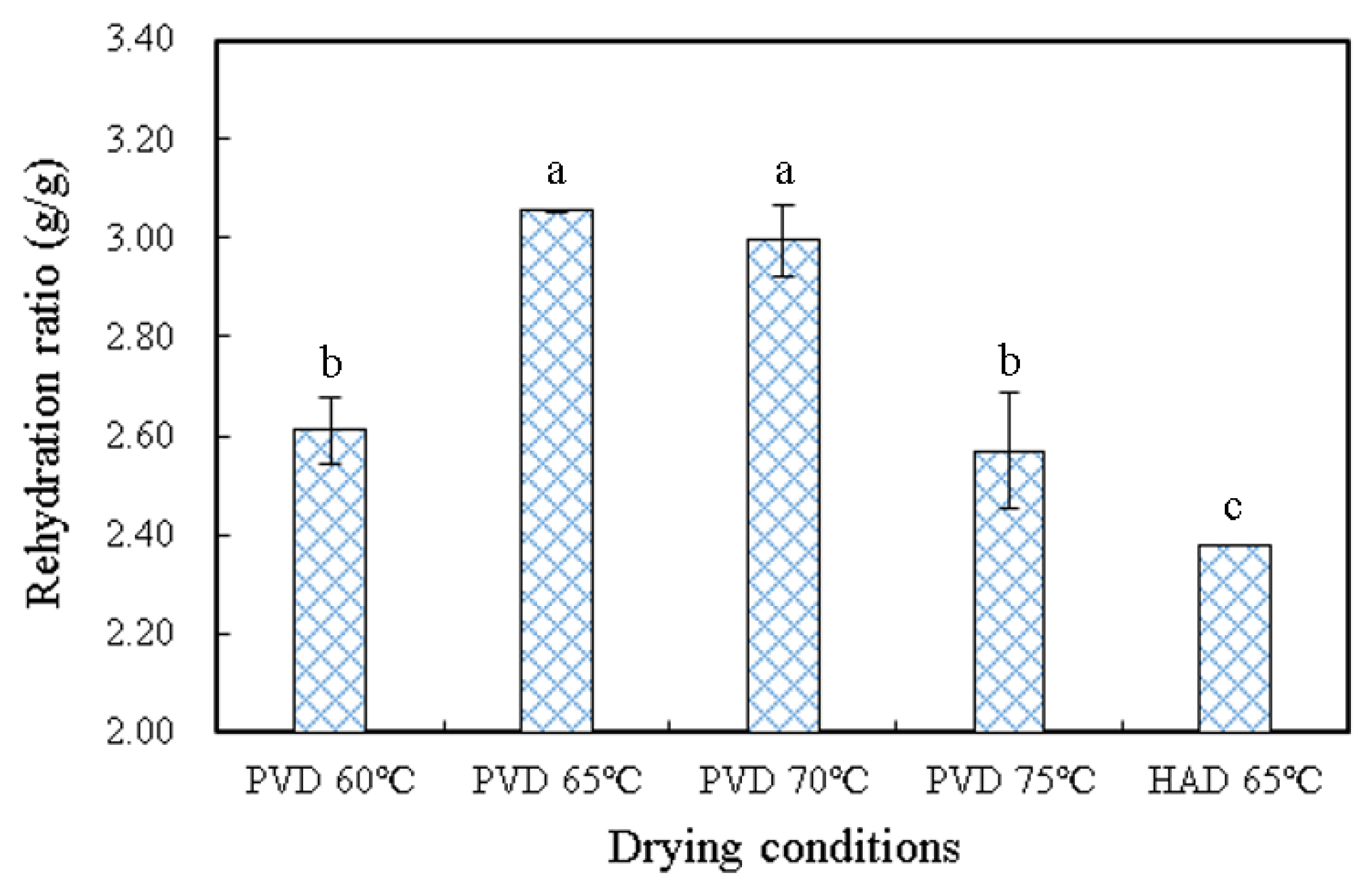
3.3. Rehydration Ratio
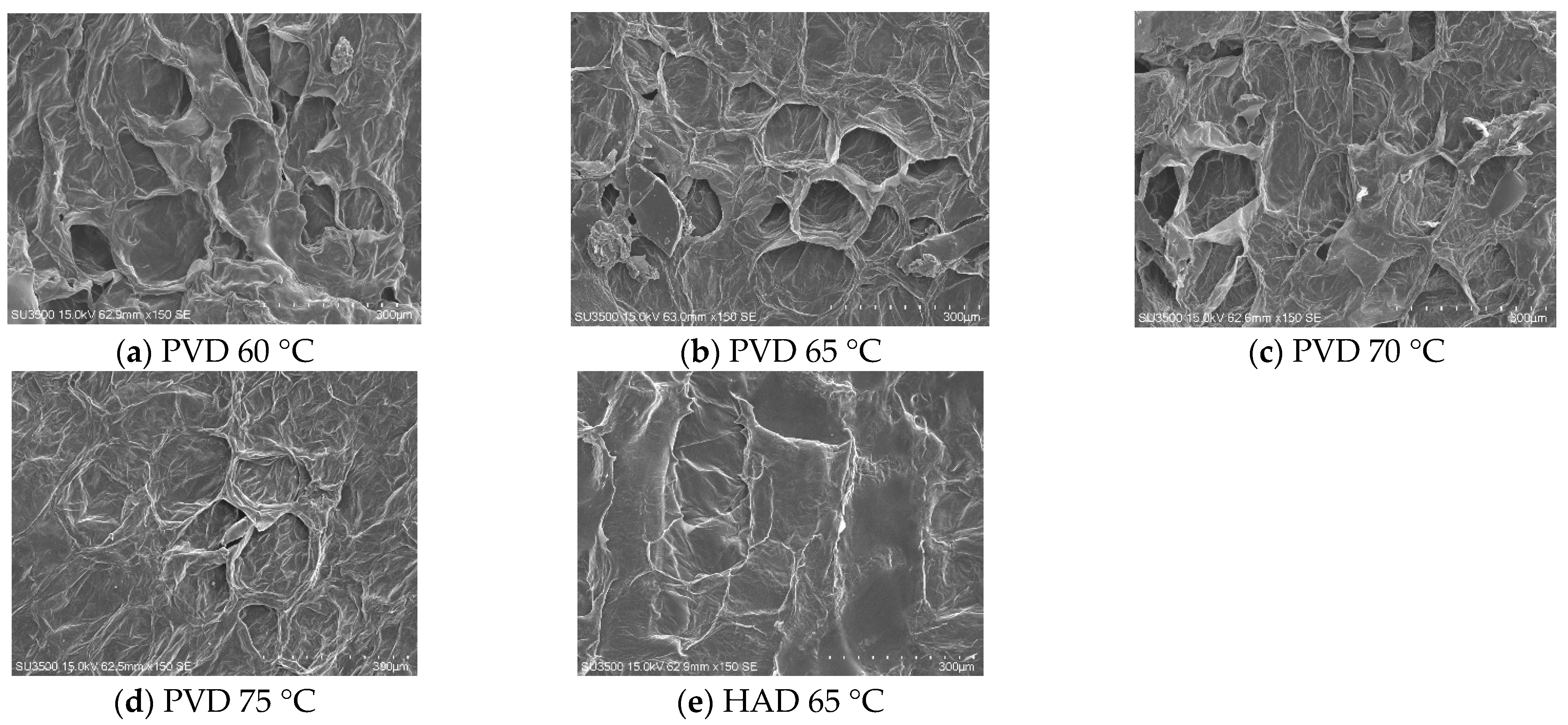
3.4. Scanning Electron Microscopy (SEM) Evaluation
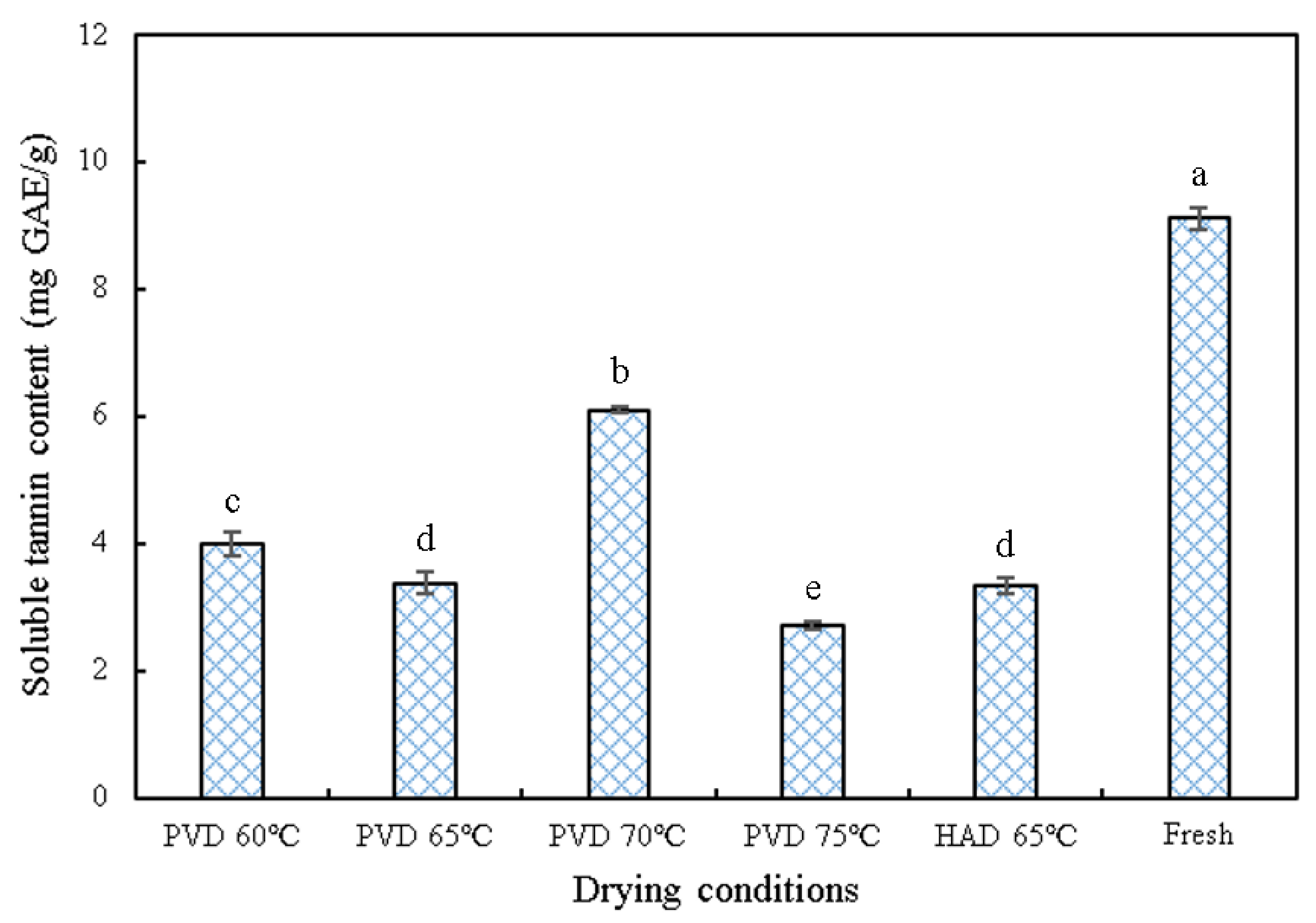
3.5. Soluble Tannin Content
3.6. Total Phenolic Content (TPC)
3.7. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.X.; Jiao, Y.L.; Liu, B.; Chao, W.H.; Duan, X.C.; Yue, T.L. Using hyperspectral imaging technology for assessing internal quality parameters of persimmon fruits during the drying process. Food Chem. 2022, 386, 132774. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yan, H.H.; Yin, Y.X.; Li, X.; Li, H.R.; Ren, D.F. Effect of microwave-assisted hydrothermal extraction on the bioactive compounds and antioxidant activities of dateplum persimmon juice and vinegar. LWT 2022, 154, 112642. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Vendrell-Calatayud, M.; Méndez-Albiñana, P.; Moreno, R.; Cano, M.P.; Villamiel, M. Extraction optimization and structural characterization of pectin from persimmon fruit (Diospyros kaki Thunb. var. Rojo brillante). Carbohydr. Polym. 2021, 272, 118411. [Google Scholar] [CrossRef]
- Zhao, C.C.; Ameer, K.; Eun, J.B. Effects of various drying conditions and methods on drying kinetics and retention of bioactive compounds in sliced persimmon. LWT 2021, 143, 111149. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pang, C.L.; Wang, L.H.; Bai, J.W.; Yang, W.X.; Gao, Z.J. Drying kinetics and quality of Monukka seedless grapes dried in an air-impingement jet dryer. Biosyst. Eng. 2010, 105, 233–240. [Google Scholar] [CrossRef]
- Karaman, S.; Toker, O.S.; Çam, M.; Hayta, M.; Doğan, M.; Kayacier, A. Bioactive and Physicochemical Properties of Persimmon as Affected by Drying Methods. Dry. Technol. 2014, 32, 258–267. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zhang, X.C.; Luo, Z.S.; Sun, J.; Li, L.; Yin, X.R.; Li, J.K.; Xu, Y.Q. Effects of inside-out heat-shock via microwave on the fruit softening and quality of persimmon during postharvest storage. Food Chem. 2021, 349, 129161. [Google Scholar] [CrossRef]
- Sampaio, R.M.; Neto, J.P.M.; Perez, V.H.; Marcos, S.K.; Boizan, M.A.; Silva, L.R.D. Mathematical modeling of drying kinetics of kinetics of persimmon fruits (Diospyros kaki CV. Fuyu). J. Food Process. Preserv. 2017, 41, e12789. [Google Scholar] [CrossRef]
- Doymaz, I. Evaluation of some thin-layer drying models of persimmon slices (Diospyros kaki L.). Energy Convers. Manag. 2012, 56, 199–205. [Google Scholar] [CrossRef]
- González, C.M.; García, A.L.; Llorca, E.; Hernando, I.; Atienzar, P.; Bermejo, A.; Moraga, G.; Quiles, A. Carotenoids in dehydrated persimmon: Antioxidant activity, structure, and photoluminescence. LWT 2021, 142, 111007. [Google Scholar] [CrossRef]
- Deng, L.Z.; Yang, X.H.; Mujumdar, A.S.; Zhao, J.H.; Wang, D.; Zhang, Q.; Wang, J.; Gao, Z.J.; Xiao, H.W. Red pepper (Capsicum annuum L.) drying: Effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure. Dry. Technol. 2018, 36, 893–907. [Google Scholar] [CrossRef]
- Senadeera, W.; Adiletta, G.; Önal, B.; Matteo, M.D.; Russo, P. Influence of Different Hot Air Drying Temperatures on Drying Kinetics, Shrinkage, and Colour of Persimmon Slices. Foods 2020, 9, 101. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Khalifa, I.; Hu, L.L.; Zhu, W.; Li, J.; Li, K.; Li, C.M. Influence of three different drying techniques on persimmon chips’ characteristics: A comparison study among hot-air, combined hot-air-microwave, and vacuum-freeze drying techniques. Food Bioprod. Process. 2019, 118, 67–76. [Google Scholar] [CrossRef]
- Xie, Y.C.; Gao, Z.J.; Liu, Y.H.; Xiao, H.W. Pulsed vacuum drying of rhizoma dioscoreae slices. LWT 2017, 80, 237–249. [Google Scholar] [CrossRef]
- Xie, L.; Mujumdar, A.S.; Fang, X.M.; Wang, J.; Dai, J.W.; Du, Z.L.; Xiao, H.W.; Liu, Y.H.; Gao, Z.J. Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food Bioprod. Process. 2017, 102, 320–331. [Google Scholar] [CrossRef]
- Wang, J.; Law, C.L.; Nema, P.K.; Zhao, J.H.; Liu, Z.L.; Deng, L.Z.; Gao, Z.J.; Xiao, H.W. Pulsed vacuum drying enhances drying kinetics and quality of lemon slices. J. Food Eng. 2018, 224, 129–138. [Google Scholar] [CrossRef]
- Wang, J.; Bai, T.Y.; Wang, D.; Fang, X.M.; Xue, L.Y.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Pulsed vacuum drying of Chinese ginger (Zingiber officinale Roscoe) slices: Effects on drying characteristics, rehydration ratio, water holding capacity, and microstructure. Dry. Technol. 2019, 37, 301–311. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wei, Z.Y.; Vidyarthi, S.K.; Pan, Z.L.; Zielinska, M.; Deng, L.Z.; Wang, Q.H.; Wei, Q.; Xiao, H.W. Pulsed vacuum drying of kiwifruit slices and drying process optimization based on artificial neural network. Dry. Technol. 2020, 39, 405–417. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xie, L.; Zielinska, M.; Pan, Z.L.; Wang, J.; Deng, L.Z.; Wang, H.; Xiao, H.W. Pulsed vacuum drying enhances drying of blueberry by altering micro-, ultrastructure and water status and distribution. LWT 2021, 142, 111013. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xie, L.; Zielinska, M.; Pan, Z.L.; Deng, L.Z.; Zhang, J.S.; Gao, L.; Wang, S.Y.; Zheng, Z.A.; Xiao, H.W. Improvement of drying efficiency and quality attributes of blueberries using innovative far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD). Innov. Food Sci. Emerg. Technol. 2022, 77, 102948. [Google Scholar] [CrossRef]
- Bai, J.W.; Cai, J.R.; Tian, X.Y. Crust Formation and Microstructural Changes of Gingko Biloba Seeds During Drying. Food Bioprocess Technol. 2019, 12, 1041–1051. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Rocculi, P.; Rosa, M.D. Strategies to improve food functionality: Structure-property relationships on high pressures homogenization, vacuum impregnation and drying technologies. Trends Food Sci. Technol. 2015, 46, 1–12. [Google Scholar] [CrossRef]
- Wang, H.; Fang, X.M.; Sutar, P.P.; Meng, J.S.; Wang, J.; Yu, X.L.; Xiao, H.W. Effects of vacuum-steam pulsed blanching on drying kinetics, colour, phytochemical contents, antioxidant capacity of carrot and the mechanism of carrot quality changes revealed by texture, microstructure and ultrastructure. Food Chem. 2021, 338, 127799. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zheng, Z.A.; Mujumdar, A.S.; Fang, X.M.; Wang, J.; Zhang, Q.; Ma, Q.; Xiao, H.W.; Liu, Y.H.; Gao, Z.J. Pulsed vacuum drying (PVD) of wolfberry: Drying kinetics and quality attributes. Dry. Technol. 2018, 36, 1501–1514. [Google Scholar] [CrossRef]
- Wang, H.; Torki, M.; Xiao, H.W.; Orsat, V.; Raghavan, G.S.V.; Liu, Z.L.; Peng, W.J.; Fang, X.M. Multi-objective analysis of evacuated tube solar-electric hybrid drying setup for drying lotus bee pollen. Renew. Sustain. Energy Rev. 2022, 168, 112822. [Google Scholar] [CrossRef]
- Deng, L.Z.; Pan, Z.L.; Mujumdar, A.S.; Zhao, J.H.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. High-humidity hot air impingement blanching (HHAIB) enhances drying quality of apricots by inactivating the enzymes, reducing drying time and altering cellular structure. Food Control 2019, 96, 104–111. [Google Scholar] [CrossRef]
- Yang, X.H.; Deng, L.Z.; Mujumdar, A.S.; Xiao, H.W.; Zhang, Q.; Kan, Z. Evolution and modeling of colour changes of red pepper (Capsicum annuum L.) during hot air drying. J. Food Eng. 2018, 231, 101–108. [Google Scholar] [CrossRef]
- Xiao, H.W.; Bai, J.W.; Xie, L.; Sun, D.W.; Gao, Z.J. Thin-layer air impingement drying enhances drying rate of American ginseng (Panax quinquefolium L.) slices with quality attributes considered. Food Bioprod. Process. 2015, 94, 581–591. [Google Scholar] [CrossRef]
- Chira, K.; Jourdes, M.; Teissedre, P.L. Cabernet sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. Eur. Food Res. Technol. 2012, 234, 253–261. [Google Scholar] [CrossRef]
- Arie, R.B.; Sonego, L. Temperate affects astringency removal and recurrence in persimmon. J. Food Sci. 1993, 58, 1397–1400. [Google Scholar] [CrossRef]
- Malakar, S.; Alam, M.; Arora, V.K. Evacuated tube solar and sun drying of beetroot slices: Comparative assessment of thermal performance, drying kinetics, and quality analysis. Solar Energy 2022, 233, 246–258. [Google Scholar] [CrossRef]
- Kayacan, S.; Karasu, S.; Akman, P.K.; Goktas, H.; Doymaz, I.; Sagdic, O. Effect of different drying methods on total bioactive compounds, phenolic profile, in vitro bioaccessibility of phenolic and HMF formation of persimmon. LWT 2020, 118, 108830. [Google Scholar] [CrossRef]
- Carmona, L.V.; Jacinto, C.P.P.; Ortega, M.H.; Navarro, M.D.H.; Villarrepthal, F.; Mondragón, H.N.; Moreno, A.O.; Reyes, G.C. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- González, C.M.; Llorca, E.; Quiles, A.; Hernando, I.; Moraga, G. An in vitro digestion study of tannins and antioxidant activity affected by drying “Rojo Brillante” persimmon. LWT 2022, 155, 112961. [Google Scholar] [CrossRef]
- Ketnawa, S.; Hamanaka, D.; Wongpoomchai, R.; Ogawa, Y.; Setha, S. Low intensity of high pressure processing increases extractable recovery of polyphenols and antioxidant activities of non-astringent persimmon fruit. LWT 2021, 151, 112162. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shon, M.S.; Kim, G.N.; Lee, S.C. Antioxidant and anti-adipogenic activities of persimmon tannins. Food Sci. Biotechnol. 2014, 23, 1689–1694. [Google Scholar] [CrossRef]
- Korus, A. Effect of preliminary processing, method of drying and storage temperature on the level of antioxidants in kale (Brassica oleracea L. var. acephala) leaves. LWT 2011, 44, 1711–1716. [Google Scholar] [CrossRef]


| Temperature (°C) | α (min) | β | χ2 (×10−4) | RSS | R2 |
|---|---|---|---|---|---|
| 60 | 233.1 | 1.422 | 9.288 | 0.009 | 0.993 |
| 65 | 228.0 | 1.294 | 7.564 | 0.007 | 0.994 |
| 70 | 173.2 | 1.453 | 13.012 | 0.009 | 0.991 |
| 75 | 141.4 | 1.387 | 0.001 | 0.006 | 0.993 |
Fresh | PVD 60 °C | PVD 65 °C | PVD 70 °C | PVD 75 °C | HAD 65 °C | |
|---|---|---|---|---|---|---|
| L* | 84.80 ± 0.73 a | 52.02 ± 1.75 e | 57.46 ± 0.40 d | 56.09 ± 0.65 d | 53.40 ± 0.96 e | 62.39 ± 0.86 b |
| a* | 7.70 ± 1.09 b | 13.48 ± 0.67 a | 12.85 ± 0.55 a | 13.61 ± 0.16 a | 13.71 ± 1.25 a | 14.60 ± 0.22 a |
| b* | 36.49 ± 1.81 d | 38.31 ± 0.37 c | 41.74 ± 0.83 b | 42.27 ± 0.64 b | 42.29 ± 1.19 b | 45.52 ± 0.38 a |
| ΔE | - | 33.34 ± 1.65 a | 28.32 ± 0.25 b | 29.88 ± 0.62 b | 32.52 ± 1.14 a | 25.13 ± 0.86 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-W.; Wang, D.; Vidyarthi, S.K.; Li, S.-B.; Liu, Z.-L.; Wang, H.; Chen, X.-J.; Xiao, H.-W. Pulsed Vacuum Drying of Persimmon Slices: Drying Kinetics, Physicochemical Properties, Microstructure and Antioxidant Capacity. Plants 2022, 11, 2500. https://doi.org/10.3390/plants11192500
Yang K-W, Wang D, Vidyarthi SK, Li S-B, Liu Z-L, Wang H, Chen X-J, Xiao H-W. Pulsed Vacuum Drying of Persimmon Slices: Drying Kinetics, Physicochemical Properties, Microstructure and Antioxidant Capacity. Plants. 2022; 11(19):2500. https://doi.org/10.3390/plants11192500
Chicago/Turabian StyleYang, Kai-Wen, Dong Wang, Sriram K. Vidyarthi, Suo-Bin Li, Zi-Liang Liu, Hui Wang, Xian-Jun Chen, and Hong-Wei Xiao. 2022. "Pulsed Vacuum Drying of Persimmon Slices: Drying Kinetics, Physicochemical Properties, Microstructure and Antioxidant Capacity" Plants 11, no. 19: 2500. https://doi.org/10.3390/plants11192500
APA StyleYang, K.-W., Wang, D., Vidyarthi, S. K., Li, S.-B., Liu, Z.-L., Wang, H., Chen, X.-J., & Xiao, H.-W. (2022). Pulsed Vacuum Drying of Persimmon Slices: Drying Kinetics, Physicochemical Properties, Microstructure and Antioxidant Capacity. Plants, 11(19), 2500. https://doi.org/10.3390/plants11192500








