Biostimulants as an Alternative to Improve the Wine Quality from Vitis vinifera (cv. Tempranillo) in La Rioja
Abstract
:1. Introduction
2. Results
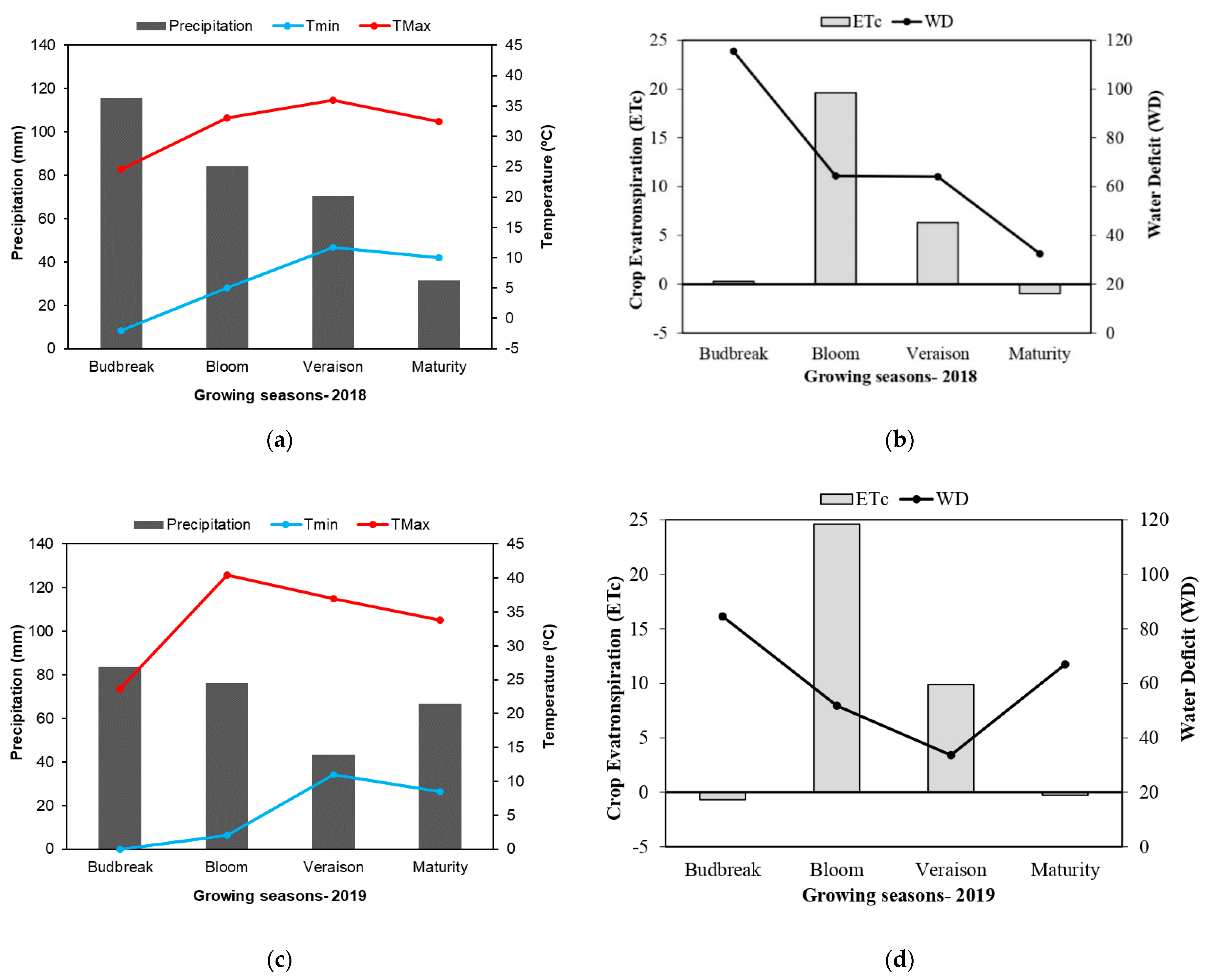
2.1. The Two Seasons Differed in Climatology and Water Availability for the Vineyard
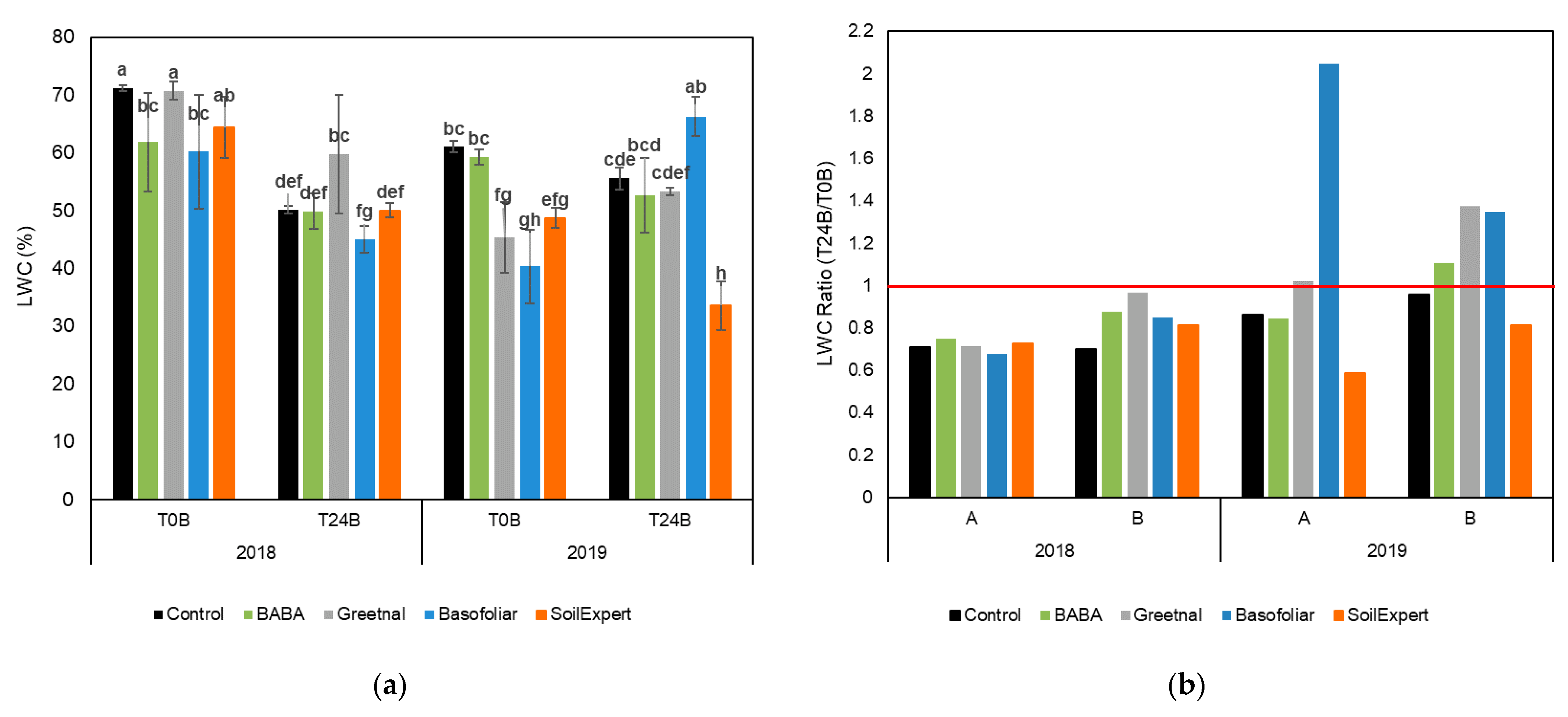
2.2. Biostimulants Modified the Water Balance of the Plants
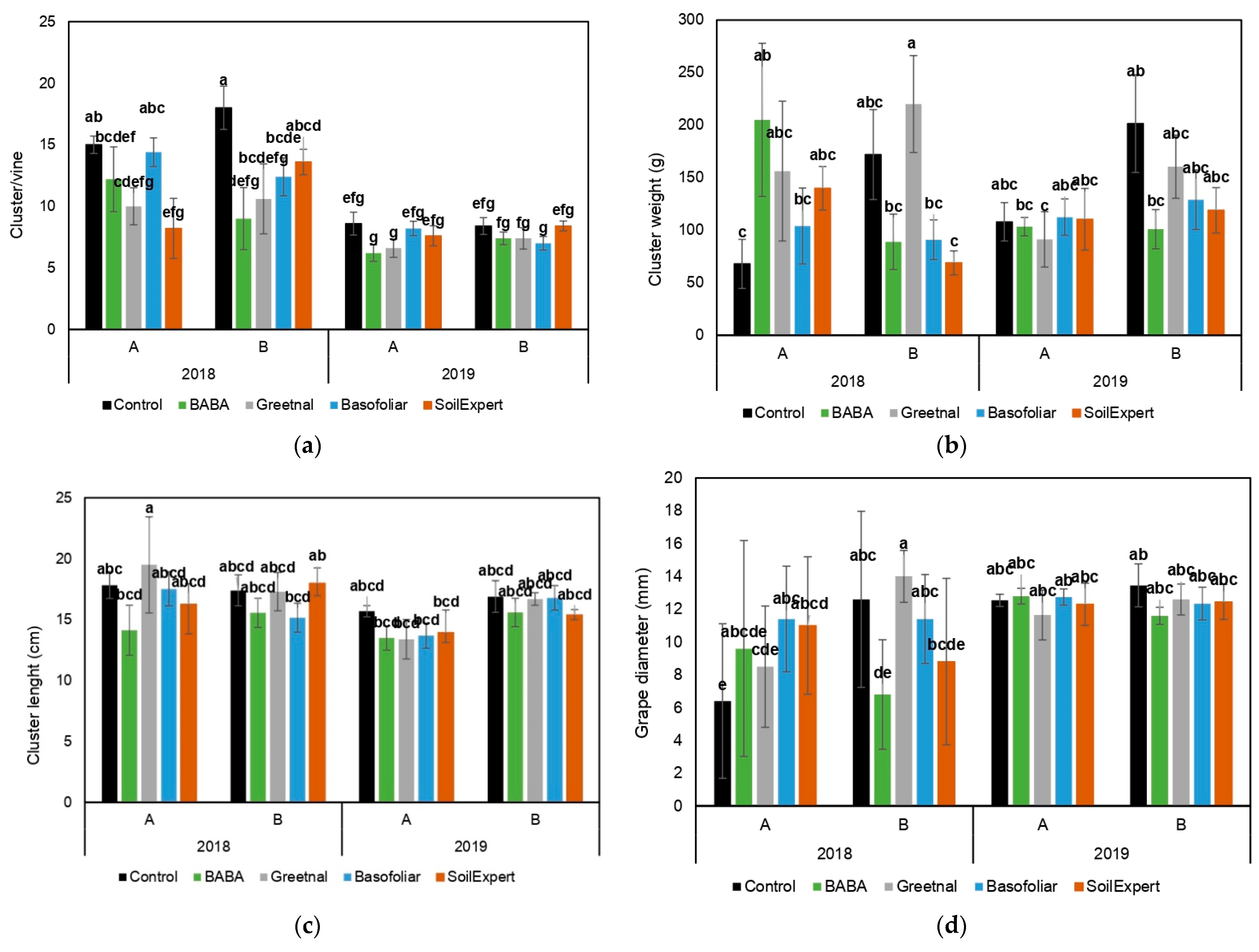
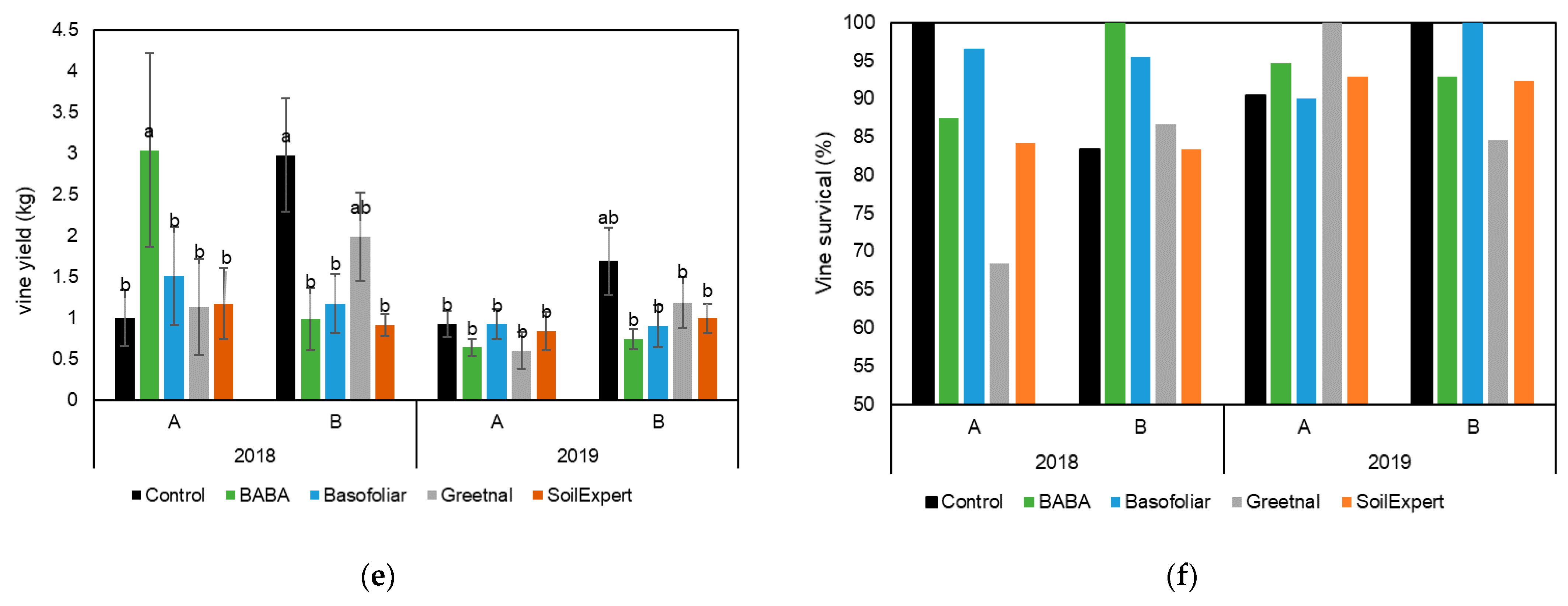
2.3. Biostimulant Applications Improved the Production under Moderate but Not Severe Stress Conditions
2.4. Biostimulant Applications Modified Water Use Efficiency Related to Production
2.5. A Better Physiological Response Supports a Higher Vine Yield
2.6. The Application of Biostimulants Altered the Metabolite Content in Vines
2.7. The Commercial Biostimulants Varied in Their Metabolic Composition
3. Discussion
4. Materials and Methods
4.1. Grapevine Field and Experimental Design
4.2. Climate Data
4.3. Leaf Water Content
4.4. Yield Related Parameters and Plant Survival
4.5. Free Amino Acids Quantification by HPLC-FLD
4.6. Organic Acids Quantification by HPLC-DAD
4.7. Carbohydrates Quantified in Berries and Leaves by HPLC-RID
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. Actualidad de La Coyuntura Del Sector Vinícola Mundial En 2019. 2020. Available online: http://www.oiv.int/public/medias/7304/es-actualidad-de-la-coyuntura-del-sector-vitivin-cola-mundia.pdf (accessed on 24 April 2022).
- Consejo Regulador de la Denominación de Origen Calificada Rioja. Estadísticas Rioja 2019. Available online: https://www.riojawine.com/wp-content/uploads/2020/05/ESTADISTICAS_RIOJA2019.pdf (accessed on 25 November 2020).
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 16. [Google Scholar] [CrossRef] [PubMed]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A Review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Soleimani, A. Effect of chilling on budbreak in ‘Thompson Seedless’ and ‘Carignane’ grapevines. Am. J. Enol. Vitic. 1972, 23, 31–34. [Google Scholar]
- Greer, D.H.; Weedon, M.M. The impact of high temperatures on Vitis vinifera Cv. Semillon grapevine performance and berry ripening. Front. Plant Sci. 2013, 4, 491. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Bonada, M.; Jeffery, D.W.; Petrie, P.R.; Moran, M.A.; Sadrás, V.O. Impact of elevated temperature and water deficit on the chemical and sensory profiles of Barossa Shiraz grapes and wines. Aust. J. Grape Wine Res. 2015, 21, 240–253. [Google Scholar] [CrossRef]
- Ramos, M.C.; Martínez de Toda, F. Variability in the potential effects of climate change on phenology and on grape composition of tempranillo in three zones of the Rioja DOCa (Spain). Eur. J. Agron. 2020, 115, 126014. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Pérez, D.; Risco, D.; Yeves, A.; Castel, J.R. Yield components and grape composition responses to seasonal water deficits in Tempranillo grapevines. Irrig. Sci. 2012, 30, 339–349. [Google Scholar] [CrossRef]
- Torres, N.; Hilbert, G.; Luquin, J.; Goicoechea, N.; Antolín, M.C. Flavonoid and amino acid profiling on Vitis vinifera L. Cv Tempranillo subjected to deficit irrigation under elevated temperatures. J. Food Compos. Anal. 2017, 62, 51–62. [Google Scholar] [CrossRef]
- Cooley, N.M.; Clingeleffer, P.R.; Walker, R.R. Effect of water deficits and season on berry development and composition of Cabernet Sauvignon (Vitis vinifera L.) grown in a hot climate. Aust. J. Grape Wine Res. 2017, 23, 260–272. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera Cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E. Effects of applied water amounts at various fractions of evapotranspiration (ETc) on leaf gas exchange of Thompson Seedless grapevines. Aust. J. Grape Wine Res. 2012, 10, 100–108. [Google Scholar] [CrossRef]
- Scholasch, T.; Rienth, M. Review of water deficit mediated changes in vine and berry physiology; Consequences for the optimization of irrigation strategies. OENO One 2019, 53, 423–444. [Google Scholar] [CrossRef]
- Ma, X.; Sanguinet, K.A.; Jacoby, P.W. Direct root-zone irrigation outperforms surface drip irrigation for grape yield and crop water use efficiency while restricting root growth. Agric. Water Manag. 2020, 231, 105993. [Google Scholar] [CrossRef]
- Ramos, M.C.; Martínez-Casasnovas, J.A. Soil water balance in rainfed vineyards of the Penedès Region (Northeastern Spain) affected by rainfall characteristics and land levelling: Influence on grape yield. Plant Soil 2010, 333, 375–389. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Pelsy, F. Molecular and cellular mechanisms of diversity within grapevine varieties. Heredity 2010, 104, 331–340. [Google Scholar] [CrossRef]
- White, M.A.; Diffenbaugh, N.S.; Jones, G.V.; Pal, J.S.; Giorgi, F. Extreme heat reduces and shifts United States premium wine production in the 21st century. Proc. Natl. Acad. Sci. USA 2006, 103, 11217–11222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriondo, M.; Jones, G.V.; Bois, B.; Dibari, C.; Ferrise, R.; Trombi, G.; Bindi, M. Projected shifts of wine regions in response to climate change. Clim. Chang. 2013, 119, 825–839. [Google Scholar] [CrossRef]
- Helay, A.A. Strategies for improvement of horticultural crops against abiotic stresses. J. Hortic. 2017, 4, e107. [Google Scholar] [CrossRef] [Green Version]
- Balestrini, R.; Magurno, F.; Walker, C.; Lumini, E.; Bianciotto, V. Cohorts of arbuscular mycorrhizal fungi (A.M.F.) in Vitis vinifera, a typical Mediterranean fruit crop. Environ. Microbiol. Rep. 2010, 2, 594–604. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Boller, T.; Felix, G. A Renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef] [Green Version]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Mancuso, S.; Azzarello, E.; Mugnai, S.; Briand, X. Marine bioactive substances (I.P.A. Extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv. Hortic. Sci. 2006, 20, 156–161. [Google Scholar] [CrossRef]
- Mugnai, S.; Azzarello, E.; Pandolfi, C.; Salamagne, S.; Briand, X.; Mancuso, S. Enhancement of ammonium and potassium root influxes by the application of marine bioactive substances positively affects Vitis vinifera plant growth. J. Appl. Phycol. 2008, 20, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physicochemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Storchi, P.; Mattii, G.B. Eco-physiological traits and phenylpropanoid profiling on potted Vitis vinifera L. Cv Pinot Noir subjected to Ascophyllum nodosum treatments under post-veraison low water availability. Appl. Sci. 2020, 10, 4473. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [Green Version]
- De Diego, N.; Sampedro, M.C.; Barrio, R.J.; Saiz-Fernández, I.; Moncaleán, P.; Lacuesta, M. Solute accumulation and elastic modulus changes in six radiata pine breeds exposed to drought. Tree Physiol. 2013, 33, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovadia, R.; Oren-Shamir, M.; Kaplunov, T.; Zutahy, Y.; Lichter, A.; Lurie, S. Effects of plant growth regulators and high temperature on colour development in “Crimson Seedless” grapes. J. Hortic. Sci. Biotechnol. 2013, 88, 387–392. [Google Scholar] [CrossRef]
- Keller, M. Managing Grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Drincovich, M.F.; Voll, L.M.; Maurino, V.G. Editorial: On the diversity of roles of organic acids. Front. Plant Sci. 2016, 7, 1592. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Zotou, A.; Loukou, Z.; Karava, O. Method development for the determination of seven organic acids in wines by reversed-phase high performance liquid chromatography. Chromatographia 2004, 60, 39–44. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Martínez-Vidaurre, J.M.; García-Escudero, E.; Garde-Cerdán, T. Amino acids content in “Tempranillo” must from three soil types over four vintages. Vitis J. Grapevine Res. 2019, 58, 3–12. [Google Scholar] [CrossRef]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. N. Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N.; et al. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef] [PubMed]
- Hamiduzzaman, M. β-Aminobutyric Acid-Induced Resistance in Grapevine Against Downy Mildew (Plasmopara Viticola). Ph.D. Thesis, University of Neuchâtel, Neuchâtel, Switzerland, 2005. [Google Scholar]
- Baccelli, I.; Mauch-Mani, B. Beta-Aminobutyric acid priming of plant defense: The role of ABA and other hormones. Plant Mol. Biol. 2016, 91, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Ugena, L.; Hýlová, A.; Podlešáková, K.; Humplík, J.F.; Doležal, K.; De Diego, N.; Spíchal, L. Characterization of biostimulant mode of action using novel multi-trait high-throughput screening of arabidopsis germination and rosette growth. Front. Plant Sci. 2018, 9, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The role of biostimulants as alleviators of biotic and abiotic stresses in grapevine: A review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Samuels, L.J.; Setati, M.E.; Blancquaert, E.H. Towards a better understanding of the potential benefits of seaweed based biostimulants in Vitis vinifera L. cultivars. Plants 2022, 11, 348. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Quaglia, M.; Calderini, O.; Moretti, C.; Poni, S.; Gatti, M.; Moncalvo, A.; Sabbatini, P.; Berrìos, J.G.; et al. Metabolic and transcriptional changes associated with the use of Ascophyllum nodosum extracts as tools to improve the quality of wine grapes (Vitis vinifera Cv. Sangiovese) and their tolerance to biotic stress. J. Sci. Food Agric. 2019, 99, 6350–6363. [Google Scholar] [CrossRef]
- Taskos, D.; Stamatiadis, S.; Yvin, J.C.; Jamois, F. Effects of an Ascophyllum nodosum (L.) Le Jol. extract on grapevine yield and berry composition of a merlot vineyard. Sci. Hortic. 2019, 250, 27–32. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Costa, B.S.-D.; Moreno-Simunovic, Y. Strategies for the improvement of fruit set in Vitis vinifera L. cv. “carménère” through different foliar biostimulants in two different locations. Cienc. Tec. Vitivinic. 2018, 33, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.J.; Volschenk, C.G.; Zorer, R. Vineyard row orientation of Vitis vinifera L. cv. Shiraz/101-14 Mgt: Climatic profiles and vine physiological status. Agric. For. Meteorol. 2016, 228–229, 104–119. [Google Scholar] [CrossRef]
- Da Mota, R.V.; Peregrino, I.; Silva, C.P.C.; Raimundo, R.H.P.; Fernandes, F.D.P.; de Souza, C.R. Row orientation effects on chemical composition and aromatic profile of Syrah winter wines. Food Sci. Technol. 2021, 41, 412–417. [Google Scholar] [CrossRef]
- Gouveia, J.; Lopes, C.M.; Pedroso, V.; Martins, S.; Rodrigues, P.; Alves, I. Effect of irrigation on soil water depletion, vegetative growth, yield and berry composition of the grapevine variety Touriga Nacional. Cienc. Tec. Vitivinic. 2013, 27, 115–122. [Google Scholar]
- Cohen, Y.; Vaknin, M.; Mauch-Mani, B. BABA-induced resistance: Milestones along a 55-year journey. Phytoparasitica 2016, 44, 513–538. [Google Scholar] [CrossRef]
- Li, C.; Cao, S.; Wang, K.; Lei, C.; Ji, N.; Xu, F.; Jiang, Y.; Qiu, L.; Zheng, Y. Heat shock protein HSP24 is involved in the BABA-induced resistance to fungal pathogen in postharvest grapes underlying an NPR1-dependent manner. Front. Plant Sci. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, M.; Csikász-Krizsics, A.; Szata, B.; Kovács, S.; Nagy; Mátai, A.; Jakab, G. Regulation of cluster compactness and resistance to Botrytis Cinerea with β-aminobutyric acid treatment in field-grown grapevine. Vitis J. Grapevine Res. 2018, 57, 35–40. [Google Scholar] [CrossRef]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef]
- Rizza, F.; Ghashghaie, J.; Meyer, S.; Matteu, L.; Mastrangelo, A.M.; Badeck, F.W. Constitutive differences in water use efficiency between two durum wheat cultivars. Field Crops Res. 2012, 125, 49–60. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Schultz, H.R. Global climate change, sustainability, and some challenges for grape and wine production. J. Wine Econ. 2016, 11, 181–200. [Google Scholar] [CrossRef]
- Núñez, L.; Serratosa, M.P.; Godoy, A.; Fariña, L.; Dellacassa, E.; Moyano, L. Comparison of physicochemical properties, amino acids, mineral elements, total phenolic compounds, and antioxidant capacity of cuban fruit and rice wines. Food Sci. Nutr. 2021, 9, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Benoît Bach, B.; Sauvage, F.X.; Dequin, S.; Camarasa, C. Role of γ-aminobutyric acid as a source of nitrogen and succinate in wine. Am. J. Enol. Vitic. 2009, 60, 508–516. [Google Scholar]
- Bayraktar, V.N. Organic acids concentration in wine stocks after Saccharomyces Cerevisiae fermentation. Biotechnol. Acta 2013, 6, 97–106. [Google Scholar] [CrossRef]
- Song, C.; Zhu, L.; Shao, Y.; Chen, F. Enhancement of GABA Content in Hongqu wine by optimisation of fermentation conditions using response surface methodology. Czech J. Food Sci. 2021, 39, 297–304. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Samani, Z.A. Reference crop evapotranspiration from ambient air temperature. Am. Soc. Agric. Eng. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines For Computing Crop Water Requirements-FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 1998; Volume 56. [Google Scholar]
- Kelly, M.T.; Blaise, A.; Larroque, M. Rapid automated high performance liquid chromatography method for simultaneous determination of amino acids and biogenic amines in wine, fruit and honey. J. Chromatogr. A 2010, 1217, 7385–7392. [Google Scholar] [CrossRef]
- Long, W. Automated amino acid Analysis using an Agilent Poroshell HPH-C18 column. Appl. Note Agil. Technol. Inc. 2017, 2, 1–10. [Google Scholar]
- Hazer, O.; Akkbik, M.; Güngör, K. Simultaneous determination of the main organic acids in Anatolian Black Pine by HPLC with DAD detector. Eurasian J. Anal. Chem. 2016, 11, 181–195. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Duan, Y.T.; Zhang, Y.F.; Pan, Q.H.; Li, J.M.; Huang, W.D. Determination of organic acids in red wine and must on only one RP-LC-column directly after sample dilution and filtration. Chromatographia 2009, 69, 1391–1395. [Google Scholar] [CrossRef]
- Lunn, J.E.; Feil, R.; Hendriks, J.H.M.; Gibon, Y.; Morcuende, R.; Osuna, D.; Scheible, W.R.; Carillo, P.; Hajirezaei, M.R.; Stitt, M. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem. J. 2006, 397, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.T.; Cai, B.D.; Jiang, H.P.; Xiao, H.M.; Yuan, B.F.; Feng, Y.Q. Sensitive analysis of trehalose-6-phosphate and related sugar phosphates in plant tissues by chemical derivatization combined with hydrophilic interaction liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1592, 82–90. [Google Scholar] [CrossRef] [PubMed]

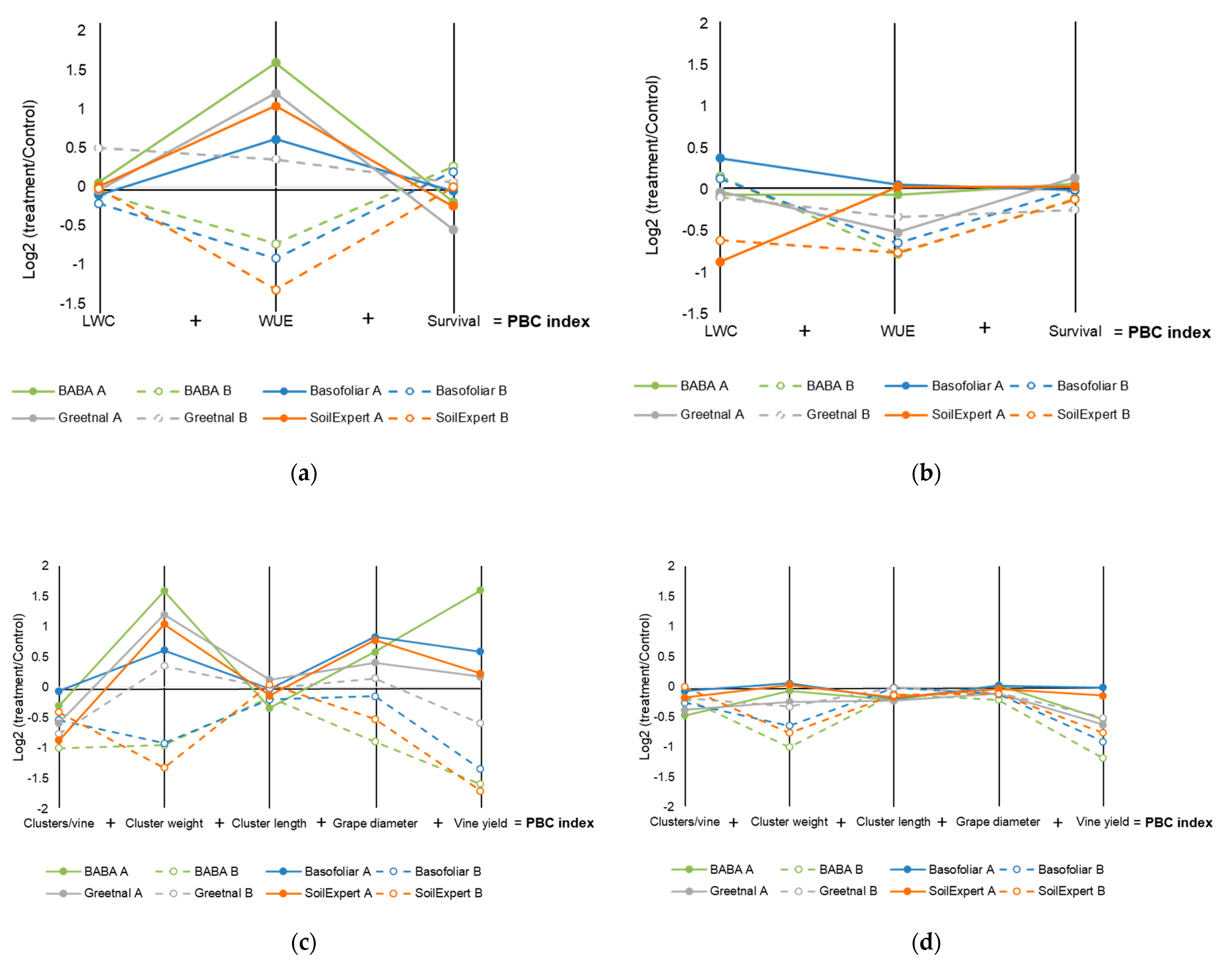
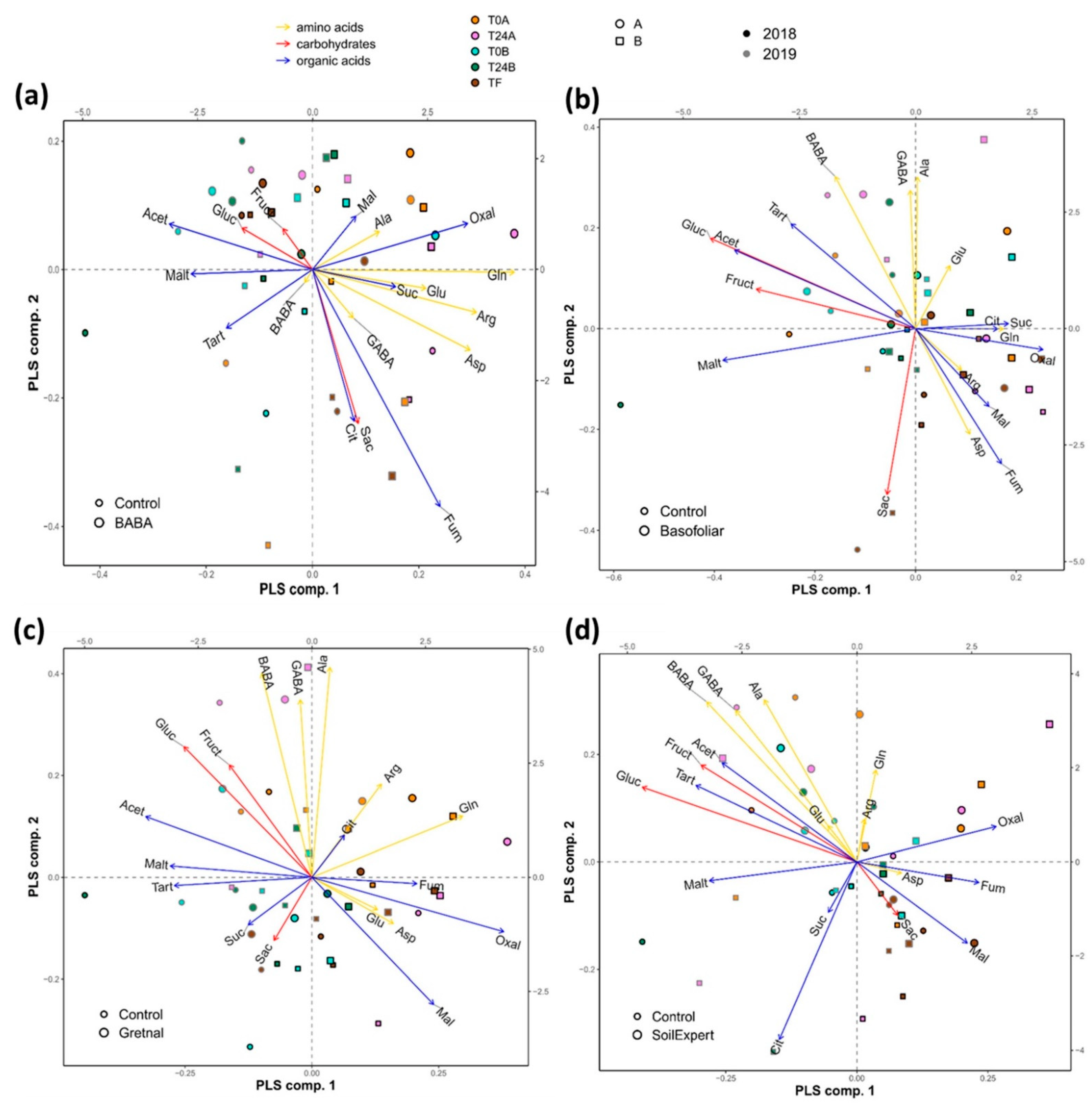
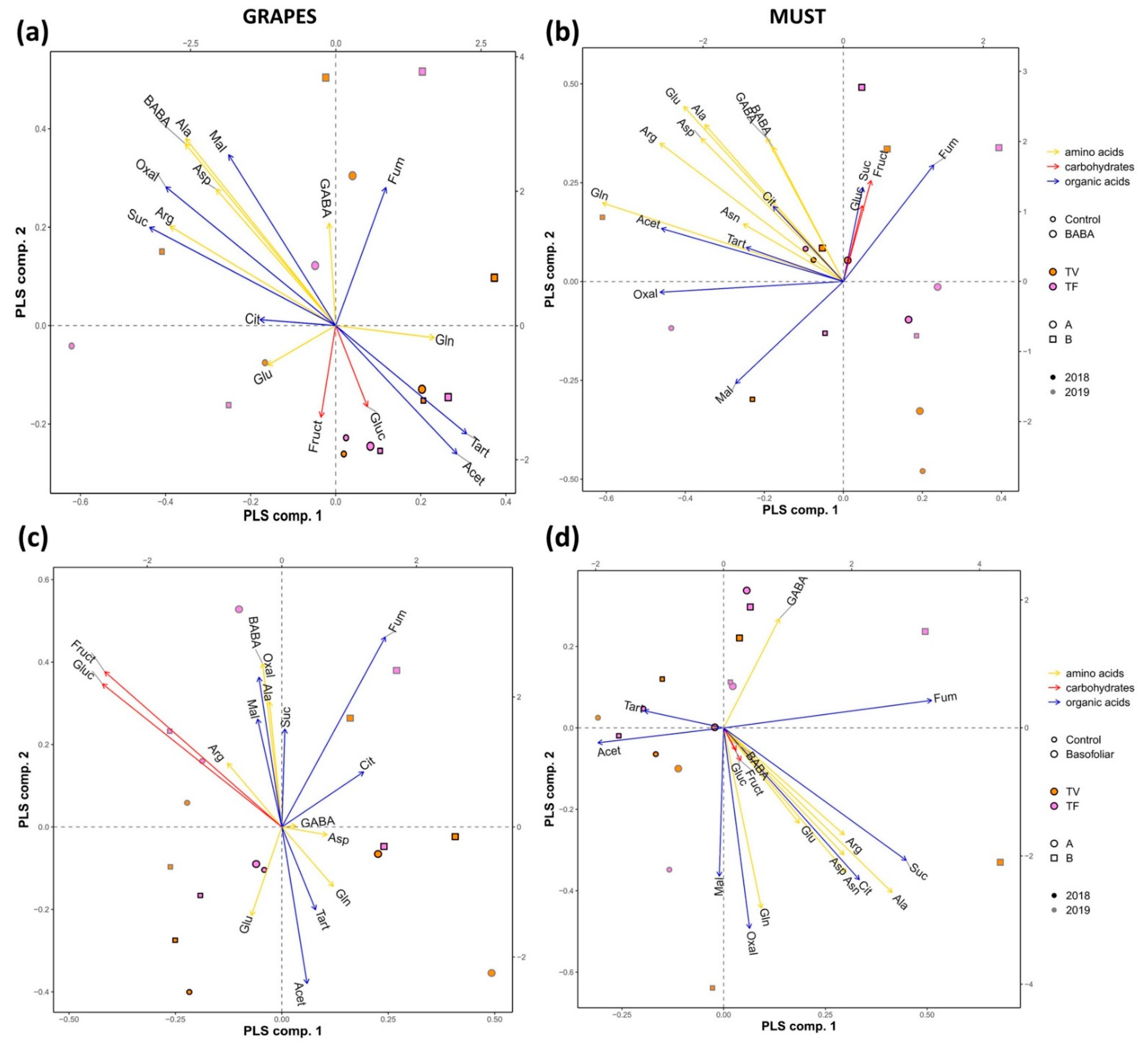
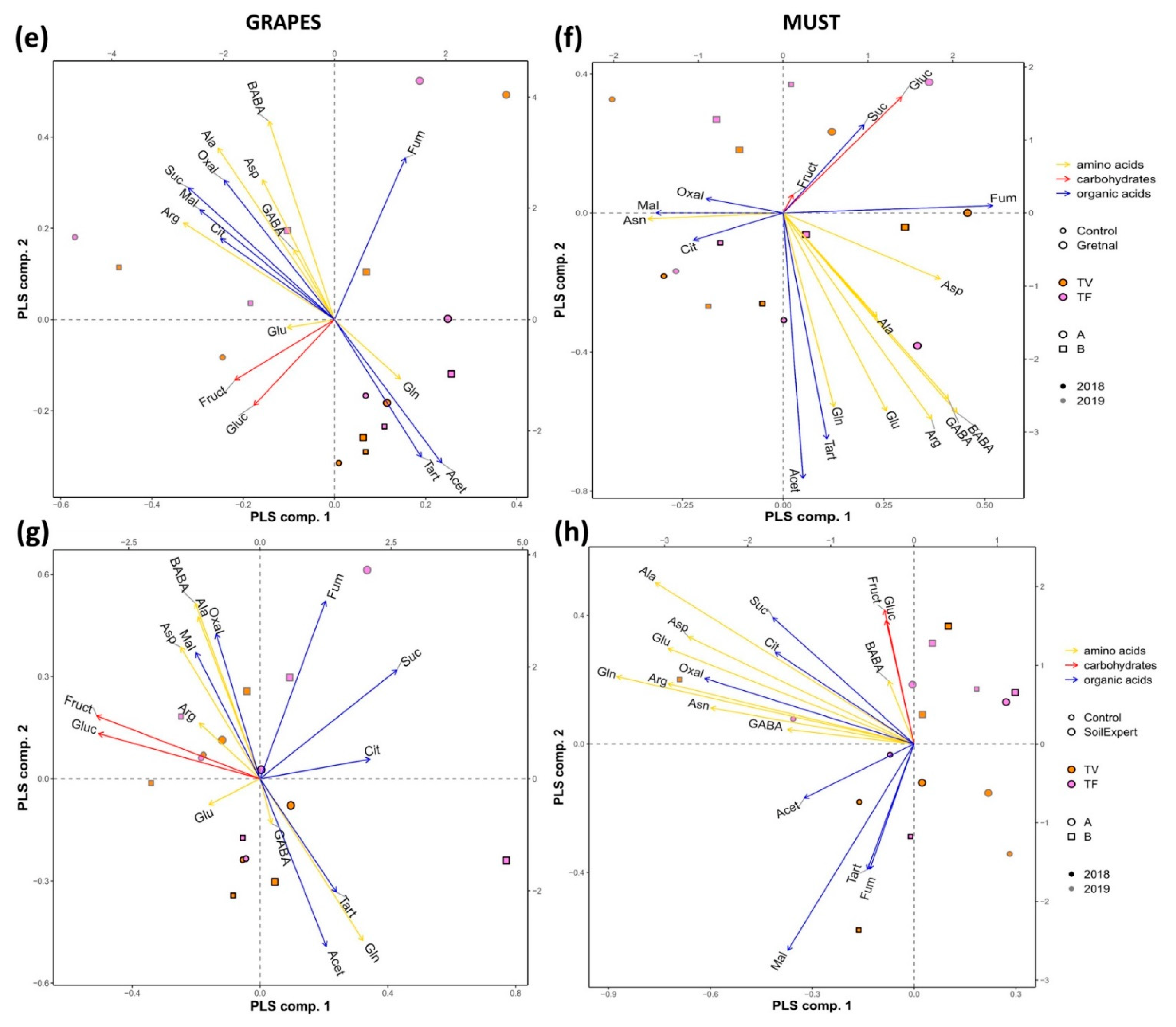




| Biostimulant | PBC Index (Physiology Survival) | PBC Index (Yield) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | |||||
| A | B | A | B | A | B | A | B | |
| GT | 0.61 | 0.91 | −0.40 | −0.66 | 1.33 | −0.85 | −1.13 | −0.95 |
| BF | 0.46 | −0.93 | 0.43 | −0.51 | 1.96 | −3.14 | −0.12 | −1.68 |
| ST | 0.80 | −1.34 | −0.80 | −1.48 | 1.05 | −3.89 | −0.31 | −1.77 |
| BABA | 1.46 | −0.55 | −0.06 | −0.72 | 3.15 | −4.59 | −0.78 | −2.50 |
| Free AAs (mg L−1) | AcOrg (mg L−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | Glu | Asn | Gln | Arg | Ala | GABA | BABA | Oxal | Tart | Mal | Acet | Cit | Suc | Fum | |
| GT | 14.7 ± 0.1 | 78.6 ± 0.0 | 0.3 ± 0.0 | 1.5 ± 0.0 | 1.0 ± 0.0 | 16.5 ± 0.1 | 2.1 ± 0.1 | 0.5 ± 0.0 | 3201.3 ± 28.4 | 440.1 ± 0.1 | <LOQ | 42.8 ± 0.2 | 172.2 ± 0.1 | <LOQ | 5.6 ± 0.1 |
| BF | 104.6 ± 0.1 | 405.1 ± 0.2 | 1.2 ± 0.1 | 0.8 ± 0.1 | 89.6 ± 0.1 | 116.5 ± 0.0 | 14.9 ± 0.0 | 204.1 ± 0.1 | 2123.7 ± 15.2 | 606.1 ± 0.3 | 612.2 ± 0.1 | 20.2 ± 0.1 | 993.0 ± 0.0 | <LOQ | 9.5 ± 0.1 |
| ST | 12.9 ± 0.1 | 100.7 ± 0.1 | 0.2 ± 0.1 | 0.9 ± 0.0 | 0.8 ± 0.1 | 15.5 ± 0.1 | 1.6 ± 0.1 | 0.3 ± 0.0 | 4507.4 ± 5.8 | 1060.5 ± 23.5 | 97.1 ± 0.1 | 18.3 ± 0.1 | 8.5 ± 0.3 | <LOQ | 22.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olavarrieta, C.E.; Sampedro, M.C.; Vallejo, A.; Štefelová, N.; Barrio, R.J.; De Diego, N. Biostimulants as an Alternative to Improve the Wine Quality from Vitis vinifera (cv. Tempranillo) in La Rioja. Plants 2022, 11, 1594. https://doi.org/10.3390/plants11121594
Olavarrieta CE, Sampedro MC, Vallejo A, Štefelová N, Barrio RJ, De Diego N. Biostimulants as an Alternative to Improve the Wine Quality from Vitis vinifera (cv. Tempranillo) in La Rioja. Plants. 2022; 11(12):1594. https://doi.org/10.3390/plants11121594
Chicago/Turabian StyleOlavarrieta, Cristina E., Maria Carmen Sampedro, Asier Vallejo, Nikola Štefelová, Ramón J. Barrio, and Nuria De Diego. 2022. "Biostimulants as an Alternative to Improve the Wine Quality from Vitis vinifera (cv. Tempranillo) in La Rioja" Plants 11, no. 12: 1594. https://doi.org/10.3390/plants11121594
APA StyleOlavarrieta, C. E., Sampedro, M. C., Vallejo, A., Štefelová, N., Barrio, R. J., & De Diego, N. (2022). Biostimulants as an Alternative to Improve the Wine Quality from Vitis vinifera (cv. Tempranillo) in La Rioja. Plants, 11(12), 1594. https://doi.org/10.3390/plants11121594






