Selected Indices to Identify Water-Stress-Tolerant Tropical Forage Grasses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Variance
2.2. Morphological Responses of Forage Grasses to Water Stress
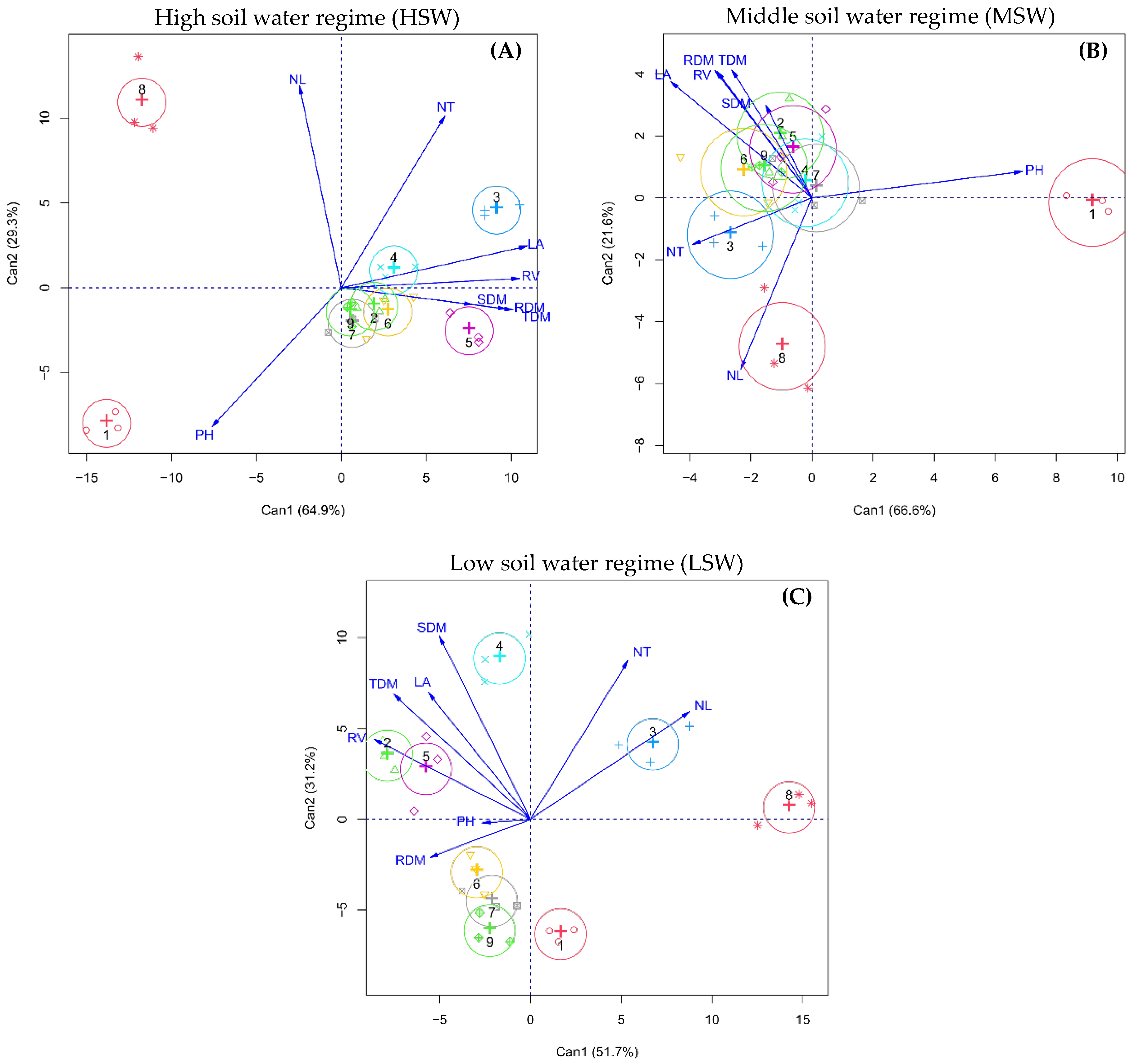
2.3. Interrelationship between Morphological Traits and Forage Grass Cultivars
2.4. Water Stress Tolerance Indices
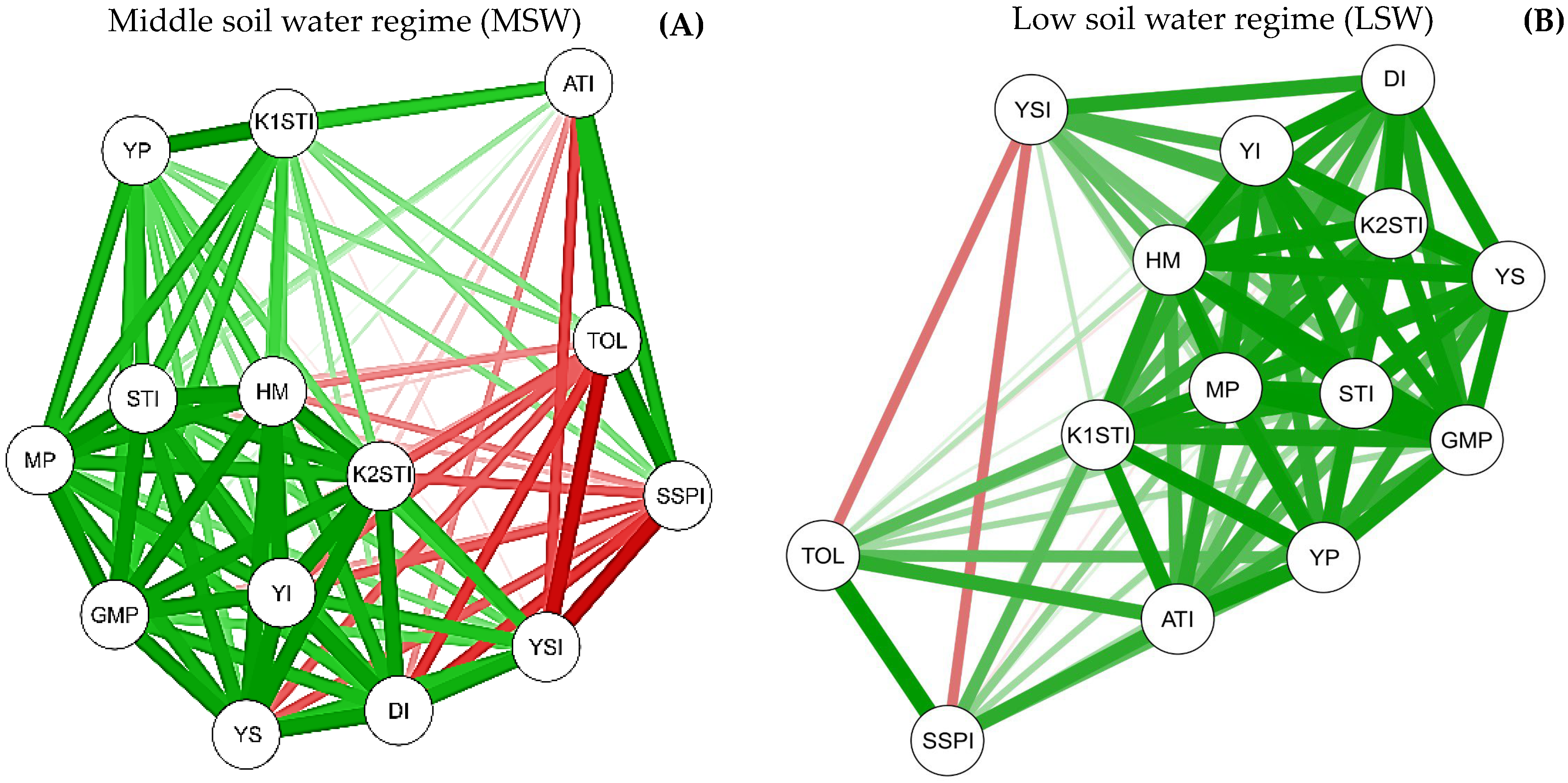
2.5. Interrelationship between Biomass Production and Water Stress Tolerance Indices
2.6. Ranking
3. Materials and Methods
3.1. Plant Growth Conditions
3.2. Experimental Design and Treatments
3.3. Plant Material and Irrigation
3.4. Measurement of Morphological Traits
3.5. Calculation of Water Stress Tolerance Indices
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- USDA—United States Department of Agriculture. Foreign Agricultural Service. Available online: https://www.usda.gov/ (accessed on 5 January 2022).
- IBGE—Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br/ (accessed on 5 January 2022).
- Feltran-Barbieri, R.; Féres, J.G. Degraded pastures in Brazil: Improving livestock production and forest restoration. Royal Soc. Open Sci. 2021, 8, 201854. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.; Zuffo, A.M.; Silva, K.C.; Lima, I.M.O.; Ardon, H.J.V. Cotton response to nitrogen fertilization in the integrated crop-livestock system. Sci. Agrar. Paranaensis 2020, 19, 211–220. [Google Scholar] [CrossRef]
- Fonseca, D.M.; Martusello, J.A. Plantas Forrageiras, 2nd ed.; Editora UFV: Viçosa, Brazil, 2022; 591p. [Google Scholar]
- Petter, F.A.; Pacheco, L.P.; Zuffo, A.M.; Piauilino, A.C.; Xavier, Z.F.; Santos, J.M.; Miranda, J.M.S. Performance of cover crops submitted to water deficit. Semin-Cienc. Agrar. 2013, 34, 3307–3319. [Google Scholar] [CrossRef]
- Pacheco, L.P.; Petter, F.A.; Lima, M.P.D.; Alixandre, T.F.; Silva, L.M.A.; Silva, R.R.; Ribeiro, W.R.M. Development of cover crops under different water levels in the soil. Afr. J. Agric. Res. 2013, 8, 2216–2223. [Google Scholar] [CrossRef]
- Staniak, M.; Kocoń, A. Forage grasses under drought stress in conditions of Poland. Acta Physiol. Plant. 2015, 37, 116. [Google Scholar] [CrossRef]
- Fariaszewska, A.; Aper, J.; Huylenbroeck, J.; Swaef, T.; Baert, J.; Pecio, L. Physiological and biochemical responses of forage grass varieties to mild drought stress under field conditions. Int. J. Plant Prod. 2020, 14, 335–353. [Google Scholar] [CrossRef]
- Silva, T.R.; Cazetta, J.O.; Carlin, S.D.; Telles, B.R. Drought-induced alterations in the uptake of nitrogen, phosphorus and potassium, and the relation with drought tolerance in sugar cane. Cienc. Agrotec. 2017, 41, 117–127. [Google Scholar] [CrossRef]
- Macedo, L.C.P.; Dornelles, S.H.B.; Peripolli, M.; Trivisiol, V.S.; Conceição, D.Q.; Pivetta, M.; Essi, L. Phenology and dry mass production of Urochloa plantaginea and Urochloa platyphylla submitted to different water quantities in the soil. Acta Sci. Biol. Sci. 2019, 41, 46127. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B. Physiological and morphometric response of forage grass species and their biomass distribution depending on the term and frequency of water deficiency. Agronomy 2021, 11, 2471. [Google Scholar] [CrossRef]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of drought damages by exogenous chitosan and yeast extract with modulating the photosynthetic pigments, antioxidant defense system and improving the productivity of garlic plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Res 2016, 30, 1554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev Genet 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Purbajanti, E.D.; Anwar, S.; Wydiati, N.; Kusmiyati, F. Drought stress effect on morphology characters, water use efficiency, growth and yield of guinea and napier grasses. Int. Res. J. Plant Sci. 2012, 3, 47–53. [Google Scholar]
- Mohammadi, R.; Armion, M.; Kahrizi, D.; Amri, A. Efficiency of screening techniques for evaluating durum wheat genotypes under mild drought conditions. Int. J. Plant Prod. 2010, 4, 11–24. [Google Scholar] [CrossRef]
- Menezes, C.B.; Ticona-Benavente, C.A.; Tardin, F.D.; Cardoso, M.J.; Bastos, E.A.; Nogueira, D.W.; Portugal, A.F.; Santos, C.V.; Schaffert, R.E. Selection indices to identify drought-tolerant grain sorghum cultivars. Genet. Mol. Res. 2014, 13, 9817–9827. [Google Scholar] [CrossRef]
- Cabral, R.C.; Maekawa, S.C.E.; Zuffo, A.M.; Steiner, F. Selection indices to identify drought-tolerant soybean cultivars. Res., Soc. Dev. 2020, 9, 1–25. [Google Scholar] [CrossRef]
- Naghavi, M.R.; Pour-Aboughadareh, A.; Khalili, M. Evaluation of drought tolerance indices for screening some of corn (Zea mays L.) cultivars under environmental conditions. Not. Sci. Biol. 2013, 5, 388–393. [Google Scholar] [CrossRef]
- Farshadfar, E.; PoursiahbidI, M.M.; Abooghadareh, A.R.P. Repeatability of drought tolerance indices in bread wheat genotypes. Intl. J. Agri. Crop Sci. 2012, 4, 891–903. [Google Scholar]
- El-Rawy, M.A.; Hassan, M.I. Effectiveness of drought tolerance indices to identify tolerant genotypes in bread wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 2014, 17, 255–266. [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R.; Bernousi, I. Evaluation of drought tolerance indices for selection of confectionery sunflower (Helianthus anuus L.) landraces under various environmental conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 187–201. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Evaluation of drought indices to identify tolerant genotypes in common bean bush (Phaseolus vulgaris L.). J. Integr. Agric. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Zuffo, A.M.; Ratke, R.F.; Steiner, F.; Oliveira, A.M.; Aguilera, J.G.; Lima, R.E. Silicon mitigates the effects of moderate drought stress in cover crops. J. Agron. Crop Sci. 2022, 208, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, C.S.; Nascimento-Júnior, D.; Silva, S.C.; Silveira, M.C.T.; Sousa, B.M.L.; Detmann, E. Characterization of tropical forage grass development pattern through the morphogenetic and structural characteristics. Rev. Bras. Zootec. 2011, 40, 527–534. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Mingoti, A.S. Análise de Dados Através de Métodos de Estatística Multivariada: Uma Abordagem Aplicada, 1st ed.; Editora UFMG: Belo Horizonte, Brazil, 2005; pp. 127–155. [Google Scholar]
- Silva, S.C.; Sbrissia, A.F.; Pereira, L.E.T. Ecophysiology of C4 forage grasses: Understanding plant growth for optimizing their use and management. Agriculture 2015, 5, 598–625. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Z.Q.; Yang, Z.P.; He, L.H. Absorption and accumulation characteristics of nitrogen in different wheat cultivars under irrigated and dryland conditions. Aust. J. Crop Sci. 2012, 6, 613–617. [Google Scholar]
- Ashraf, M.; Shahbaz, M.; Ali, Q. Drought-induced modulation in growth and mineral nutrients in canola (Brassica napus L.). Pak. J. Bot. 2013, 45, 93–98. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.G.; Cunha, T. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2018; 595p. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análises de Solos, 3rd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2017; 279p. [Google Scholar]
- Casaroli, D.; Lier, Q.J. Critérios para determinação da capacidade de vaso. Rev. Bras. Ci. Solo 2008, 32, 59–66. [Google Scholar] [CrossRef]
- Imakumbili, M.L.E. Making Water Stress Treatments in Pot Experiments: An Illustrated Step-by-Step Guide; Sokoine University of Agriculture: Morogoro, Tanzania, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Benincasa, M.P.M. Análise de Crescimento de Plantas: Noções Básicas, 1st ed.; Editora FUNEP: Jaboticabal, Brazil, 2003; pp. 1–42. [Google Scholar]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environments. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybean. Part 1: Evaluation of three screening techniques for heat and drought tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Blum, A. Plant Breeding for Stress Environments; CRC Press: Boca Raton, FL, USA, 1988; pp. 1–12. [Google Scholar]
- Fernández, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–18 August 1992; pp. 257–270. [Google Scholar]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campaline, R.G.; Ricciardi, G.L.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Farshadfar, E.; Sutka, J. Multivariate analysis of drought tolerance in wheat substitution lines. Cereal Res. Commun. 2003, 31, 33–39. [Google Scholar] [CrossRef]
- Moosavi, S.S.; Samadi, B.Y.; Naghavi, M.R.; Zali, A.A.; Dashti, H.; Pourshahbazi, A. Introduction of new indices to identify relative drought tolerance and resistance in wheat genotypes. Desert 2008, 12, 165–178. [Google Scholar]
- Jafari, A.; Paknejad, F.; Jami Al-ahmadi, M. Evaluation of selection indices for drought tolerance of corn (Zea mays L.) hybrids. Int. J. Plant Prod. 2009, 3, 33–38. [Google Scholar] [CrossRef]
- Zuffo, A.M.; Steiner, F.; Sousa, T.D.O.; Aguilera, J.G.; Teodoro, P.E.; Alcântara-Neto, F.; Ratke, R.F. How does water and salt stress affect the germination and initial growth of Brazilian soya bean cultivars? J. Agron. Crop Sci. 2020, 206, 837–850. [Google Scholar] [CrossRef]
| Causes of Variation | Probability > F | |||||||
|---|---|---|---|---|---|---|---|---|
| PH | NT | NL | LA | SDM | RDM | TDM | RV | |
| Forage cultivar (C) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Soil water regime (W) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| C × W | <0.01 | 0.914 | <0.01 | 0.045 | 0.518 | 0.020 | 0.029 | <0.01 |
| CV (%) | 13.65 | 16.41 | 18.61 | 17.82 | 15.99 | 20.66 | 15.70 | 21.92 |
| Forage Grass Cultivar | Soil Water Regime | ||
|---|---|---|---|
| High | Middle | Low | |
| Plant Height (cm) | |||
| U. brizantha cv. BRS Piatã | 74.0 ± 5.0 bA | 63.7 ± 8.1 bB | 53.3 ± 4.7 bC |
| U. brizantha cv. Marandu | 62.7 ± 10.1 cA | 51.3 ± 4.8 cA | 50.0 ± 2.5 bA |
| U. brizantha cv. Xaraés | 62.0 ± 9.9 cA | 56.0 ± 2.0 cA | 50.7 ± 2.4 bA |
| U. ruziziensis cv. Comum | 47.7 ± 6.4 cA | 47.3 ± 2.4 cA | 43.3 ± 2.9 bA |
| P. glaucum cv. ADR 300 | 154.1 ± 5.3 aA | 124.6 ± 5.1 aB | 86.2 ± 3.3 aC |
| P. maximum cv. Aruana | 72.7 ± 3.7 bA | 71.0 ± 7.5 bA | 71.3 ± 1.8 aA |
| P. maximum cv. Mombaça | 79.7 ± 6.4 bA | 62.7 ± 4.1 bB | 62.0 ± 2.0 bB |
| P. maximum cv. Tanzânia | 68.7 ± 5.8 bA | 63.7 ± 5.3 bA | 63.7 ± 1.3 bA |
| P. atratum cv. Pojuca | 62.0 ± 9.9 cA | 52.3 ± 1.4 cA | 52.7 ± 3.7 bA |
| Number of leaves per plant | |||
| U. brizantha cv. BRS Piatã | 40 ± 1 dA | 33 ± 3 bA | 20 ± 1 bA |
| U. brizantha cv. Marandu | 41 ± 4 dA | 32 ± 3 bA | 24 ± 1 bA |
| U. brizantha cv. Xaraés | 36 ± 2 dA | 26 ± 1 bA | 18 ± 1 bA |
| U. ruziziensis cv. Comum | 119 ± 6 bA | 88 ± 2 aB | 51 ± 3 aC |
| P. glaucum cv. ADR 300 | 26 ± 2 dA | 21 ± 1 bA | 15 ± 2 bA |
| P. maximum cv. Aruana | 75 ± 5 cA | 47 ± 5 bB | 38 ± 3 bB |
| P. maximum cv. Mombaça | 52 ± 6 dA | 30 ± 2 bB | 23 ± 3 bC |
| P. maximum cv. Tanzânia | 53 ± 2 dA | 32 ± 1 bA | 32 ± 5 bA |
| P. atratum cv. Pojuca | 195 ± 20 aA | 94 ± 19 aB | 70 ± 10 aC |
| Leaf area (dm2/plant) | |||
| U. brizantha cv. BRS Piatã | 16.9 ± 1.2 bA | 10.2 ± 0.3 bB | 7.6 ± 0.5 aB |
| U. brizantha cv. Marandu | 20.8 ± 2.2 bA | 17.5 ± 5.1 aA | 9.9 ± 0.9 aB |
| U. brizantha cv. Xaraés | 21.0 ± 1.2 bA | 14.8 ± 1.5 aB | 8.8 ± 1.5 aC |
| U. ruziziensis cv. Comum | 31.6 ± 6.8 aA | 18.9 ± 2.9 aB | 11.9 ± 1.6 aC |
| P. glaucum cv. ADR 300 | 1.6 ± 0.2 dA | 1.4 ± 0.1 bA | 1.3 ± 0.2 bA |
| P. maximum cv. Aruana | 28.9 ± 1.6 aA | 17.0 ± 2.0 aB | 11.9 ± 1.8 aB |
| P. maximum cv. Mombaça | 28.6 ± 2.7 aA | 18.8 ± 3.7 aB | 12.0 ± 1.3 aC |
| P. maximum cv. Tanzânia | 23.3 ± 2.3 bA | 21.1 ± 2.3 aA | 12.9 ± 0.8 aB |
| P. atratum cv. Pojuca | 10.8 ± 1.6 cA | 5.5 ± 0.8 bA | 4.4 ± 1.3 bA |
| Forage Grass Cultivar | Soil Water Regimes | ||
|---|---|---|---|
| High | Middle | Low | |
| Root dry matter (g plant–1) | |||
| U. brizantha cv. BRS Piatã | 23.3 ± 3.4 aA | 15.0 ± 5.5 aB | 5.5 ± 1.0 aC |
| U. brizantha cv. Marandu | 19.5 ± 1.3 aA | 12.5 ± 1.2 aB | 7.5 ± 1.0 aB |
| U. brizantha cv. Xaraés | 14.0 ± 1.4 bA | 12.2 ± 1.1 aA | 8.8 ± 1.1 aA |
| U. ruziziensis cv. Comum | 22.8 ± 1.0 aA | 14.4 ± 0.3 aB | 7.1 ± 0.6 aC |
| P. glaucum cv. ADR 300 | 5.4 ± 0.6 cA | 5.2 ± 0.4 bA | 3.6 ± 0.2 aA |
| P. maximum cv. Aruana | 17.5 ± 3.9 aA | 8.3 ± 1.1 bB | 1.2 ± 0.1 aC |
| P. maximum cv. Mombaça | 28.4 ± 6.7 aA | 13.1 ± 0.6 aB | 8.3 ± 0.9 aB |
| P. maximum cv. Tanzânia | 21.0 ± 3.6 aA | 15.0 ± 3.4 aB | 12.3 ± 1.2 aB |
| P. atratum cv. Pojuca | 7.0 ± 1.5 cA | 3.6 ± 0.7 bA | 2.9 ± 0.3 aA |
| Total dry matter (g plant–1) | |||
| U. brizantha cv. BRS Piatã | 55.6 ± 4.3 bA | 36.0 ± 1.3 bB | 23.0 ± 5.9 bC |
| U. brizantha cv. Marandu | 50.7 ± 1.6 bA | 35.3 ± 2.0 bB | 23.2 ± 1.7 bC |
| U. brizantha cv. Xaraés | 42.4 ± 3.9 cA | 32.5 ± 1.8 bB | 22.6 ± 0.7 bC |
| U. ruziziensis cv. Comum | 56.8 ± 1.0 bA | 45.9 ± 3.5 aB | 26.1 ± 1.3 aC |
| P. glaucum cv. ADR 300 | 33.3 ± 0.3 cA | 25.3 ± 1.1 cB | 17.7 ± 0.2 bB |
| P. maximum cv. Aruana | 58.1 ± 4.3 bA | 34.0 ± 7.4 bB | 26.8 ± 0.2 aB |
| P. maximum cv. Mombaça | 69.3 ± 7.1 aA | 42.1 ± 1.2 aB | 29.9 ± 0.7 aC |
| P. maximum cv. Tanzânia | 56.3 ± 4.8 bA | 43.2 ± 4.3 aB | 33.7 ± 1.4 aB |
| P. atratum cv. Pojuca | 34.4 ± 1.8 cA | 18.0 ± 4.2 cB | 17.3 ± 1.2 bB |
| Root volume (cm3 plant–1) | |||
| U. brizantha cv. BRS Piatã | 88.9 ± 19.8 dA | 64.4 ± 6.8 aB | 38.9 ± 5.6 aC |
| U. brizantha cv. Marandu | 106.7 ± 3.3 cA | 61.8 ± 6.7 aB | 37.8 ± 4.0 aB |
| U. brizantha cv. Xaraés | 94.0 ± 6.8 cA | 58.9 ± 9.5 aB | 32.9 ± 0.4 aC |
| U. ruziziensis cv. Comum | 154.4 ± 14.6 aA | 85.0 ± 8.7 aB | 33.2 ± 1.9 aC |
| P. glaucum cv. ADR 300 | 53.3 ± 5.7 dA | 31.7 ± 1.0 bB | 23.3 ± 1.9 aB |
| P. maximum cv. Aruana | 113.0 ± 3.5 cA | 85.0 ± 16.0 aB | 38.9 ± 2.2 aC |
| P. maximum cv. Mombaça | 162.8 ± 11.4 aA | 71.1 ± 8.7 aB | 52.2 ± 1.1 aB |
| P. maximum cv. Tanzânia | 133.3 ± 19.0 bA | 83.3 ± 3.8 aB | 58.9 ± 6.8 aC |
| P. atratum cv. Pojuca | 64.4 ± 2.2 dA | 22.1 ± 9.0 bB | 21.1 ± 4.0 aB |
| Causes of Variation | Number of Tillers (Units) | Shoot Dry Matter (g plant–1) |
|---|---|---|
| Forage grass cultivars | ||
| U. brizantha cv. BRS Piatã | 10.8 ± 0.8 d | 20.8 ± 2.6 b |
| U. brizantha cv. Marandu | 12.3 ± 0.9 d | 23.2 ± 2.3 b |
| U. brizantha cv. Xaraés | 11.1 ± 0.7 d | 20.8 ± 2.2 b |
| U. ruziziensis cv. Comum | 26.1 ± 3.0 a | 28.2 ± 2.6 a |
| P. glaucum cv. ADR 300 | 4.2 ± 0.3 e | 20.8 ± 2.0 b |
| P. maximum cv. Aruana | 18.4 ± 1.2 b | 30.6 ± 3.4 a |
| P. maximum cv. Mombaça | 15.8 ± 1.0 c | 30.5 ± 3.1 a |
| P. maximum cv. Tanzânia | 15.6 ± 1.0 c | 28.3 ± 2.2 a |
| P. atratum cv. Pojuca | 19.8 ± 1.4 b | 18.2 ± 2.6 b |
| Soil water regimes | ||
| High | 16.4 ± 1.2 a | 33.1 ± 1.1 a |
| Middle | 15.6 ± 1.6 a | 23.6 ± 1.4 b |
| Low | 12.6 ± 1.1 b | 17.8 ± 0.8 c |
| Forage Grass Cultivar | YP † | YS †† | Water Stress Tolerance Indices | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOL | MP | YSI | DI | STI | GMP | YI | k1STI | k2STI | SSPI | ATI | HM | |||
| Middle soil water regime (MSW) | ||||||||||||||
| U. brizantha cv. BRS Piatã | 32.3 b | 21.0 a | 11.3 a | 26.6 b | 0.65 a | 0.58 a | 0.62 b | 26.0 b | 0.89 a | 0.95 b | 0.89 a | 23.9 a | 210.4 a | 25.4 b |
| U. brizantha cv. Marandu | 31.3 b | 22.8 a | 8.5 a | 27.0 b | 0.73 a | 0.71 a | 0.65 b | 26.7 b | 0.96 a | 0.89 b | 0.96 a | 17.9 a | 161.1 a | 26.3 b |
| U. brizantha cv. Xaraés | 28.4 b | 20.2 a | 8.2 a | 24.3 b | 0.72 a | 0.61 a | 0.53 b | 240 b | 0.86 a | 0.75 b | 0.86 a | 17.3 a | 143.5 a | 23.6 b |
| U. ruziziensis cv. Comum | 34.0 b | 28.1 a | 5.9 a | 31.1 a | 0.83 a | 0.99 a | 0.88 a | 30.9 a | 1.19 a | 1.06 b | 1.19 a | 12.4 a | 131.5 a | 30.8 a |
| P. glaucum cv. ADR 300 | 28.0 b | 20.1 a | 7.8 a | 24.0 b | 0.72 a | 0.61 a | 0.51 b | 23.7 b | 0.85 a | 0.71 b | 0.85 a | 16.5 a | 132.6 a | 23.4 b |
| P. maximum cv. Aruana | 40.5 a | 25.7 a | 14.8 a | 33.1 a | 0.63 a | 0.82 a | 0.95 a | 31.3 a | 1.09 a | 1.50 a | 1.09 a | 31.4 a | 270.3 a | 29.8 a |
| P. maximum cv. Mombaça | 41.0 a | 29.0 a | 12.0 a | 35.0 a | 0.72 a | 0.89 a | 1.08 a | 34.3 a | 1.23 a | 1.57 a | 1.23 a | 25.4 a | 305.4 a | 33.7 a |
| P. maximum cv. Tanzânia | 35.3 a | 28.2 a | 7.1 a | 31.8 a | 0.80 a | 0.96 a | 0.91 a | 31.5 a | 1.19 a | 1.14 b | 1.19 a | 15.1 a | 157.2 a | 31.2 a |
| P. atratum cv. Pojuca | 27.4 b | 14.3 a | 13.0 a | 20.8 b | 0.53 a | 0.36 a | 0.36 b | 19.5 b | 0.61 a | 0.68 b | 0.61 a | 27.6 a | 169.6 a | 18.3 b |
| Mean | 5.11 | 4.41 | 1.83 | 4.68 | 0.06 | 0.19 | 0.22 | 4.80 | 0.25 | 0.33 | 0.25 | 5.19 | 55.79 | 4.91 |
| CV (%) | 15.41 | 24.93 | 11.88 | 18.43 | 11.02 | 35.71 | 40.32 | 19.88 | 24.93 | 31.60 | 24.93 | 11.88 | 27.64 | 21.38 |
| Low soil water regime (LSW) | ||||||||||||||
| U. brizantha cv. BRS Piatã | 32.3 b | 14.8 d | 17.5 a | 23.6 c | 0.46 a | 0.39 d | 0.44 d | 21.9 c | 0.84 d | 0.95 b | 0.84 c | 49.4 a | 203.9 b | 20.3 d |
| U. brizantha cv. Marandu | 31.3 b | 15.6 d | 15.6 a | 23.4 c | 0.50 a | 0.44 d | 0.45 d | 22.1 c | 0.88 d | 0.89 b | 0.88 c | 44.2 a | 184.6 b | 20.8 d |
| U. brizantha cv. Xaraés | 28.4 b | 13.8 d | 14.6 a | 21.1 c | 0.49 a | 0.39 d | 0.36 d | 19.8 c | 0.78 d | 0.75 b | 0.78 c | 41.2 a | 156.4 b | 18.5 d |
| U. ruziziensis cv. Comum | 34.0 b | 19.0 c | 15.0 a | 26.5 b | 0.56 a | 0.61 c | 0.59 c | 25.4 b | 1.08 c | 1.06 b | 1.08 c | 42.3 a | 204.6 b | 24.4 c |
| P. glaucum cv. ADR 300 | 28.0 b | 14.2 d | 13.8 a | 21.1 c | 0.51 a | 0.41 d | 0.36 d | 19.9 c | 0.80 d | 0.71 b | 0.80 c | 39.1 a | 146.9 b | 18.8 d |
| P. maximum cv. Aruana | 40.5 a | 25.6 a | 14.9 a | 33.1 a | 0.63 a | 0.91 a | 0.95 a | 32.2 a | 1.45 a | 1.50 a | 1.45 a | 42.3 a | 257.0 a | 31.4 a |
| P. maximum cv. Mombaça | 41.0 a | 21.6 b | 19.4 a | 31.3 a | 0.53 a | 0.65 c | 0.82 b | 29.7 a | 1.22 b | 1.57 a | 1.22 b | 54.7 a | 315.7 a | 28.3 b |
| P. maximum cv. Tanzânia | 35.3 a | 21.4 b | 13.9 a | 28.4 b | 0.61 a | 0.74 b | 0.69 b | 27.5 b | 1.21 b | 1.14 b | 1.21 b | 39.3 a | 204.7 b | 26.7 c |
| P. atratum cv. Pojuca | 27.4 b | 13.0 d | 14.3 a | 20.2 c | 0.48 a | 0.36 d | 0.32 d | 18.8 c | 0.74 d | 0.68 b | 0.74 c | 40.6 a | 142.8 b | 17.5 d |
| Mean | 5.11 | 4.88 | 3.04 | 4.76 | 0.09 | 0.21 | 0.24 | 4.78 | 0.21 | 0.33 | 0.21 | 6.43 | 62.54 | 4.86 |
| CV (%) | 15.41 | 20.98 | 30.89 | 16.88 | 12.90 | 28.55 | 33.69 | 17.35 | 20.98 | 31.60 | 20.98 | 30.89 | 33.47 | 18.03 |
| Forage Grass Cultivar | YP | YS | Stress Tolerance Indices | (±SD) | Tolerance Level † | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOL | MP | YSI | DI | STI | GMP | YI | k1STI | k2STI | SSPI | ATI | HM | |||||
| Middle Soil Water Regime | ||||||||||||||||
| U. brizantha cv. BRS Piatã | 5 | 6 | 6 | 6 | 7 | 8 | 6 | 6 | 6 | 5 | 6 | 4 | 3 | 6 | 5.7 (±0.8) | MS |
| U. brizantha cv. Marandu | 6 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 5 | 6 | 5 | 5 | 5 | 5 | 5.0 (±0.3) | MT |
| U. brizantha cv. Xaraés | 7 | 7 | 4 | 7 | 6 | 7 | 7 | 7 | 7 | 7 | 7 | 6 | 7 | 7 | 6.6 (±0.6) | MS |
| U. ruziziensis cv. Comum | 4 | 3 | 1 | 4 | 1 | 1 | 4 | 4 | 3 | 4 | 3 | 9 | 9 | 3 | 3.8 (±1.6) | MT |
| P. glaucum cv. ADR 300 | 8 | 8 | 3 | 8 | 5 | 6 | 8 | 8 | 8 | 8 | 8 | 7 | 8 | 8 | 7.2 (±1.1) | S |
| P. maximum cv. Aruana | 2 | 4 | 9 | 2 | 8 | 4 | 2 | 3 | 4 | 2 | 4 | 1 | 2 | 4 | 3.6 (±1.6) | MT |
| P. maximum cv. Mombaça | 1 | 1 | 7 | 1 | 4 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1.9 (±1.3) | T |
| P. maximum cv. Tanzânia | 3 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 8 | 6 | 2 | 3.0 (±1.1) | T |
| P. atratum cv. Pojuca | 9 | 9 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 2 | 4 | 9 | 8.1 (±1.5) | S |
| Low Soil Water Regime | ||||||||||||||||
| U. brizantha cv. BRS Piatã | 5 | 6 | 8 | 5 | 9 | 7 | 6 | 6 | 6 | 5 | 6 | 2 | 5 | 4 | 5.7 (±1.2) | MS |
| U. brizantha cv. Marandu | 6 | 5 | 7 | 6 | 6 | 5 | 5 | 5 | 5 | 6 | 5 | 3 | 6 | 5 | 5.4 (±0.7) | MS |
| U. brizantha cv. Xaraés | 7 | 8 | 4 | 7 | 7 | 8 | 8 | 8 | 8 | 7 | 8 | 6 | 7 | 2 | 6.8 (±1.2) | MS |
| U. ruziziensis cv. Comum | 4 | 4 | 6 | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 6 | 4.2 (±0.5) | MT |
| P. glaucum cv. ADR 300 | 8 | 7 | 1 | 8 | 5 | 6 | 7 | 7 | 7 | 8 | 7 | 9 | 8 | 3 | 6.5 (±1.6) | MS |
| P. maximum cv. Aruana | 2 | 1 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 5 | 2 | 9 | 2.4 (±1.7) | T |
| P. maximum cv. Mombaça | 1 | 2 | 9 | 2 | 4 | 3 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 8 | 2.9 (±1.8) | T |
| P. maximum cv. Tanzânia | 3 | 3 | 2 | 3 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 8 | 3 | 7 | 3.4 (±1.2) | MT |
| P. atratum cv. Pojuca | 9 | 9 | 3 | 9 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | 7 | 9 | 1 | 7.8 (±1.8) | S |
| Forage Grass Cultivar | Common Name | Cultivar | Growth Habit | Soil Fertility Requirement | Forage Yield | Drought Tolerance |
|---|---|---|---|---|---|---|
| Urochloa brizantha | Palisade grass | BRS Piatã | Semierect | Medium | High | High |
| Urochloa brizantha | Palisade grass | Marandu | Erect | Medium | Medium | Medium |
| Urochloa brizantha | Palisade grass | Xaraés | Semierect | Medium | High | Medium |
| Panicum maximum | Guinea grass | Aruana | Erect | Medium/High | High | Medium/Low |
| Panicum maximum | Guinea grass | Mombaça | Erect | Medium/High | High | Medium/Low |
| Panicum maximum | Guinea grass | Tanzânia | Erect | Medium/High | High | Medium/Low |
| Pennisetum glaucum | Pearl millet | ADR 300 | Erect | Medium | High | Medium/High |
| Urochloa ruziziensis | Ruzigrass | Comum | Semierect | Medium/High | High | Low |
| Paspalum atratum | Atratum | Pojuca | Erect | Low | High | Low |
| Water Stress Tolerance Index | Equation † | Reference |
|---|---|---|
| 1. Tolerance | TOL = YP − YS | [37] |
| 2. Mean productivity | MP = (YS + YP)/2 | [37] |
| 3. Yield stability index | YSI = YS/YP | [38] |
| 4. Drought resistance index | DI = [YS × (YS/YP)]/ȲS | [39] |
| 5. Stress tolerance index | STI = (YS × YP)/(ȲP)2 | [40] |
| 6. Geometric mean productivity | GMP = √(YS × YP) | [40] |
| 7. Yield index | YI = YS/ȲS | [41] |
| 8. Modified stress tolerance (k1) | k1STI = YP2/ȲP2 | [42] |
| 9. Modified stress tolerance (k2) | k2STI = YS2/ȲS2 | [42] |
| 10. Stress susceptibility percentage index | SSPI = [(YP − YS)/2 × ȲP] × 100 | [43] |
| 11. Abiotic tolerance index | ATI = [(YP–YS)/(ȲP/ȲS)] × √(YP × YS) | [43] |
| 12. Harmonic mean | HM = [2 × (YS × YP)]/(YS + YP) | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuffo, A.M.; Steiner, F.; Aguilera, J.G.; Ratke, R.F.; Barrozo, L.M.; Mezzomo, R.; Santos, A.S.d.; Gonzales, H.H.S.; Cubillas, P.A.; Ancca, S.M. Selected Indices to Identify Water-Stress-Tolerant Tropical Forage Grasses. Plants 2022, 11, 2444. https://doi.org/10.3390/plants11182444
Zuffo AM, Steiner F, Aguilera JG, Ratke RF, Barrozo LM, Mezzomo R, Santos ASd, Gonzales HHS, Cubillas PA, Ancca SM. Selected Indices to Identify Water-Stress-Tolerant Tropical Forage Grasses. Plants. 2022; 11(18):2444. https://doi.org/10.3390/plants11182444
Chicago/Turabian StyleZuffo, Alan Mario, Fábio Steiner, Jorge González Aguilera, Rafael Felippe Ratke, Leandra Matos Barrozo, Ricardo Mezzomo, Adaniel Sousa dos Santos, Hebert Hernán Soto Gonzales, Pedro Arias Cubillas, and Sheda Méndez Ancca. 2022. "Selected Indices to Identify Water-Stress-Tolerant Tropical Forage Grasses" Plants 11, no. 18: 2444. https://doi.org/10.3390/plants11182444
APA StyleZuffo, A. M., Steiner, F., Aguilera, J. G., Ratke, R. F., Barrozo, L. M., Mezzomo, R., Santos, A. S. d., Gonzales, H. H. S., Cubillas, P. A., & Ancca, S. M. (2022). Selected Indices to Identify Water-Stress-Tolerant Tropical Forage Grasses. Plants, 11(18), 2444. https://doi.org/10.3390/plants11182444










