Effect of Extracts from Dominant Forest Floor Species of Clear-Cuts on the Regeneration and Initial Growth of Pinus sylvestris L. with Respect to Climate Change
Abstract
1. Introduction
2. Results
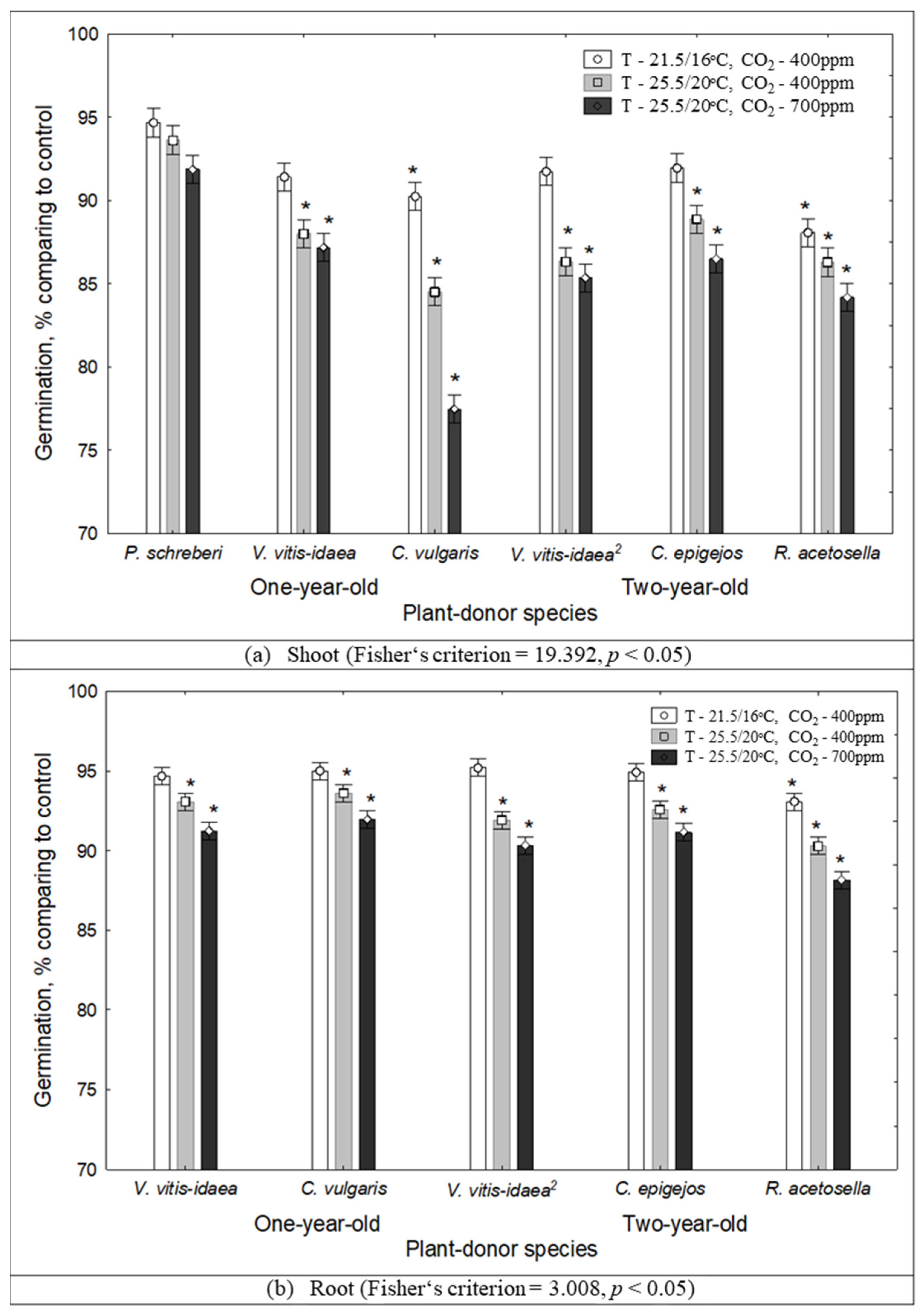
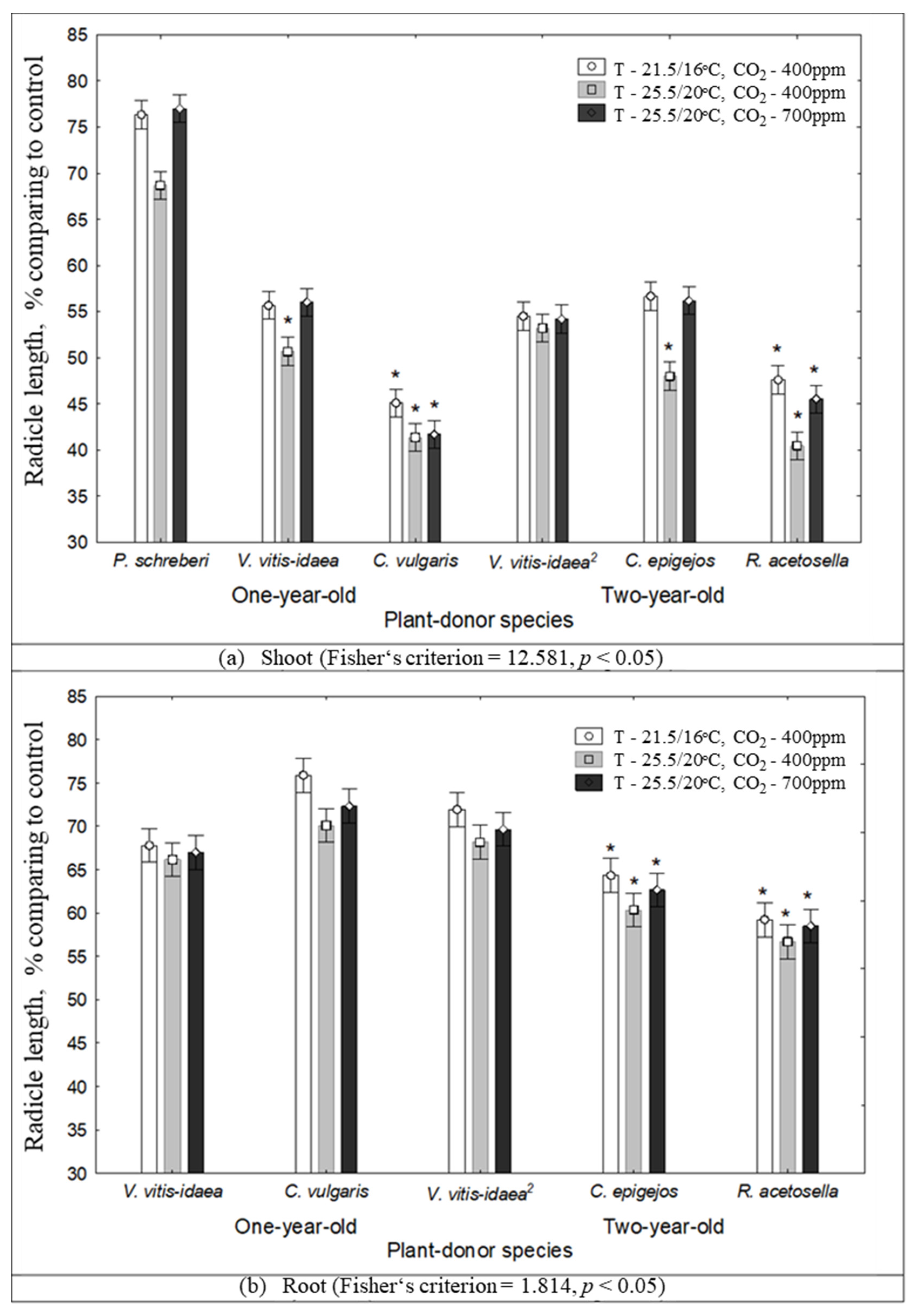
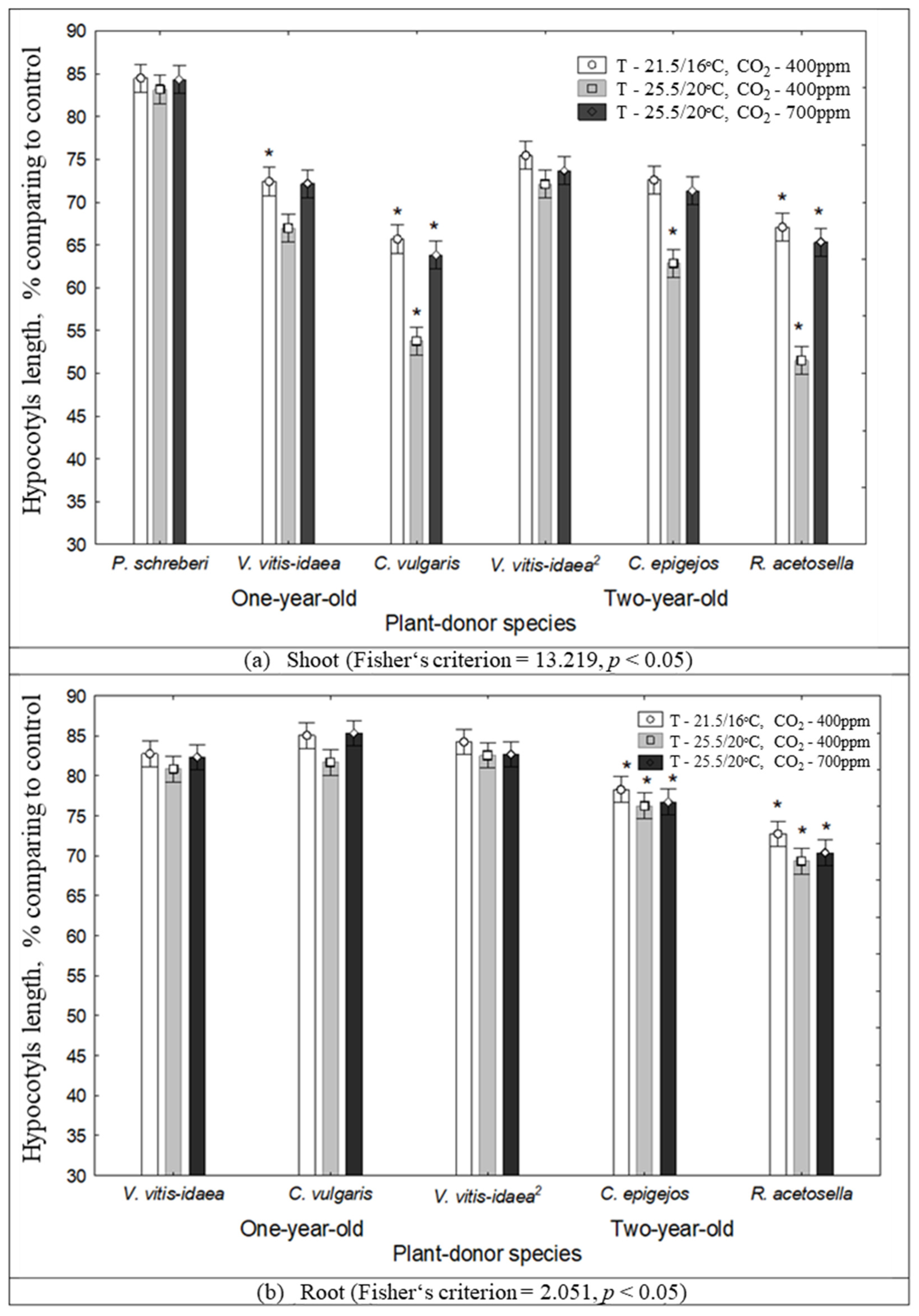
2.1. The Impact of Environmental Conditions on Seed Germination and Seedling Growth under the Effect of Plant Donors
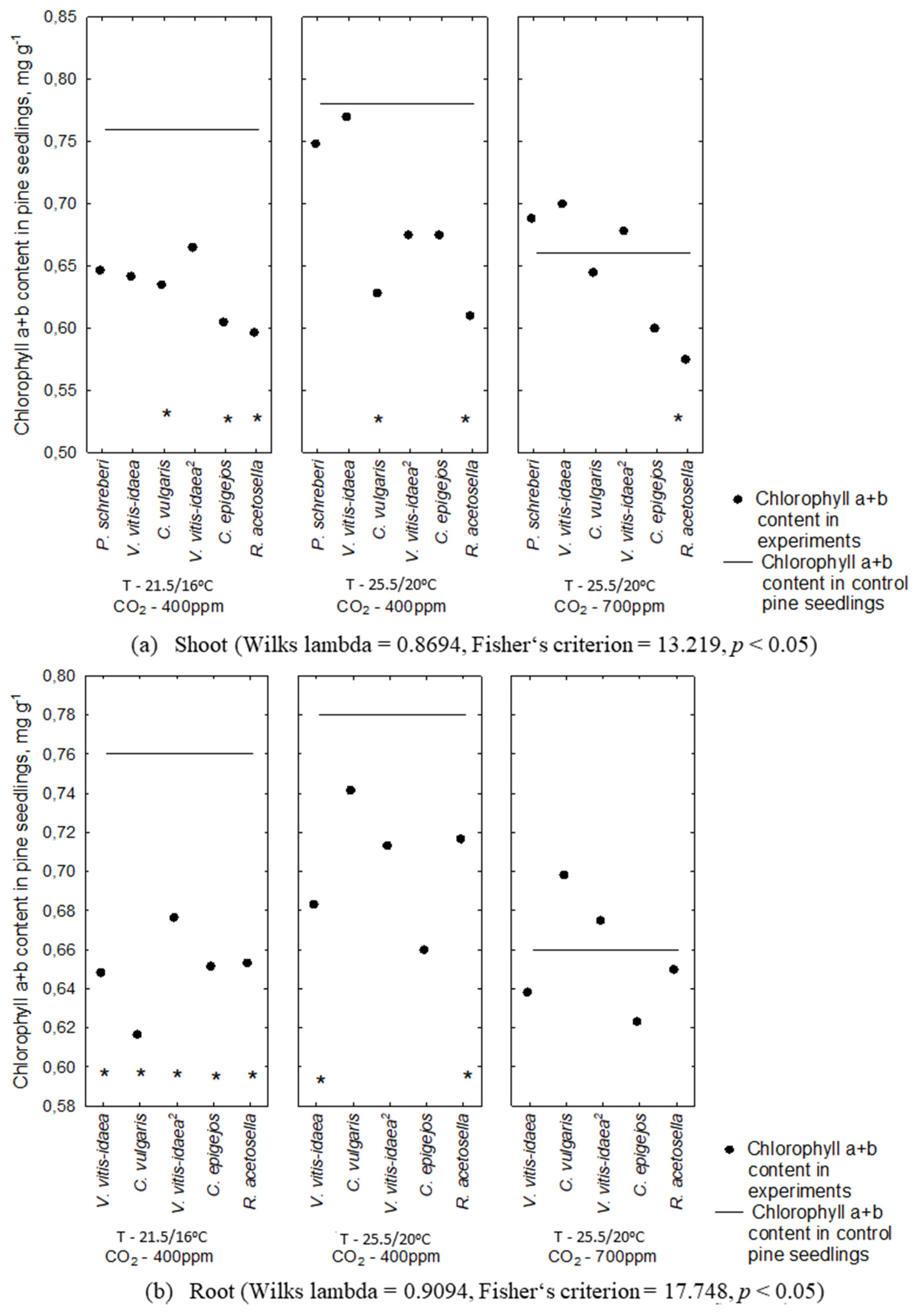
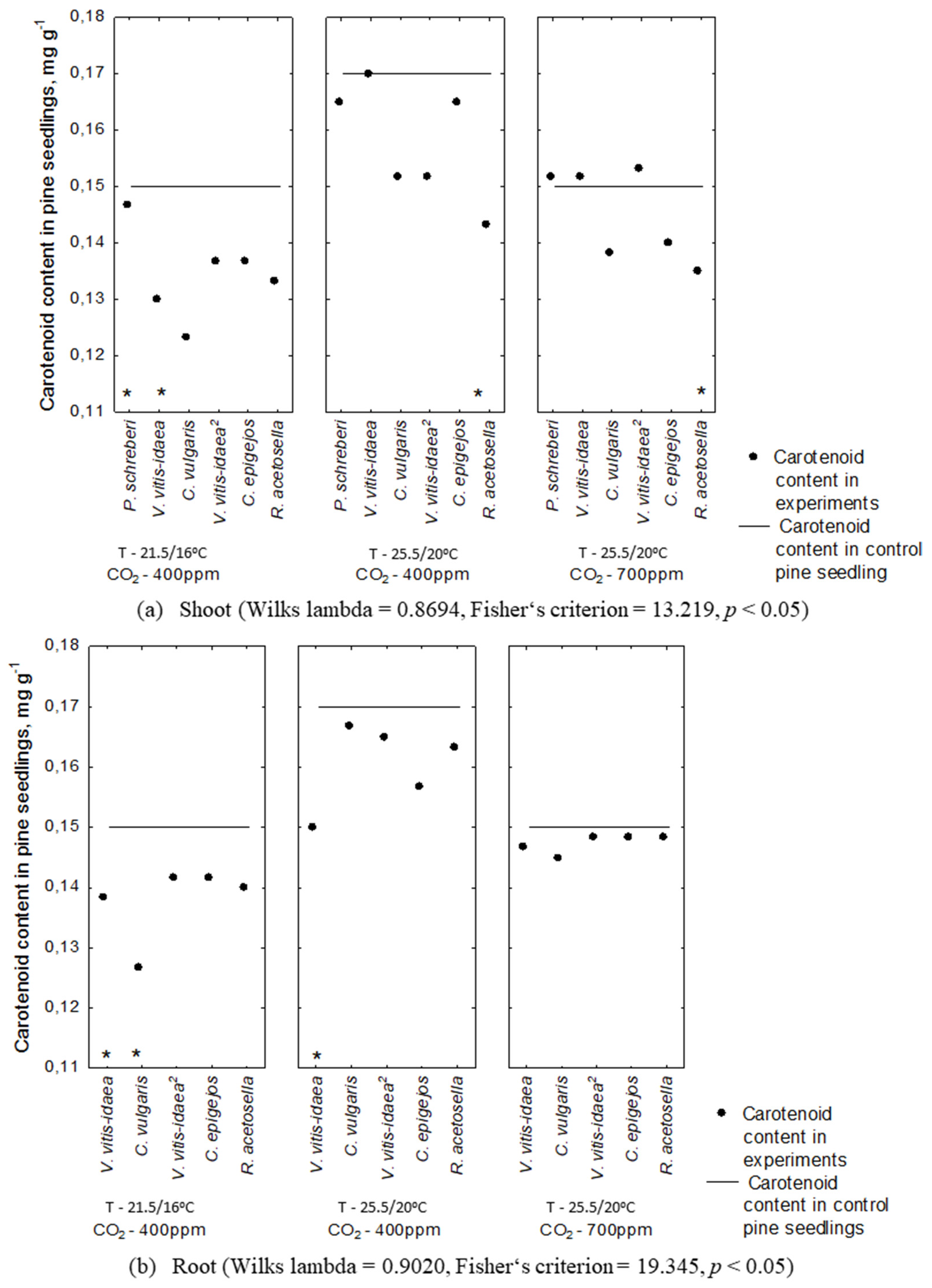
2.2. Allelopathic Effect on Photosynthetic Pigment Content under Different Environmental Conditions
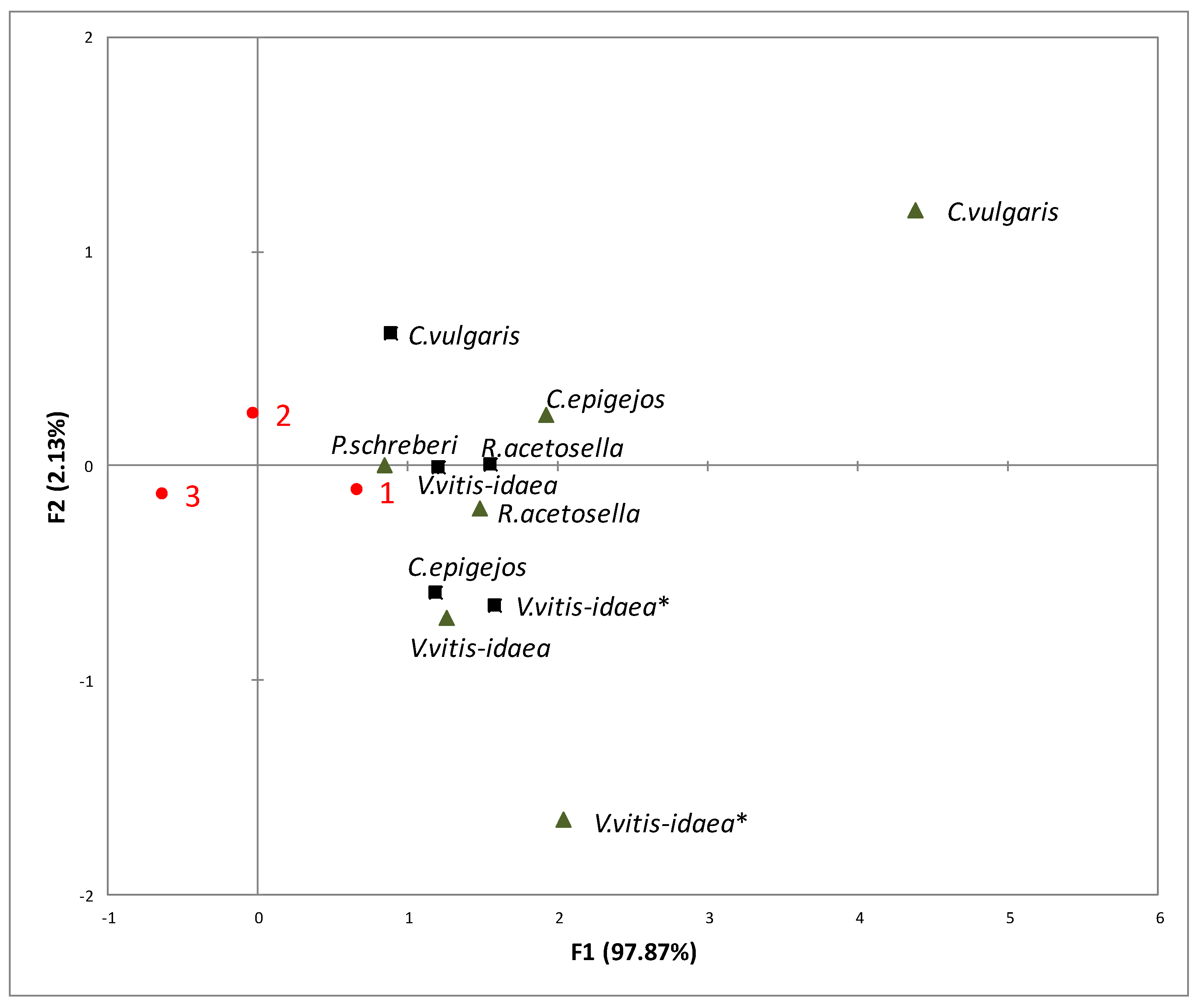
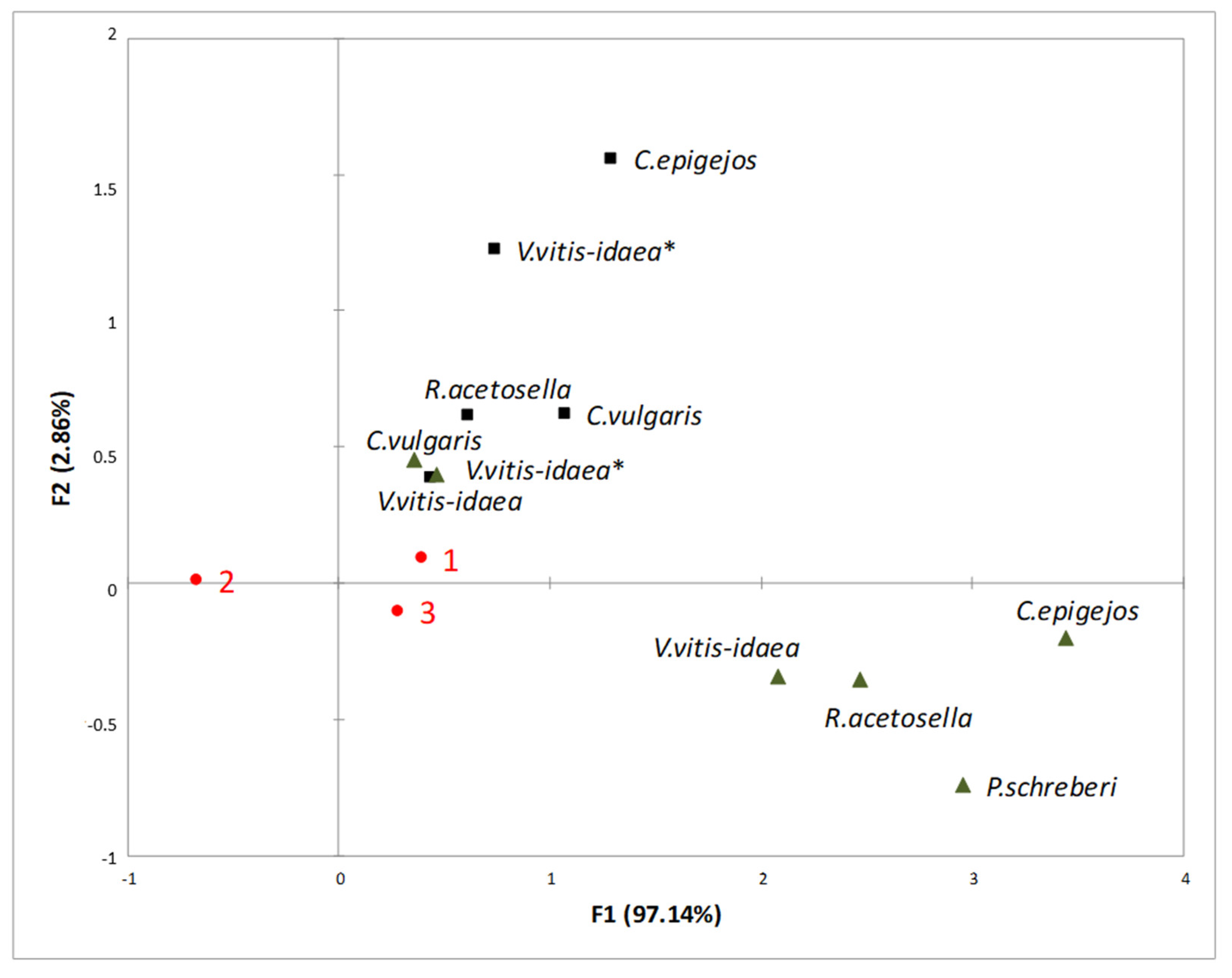
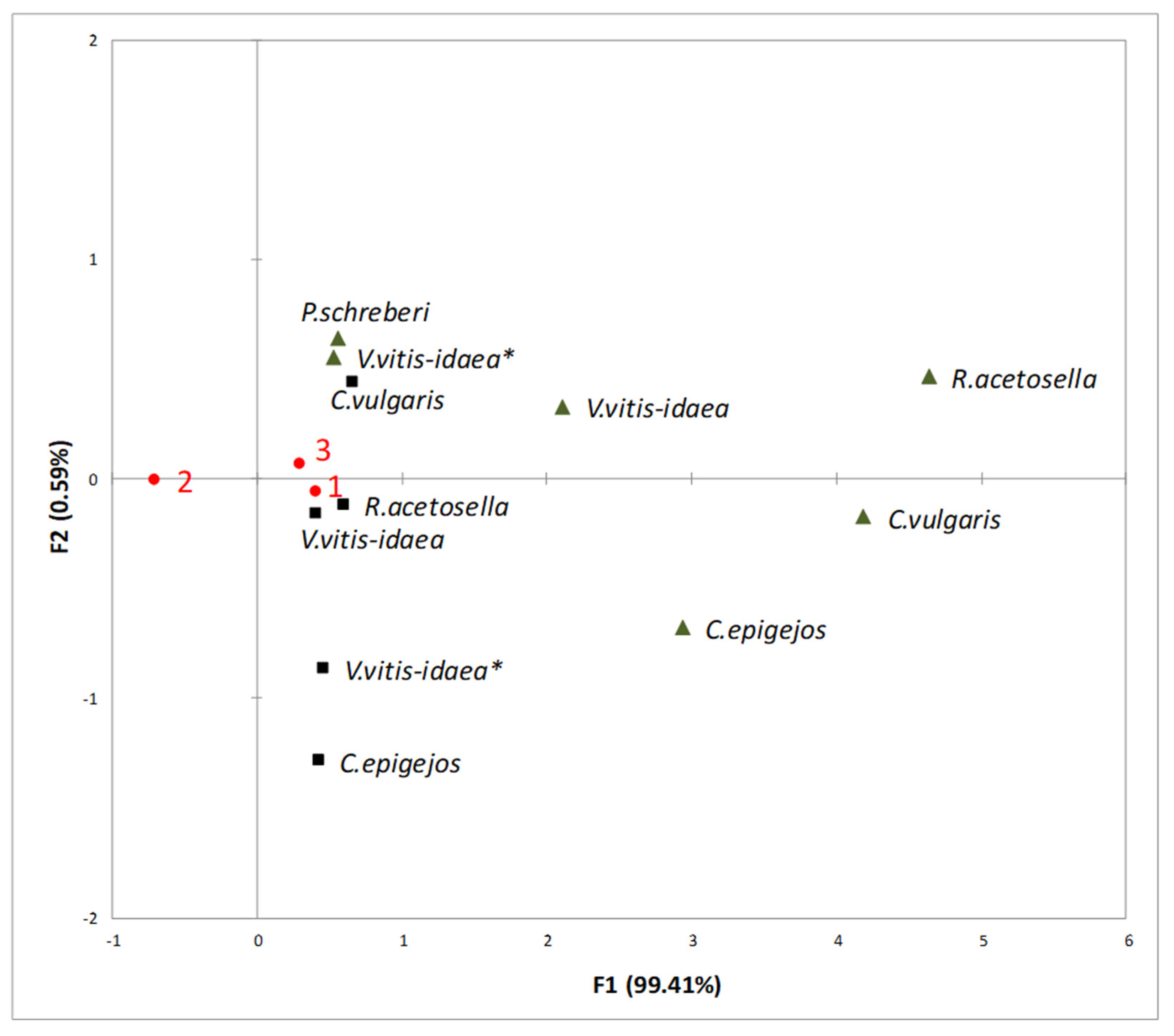
2.3. Interaction between Plant-Donor Species and Environmental Conditions
3. Discussion
4. Materials and Methods
4.1. Dominant Species
4.2. Laboratory Bioassays
- the first three chambers (current environmental conditions)—temperature of 21.5/16 °C at day/night with 400 ppm CO2,
- the second three chambers (increasing temperature (+4 °C) conditions)—25.5/20 °C with 400 ppm CO2,
- the third three chambers (increasing temperature with higher CO2 concentration conditions)—25.5/20 °C with 700 ppm CO2.
4.3. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dalgleish, H.J.; Koons, D.N.; Adler, P.B. Can life history traits predict the response of forb populations to changes in climate variability? J. Ecol. 2010, 98, 209–217. [Google Scholar] [CrossRef]
- Donohue, K.R.; De Casas, R.; Burghardt, L.; Kovach, K.; Willis, C. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Ruan, X.; Pan, C.D.; Liu, R.; Li, Z.H.; LI, S.L.; Jiang, D.A.; Zhang, J.C.; Wang, G.; Zhao, Y.X.; Wang, Q. Effects of climate warming on plant autotoxicity in forest evolution: A case simulation analysis for Picea schrenkiana regeneration. Ecol. Evol. 2016, 6, 5854–5866. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Evans, A.S.; Cabin, R.J. Can Dormancy Affect the Evolution of Post-Germination Traits? The Case of Lesquerella Fendleri. Ecology 1995, 76, 344–356. [Google Scholar] [CrossRef]
- Hovenden, M.J.; Wills, K.E.; Chaplin, R.E.; Vander Schoor, J.K.; Williams, A.L.; Osanai, Y.U.I.; Newton, P.C. Warming and elevated CO2 affect the relationship between seed mass, germinability, and seedling growth in Austrodanthonia caespitosa, a dominant Australian grass. Glob. Chang. Biol. 2008, 14, 1633–1641. [Google Scholar] [CrossRef]
- Ibanez, I.; Clark, J.S.; LaDeau, S.; Hille Ris Lambers, J. Exploiting temporal variability to understand tree recruitment response to climate change. Ecol. Monogr. 2007, 77, 163–177. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Logant, B.; Pieters, A.B.; Andrewg, B.; Scott, J.G. Plant response to climate change along the forest-tundra ecotone in northeastern Siberia. Glob. Chang. Biol. 2013, 19, 3449–3462. [Google Scholar]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, G.E.; Tchebakova, N.M.; Parfenova, Y.I.; Wykoff, W.R.; Kuzmina, N.A.; Milyutin, L.I. Intraspecific responses to climate in Pinus sylvestris. Glob. Chang. Biol. 2002, 8, 912–929. [Google Scholar] [CrossRef]
- Jump, A.S.; Mátyás, C.; Peńuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef]
- Matias, L.; Jump, A.S. Interactions between growth, demography and biotic interactions in determining species range limits in a warming world: The case of Pinus sylvestris. For. Ecol. Manag. 2014, 282, 10–22. [Google Scholar] [CrossRef]
- Mason, W.L.; Alia, R. Current and future status of Scots pine (Pinus sylvestris L.) (Eds). Silviculture and Biodiversity of Scots pine Forests in Europe. For. Syst. 2000, 1-2000, 317–333. [Google Scholar]
- Lovett, J.H. Allelopathy: The Australian experience. In The Science of Allelopathy; Putnam, A.R., Tang, C.-S., Eds.; John Wiley & Sons: New York, NY, USA, 1986; pp. 75–99. [Google Scholar]
- Mallik, A.U. Conifer regeneration problems in boreal and temperate forest with ericaceous understory: Role of disturbance, seedbed limitation, and keystone change. Crit. Rev. Plant Sci. 2003, 22, 341–366. [Google Scholar] [CrossRef]
- McKey, D.; Waterman, P.G.; Mbi, C.N.; Gartlon, J.S.; Strubraker, T.T. Phenolic content of vegetation in two African rain forests: Ecological implications. Science 1978, 202, 61–63. [Google Scholar]
- Willianson, G.B.; Richardson, D.R.; Fischer, N.H. Allelopathic mechanism in fire-prone communities. In Allelopathy: Basic and Applied Aspects; Rizvi, S.J.H., Rizvi, V., Eds.; Chapman & Hall: London, UK, 1992; pp. 57–75. [Google Scholar]
- Van Rooyen, M.W.; Theron, G.K.; van Rooyen, N.; Jankowitz, W.J.; Matthews, W.S. Mysterious circles in the Namib Desert: Review of hypotheses on their origin. J. Arid. Env. 2004, 57, 467–485. [Google Scholar] [CrossRef]
- Čaboun, V. Results of allelopathy research in Slovak forest ecosystems. In Proceedings of the Second European Allelopathy Symposium “Allelopathy—From understanding to application”, Pulawy, Poland, 3–5 June 2004; p. 24. [Google Scholar]
- Reigosa, M.J.; Pedrol, N.; Gonzalez, L. Allelopathy: A Physiological Process with Ecological Implications; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Tausz, M.; Tausz-Posch, S.; Norton, R.M.; Fitzgerald, G.J.; Nicolas, M.E.; Seneweera, S. Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations. Environ. Exper. Bot. 2013, 88, 71–80. [Google Scholar] [CrossRef]
- Goufo, P.; Pereira, J.; Moutinho-Pereira, J.; Correia, C.M.; Figueiredo, N.; Carranca, C.; Rosa, E.A.S.; Trindade, H. Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ. Exper. Bot. 2014, 99, 28–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Peng, S.; Zobel, K. Climate Warming May Facilitate Invasion of the Exotic Shrub Lantana camara. PLoS ONE 2014, 9, e105500. [Google Scholar] [CrossRef]
- Sirgedaitė-Šėžienė, V.; Baležentienė, L.; Varnagirytė-Kabašinskienė, I.; Stakėnas, V.; Baliuckas, V. Allelopathic effects of dominant ground vegetation species on initial growth of Pinus sylvestris L. seedlings in response to different temperature scenarios. iForest Biogeosci. For. 2019, 12, 132–140. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Lütz, C.; Anegg, S.; Gerant, D.; Alaoui-Sosse, B.; Gerard, J.; Dizengremel, P. Beech treles exposed to high CO2 and to simulated summer ozone levels: Effects on photosyntesis, chloroplast components and leaf enzime activity. Physiol. Plant. 2000, 109, 252–259. [Google Scholar] [CrossRef]
- Alexieva, V.; Ivanov, S.; Sergiev, I.; Karanov, E. Interaction between stresses. Bulg. J. Plant Physiol. 2003, 29, 1–17. [Google Scholar]
- Mahmoodzadeh, H.; Ghasemi, M.; Zanganeh, H. Allelopathic effect of medicinal plant Cannabis sativa L. on Lactuca sativa L. seed germination. Acta Agric. Slov. 2015, 105, 233–239. [Google Scholar] [CrossRef]
- Blanco, J.A. The representation of allelopathy in ecosystem level forest models. Ecol. Model. 2007, 209, 65–77. [Google Scholar] [CrossRef]
- Quader, M.; Daggard, G.; Barrow, R.; Walker, S.; Sutherland, M.W. Allelopathy, DIMBOA production and genetic variability in accessions of Triticum speltoides. J. Chem. Ecol. 2001, 27, 747–760. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Žemaitis, P.; Baliuckas, V. The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum. Forests 2019, 10, 287. [Google Scholar] [CrossRef]
- Wolfson, J.L.; Murdock, L.L. Growth of Manduca sexta on wounded tomato plants: Role of induced proteinase inhibitors. Entomol. Exp. Appl. 1990, 54, 257–264. [Google Scholar] [CrossRef]
- Šėžienė, V.; Baležentienė, L.; Maruška, A. Identification and Biological Activity of Phenolic Compounds in Dominant’ Extracts of Pine Forest Clear-Cuts. iForestry 2017, 10, 309–314. [Google Scholar] [CrossRef]
- Yamasaki, S.H.; Fyles, J.W.; Egger, N.E.; Titus, B.D. The effect of Kalmia angustifolia on growth, nutrition, and ectomycorrhizal symbiont community of black spruce. For. Ecol. Manag. 1998, 105, 197–207. [Google Scholar] [CrossRef]
- Mallik, A.U. Growth and physiological responses of black spruce (Picea mariana) to sites dominated by Ledum groenlandicum. J. Chem. Ecol. 1996, 22, 575–585. [Google Scholar]
- Messier, C. Factors limiting early growth of western red cedar, western hemlock and Sitka spruce seedlings on ericaceous–dominated clearcut sites in coastal British Columbia. For. Ecol. Manag. 1993, 60, 181–206. [Google Scholar] [CrossRef]
- Prescott, C.E.; Weetman, G.F. Salal Cedar Hemlock Integrated Research Program: Research Update # 1; Faculty of Forestry, University of British Columbia: Vancouver, BC, Canada, 1996; p. 49. [Google Scholar]
- Fraser, L.; Chanway, C.P.; Turkington, R. The competitive role of Gaultheria shallon on planted western hemlock and western red cedar saplings on northern Vancouver Island. For. Ecol. Manag. 1995, 75, 27–39. [Google Scholar] [CrossRef]
- Jaderlund, A.; Zackrisson, O.; Nilsson, M.-C. Effects of bilberry (Vaccinium myrtillus L.) litter on seed germination and early seedling growth of four boreal tree species. J. Chem. Ecol. 1996, 22, 973–986. [Google Scholar] [CrossRef]
- Gallet, C.; Lebreton, P. Evolution of phenolic patterns and associated litter and humus of a mountain forest ecosystem. Soil Biol. Biochem. 1995, 27, 157–165. [Google Scholar] [CrossRef]
- Gallet, C.; Nilsson, M.-C.; Zackrisson, O. Phenolic metabolites of allelopathic significance in Empetrun hermaphroditum leaves and associated humus. Plant Soil 1999, 210, 1–9. [Google Scholar] [CrossRef]
- Nilsson, M.-C. Separation of allelopathy and resource competition by the boreal dwarf shrub Empetrum hermaphroditum Hagerup. Oecologia 1997, 98, 1–7. [Google Scholar] [CrossRef]
- Jäderlund, A.; Zackrisson, O.; Dahlberg, A.; Nilsson, M.C. Interference of Vaccinium myrtillus on establishment, growth, and nutrition of Picea abies seedlings in a northern boreal site. Can. J. For. Res. 1997, 27, 2017–2025. [Google Scholar] [CrossRef]
- Becklin, K.M.; Anderson, J.T.; Gerhart, L.M.; Wadgymar, S.M.; Wessinger, C.A.; Ward, J.K. Examining Plant Physiological Responses to Climate Change through an Evolutionary Lens. Plant Physiol. 2016, 172, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Siddiqui, M.B. Allelopathic effect of aqueous extracts of different part of Eclipta alba (L.) Hassk. on some crop and weed plants. J. Agric. Ext. Rural Dev. 2014, 6, 55–60. [Google Scholar]
- Heidarzadeh, A.; Pirdashti, H.; Esmaeili, M.A.; Asghari, J. Inhibitory Activity of Allelochemicals on Barnyardgrass (Echinochloa crus-galli L.) Seed and Seedlings Parameters. World Appl. Sci. J. 2012, 17, 1535–1540. [Google Scholar]
- Rashedmohasel, M.J.; Mousvi, S.K. Principles of Weed Management; Ferdowsi University of Mashhad Publication: Mashhad, Iran, 2007; pp. 273–290. [Google Scholar]
- Wang, R.L.; Zeng, R.S.; Peng, S.L.; Chen, B.M.; Liang, X.T.; Xin, X.W. Elevated temperature may accelerate invasive expansion of the liana plant Ipomoea cairica. Weed Res. 2011, 51, 574–580. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Gomes, M.P.; Garcia, Q.S.; Barreto, L.C.; Pimenta, L.P.S.P.; Matheus, M.T. Allelopathy: An overview from micro- to macroscopic organisms, from cells to environments, and the perspectives in a climate-changing world. Biologia 2017, 72, 113–129. [Google Scholar] [CrossRef]
- Balčiūnas, M.; Jankauskienė, Z.; Brazaitytė, A.; Duchovskis, P. The effect of plant stand density on plant leaf area index and content of photosynthetic. Zemdirb. Agric. 2008, 95, 97–109. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde, 3rd ed.; Springer Wien: New York, NY, USA, 1964; p. 865. [Google Scholar]
- WRB. World Reference Base for Soil Resources; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014; p. 191. [Google Scholar]
- Vaičys, M.; Beniušis, R.; Karazija, S.; Kuliešis, A.; Raguoti, A.; Rutkauskas, A. Classification of forest sites. Forest Site Types; LUTUTĖ: Kaunas, Lithuania, 2006; p. 95. [Google Scholar]
- ISTA. Method Validation for Seed Testing; The International Seed Testing Association (ISTA): Basedorf, Switzerland, 2007; p. 66. [Google Scholar]
- Wettstein, D. Chlorophyll Letale und der submikroskopishe Formveschsel der Plastiden. Exp. Cell Res. 1957, 12, 427. [Google Scholar] [CrossRef]
- Sirgedaitė-Šėžienė, V.; Mildažienė, V.; Žemaitis, P.; Ivankov, A.; Koga, K.; Shiratani, M.; Baliuckas, V. Long-term response of Norway spruce to seed treatment with cold plasma: Dependence of the effects on the genotype. Plasma Process. Polym. 2021, 18, 2000159. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® User’s Guide; Version 9.3; SAS Institute Incorporated: Cary, NC, USA, 2010. [Google Scholar]
| Age of Clear-Cuts | Extract | Plant-Donor | Pr > Chi-Square | Climate Conditions | ||
|---|---|---|---|---|---|---|
| 1/3 1 | 1/2 | 3/2 | ||||
| 1-year-old | Shoot | P. schreberi | 0.2380 | |||
| V. vitis-idaea | 0.0156 | |||||
| C. vulgaris | 0.0066 | ** | * | |||
| Root | V. vitis-idaea | 0.0581 | ||||
| C. vulgaris | 0.1275 | |||||
| 2-year-old | Shoot | V. vitis-idaea | 0.0007 | *** | * | |
| C. epigejos | 0.0012 | * | * | |||
| R. acetosella | 0.0144 | * | ||||
| Root | V. vitis-idaea | 0.0002 | ** | |||
| C. epigejos | 0.0668 | |||||
| R. acetosella | 0.0147 | ** | ||||
| Age of Clear-Cuts | Extract | Plant-Donor | Pr > Chi-Square | Comparison of Treatments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/3 1 | 1/2 | 3/2 | 1/3 | 1/2 | 3/2 | |||||
| Radicle length | Hypocotyl length | Radicle length | Hypocotyl length | |||||||
| 1-year-old | Shoot | P. schreberi | 0.0477 | 0.8503 | * | |||||
| V. vitis-idaea | 0.8441 | 0.4291 | * | |||||||
| C. vulgaris | 0.9333 | 0.0903 | ||||||||
| Root | V. vitis-idaea | 0.8630 | 0.8708 | |||||||
| C. vulgaris | 0.8179 | 0.6439 | ||||||||
| 2-year-old | Shoot | V. vitis-idaea | 0.2528 | 0.9828 | ||||||
| C. epigejos | 0.1589 | 0.6139 | ||||||||
| R. acetosella | 0.0497 | 0.0012 | * | * | *** | ** | ||||
| Root | V. vitis-idaea | 0.9521 | 0.8082 | |||||||
| C. epigejos | 0.8655 | 0.8391 | ||||||||
| R. acetosella | 0.9554 | 0.9176 | ||||||||
| Age of Clear-Cuts | Extract | Plant-Donor | Pr > Chi-Square | Comparison of Treatments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/3 1 | 1/2 | 3/2 | 1/3 1 | 1/2 | 3/2 | |||||
| Chlorophyll a + b | Carotenoid | Chlorophyll a + b | Carotenoid | |||||||
| 1-year-old | Shoot | P. schreberi | 0.0008 | 0.0008 | ** | ** | ** | ** | ||
| V. vitis-idaea | 0.0042 | <.0001 | * | * | ** | *** | ||||
| C. vulgaris | 0.7602 | 0.0150 | * | |||||||
| Root | V. vitis-idaea | 0.0950 | 0.0638 | |||||||
| C. vulgaris | 0.0003 | <.0001 | ** | ** | ** | *** | ** | |||
| 2-year-old | Shoot | V. vitis-idaea | 0.7241 | 0.0414 | * | |||||
| C. epigejos | 0.0599 | 0.0003 | ** | ** | ||||||
| R. acetosella | 0.8345 | 0.6707 | ||||||||
| Root | V. vitis-idaea | 0.0095 | 0.0010 | ** | ** | ** | ||||
| C. epigejos | 0.2013 | 0.0495 | ||||||||
| R. acetosella | 0.0837 | 0.0108 | * | |||||||
| Parameter | Variance Component of Interaction between Dominant Species and Climate Conditions (%±SE) | Pr > Z | Climate Conditions | pH | |||
|---|---|---|---|---|---|---|---|
| F criterion | p-value | F criterion | p-value | ||||
| Germination | 17.42 | ±9.57 | * | 7.41 | ** | 7.01 | *** |
| Radicle length | 38.67 | ±17.06 | ** | 0.33 | - | 13.03 | *** |
| Hypocotyl length | 22.49 | ±11.14 | * | 0.87 | - | 8.08 | *** |
| Carotenoid | 9.17 | ±5.16 | * | 12.23 | *** | 3.89 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirgedaitė-Šėžienė, V.; Marčiulynas, A.; Baliuckas, V. Effect of Extracts from Dominant Forest Floor Species of Clear-Cuts on the Regeneration and Initial Growth of Pinus sylvestris L. with Respect to Climate Change. Plants 2021, 10, 916. https://doi.org/10.3390/plants10050916
Sirgedaitė-Šėžienė V, Marčiulynas A, Baliuckas V. Effect of Extracts from Dominant Forest Floor Species of Clear-Cuts on the Regeneration and Initial Growth of Pinus sylvestris L. with Respect to Climate Change. Plants. 2021; 10(5):916. https://doi.org/10.3390/plants10050916
Chicago/Turabian StyleSirgedaitė-Šėžienė, Vaida, Adas Marčiulynas, and Virgilijus Baliuckas. 2021. "Effect of Extracts from Dominant Forest Floor Species of Clear-Cuts on the Regeneration and Initial Growth of Pinus sylvestris L. with Respect to Climate Change" Plants 10, no. 5: 916. https://doi.org/10.3390/plants10050916
APA StyleSirgedaitė-Šėžienė, V., Marčiulynas, A., & Baliuckas, V. (2021). Effect of Extracts from Dominant Forest Floor Species of Clear-Cuts on the Regeneration and Initial Growth of Pinus sylvestris L. with Respect to Climate Change. Plants, 10(5), 916. https://doi.org/10.3390/plants10050916





