Evaluation of the Conditions for the Cultivation of Callus Cultures of Hyssopus officinalis Regarding the Yield of Polyphenolic Compounds
Abstract
1. Introduction
2. Results
2.1. Callus Growth Curve
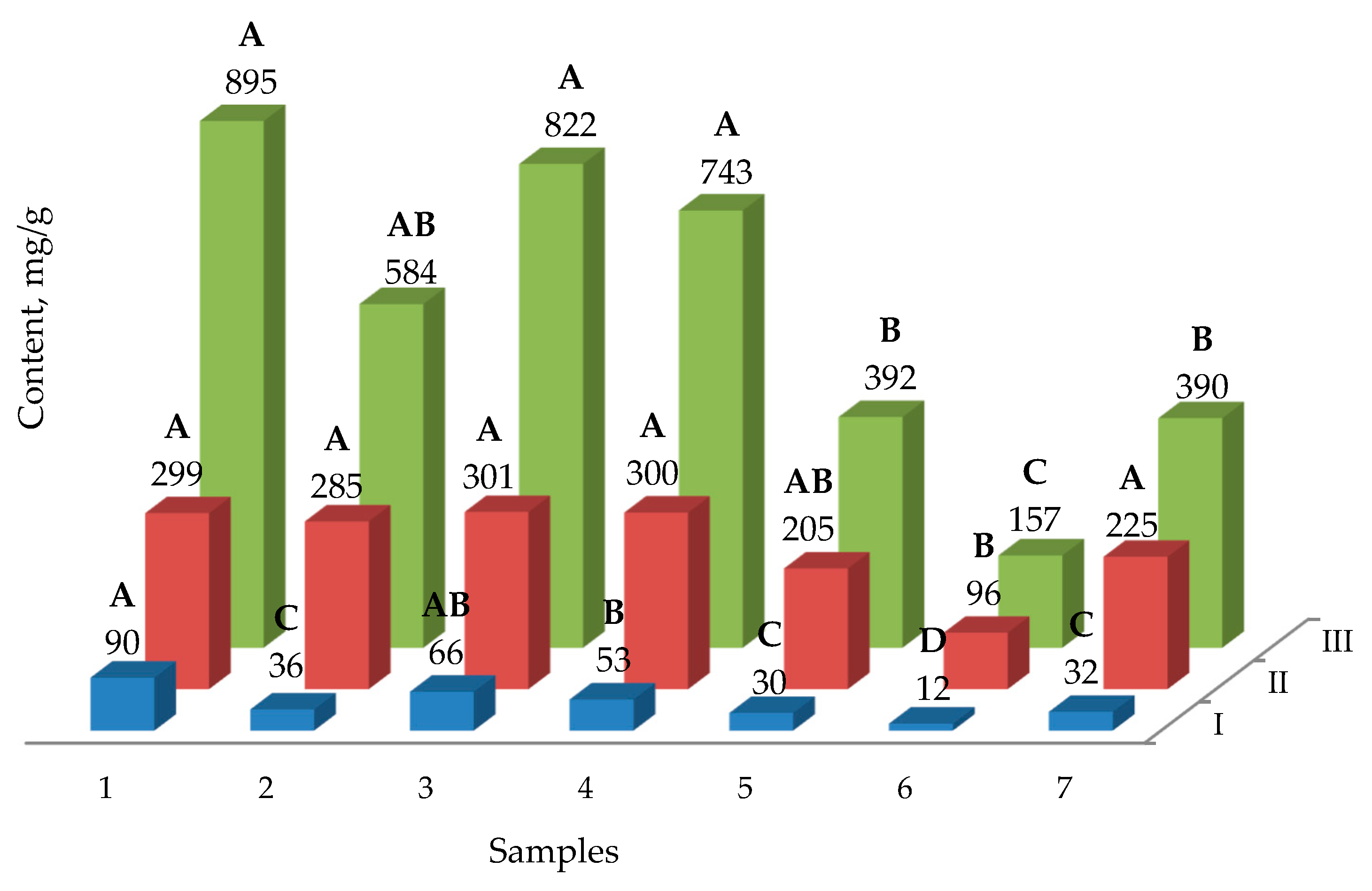
2.2. The Yield of Phenolic Compounds
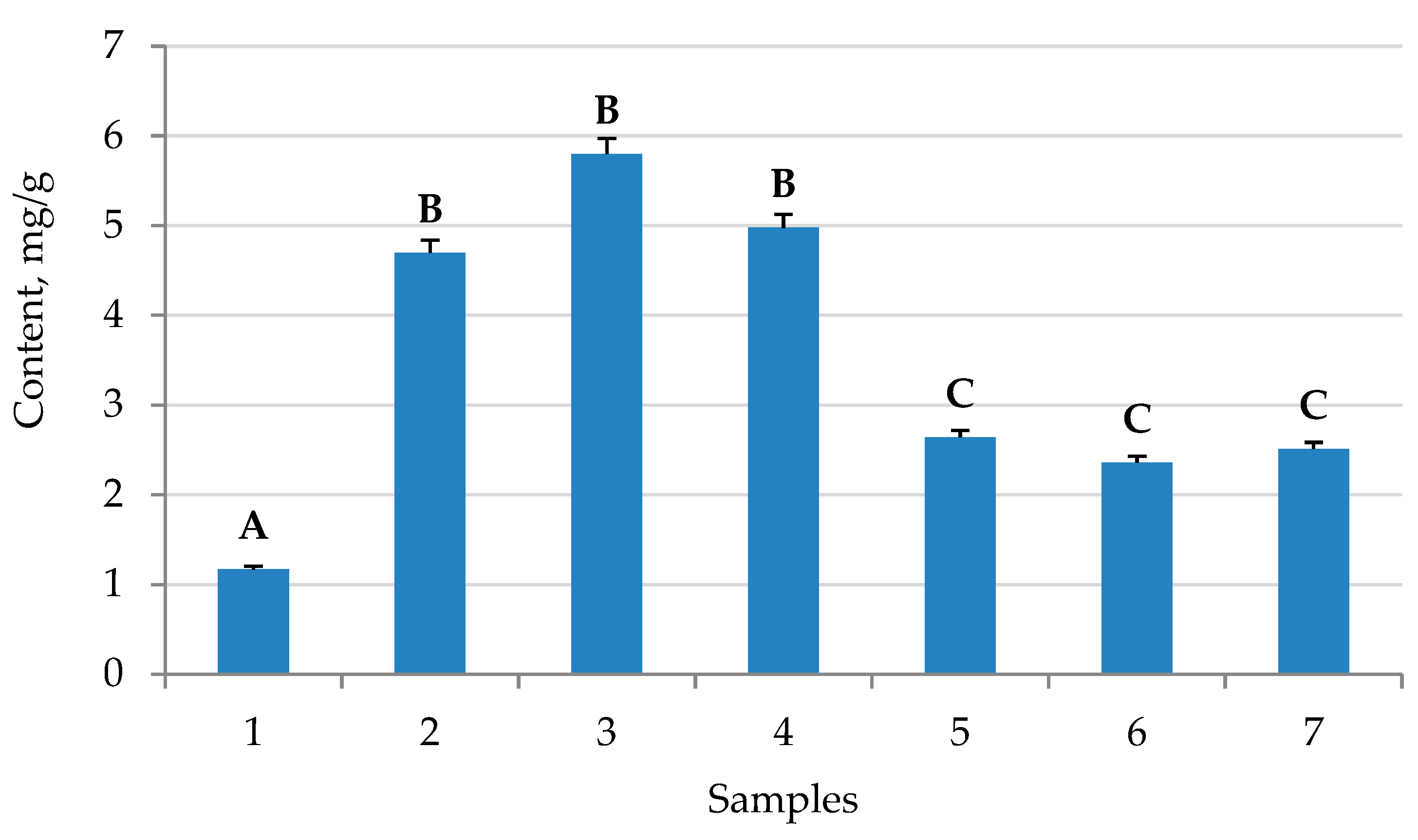
2.3. The Yield of Saponins
3. Discussion
4. Materials and Methods
4.1. Obtaining Plant Callus Cell Cultures
4.2. Cultivation of Callus Cell Cultures
4.3. Study of Growth Characteristics of Callus Cell Cultures (In Vitro)
4.4. Drying of Callus Cell Culture Biomass
4.5. Preparation of Extract Samples
4.6. Quantitative Determination of the Contents of Individual Phenolic Compounds by HPLC
4.7. Determination of the Total Polyphenol Compounds
4.8. Determination of Total Flavonoids
4.9. Qualitative and Quantitative Determination of Saponins
4.10. Antioxidant Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kubica, P.; Snoch, A.; Ekiert, H. High production of bioactive depsides in shoot and callus cultures of Aronia arbutifolia and Aronia prunifolia. Acta Physiol. Plant 2018, 40, 48. [Google Scholar] [CrossRef]
- Castro, A.H.F.; de Braga, K.Q.; de Sousa, F.M.; Coimbra, M.C.; Chagas, R.C.R. Callus induction and bioactive phenolic compounds production from Byrsonima verbascifolia (L.) DC. (Malpighiaceae). Rev. Rev. Ciênc. Agron. 2016, 47, 143–151. [Google Scholar]
- Ahmad, W.; Zahir, A.; Nadeem, M.; Zia, M.; Hano, C.; Abbasi, B.H. Thidiazuron-induced efficient biosynthesis of phenolic compounds in callus culture of Ipomoea turbinata Lagasca and Segura. Vitr. Cell Dev. Biol. Plant 2019, 55, 710–719. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-L.; Li, J.; Li, J.-X.; Liu, S.; Huang, L.; Gao, W. Production of active compounds in medicinal plants: From plant tissue culture to biosynthesis. Chin. Herb. Med. 2017, 9, 115–125. [Google Scholar] [CrossRef]
- Hendrawati, O.; Woerdenbag, H.J.; Hille, J.; Kayser, O. Metabolic engineering of medicinal plants and microorganisms for the production of natural products. In Pharmaceutical Biotechnology: Drug Discovery and Clinical Applications, 2nd ed.; Kayser, O., Warzecha, H., Eds.; John Wiley & Sons Limited: Weinhein, Germany, 2012; pp. 491–526. [Google Scholar]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Gureev, M.; Krasavin, M. Plants of the Russian Federation pharmacopeia: An unexhausted natural products research opportunity? Nat. Prod. Res. 2020, 1–3. [Google Scholar] [CrossRef]
- Babich, O.; Prosekov, A.; Zaushintsena, A.; Sukhikh, A.; Dyshlyuk, L.; Ivanova, S. Identification and quantification of phenolic compounds of Western Siberia Astragalus danicus in different regions. Heliyon 2019, 5, e02245. [Google Scholar] [CrossRef]
- Zaushintsena, A.V.; Bruhachev, E.N.; Belashova, O.V.; Asyakina, L.K.; Kurbanova, M.G.; Vesnina, A.D.; Fotina, N.V. Extracts of Rhodiola rosea L. and Scutellaria galericulata L. in functional dairy products. Foods Raw Mater. 2020, 8, 163–170. [Google Scholar] [CrossRef]
- Fathiazad, F.; Mazandarani, M.; Hamedeyazdan, S. Phytochemical analysis and antioxidant activity of Hyssop officinalis L. from Iran. Adv. Pharm. Bull. 2011, 1, 63–67. [Google Scholar] [PubMed]
- Hatipoğlu, G.; Sökmen, M.; Bektaş, E.; Daferera, D.; Sökmen, A.; Demir, E.; Şahin, H. Automated and standard extraction of antioxidant phenolic compounds of Hyssop officinalis L. ssp. Angustifolius. Ind. Crops Prod. 2013, 43, 427–433. [Google Scholar] [CrossRef]
- Zielińska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, Z.; Wysokińska, H. Sterols and triterpenes in cell culture of Hyssop officinalis L. Z. Nat. C 2003, 58, 308–312. [Google Scholar]
- Aghaei, K.; Ghasemi Pirbalouti, A.; Mousavi, A.; Badi, H.N.; Mehnatkesh, A. Effects of foliar spraying of l-phenylalanine and application of bio-fertilizers on growth, yield, and essential oil of hyssop [Hyssop officinalis L. subsp. Angustifolius (Bieb.)]. Biocatal. Agric. Biotechnol. 2019, 21, 101318. [Google Scholar] [CrossRef]
- Toma, I.; Toma, C.; Ghiorghita, G. Histo-anatomy and in vitro morphogenesis in Hyssopus officinalis L. (Lamiaceae). Acta Bot. Croat. 2004, 63, 59–68. [Google Scholar]
- Meyer, H.J.; Van Staden, J. The in vitro production of an anthocyanin from callus cultures of Oxalis linearis. Plant Cell Tissue Organ Cult. 1995, 40, 55–58. [Google Scholar] [CrossRef]
- Sangwan, R.S.; Norreel, B.; Harada, H. Effects of kinetin and gibberellin a3 on callus growth and organ formation in Limnophila chinensis tissue culture. Biol. Plant 1976, 18, 126–131. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssop officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Maneechai, S.; De-Eknamkul, W.; Umehara, K.; Noguchi, H.; Likhitwitayawuid, K. Flavonoid and stilbenoid production in callus cultures of Artocarpus lakoocha. Phytochemistry 2012, 81, 42–49. [Google Scholar] [CrossRef]
- Tan, S.H.; Musa, R.; Ariff, A.; Maziah, M. Effect of plant growth regulators on callus, cell suspension and cell line selection for flavonoid production from pegaga (Centella Asiatica L. urban). Am. J. Biochem. Biotechnol. 2010, 6. [Google Scholar] [CrossRef]
- Luczkiewicz, M.; Kokotkiewicz, A.; Glod, D. Plant growth regulators affect biosynthesis and accumulation profile of isoflavone phytoestrogens in high-productive in vitro cultures of Genista tinctoria. Plant Cell Tissue Organ Cult. 2014, 118, 419–429. [Google Scholar] [CrossRef]
- Park, J.-S.; Seong, Z.-K.; Kim, M.-S.; Ha, J.-H.; Moon, K.-B.; Lee, H.-J.; Lee, H.-K.; Jeon, J.-H.; Park, S.U.; Kim, H.-S. Production of flavonoids in callus cultures of Sophora Flavescens Aiton. Plants 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Appelhagen, I.; Wulff-Vester, A.K.; Wendell, M.; Hvoslef-Eide, A.-K.; Russell, J.; Oertel, A.; Martens, S.; Mock, H.-P.; Martin, C.; Matros, A. Colour bio-factories: Towards scale-up production of anthocyanins in plant cell cultures. Metab. Eng. 2018, 48, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, A.; Raven, M.; Blind, K. The role of standardization at the interface of product and process development in biotechnology. J. Technol. Transf. 2019, 44, 1097–1133. [Google Scholar] [CrossRef]
- Yang, Y.; Asyakina, L.K.; Babich, O.O.; Dyshlyuk, L.S.; Sukhikh, S.A.; Popov, A.D.; Kostyushina, N.V. Physicochemical properties and biological activity of extracts of dried biomass of callus and suspension cells and in vitro root cultures. Food Process. Tech. Technol. 2020, 50, 480–492. [Google Scholar] [CrossRef]
- Kahrizi, D. Study of callus induction and cell culture to secondary metabolite production in Hyssop officinalis L. J. Rep. Pharm. Sci. 2016, 5, 104–111. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Bibi, A.; Adil, M.; Mashwani, Z.-R. Production of callus biomass and antioxidant secondary metabolites in black cumin. J. Anim. Plant Sci. 2018, 28, 1321–1328. [Google Scholar]
- Asyakina, L.K.; Babich, O.O.; Pungin, A.V.; Prosekov, A.Y.; Popov, A.D.; Voblikova, T.V. Optimization of extraction parameters of biologically active substances from dried biomass of callus, suspension cells and root cultures in vitro. IOP Conf. Ser. Earth Environ. Sci. 2020, 613, 012008. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.; Gillespie, K. Estimation of total phenolic and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Pirogov, A.; Sokolova, L.; Sokerina, E.; Tataurova, O.; Shpigun, O. Determination of flavonoids as complexes with Al3+ in microemulsion media by HPLC method with fluorescence detection. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 220–224. [Google Scholar] [CrossRef]
- Desbène, S.; Hanquet, B.; Shoyama, Y.; Wagner, H.; Lacaille-Dubois, M.-A. Biologically active triterpene saponins from callus tissue of Polygala amarella. J. Nat. Prod. 1999, 62, 923–926. [Google Scholar] [CrossRef]
- Rasool, R.; Ganai, B.A.; Kamili, A.N.; Akbar, S. Antioxidant potential in callus culture of Artemisia amygdalina Decne. Nat. Prod. Res. 2012, 26, 2103–2106. [Google Scholar] [PubMed]
- Rasool, R.; Ahmad, B.; Azra, G.; Kamili, N. antioxidant and antibacterial activities of extracts from wild and in vitro- raised cultures of Prunella vulgaris L. Med. Aromat. Plant Sci. Biotechnol. 2010, 4, 20–27. [Google Scholar]

| Name of Phenolic Compound | Rt (min) ± 0.5 | SD | NP | Culture Media | |||||
|---|---|---|---|---|---|---|---|---|---|
| MS-1 | MS-2 | MS-3 | B5-1 | B5-2 | B5-3 | ||||
| Ferulic acid | 17.60 | 1.00 | 36.92 | 28.62 | 31.15 | 29.17 | 22.37 | 11.34 | 20.43 |
| Isoquercitrin | 27.80 | 0.50 | 32.78 | 22.83 | 27.62 | 25.14 | 17.13 | 9.76 | 19.71 |
| Rutin | 28.50 | 1.00 | 21.93 | 17.43 | 19.75 | 18.19 | 12.45 | 8.34 | 13.43 |
| Quercetin | 32.00 | 0.10 | 1.79 | 0.97 | 1.14 | 1.03 | 0.54 | 0.41 | 0.50 |
| Quercetin-7-O-glucoside | 35.20 | 0.10 | 0.89 | 0.45 | 0.67 | 0.57 | 0.24 | <0.2 | 0.40 |
| Luteolin | 48.20 | 0.10 | 2.25 | 1.14 | 1.98 | 1.60 | 0.76 | 0.31 | 0.82 |
| Components | MS-1 | MS-2 | MS-3 | B5-1 | B5-2 | B5-3 |
|---|---|---|---|---|---|---|
| Sucrose, g | 30 | 30 | 30 | 30 | 30 | 30 |
| Casein Hydrolyzate, mg | 500 | - | - | 500 | - | - |
| Myo-inositol, mg | 100 | 100 | 100 | 100 | 100 | 100 |
| Thiamine, mg | 0.1 | 0.1 | 0.1 | 10.0 | 10.0 | 10.0 |
| Pyridoxine, mg | 0.5 | 0.5 | 0.5 | 1.0 | 1.0 | 1.0 |
| Nicotinic acid, mg | 0.5 | 0.5 | 0.5 | 1.0 | 1.0 | 1.0 |
| Kinetin, mg | - | 2 | - | - | 2 | - |
| 6-benzylaminopurine, mg | 0.5 | - | 1.0 | 0.5 | - | 1.0 |
| Indolylacetic acid, mg | - | - | 2 | - | - | 2 |
| 1-naphthylacetic acid, mg | - | 3 | - | - | 3 | - |
| 2,4-dichlorophenoxyacetic acid, mg | 2.0 | - | - | 2.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babich, O.; Sukhikh, S.; Pungin, A.; Astahova, L.; Chupakhin, E.; Belova, D.; Prosekov, A.; Ivanova, S. Evaluation of the Conditions for the Cultivation of Callus Cultures of Hyssopus officinalis Regarding the Yield of Polyphenolic Compounds. Plants 2021, 10, 915. https://doi.org/10.3390/plants10050915
Babich O, Sukhikh S, Pungin A, Astahova L, Chupakhin E, Belova D, Prosekov A, Ivanova S. Evaluation of the Conditions for the Cultivation of Callus Cultures of Hyssopus officinalis Regarding the Yield of Polyphenolic Compounds. Plants. 2021; 10(5):915. https://doi.org/10.3390/plants10050915
Chicago/Turabian StyleBabich, Olga, Stanislav Sukhikh, Artem Pungin, Lidiya Astahova, Evgeny Chupakhin, Daria Belova, Alexander Prosekov, and Svetlana Ivanova. 2021. "Evaluation of the Conditions for the Cultivation of Callus Cultures of Hyssopus officinalis Regarding the Yield of Polyphenolic Compounds" Plants 10, no. 5: 915. https://doi.org/10.3390/plants10050915
APA StyleBabich, O., Sukhikh, S., Pungin, A., Astahova, L., Chupakhin, E., Belova, D., Prosekov, A., & Ivanova, S. (2021). Evaluation of the Conditions for the Cultivation of Callus Cultures of Hyssopus officinalis Regarding the Yield of Polyphenolic Compounds. Plants, 10(5), 915. https://doi.org/10.3390/plants10050915










