Priming with Small Molecule-Based Biostimulants to Improve Abiotic Stress Tolerance in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
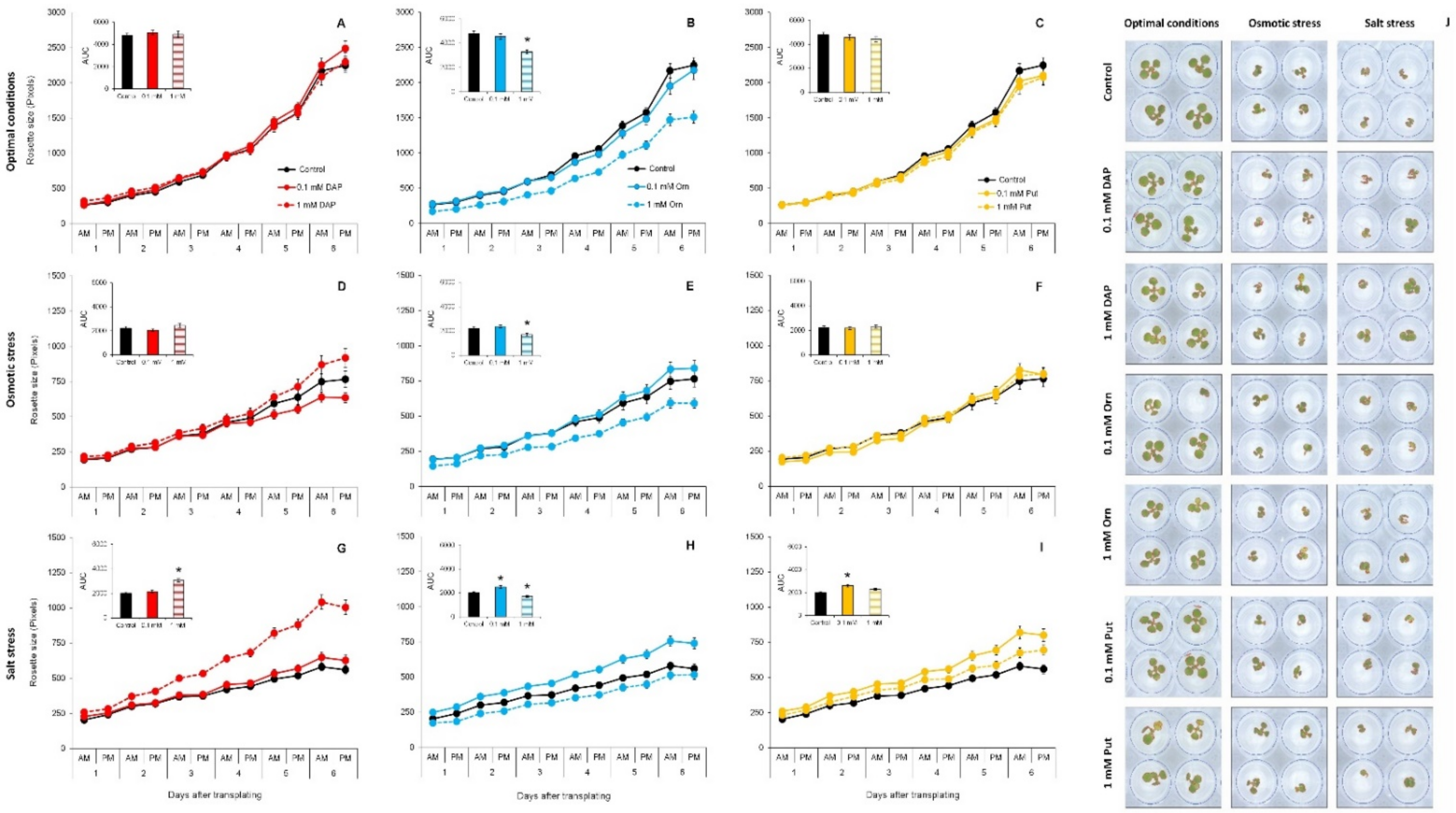
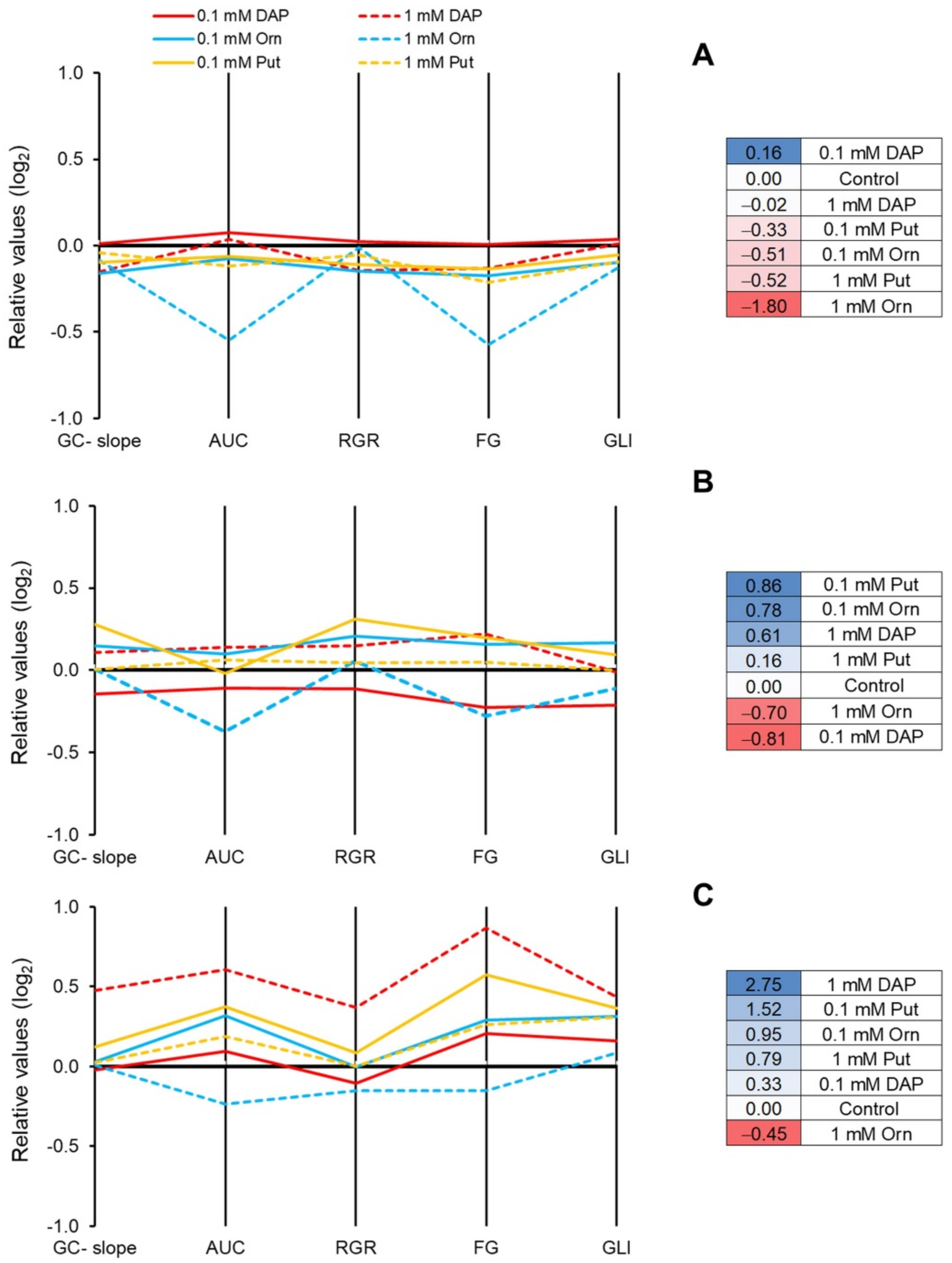
2.1. The Priming with DAP, Orn, and Put Improved Plant Performance under Abiotic Stress but Not under Optimal Growth Conditions
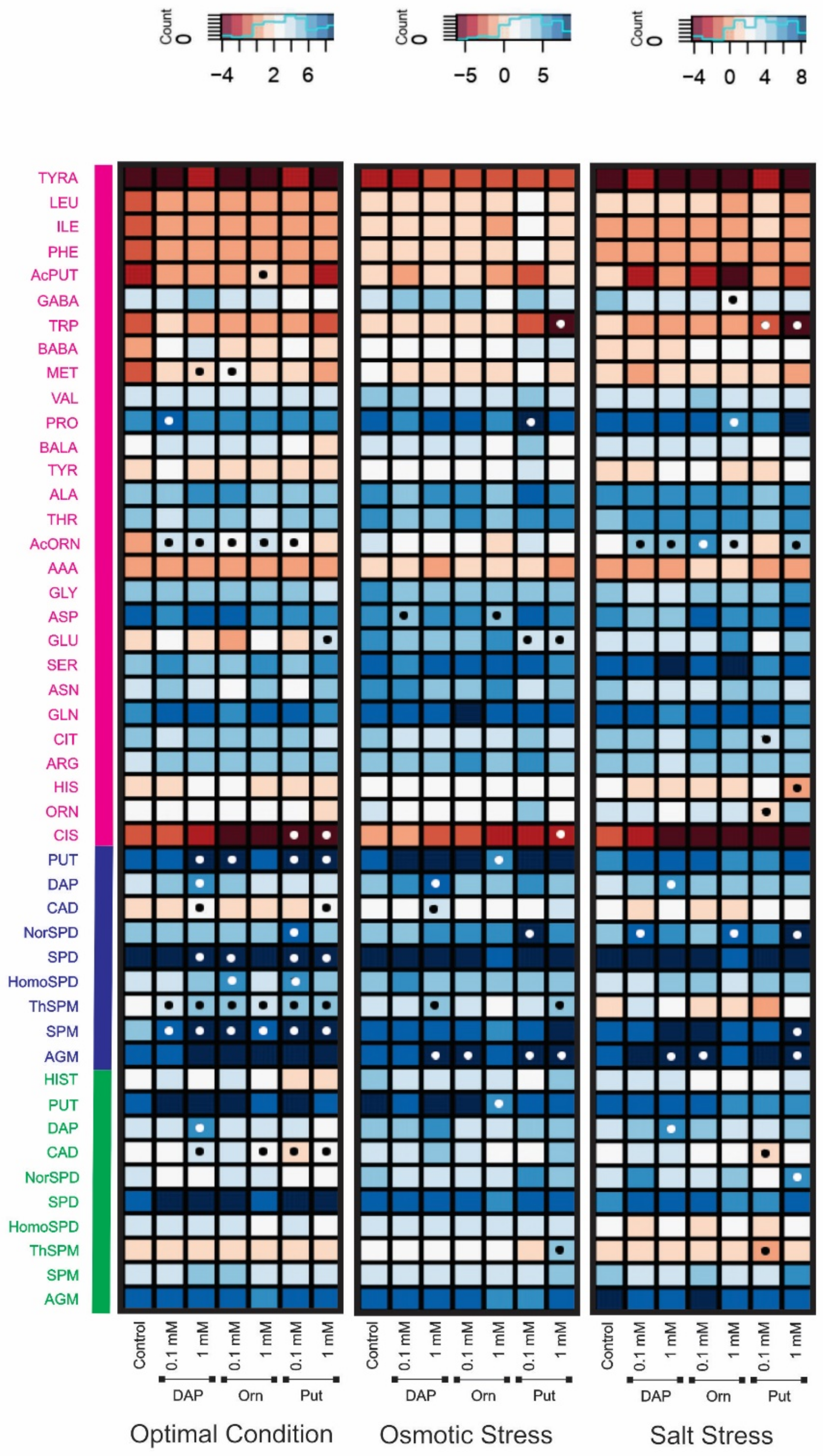
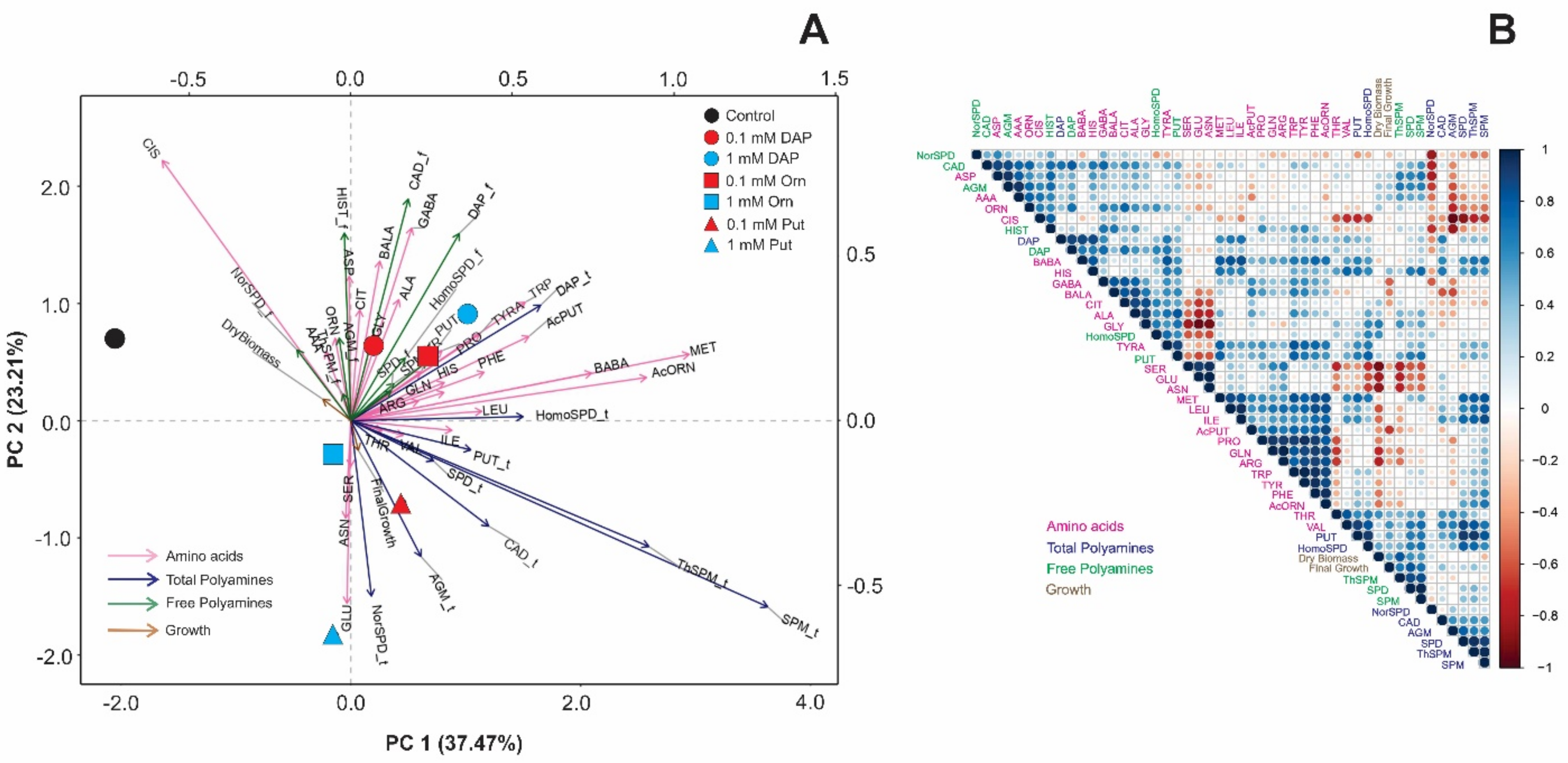
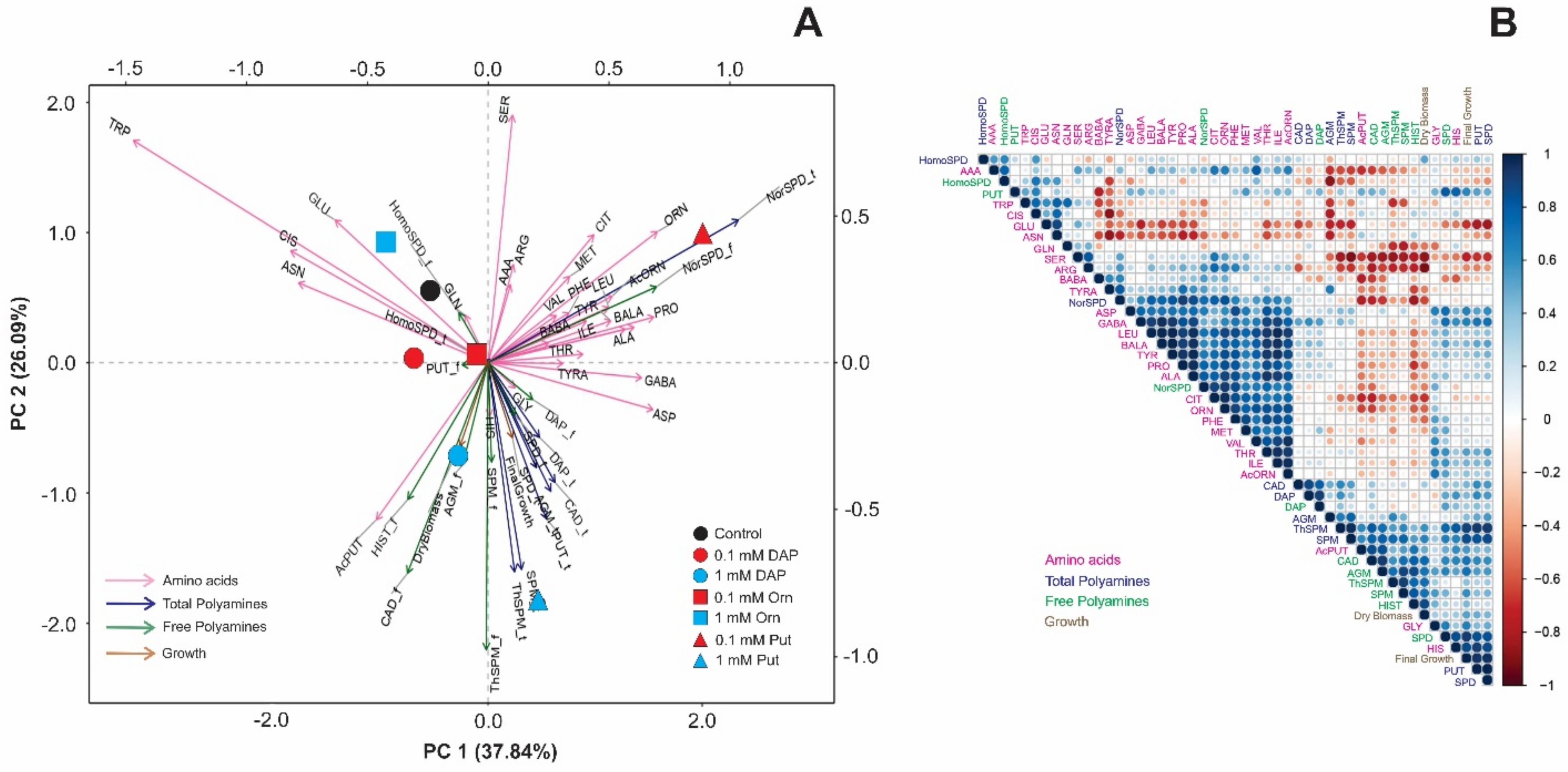
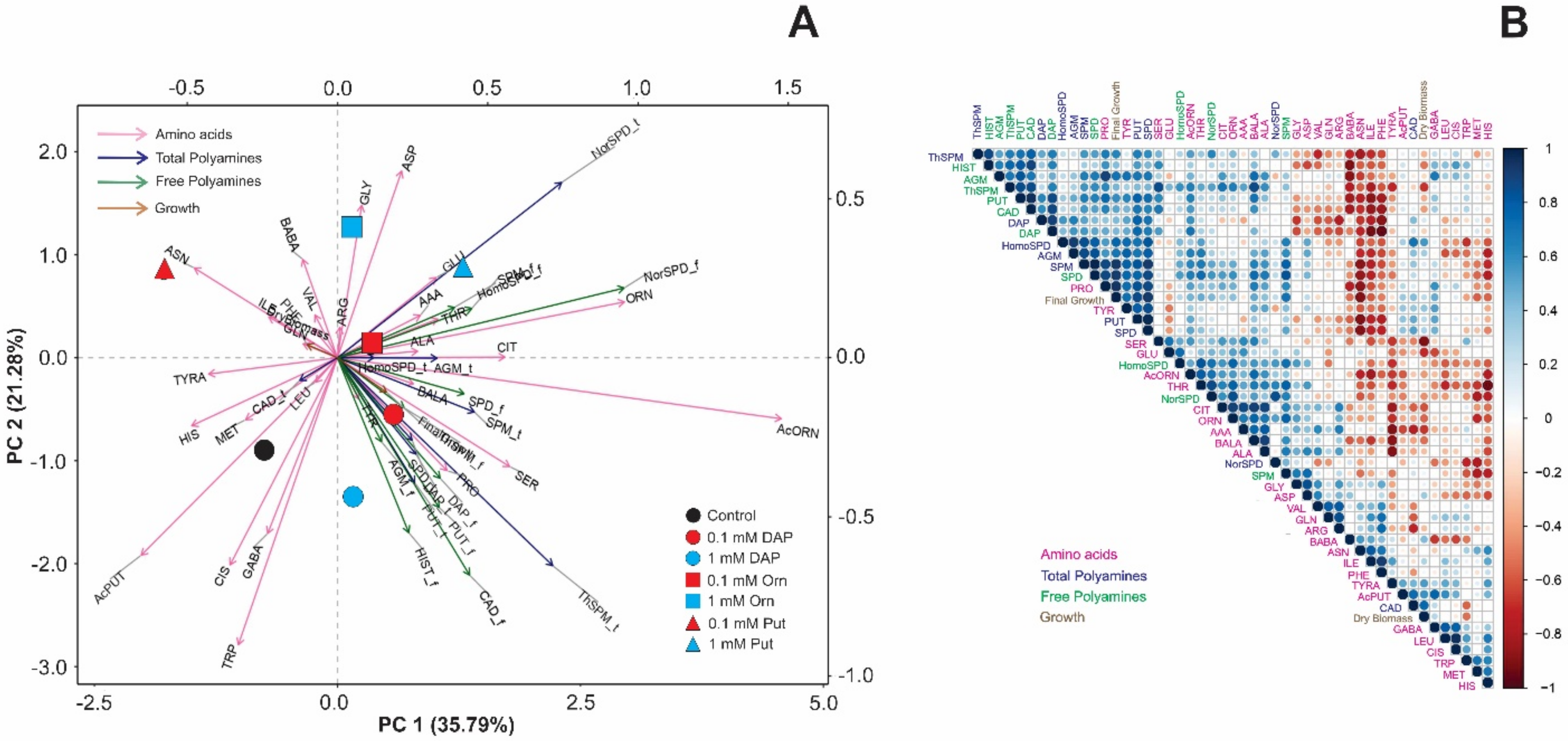
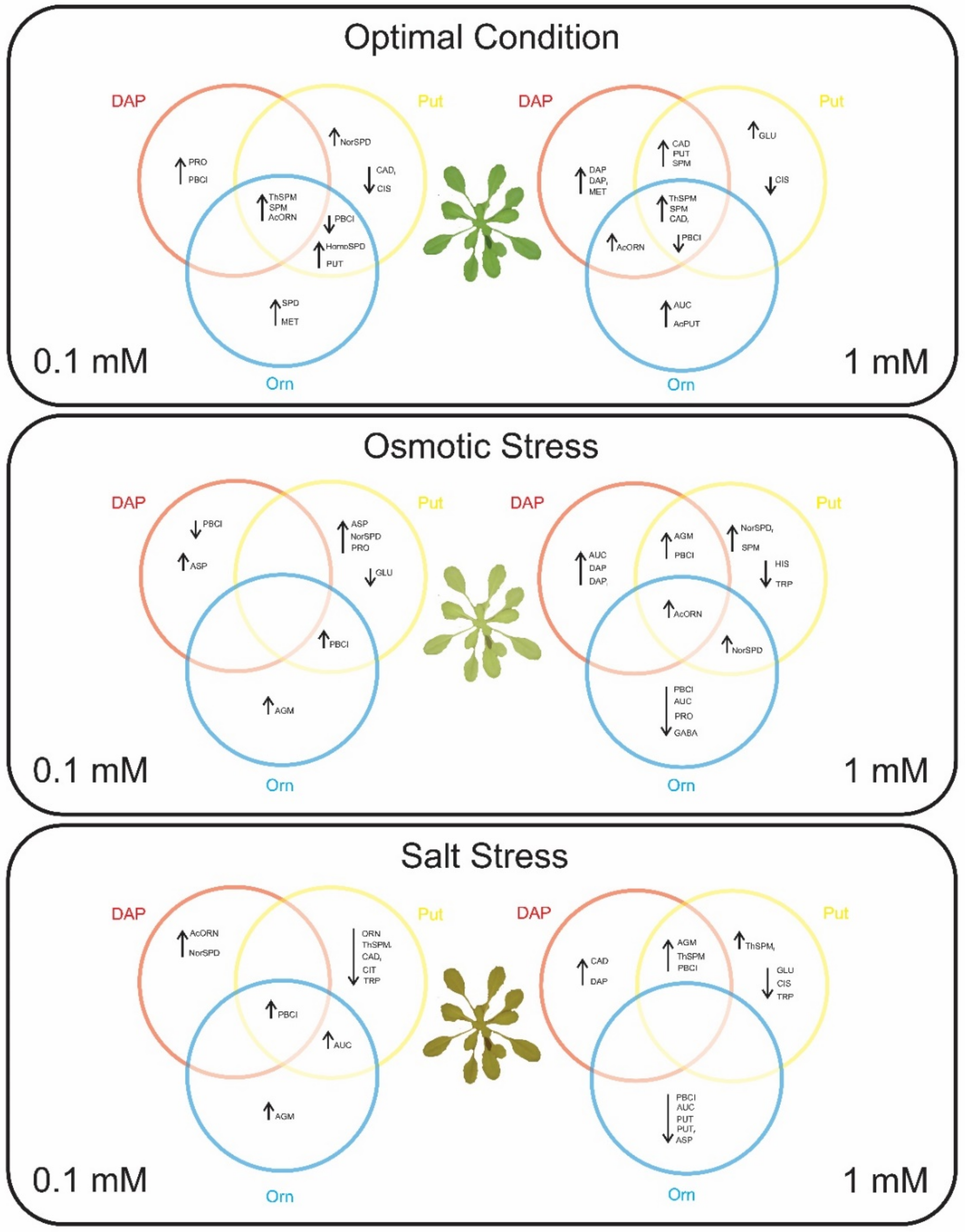
2.2. Seed Priming Induced Changes in the N-Related Metabolites, and the Changes Were Due to the Interaction between the Priming Agent, Its Concentration, and Growth Conditions
2.3. Seed Priming Induced Changes in the N-Related Metabolites, and the Changes Were Due to the Interaction between the Priming Agent, Its Concentration, and Growth Conditions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material, Priming, and Growth Conditions
5.2. Plant Growth under Optimal Conditions and Osmotic and Salt Stresses
5.3. Plant Phenotyping Determinations
5.3.1. Rosette Growth Analysis
5.3.2. Rosette Color Indices
5.4. Targeted Metabolomic Analysis
5.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouras, E.; Jarlan, L.; Khabba, S.; Er-Raki, S.; Dezetter, A.; Sghir, F.; Tramblay, Y. Assessing the Impact of Global Climate Changes on Irrigated Wheat Yields and Water Requirements in a Semi-Arid Environment of Morocco. Sci. Rep. 2019, 9, 19142. [Google Scholar] [CrossRef] [PubMed]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for Drought Assessment in the Mediterranean Region under Future Climate Scenarios. Earth Sci. Rev. 2020, 210, 103348. [Google Scholar]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of Salt-Induced Land Degradation and Restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- FAO. Land and Plant Nutrition Management Service. Available online: https://scholar.google.com/scholar_lookup?title=FAOLandandPlantNutritionManagementService&publication_year=2010&author=FAO (accessed on 19 April 2022).
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and Combined Effects of Transient Drought and Heat Stress on Carbon Assimilation and Seed Filling in Chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar]
- Lyon, C.; Saupe, E.E.; Smith, C.J.; Hill, D.J.; Beckerman, A.P.; Stringer, L.C.; Marchant, R.; McKay, J.; Burke, A.; O’Higgins, P.; et al. Climate Change Research and Action Must Look beyond 2100. Glob. Chang. Biol. 2022, 28, 349–361. [Google Scholar] [CrossRef]
- Le Mouël, C.; Forslund, A. How Can We Feed the World in 2050? A Review of the Responses from Global Scenario Studies. Eur. Rev. Agric. Econ. 2017, 44, 541–591. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Roddy, A.B.; Wason, J.W.; McElrone, A.J. Functional Status of Xylem Through Time. Annu. Rev. Plant Biol. 2019, 70, 407–433. [Google Scholar]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; et al. Plant Breeding and Climate Changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Brummer, E.C.; Barber, W.T.; Collier, S.M.; Cox, T.S.; Johnson, R.; Murray, S.C.; Olsen, R.T.; Pratt, R.C.; Thro, A.M. Plant Breeding for Harmony between Agriculture and the Environment. Front. Ecol. Environ. 2011, 9, 561–568. [Google Scholar]
- Schaart, J.G.; van de Wiel, C.C.M.; Lotz, L.A.P.; Smulders, M.J.M. Opportunities for Products of New Plant Breeding Techniques. Trends Plant Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Geelen, D. Developing Biostimulants from Agro-Food and Industrial by-Products. Front. Plant Sci. 2018, 871, 1567. [Google Scholar]
- Lee, J.K.; Patel, S.K.S.; Sung, B.H.; Kalia, V.C. Biomolecules from Municipal and Food Industry Wastes: An Overview. Bioresour. Technol. 2020, 298, 122346. [Google Scholar] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Diego, N.; Spíchal, L. Use of Plant Metabolites to Mitigate Stress Effects in Crops. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 261–300. [Google Scholar]
- Patel, S.K.S.; Kalia, V.C. Advancements in the Nanobiotechnological Applications. Indian J. Microbiol. 2021, 61, 401–403. [Google Scholar] [CrossRef]
- Kalia, V.C.; Gong, C.; Patel, S.K.S.; Lee, J.K. Regulation of Plant Mineral Nutrition by Signal Molecules. Microorganisms 2021, 9, 774. [Google Scholar]
- Beckers, G.J.; Conrath, U. Priming for Stress Resistance: From the Lab to the Field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- Majumdar, R.; Minocha, R.; Minocha, S.C. Ornithine: At the Crossroads of Multiple Paths to Amino Acids and Polyamines. In Amino Acids in Higher Plants; Northern Research Station: Osfordshire, UK, 2015; pp. 156–176. [Google Scholar]
- Hussein, H.A.A.; Mekki, B.B.; El-Sadek, M.E.A.; El Lateef, E.E. Effect of L-Ornithine Application on Improving Drought Tolerance in Sugar Beet Plants. Heliyon 2019, 5, e02631. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Dey, A.; Gupta, B. Plant Polyamines in Abiotic Stress Responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and Polyamines Regulate Plant Stress Responses by Altering GABA Pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A. The Di- and Poly-Amine Oxidases of Higher Plants. Biochem. Soc. Trans. 1985, 13, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jammes, F.; Leonhardt, N.; Tran, D.; Bousserouel, H.; Véry, A.A.; Renou, J.P.; Vavasseur, A.; Kwak, J.M.; Sentenac, H.; Bouteau, F.; et al. Acetylated 1,3-Diaminopropane Antagonizes Abscisic Acid-Mediated Stomatal Closing in Arabidopsis. Plant J. 2014, 79, 322–333. [Google Scholar] [CrossRef] [Green Version]
- De Diego, N.; Fürst, T.; Humplík, J.F.; Ugena, L.; Podlešáková, K.; Spíchal, L. An Automated Method for High-Throughput Screening of Arabidopsis Rosette Growth in Multi-Well Plates and Its Validation in Stress Conditions. Front. Plant Sci. 2017, 8, 1702. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, M.; De Diego, N.; Ugena, L.; Spíchal, L.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Rouphael, Y.; Colla, G.; Panzarová, K. Seed Priming with Protein Hydrolysates Improves Arabidopsis Growth and Stress Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 626301. [Google Scholar] [CrossRef]
- Nagabhushan Arun, M.; Shankara Hebbar, S.; Bhanuprakash; Senthivel, T.; Kumar Nair, A.; Padmavathi, G.; Pandey, P.; Singh, A. Seed Priming: The Way Forward to Mitigate Abiotic Stress in Crops. In Plant Stress Physiology—Perspectives in Agriculture; IntechOpen: London, UK, 2022. [Google Scholar]
- Ugena, L.; Hýlová, A.; Podlešáková, K.; Humplík, J.F.; Doležal, K.; De Diego, N.; Spíchal, L. Characterization of Biostimulant Mode of Action Using Novel Multi-Trait High-Throughput Screening of Arabidopsis Germination and Rosette Growth. Front. Plant Sci. 2018, 9, 1327. [Google Scholar] [CrossRef] [Green Version]
- García-García, A.L.; García-Machado, F.J.; Borges, A.A.; Morales-Sierra, S.; Boto, A.; Jiménez-Arias, D. Pure Organic Active Compounds Against Abiotic Stress: A Biostimulant Overview. Front. Plant Sci. 2020, 11, 1839. [Google Scholar]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Westman, S.M.; Kloth, K.J.; Hanson, J.; Ohlsson, A.B.; Albrectsen, B.R. Defence Priming in Arabidopsis—A Meta-Analysis. Sci. Rep. 2019, 9, 13309. [Google Scholar] [CrossRef]
- Bryksová, M.; Hybenová, A.; Hernándiz, A.E.; Novák, O.; Pěnčík, A.; Spíchal, L.; De Diego, N.; Doležal, K. Hormopriming to Mitigate Abiotic Stress Effects: A Case Study of N9-Substituted Cytokinin Derivatives with a Fluorinated Carbohydrate Moiety. Front. Plant Sci. 2020, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Nisler, J.; Kopečný, D.; Pěkná, Z.; Končitíková, R.; Koprna, R.; Murvanidze, N.; Werbrouck, S.P.O.; Havlíček, L.; De Diego, N.; Kopečná, M.; et al. Diphenylurea-Derived Cytokinin Oxidase/Dehydrogenase Inhibitors for Biotechnology and Agriculture. J. Exp. Bot. 2021, 72, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Sierra, A.; Tiznado-Hernández, M.E.; Jha, D.K.; Janmeja, N.; Arul, J. Abiotic Stress Hormesis: An Approach to Maintain Quality, Extend Storability, and Enhance Phytochemicals on Fresh Produce during Postharvest. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3659–3682. [Google Scholar] [CrossRef] [PubMed]
- De Diego, N.; Sampedro, M.C.; Barrio, R.J.; Saiz-Fernández, I.; Moncaleán, P.; Lacuesta, M. Solute Accumulation and Elastic Modulus Changes in Six Radiata Pine Breeds Exposed to Drought. Tree Physiol. 2013, 33, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, C.F.; Ugena, L.; Humplík, J.F.; Polák, M.; Ćavar Zeljković, S.; Podlešáková, K.; Fürst, T.; De Diego, N.; Spíchal, L. A Novel Image-Based Screening Method to Study Water-Deficit Response and Recovery of Barley Populations Using Canopy Dynamics Phenotyping and Simple Metabolite Profiling. Front. Plant Sci. 2019, 10, 1252. [Google Scholar] [CrossRef]
- Adio, A.M.; Casteel, C.L.; de Vos, M.; Kim, J.H.; Joshi, V.; Li, B.; Juéry, C.; Daron, J.; Kliebenstein, D.J.; Jandera, G. Biosynthesis and Defensive Function of Nδ-Acetylornithine, a Jasmonate-Induced Arabidopsis Metabolite. Plant Cell 2011, 23, 3303–3318. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.R.; Ahmed, S.; Yan, J.; Adio, A.M.; Powell, H.M.; Morris, P.F.; Jander, G. Arabidopsis ADC1 Functions as an Nδ-Acetylornithine Decarboxylase. J. Integr. Plant Biol. 2020, 62, 601–613. [Google Scholar] [CrossRef]
- Abdelhakim, L.O.A.; Mendanha, T.; Palma, C.F.F.; Vrobel, O.; Štefelová, N.; Ćavar Zeljković, S.; Tarkowski, P.; De Diego, N.; Wollenweber, B.; Rosenqvist, E.; et al. Elevated CO2 Improves the Physiology but Not the Final Yield in Spring Wheat Genotypes Subjected to Heat and Drought Stress During Anthesis. Front. Plant Sci. 2022, 13, 379. [Google Scholar] [CrossRef]
- Slocum, R.D. Genes, Enzymes and Regulation of Arginine Biosynthesis in Plants. Plant Physiol. Biochem. 2005, 43, 729–745. [Google Scholar] [CrossRef]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Unfinished Story of Polyamines: Role of Conjugation, Transport and Light-Related Regulation in the Polyamine Metabolism in Plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef]
- Peng, H.; Meyer, R.S.; Yang, T.; Whitaker, B.D.; Trouth, F.; Shangguan, L.; Huang, J.; Litt, A.; Little, D.P.; Ke, H.; et al. A Novel Hydroxycinnamoyl Transferase for Synthesis of Hydroxycinnamoyl Spermine Conjugates in Plants. BMC Plant Biol. 2019, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Bassard, J.E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging Polyamines to the Phenolic Metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, M.; Lübken, T.; Eschen-Lippold, L.; Gorzolka, K.; Blum, E.; Matern, A.; Marillonnet, S.; Böttcher, C.; Dräger, B.; Rosahl, S. MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides. Plant Cell 2015, 28, 583–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muroi, A.; Ishihara, A.; Tanaka, C.; Ishizuka, A.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Accumulation of Hydroxycinnamic Acid Amides Induced by Pathogen Infection and Identification of Agmatine Coumaroyltransferase in Arabidopsis Thaliana. Planta 2009, 230, 517–527. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Meng, Y.; Hu, J.; Ding, M.; Bian, J.; Yan, M.; Han, J.; Zhou, M. Jasmonic Acid/Ethylene Signaling Coordinates Hydroxycinnamic Acid Amides Biosynthesis through ORA59 Transcription Factor. Plant J. 2018, 95, 444–457. [Google Scholar] [CrossRef]
- Del Duca, S.; Dondini, L.; Della Mea, M.; Munoz De Rueda, P.; Serafini-Fracassini, D. Factors Affecting Transglutaminase Activity Catalysing Polyamine Conjugation to Endogenous Substrates in the Entire Chloroplast. Plant Physiol. Biochem. 2000, 38, 429–439. [Google Scholar] [CrossRef]
- Poidevin, L.; Unal, D.; Belda-Palazón, B.; Ferrando, A. Polyamines as Quality Control Metabolites Operating at the Post-Transcriptional Level. Plants 2019, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel Algorithms for Remote Estimation of Vegetation Fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Perry, E.M.; Roberts, D.A. Sensitivity of Narrow-Band and Broad-Band Indices for Assessing Nitrogen Availability and Water Stress in an Annual Crop. Agron. J. 2008, 100, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A Visible Band Index for Remote Sensing Leaf Chlorophyll Content at the Canopy Scale. Int. J. Appl. Earth Obs. Geoinf. 2012, 21, 103–112. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernándiz, A.E.; Aucique-Perez, C.E.; Ćavar Zeljković, S.; Štefelová, N.; Salcedo Sarmiento, S.; Spíchal, L.; De Diego, N. Priming with Small Molecule-Based Biostimulants to Improve Abiotic Stress Tolerance in Arabidopsis thaliana. Plants 2022, 11, 1287. https://doi.org/10.3390/plants11101287
Hernándiz AE, Aucique-Perez CE, Ćavar Zeljković S, Štefelová N, Salcedo Sarmiento S, Spíchal L, De Diego N. Priming with Small Molecule-Based Biostimulants to Improve Abiotic Stress Tolerance in Arabidopsis thaliana. Plants. 2022; 11(10):1287. https://doi.org/10.3390/plants11101287
Chicago/Turabian StyleHernándiz, Alba E., Carlos Eduardo Aucique-Perez, Sanja Ćavar Zeljković, Nikola Štefelová, Sara Salcedo Sarmiento, Lukáš Spíchal, and Nuria De Diego. 2022. "Priming with Small Molecule-Based Biostimulants to Improve Abiotic Stress Tolerance in Arabidopsis thaliana" Plants 11, no. 10: 1287. https://doi.org/10.3390/plants11101287
APA StyleHernándiz, A. E., Aucique-Perez, C. E., Ćavar Zeljković, S., Štefelová, N., Salcedo Sarmiento, S., Spíchal, L., & De Diego, N. (2022). Priming with Small Molecule-Based Biostimulants to Improve Abiotic Stress Tolerance in Arabidopsis thaliana. Plants, 11(10), 1287. https://doi.org/10.3390/plants11101287






