Antifungal Activity and Biochemical Profiling of Exudates from Germinating Maize Nostrano di Storo Local Variety
Abstract
1. Introduction
2. Results and Discussion
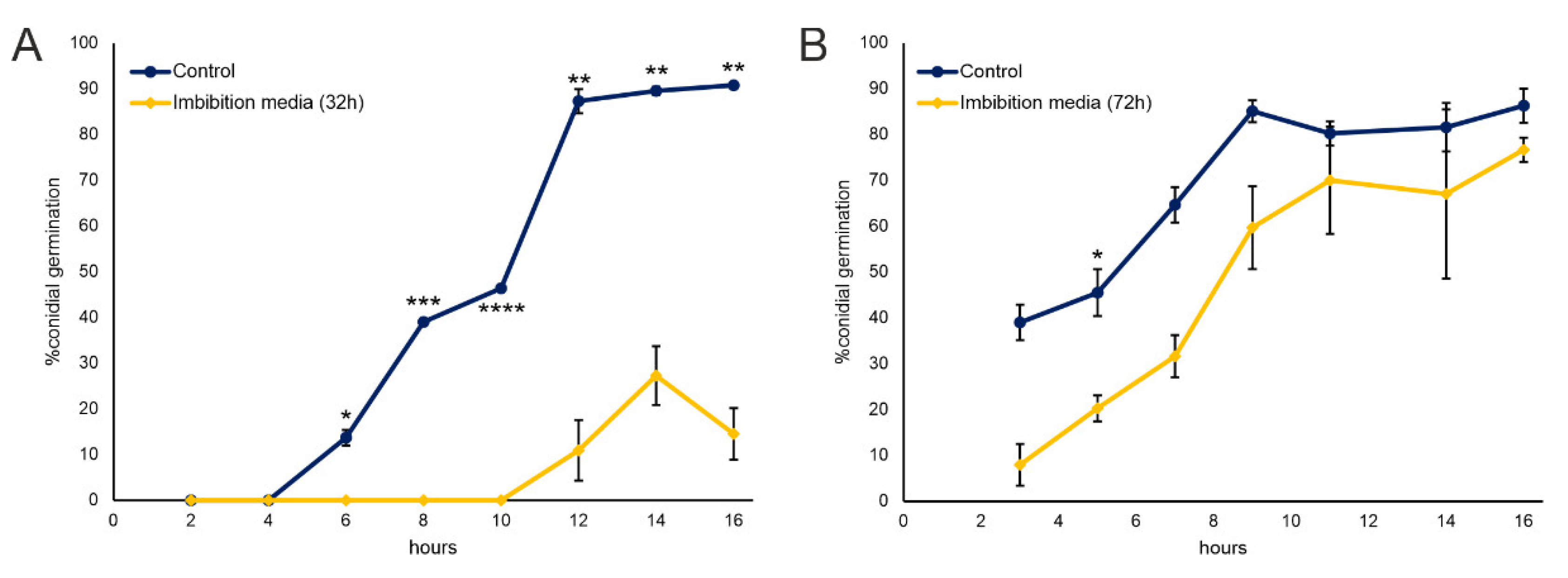
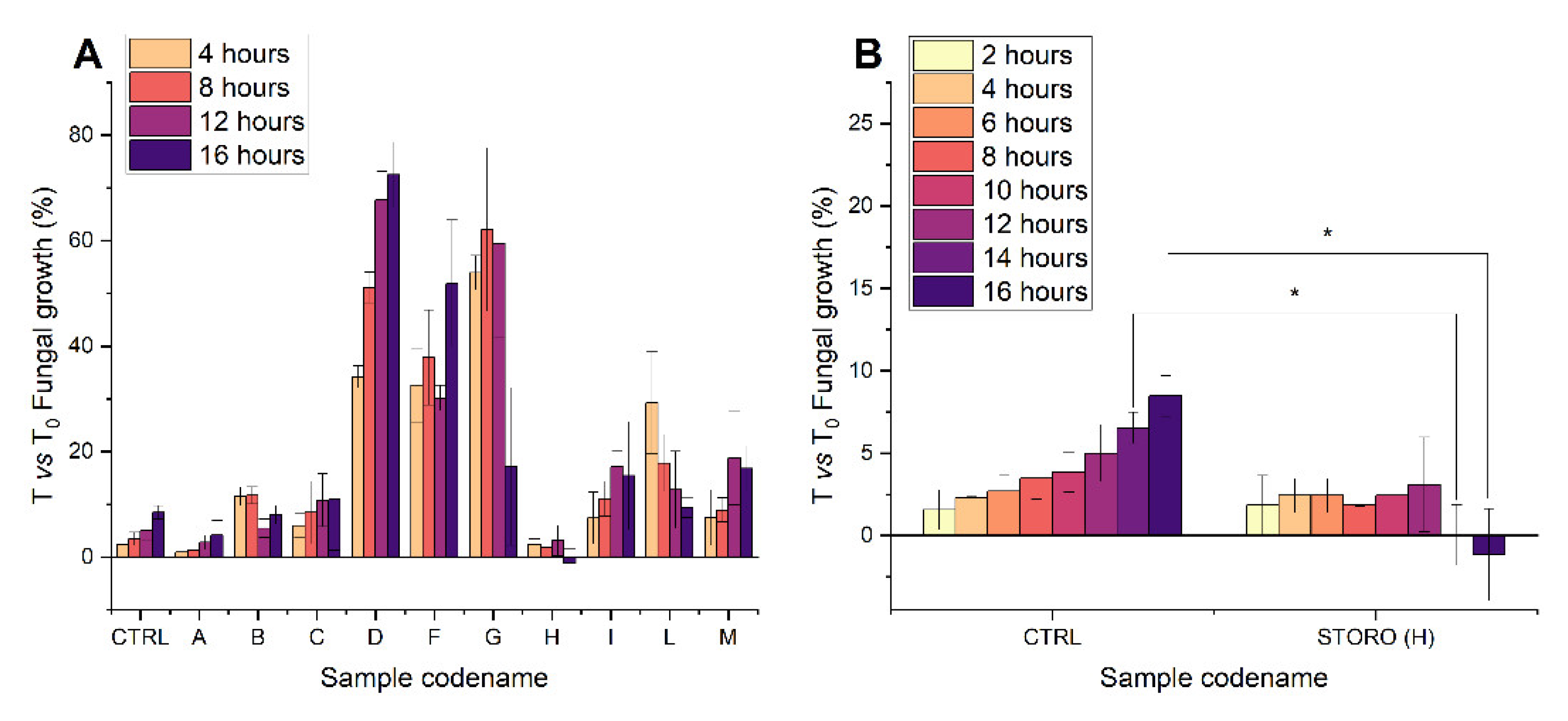
2.1. Nostrano di Storo Seed Exudates during Early Germination Inhibit F. verticilloides Conidial Germination
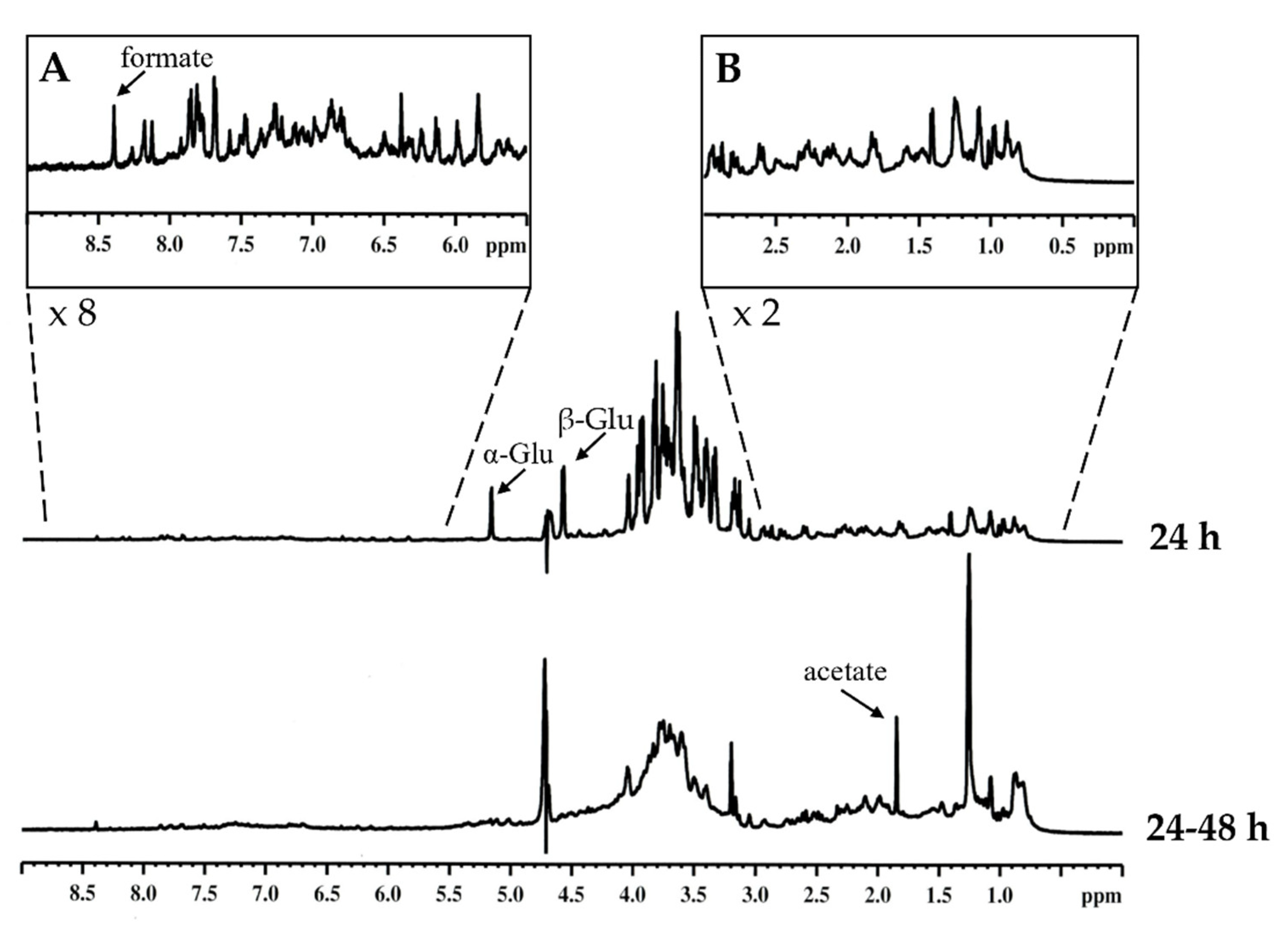
2.2. NMR Nostrano di Storo Exudation Profiles Over-Time
2.3. Free Fatty Acid Analysis of Maize Seeds and Antifungal Active Exudate
2.4. Characterization of the Protein Fraction
3. Materials and Methods
3.1. Seed Samples and Imbibition Media Collection Procedures
3.2. Antifungal Activity Assay
3.3. NMR Analyses
3.4. Lipid Extraction and Analyses
3.5. Mass Spectrometry
3.6. Statistical Analyses and Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, E.B. Microbial Dynamics and Interactions in the Spermosphere. Annu. Rev. Phytopathol. 2004, 42, 271–309. [Google Scholar] [CrossRef] [PubMed]
- Schiltz, S.; Gaillard, I.; Pawlicki-Jullian, N.; Thiombiano, B.; Mesnard, F.; Gontier, E. A Review: What Is the Spermosphere and How Can It Be Studied? J. Appl. Microbiol. 2015, 119, 1467–1481. [Google Scholar] [CrossRef] [PubMed]
- Scarafoni, A.; Ronchi, A.; Prinsi, B.; Espen, L.; Assante, G.; Venturini, G.; Duranti, M. The Proteome of Exudates from Germinating Lupinus Albus Seeds Is Secreted through a Selective Dual-Step Process and Contains Proteins Involved in Plant Defence. FEBS J. 2013, 280, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Bush, B.J.; Carson, M.L.; Cubeta, M.A.; Hagler, W.M.; Payne, G.A. Infection and Fumonisin Production by Fusarium Verticillioides in Developing Maize Kernels. Phytopathology 2004, 94, 88–93. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of Crop Residues on Soil Health: A Review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Ferreira, A.; Quecine, M.C.; Lacava, P.T.; Oda, S.; Azevedo, J.L.; Araújo, W.L. Diversity of Endophytic Bacteria from Eucalyptus Species Seeds and Colonization of Seedlings by Pantoea Agglomerans. FEMS Microbiol. Lett. 2008, 287, 8–14. [Google Scholar] [CrossRef]
- De Azevedo, J.L.; de Araujo, W.L. Diversity and applications of Endophytic fungi isolated from tropical plants. In Fungi: Multifaceted Microbes; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gamalero, E.; Bona, E.; Glick, B.R. Current Techniques to Study Beneficial Plant-Microbe Interactions. Microorganisms 2022, 10, 1380. [Google Scholar] [CrossRef]
- Majewska-Sawka, A.; Nakashima, H. Endophyte Transmission via Seeds of Lolium perenne L.: Immunodetection of Fungal Antigens. Fungal Genet. Biol. 2004, 41, 534–541. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Koslow, J.M.; Clay, K. Endophytic Fungi Alter Relationships between Diversity and Ecosystem Properties. Ecol. Lett. 2004, 7, 42–51. [Google Scholar] [CrossRef]
- Jordaan, A.; Taylor, J.E.; Rossenkhan, R. Occurrence and Possible Role of Endophytic Fungi Associated with Seed Pods of Colophospermum Mopane (Fabaceae) in Botswana. S. Afr. J. Bot. 2006, 72, 245–255. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Clay, K. Endophyte Symbiosis with Tall Fescue: How Strong Are the Impacts on Communities and Ecosystems? Fungal Biol. Rev. 2007, 21, 107–124. [Google Scholar] [CrossRef]
- Cankar, K.; Kraigher, H.; Ravnikar, M.; Rupnik, M. Bacterial Endophytes from Seeds of Norway Spruce (Picea abies L. Karst). FEMS Microbiol. Lett. 2005, 244, 341–345. [Google Scholar] [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic Bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial Effects of Endophytic Pantoea Ananatis with Ability to Promote Rice Growth under Saline Stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; van Vuurde, J.W. Natural Incidence of Endophytic Bacteria in Pea Cultivars under Field Conditions. Can. J. Microbiol. 2000, 46, 1036–1041. [Google Scholar] [CrossRef]
- Sturz, A.V.; Matheson, B.G. Populations of Endophytic Bacteria Which Influence Host-Resistance to Erwinia-Induced Bacterial Soft Rot in Potato Tubers. Plant Soil 1996, 184, 265–271. [Google Scholar] [CrossRef]
- McInroy, J.A.; Kloepper, J.W. Survey of Indigenous Bacterial Endophytes from Cotton and Sweet Corn. Plant Soil 1995, 173, 337–342. [Google Scholar] [CrossRef]
- Munif, A.; Hallmann, J.; Sikora, R.A. Induced Systemic Resistance of Selected Endophytic Bacteria against Meloidogyne Incognita on Tomato. Meded Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2001, 66, 663–669. [Google Scholar]
- Zhang, W.; Han, D.Y.; Dick, W.A.; Davis, K.R.; Hoitink, H.A. Compost and Compost Water Extract-Induced Systemic Acquired Resistance in Cucumber and Arabidopsis. Phytopathology 1998, 88, 450–455. [Google Scholar] [CrossRef]
- Han, D.Y.; Coplin, D.L.; Bauer, W.D.; Hoitink, H.A. A Rapid Bioassay for Screening Rhizosphere Microorganisms for Their Ability to Induce Systemic Resistance. Phytopathology 2000, 90, 327–332. [Google Scholar] [CrossRef]
- Huang, H.C.; Erickson, R.S. Biological Control of Botrytis Stem and Blossom Blight of Lentil. Plant Path. Bull. 2002, 11, 7–14. [Google Scholar]
- Bardin, S.D.; Huang, H.C.; Liu, L.; Yanke, L.J. Control, by Microbial Seed Treatment, of Dampingoff Caused by Pythium sp. on Canola, Safflower, Dry Pea, and Sugar Beet. Can. J. Plant Pathol. 2003, 25, 268–275. [Google Scholar] [CrossRef]
- Yuen, G.Y.; Craig, M.L.; Kerr, E.D.; Steadman, J.R. Influences of Antagonist Population Levels, Blossom Development Stage, and Canopy Temperature on the Inhibition of Sclerotinia Sclerotiorum on Dry Edible Bean by Erwinia Herbicola. Phytopathology 1994, 84, 495–501. [Google Scholar] [CrossRef]
- Yuen, G.Y.; Steadman, J.R.; Lindgren, D.T.; Schaff, D.; Jochum, C. Bean Rust Biological Control Using Bacterial Agents. Crop Prot. 2001, 20, 395–402. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Fuerst, E.P.; James, M.S.; Pollard, A.T.; Okubara, P.A. Defense Enzyme Responses in Dormant Wild Oat and Wheat Caryopses Challenged with a Seed Decay Pathogen. Front. Plant Sci. 2017, 8, 2259. [Google Scholar] [CrossRef]
- Venturini, G.; Assante, G.; Toffolatti, S.L.; Vercesi, A. Pathogenicity Variation in Fusarium Verticillioides Populations Isolated from Maize in Northern Italy. Mycoscience 2013, 54, 285–290. [Google Scholar] [CrossRef]
- Raposo, R.; Colgan, R.; Delcan, J.; Melgarejo, P. Application of an Automated Quantitative Method to Determine Fungicide Resistance in Botrytis Cinerea. Plant Dis. 1995, 79, 294–296. [Google Scholar] [CrossRef]
- Vasmatkara, P.; Kaura, K.; Pannub, P.P.S.; Kaurc, G.; Kaur, H. Unraveling the metabolite signatures of maize genotypes showing T differential response towards southern corn leaf blight by 1H-NMR and FTIR spectroscopy. Physiol. Mol. Plant Pathol. 2019, 108, 101441. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.A.; Stanley, P.D.; Holmes, H. Application of NMR-based Metabolomics to the Investigation of Salt Stress in Maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Ando, I.; Hirose, T.; Nemoto, T.; Totsune, K.; Imai, Y.; Takeuki, K.; Fujiwara, M. Quantification of molecules in 1H-NMR metabolomics with formate as a concentration standard. J. Toxicol. Sci. 2010, 35, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Stojaković, M.; Ivanović, M.; Jocković, D.; Vasić, N.A. Characteristics of reselected Mo17 and B73 inbred lines of maize. Maydica 2007, 52, 257–260. [Google Scholar]
- Xing, J.; Chin, C.-K. Modification of Fatty Acids in Eggplant Affects Its Resistance to Verticilliumdahliae. Physiol. Mol. Plant Pathol. 2000, 56, 217–225. [Google Scholar] [CrossRef]
- Xue, H.Q.; Upchurch, R.G.; Kwanyuen, P. Relationships Between Oleic and Linoleic Acid Content and Seed Colonization by Cercospora Kikuchii and Diaporthe Phaseolorum. Plant Dis. 2008, 92, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Hinze, L.L.; Gardner, H.W.; Keller, N.P. Sporogenic Effect of Polyunsaturated Fatty Acids on Development of Aspergillus spp. Appl. Environ. Microbiol. 1999, 65, 3668–3673. [Google Scholar] [CrossRef]
- Walley, J.W.; Kliebenstein, D.J.; Bostock, R.M.; Dehesh, K. Fatty Acids and Early Detection of Pathogens. Curr. Opin. Plant Biol. 2013, 16, 520–526. [Google Scholar] [CrossRef]
- Trépanier, M.; Bécard, G.; Moutoglis, P.; Willemot, C.; Gagné, S.; Avis, T.J.; Rioux, J.-A. Dependence of Arbuscular-Mycorrhizal Fungi on Their Plant Host for Palmitic Acid Synthesis. Appl. Environ. Microbiol. 2005, 71, 5341–5347. [Google Scholar] [CrossRef]
- Barros, M.; Fleuri, L.F.; Macedo, G.A. Seed Lipases: Sources, Applications and Properties—A Review. Braz. J. Chem. Eng. 2010, 27, 15–29. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.F. Oxidized Fatty Acids as Inter-Kingdom Signaling Molecules. Molecules 2014, 19, 1273–1285. [Google Scholar] [CrossRef]
- Kachroo, A.; Venugopal, S.C.; Lapchyk, L.; Falcone, D.; Hildebrand, D.; Kachroo, P. Oleic Acid Levels Regulated by Glycerolipid Metabolism Modulate Defense Gene Expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 5152–5157. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wei, Z.-Z.; Liu, C.-H.; Chen, Q.; Xu, B.-J.; Guo, Z.-R.; Cao, Y.-L.; Wang, Y.; Han, Y.-N.; Chen, C.; et al. Linoleic Acid Isomerase Gene FgLAI12 Affects Sensitivity to Salicylic Acid, Mycelial Growth and Virulence of Fusarium Graminearum. Sci. Rep. 2017, 7, 46129. [Google Scholar] [CrossRef]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Göbel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the Lipoxygenase ZmLOX3 Increases Susceptibility of Maize to Aspergillus spp. Mol. Plant Microbe Interact. 2009, 22, 222–231. [Google Scholar] [CrossRef]
- Ma, K.; Kou, J.; Khashi U Rahman, M.; Du, W.; Liang, X.; Wu, F.; Li, W.; Pan, K. Palmitic Acid Mediated Change of Rhizosphere and Alleviation of Fusarium Wilt Disease in Watermelon. Saudi J. Biol. Sci. 2021, 28, 3616–3623. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Rosa, S.; Pesaresi, P.; Mizzotti, C.; Bulone, V.; Mezzetti, B.; Baraldi, E.; Masiero, S. Game-Changing Alternatives to Conventional Fungicides: Small RNAs and Short Peptides. Trends Biotechnol. 2022, 40, 320–337. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Xu, L.; Zou, Y.; Song, W. Study on Diversity of Endophytic Bacterial Communities in Seeds of Hybrid Maize and Their Parental Lines. Arch. Microbiol. 2012, 194, 1001–1012. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, S.; Zou, Y.; Wang, J.; Song, W. Investigation on Diversity and Population Succession Dynamics of Endophytic Bacteria from Seeds of Maize (Zea mays L., Nongda108) at Different Growth Stages. Ann. Microbiol. 2013, 63, 71–79. [Google Scholar] [CrossRef]
- Kang, S.H.; Cho, H.-S.; Cheong, H.; Ryu, C.-M.; Kim, J.F.; Park, S.-H. Two Bacterial Entophytes Eliciting Both Plant Growth Promotion and Plant Defense on Pepper (Capsicum annuum L.). J. Microbiol. Biotechnol. 2007, 17, 96–103. [Google Scholar]
- Coutinho, T.A.; Venter, S.N. Pantoea Ananatis: An Unconventional Plant Pathogen. Mol. Plant Pathol. 2009, 10, 325–335. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Giddens, S.R.; Bean, D.C. Investigations into the in Vitro Antimicrobial Activity and Mode of Action of the Phenazine Antibiotic D-Alanylgriseoluteic Acid. Int. J. Antimicrob. Agents 2007, 29, 93–97. [Google Scholar] [CrossRef]
- Harvey, K.L.; Jarocki, V.M.; Charles, I.G.; Djordjevic, S.P. The Diverse Functional Roles of Elongation Factor Tu (EF-Tu) in Microbial Pathogenesis. Front. Microbiol. 2019, 10, 2351. [Google Scholar] [CrossRef]
- Dallo, S.F.; Kannan, T.R.; Blaylock, M.W.; Baseman, J.B. Elongation Factor Tu and E1 Beta Subunit of Pyruvate Dehydrogenase Complex Act as Fibronectin Binding Proteins in Mycoplasma pneumoniae. Mol. Microbiol. 2002, 46, 1041–1051. [Google Scholar] [CrossRef]
- Igarashi, D.; Bethke, G.; Xu, Y.; Tsuda, K.; Glazebrook, J.; Katagiri, F. Pattern-Triggered Immunity Suppresses Programmed Cell Death Triggered by Fumonisin B1. PLoS ONE 2013, 8, e60769. [Google Scholar] [CrossRef]
- Passera, A.; Follador, A.; Morandi, S.; Miotti, N.; Ghidoli, M.; Venturini, G.; Quaglino, F.; Brasca, M.; Casati, P.; Pilu, R.; et al. Bacterial Communities in the Embryo of Maize Landraces: Relation with Susceptibility to Fusarium Ear Rot. Microorganisms 2021, 9, 2388. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, X.; Wang, J.; Wu, L.; Zheng, Z.; Yu, Z. Antagonistic Effects of Volatiles Generated by Bacillus subtilis on Spore Germination and Hyphal Growth of the Plant Pathogen, Botrytis cinerea. Biotechnol. Lett. 2008, 30, 919–923. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hellman, U.; Wernstedt, C.; Góñez, J.; Heldin, C.H. Improvement of an “In-Gel” Digestion Procedure for the Micropreparation of Internal Protein Fragments for Amino Acid Sequencing. Anal. Biochem. 1995, 224, 451–455. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.-M.; Taly, J.-F.; Notredame, C. T-Coffee: A Web Server for the Multiple Sequence Alignment of Protein and RNA Sequences Using Structural Information and Homology Extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, S.; Girlando, V.; Pasquali, M.; Scarafoni, A. Valorization of Okara by Enzymatic Production of Anti-Fungal Compounds for Plant Protection. Molecules 2021, 26, 4858. [Google Scholar] [CrossRef] [PubMed]

| SAMPLE | NAME |
|---|---|
| A | B73/Mo17 |
| B | R2869 RP1 x BP1 |
| C | R2830 Biancoperla |
| D | R2818 Cinquantone |
| F | R3303 Pignoletto |
| G | R2826 Bianco Vitreo |
| H | R2833 Nostrano di Storo |
| I | R2828 Nostrano dell’isola |
| L | R3304 Ottofile tortonese |
| M | R2822 Marano |
| Samples | Aliphatic H (0–3.0ppm) | O-Alkyl H (3.0–5.5 ppm) | Aromatic/Olefinic H (5.5–9.0 ppm) |
|---|---|---|---|
| Nostrano di Storo | |||
| 24 h (%) | 19.00 | 80.28 | 0.72 |
| 24/48 h (%) | 35.01 | 64.51 | 0.47 |
| Fold change | 1.84 | 0.80 | 0.65 |
| B73/Mo17 | |||
| 24 h (%) | 35.83 | 59.03 | 4.13 |
| 24/48 h (%) | 40.45 | 48.41 | 11.14 |
| Fold change | 1.13 | 0.82 | 2.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, S.; De Benedetti, S.; Mazzini, S.; Borgonovo, G.; Bona, E.; Cavaletto, M.; Corsetto, P.A.; Ghidoli, M.; Pilu, S.R.; Scarafoni, A. Antifungal Activity and Biochemical Profiling of Exudates from Germinating Maize Nostrano di Storo Local Variety. Plants 2022, 11, 2435. https://doi.org/10.3390/plants11182435
Rosa S, De Benedetti S, Mazzini S, Borgonovo G, Bona E, Cavaletto M, Corsetto PA, Ghidoli M, Pilu SR, Scarafoni A. Antifungal Activity and Biochemical Profiling of Exudates from Germinating Maize Nostrano di Storo Local Variety. Plants. 2022; 11(18):2435. https://doi.org/10.3390/plants11182435
Chicago/Turabian StyleRosa, Stefano, Stefano De Benedetti, Stefania Mazzini, Gigliola Borgonovo, Elisa Bona, Maria Cavaletto, Paola Antonia Corsetto, Martina Ghidoli, Salvatore Roberto Pilu, and Alessio Scarafoni. 2022. "Antifungal Activity and Biochemical Profiling of Exudates from Germinating Maize Nostrano di Storo Local Variety" Plants 11, no. 18: 2435. https://doi.org/10.3390/plants11182435
APA StyleRosa, S., De Benedetti, S., Mazzini, S., Borgonovo, G., Bona, E., Cavaletto, M., Corsetto, P. A., Ghidoli, M., Pilu, S. R., & Scarafoni, A. (2022). Antifungal Activity and Biochemical Profiling of Exudates from Germinating Maize Nostrano di Storo Local Variety. Plants, 11(18), 2435. https://doi.org/10.3390/plants11182435











