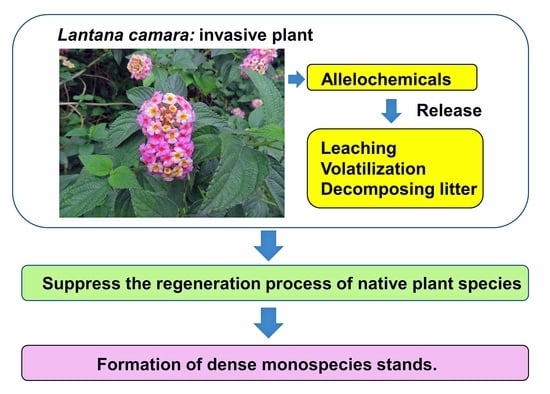
Allelopathy of Lantana camara as an Invasive Plant
Abstract
1. Introduction
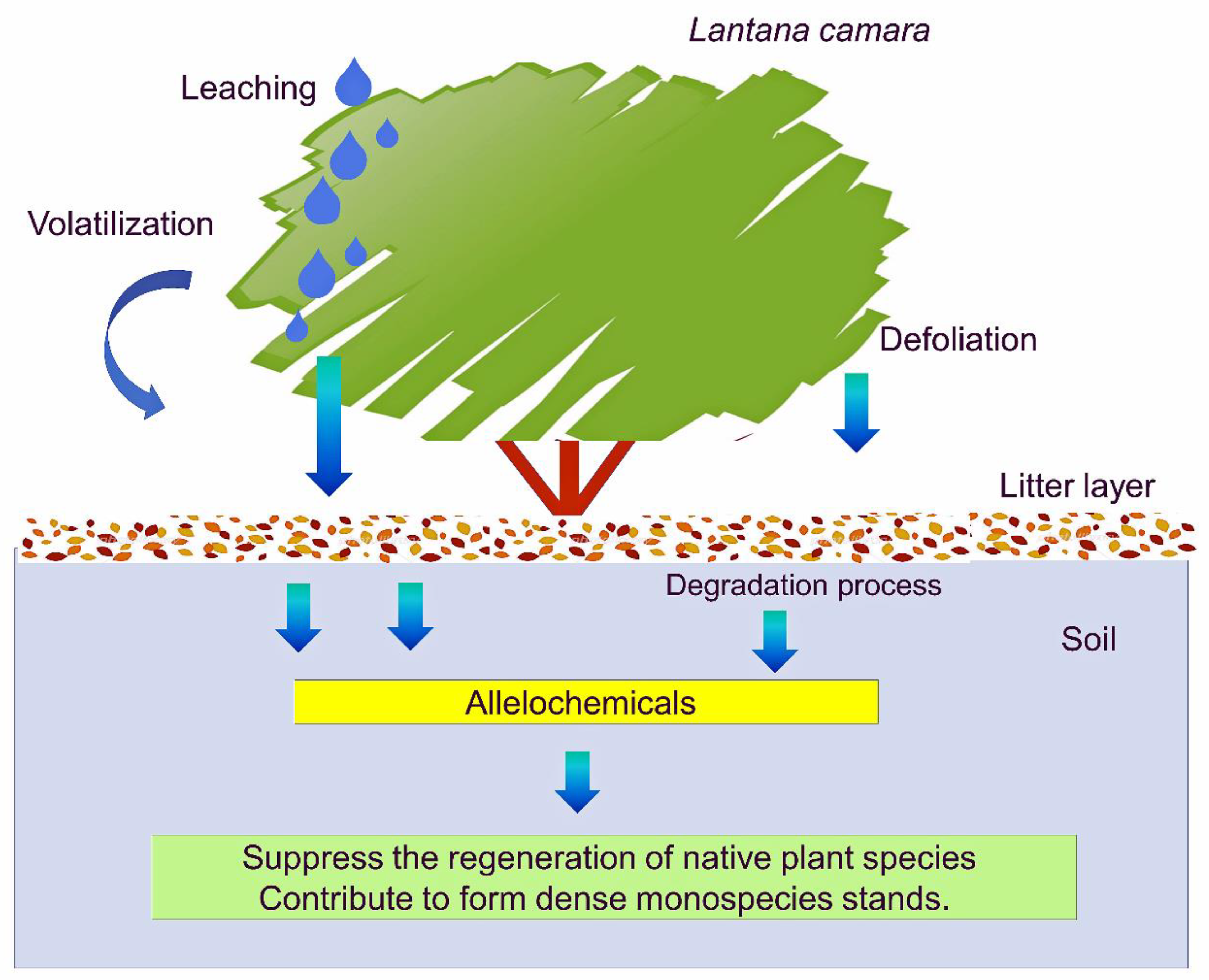
2. Allelopathy of L. camara
2.1. Extract
2.2. Leachate
2.3. Residue
2.4. Rhizosphere Soil
| Source | Target Plant Species | Inhibition | Stimulation | Reference |
|---|---|---|---|---|
| Extract | ||||
| Leaf | Lactuca sativa | Germination, cellular membrane development | Reactive oxygen form | [32] |
| Eichhornia crassipes | Development of leaf buds, catalase, leaf necrosis | SOD activity, H2O2 accumulation, membrane peroxidation | [33] | |
| Brassica juncea, Cucumis sativus, Phaseolus mungo, Raphanus sativus, Vigna unguiculata, Cicer arietinum | Germination and growth | [34] | ||
| Centroma pubescens | Germination and growth | [35] | ||
| Vigna radiata | Germination and growth | [36] | ||
| Funaria hygrometrica | Regeneration | [40] | ||
| Leaf, stem | Lolium multiflorum | Germination and growth | [43] | |
| Leaf, stem, root | Cicer arietinum, | Germination and growth | [37] | |
| Phaseolus mungo | Germination and growth | [38] | ||
| Lens esculenta | Germination and growth | [39] | ||
| Flower | Eruca sativa | Germination and growth | [41] | |
| Flower, fruit, leaf | Raphanus sativus, Lactuca sativa | Germination and growth | [42] | |
| Leachate | ||||
| Shoot, flower | Eichhornia crassipes | Growth | [44,45] | |
| Leaf | Mimosa pudica | Concentrations of insoluble carbohydrate, protein and nucleic acid. Activities of dehydrogenase, catalase and peroxidase | Concentrations of amino acid and soluble carbohydrate | [46,47] |
| Root | Growth, protein synthesis | [48] | ||
| Triticum aestivum, | Germination and growth | [49] | ||
| Fruit, leaf | Pennisetum americanum, Setaria italica, Lactuca sativa | Growth | [50] | |
| Residue | ||||
| Shoot | Triticum aestivum, Zea mays, Glycine max, Lepidium virginicum, Abutilon theophrasti | Growth | [51] | |
| Root, shoot, decomposed | Morrenia odorata | Growth | [52] | |
| Decomposed leaf litter | Raphanus sativus, Lactuca sativa, Bidens pilosa, Bidens bipinnata, Urena lobata | Growth | [53] | |
| Rhizosphere soil | Achyranthes aspera, Albizia lebbeck | Growth | [54] | |
| Avena sativa, Cicer arietinum., Hordeum vulgare, Triticum aestivum | Germination and growth | [55] |
3. Allelochemicals
4. Invasion and Allelopathy of L. camara
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Duggin, J.A.; Gentle, C.B. Experimental evidence on the importance of disturbance intensity for invasion of Lantana camara L. in dry rainforest-open forest ecotones in north-eastern NSW, Australia. For. Ecol. Manag. 1998, 109, 279–292. [Google Scholar] [CrossRef]
- Swarbrick, J.T.; Willson, B.W.; Hanna-Jones, M.A. Lantana camara L. In The Biology of Australian Weeds; Panetta, F.D., Groves, R.H., Shepherd, R.C.H., Eds.; R.G.&F.J. Richardson: Melbourne, Australia, 1998; pp. 119–136. [Google Scholar]
- Sharma, G.P.; Raghubanshi, A.S.; Singh, J.S. Lantana invasion: An overview. Weed Biol. Manag. 2005, 5, 157–165. [Google Scholar] [CrossRef]
- Priyanka, N.; Joshi, P.K. A review of Lantana camara studies in India. Int. J. Sci. Res. Pub. 2013, 3, 1–11. [Google Scholar]
- Dogra, K.S.; Kohli, R.K.; Sood, S.K. An assessment and impact of three invasive species in the Shivalik hills of Himachal Pradesh, India. Int. J. Biodivers. Conserv. 2009, 1, 4–10. [Google Scholar]
- Global Invasive Species Database. Species Profile: Lantana camara. Available online: http://www.iucngisd.org/gisd/species.php?sc=56 (accessed on 20 April 2021).
- Singh, S.P. Biological control. In 50 Years of Crop. Science Research in India; Paroda, R.S., Chadha, K.L., Eds.; Indian Council of Agricultural Research: New Delhi, India, 1996; pp. 88–116. [Google Scholar]
- Van Oosterhout, E.; Clark, A.; Day, M.D.; Menzies, E. Lantana Control Manual. Current Management and Control. Options for Lantana (Lantana camara) in Australian State of Queensland. Department of Natural Resources, Mines and Energy, Brisbane, Qld, Australia. Available online: http://www.nrm.qld.gov.au/pests/wons/Lantana (accessed on 23 November 2004).
- Vardien, W.; Richardson, M.D.; Foxcroft, L.C.; Thompson, G.D.; Wilson, J.R.U.; Le Rouxa, J.J. Invasion dynamics of Lantana camara L. (sensu lato) in South Africa. S. Afr. J. Bot. 2012, 81, 81–94. [Google Scholar] [CrossRef]
- Coutts-Smith, A.; Downey, P. Impact of Weeds on Threatened Biodiversity in New South Wales; Technical Series No.11; CRC for Australian Weed Management: Adelaide, Australia, 2006. [Google Scholar]
- Binggeli, P. Verbenaceae, Lantana camara, fankatavinakoho, fotatra, mandadrieko, rajejeka, radredreka, ramity. In The Natural History of Madagascar; Goodman, S.M., Benstead, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 415–417. [Google Scholar]
- Peng, Z.; Bhattarai, K.; Parajuli, S.; Cao, Z.; Deng, Z. Transcriptome analysis of young ovaries reveals candidate genes involved in gamete formation in Lantana camara. Plants 2019, 8, 263. [Google Scholar] [CrossRef]
- Everist, S.L. Poisonous Plants of Australia; Rev edn, Angus & Robertson: Sydney, Australia, 1981; p. 966. [Google Scholar]
- Palmer, W.A.; Pullen, K.R. The phytophagous arthropods associated with Lantana camara, L. hirsuta, L. urticifolia and L. urticoides (Verbenaceae) in North America. Biol. Control 1995, 5, 54–72. [Google Scholar] [CrossRef]
- Thompson, J.D.; McNeilly, T.; Gray, A.J. Population variation in Spartina anglica C.E. Hubbard: I. Evidence from a common garden experiment. New Phytol. 1991, 117, 115–128. [Google Scholar] [CrossRef]
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef]
- Gujral, G.S.; Vasudevan, P. Lantana camara L., a problem weed. J. Sci. Indust. Res. 1983, 42, 281–286. [Google Scholar]
- Jordaan, L.A.; Johnson, S.D.; Downs, C.T. The role of avian frugivores in germination of seeds of fleshy-fruited invasive alien plants. Biol. Inva. 2011, 13, 1917–1930. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemology, global consequences and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Swarbrick, J.T.; Willson, B.W.; Hannan-Jones, M.A. The biology of Australian weeds 25 Lantana camara L. Plant Prot. Q. 1995, 10, 82–95. [Google Scholar]
- Mallik, A.U. Allelopathy and competition in coniferous forests. Environ. For. Sci. 1998, 54, 309–315. [Google Scholar]
- Kato-Noguchi, H.; Takeshita, S.; Kimura, F.; Ohno, O.; Suenaga, K. A novel allelopathic active substance in Ginkgo biloba. J. Plant Physiol. 2013, 170, 1595–1599. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Takeshita, S. Contribution of a phytotoxic compound to the allelopathy of Ginkgo biloba. Plant Signal. Behav. 2013, 8, e26999. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelopathic substances of mango (Mangifera indica L.). Weed Biol. Manag. 2020, 20, 131–138. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Phytotoxic substances involved in teak allelopathy and agroforestry. Appl. Sci. 2021, 11, 3314. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Gindri, D.M.; Coelho, C.M.M.; Uarrota, V.G. Physiological and biochemical effects of Lantana camara L. allelochemicals on the seed germination of Avena sativa L. Pesqui. Agropecu. Trop. 2020, 50, e62546. [Google Scholar] [CrossRef]
- Zheng, H.; Wei, N.; Wang, L.; He, P. Effects of Lantana camara leaf extract on the activity of superoxide dismutase and accumulation of H2O2 in water hyacinth leaf. J. Plant Physiol. Mol. Biol. 2006, 32, 189–194. [Google Scholar]
- Ahmed, R.; Uddin, M.B.; Khan, M.A.; Mukul, S.A.; Hossain, M.K. Allelopathic effects of Lantana camara on germination and growth behavior of some agricultural crops in Bangladesh. J. For. Res. 2007, 18, 301–304. [Google Scholar] [CrossRef]
- Rusdy, M.; Ako, A. Allelopathic effect of Lantana camara and Chromolaena odorata on germination and seedling growth of Centroma pubescens. Int. J. Appl. Environ. Sci. 2017, 12, 1769–1776. [Google Scholar]
- Julio, A.; Tandoc, W.C.; Tipace, H.D.; Vendivil, Y.F.; Yanesa, Z.; Tare, M.V.R.; Lactaoen, E.J.; Clemente, K.J. Allelopathic effect of Lantana camara and Chromolaena odorata leaf extracts on plant germination. Asian J. Agric. Biol. 2019, 7, 190–196. [Google Scholar]
- Oudhia, P. Allelopathic effects of Lantana camara L. on chickpea. Ecol. Environ. Conserv. 2000, 6, 223–225. [Google Scholar]
- Vijay, B.; Jain, B.K. Allelopathic effects of Lantana camara L. on in vitro seed germination of Phaseolus mungo. Int. J. Plant Sci. 2010, 5, 43–45. [Google Scholar]
- Singh, A.; Satsangi, G.P.; Srivastava, J.N. Allelopathic aspects of Lantana camara on germination and seedling growth of Lens esculanta. Vegetos 2012, 25, 233–235. [Google Scholar]
- Choyal, R.; Sharma, S.K. Allelopathic effects of Lantana camara (Linn) on Regeneration in Funaria hygrometrica. Indian J. Fund. Appl. Life Sci. 2011, 1, 177–182. [Google Scholar]
- Labruzzo, A.; Carrubba, A.; Di Marco, G.; Ebadi, M.T. Herbicidal potential of aqueous extracts from Melia azedarach L., Artemisia arborescens L., Rhus coriaria L. and Lantana camara L. Allelopath. J. 2017, 41, 81–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, S.; Zhang, Y. Allelopathie potential of reproductive organs of exotic weed Lantana camara. Allelopath. J. 2009, 23, 213–220. [Google Scholar]
- Achhireddy, N.R.; Singh, M.; Achhireddy, L.L.; Nigg, H.N.; Nagy, S. Isolation and partial characterization of phytotoxic compounds from lantana (Lantana camara L.). J. Chem. Ecol. 1985, 11, 979–988. [Google Scholar] [CrossRef]
- Saxena, M.K. Aqueous leachate of Lantana camara kills water hyacinth. J. Chem. Ecol. 2000, 26, 2435–2447. [Google Scholar] [CrossRef]
- Motwani, G.; Golani, N.; Solanki, H. Allelopathic effects of aqueous leaf leachates of Lantana camara on Eichhorina crassipes. Lifesci. Leafl. 2013, 1, 83–90. [Google Scholar]
- Maiti, P.P.; Bhakat, R.K.; Bhattacharjee, A. Allelopathic effects of Lantana camara on physio-biochemical parameters of Mimosa pudica seeds. Allelopath. J. 2008, 22, 59–67. [Google Scholar]
- Maiti, P. Biometric evaluation of allelopathic potential of Lantana camara L. on Mimosa seeds. J. Crit. Rev. 2020, 7, 837–847. [Google Scholar]
- Romero-Romero, T.; Anaya, A.L.; Cruz-Ortega, R. Screening for effects of phytochemical variability on cytoplasmic protein synthesis pattern of crop plants. J. Chem. Ecol. 2002, 28, 617–629. [Google Scholar] [CrossRef]
- Oudhia, P. Allelopathic effects of root leachates of some obnoxious weeds on germination and seedling vigour of wheat. Ecol. Environ. Conserv. 2001, 7, 111–113. [Google Scholar]
- Hussain, F.; Ghulam, S.; Sher, Z.; Ahmad, B. Allelopathy by Lantana camara L. Pak. J. Bot. 2011, 43, 2373–2378. [Google Scholar]
- Mersie, W.; Singh, M. Allelopathic effect of lantana on some agronomic crops and weeds. Plant Soil 1987, 98, 25–30. [Google Scholar] [CrossRef]
- Achhireddy, N.R.; Singh, M. Allelopathic effects of lantana (Lantana camara) on milkweedvine (Morrenia odorata). Weed Sci. 1984, 32, 757–761. [Google Scholar] [CrossRef]
- Wang, R.; Kang, X.; Quan, G.; Zhang, J. Influence of Lantana camara on soil II. Effects of Lantana camara leaf litter on plants and soil properties. Allelopath. J. 2015, 35, 207–216. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Dogra, K.S.; Kaur, S.; Kohli, R.K.; Negi, A. Negative effect of litter of invasive weed Lantana camara on structure and composition of vegetation in the lower Siwalik Hills, northern India. Environ. Monit. Assess. 2014, 186, 3379–3389. [Google Scholar] [CrossRef] [PubMed]
- Hayyat, M.S.; Safdar, M.E.; Asif, M.; Tanveer, A.; Ali, L.; Qamar, R.; Ali, H.H.; Farooq, N.; Javeed, H.M.A.; Tarar, Z.H. Allelopathic effect of waste-land weeds on germination and growth of winter crops. Planta Daninha 2020, 38, e020173626. [Google Scholar] [CrossRef]
- Singh, M.; Tamma, R.V.; Nigg, H.N. HPLC identification of allelopathic compounds from Lantana camara. J. Chem. Ecol. 1989, 15, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Singh, M.; Dezman, D.J. Qualitative and quantitative characterization of phenolic compounds from lantana (Lantana camara) leaves. Weed Sci. 1989, 37, 302–307. [Google Scholar] [CrossRef]
- Kong, C.H.; Wang, P.; Zhang, C.X.; Zhang, M.X.; Hu, F. Herbicidal potential of allelochemicals from Lantana camara against Eichhornia crassipes and the alga Microcystis aeruginosa. Weed Res. 2006, 46, 290–295. [Google Scholar] [CrossRef]
- Barton, D.H.R.; de Mayo, P. Triterpenoids. Part XVI. Theconstitution of rehmannic acid. J. Chem. Soc. 1954, 900–903. [Google Scholar] [CrossRef]
- Hart, N.; Lamberton, J.; Sioumis, A.; Suares, H. New triterpenes of Lantana camara. A comparative study of the constituents of several taxa. Aust. J. Chem. 1976, 29, 655–671. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Lantana camara L. (Verbenaceae). Fitoterapia 2000, 71, 467–486. [Google Scholar] [CrossRef]
- Qureshi, H.; Anwar, T.; Ali, Q.; Haider, M.Z.; Habib, N.; Fatima, S.; Waseem, M.; Bibi, Y.; Arshad, M.; Adkins, S.W. Isolation of natural herbicidal compound from Lantana camara. Int. J. Environ. Anal. Chem. 2021, 101, 631–638. [Google Scholar] [CrossRef]
- Passos, J.L.; Barbosa, L.C.A.; Demuner, J.A.; Alvarenga, E.S.; da Silva, C.M.; Barreto, R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules 2012, 17, 11447–11455. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Sharma, O.P.; Sharma, S.; Pattabhi, V.; Mahato, S.B.; Sharma, P.D. A review of the hepatotoxic plant Lantana camara. Crit. Rev. Toxicol. 2007, 37, 313–352. [Google Scholar] [CrossRef]
- Mishra, A. Allelopathic properties of Lantana camara. Int. Res. J. Basic Clin. Stud. 2015, 3, 13–28. [Google Scholar]
- Gentle, C.B.; Duggin, J.A. Allelopathy as a competitive strategy in persistent thickets of Lantana camara L. in three Australian forest communities. Plant Ecol. 1997, 132, 85–95. [Google Scholar] [CrossRef]
- Mutshekwa, T.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Macroinvertebrate colonisation associated with native and invasive leaf litter decomposition. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 32. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and plant invasions: Traditional, congeneric, and bio-geographical approaches. Biol. Inva. 2008, 10, 875–890. [Google Scholar]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principals and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74. [Google Scholar]
- Einhellig, F.A. Mode of action of allelochemical action of phenolic compounds. In Chemistry and Mode of Action of Allelochemicals; Macías, F.A., Galindo, J.C.G., Molino, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 217–238. [Google Scholar]
- Lockwood, J.L.; Simberloff, D.; McKinney, M.L.; Von Holle, B. How many, and which, plants will invade natural areas. Biol. Inva. 2001, 3, 1–8. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, M.; Chen, X.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar]
- Zhang, Q.; Zhang, Y.; Peng, S.; Zobel, K. Climate warming may facilitate invasion of the exotic shrub Lantana camara. PLoS ONE 2014, 9, e105500. [Google Scholar]

| Allelochemical | Chemical Class | Source | Target Plant Species | Inhibition | Reference |
|---|---|---|---|---|---|
| Caffeic acid, gentisic acid, p-hydroxybenzolic acid, vanillic acid, salicylic acid, ferulic acid, p-coumaric acid, methyl coumarin, α-resorcylic acid, β-resorcylic acid, vanillin, quercetin. | Phenolic | Leaf | Lolium multiflorum | Growth | [56] |
| Caffeic acid, gentisic acid, p-hydoxybenzoic acid, vanilic acid, salicylic acid, ferulic acid, p-coumaric acid, m-coumaric acid, o-coumaric acid, methyl coumarin, syringic acid, protocatechuic acid, t-cinnamic acid and vanillin | Phenolic | Leaf | Lemna minor | Growth | [57] |
| Lantadene A, lantadene B | Triterpene | Leaf, rhizosphere soil | Eichhornia crassipeas, Microcystis aeruginosa | Growth | [58] |
| Vitexin | Flavonoide | Leaf | Phalaris minor, Avena fatua, Chenopodium album, Rumex dentatus | Growth * | [62] |
| E-caryophyllene, bicyclogermacrene, α-humulene | Sesquterpene | Essential oil from leaf | Corynespora cassiicola | Growth ** | [63] |
| β-Caryophyllene, α-humulene, γ-muurolene, α-curcumene, β-curcumene, γ-curcumene | Sesquterpene | Essential oil from leaf | Portulaca oleracea | Growth ** | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an Invasive Plant. Plants 2021, 10, 1028. https://doi.org/10.3390/plants10051028
Kato-Noguchi H, Kurniadie D. Allelopathy of Lantana camara as an Invasive Plant. Plants. 2021; 10(5):1028. https://doi.org/10.3390/plants10051028
Chicago/Turabian StyleKato-Noguchi, Hisashi, and Denny Kurniadie. 2021. "Allelopathy of Lantana camara as an Invasive Plant" Plants 10, no. 5: 1028. https://doi.org/10.3390/plants10051028
APA StyleKato-Noguchi, H., & Kurniadie, D. (2021). Allelopathy of Lantana camara as an Invasive Plant. Plants, 10(5), 1028. https://doi.org/10.3390/plants10051028