A Phytomelatonin-Rich Extract Obtained from Selected Herbs with Application as Plant Growth Regulator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Proximate Analysis
2.4. Biochemical Analysis
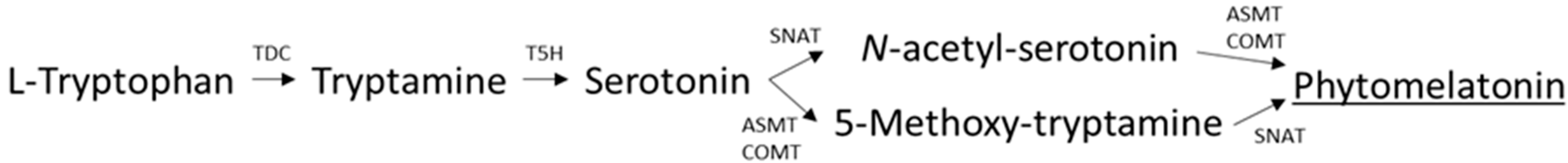
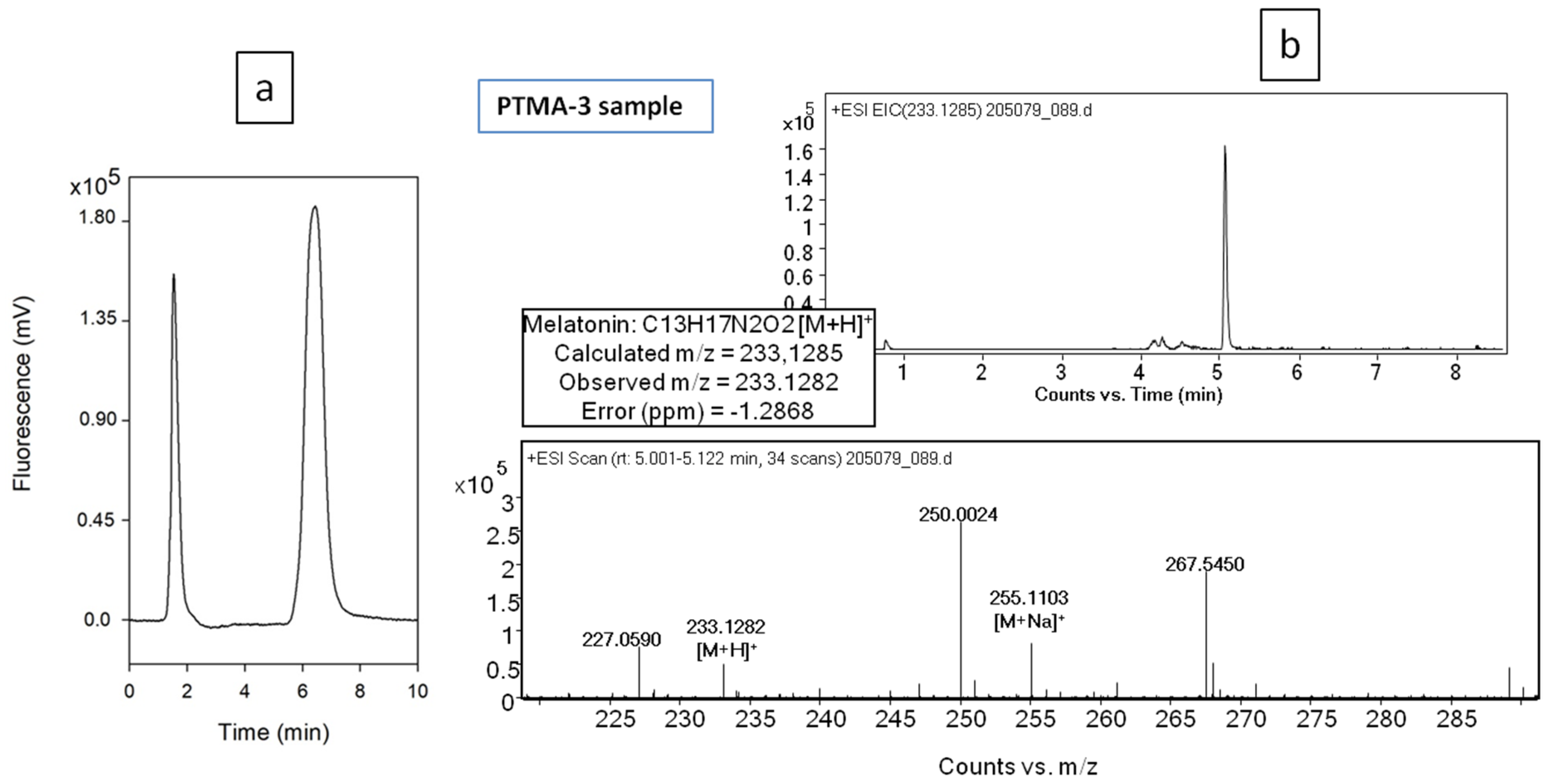
2.5. Phytomelatonin Analysis
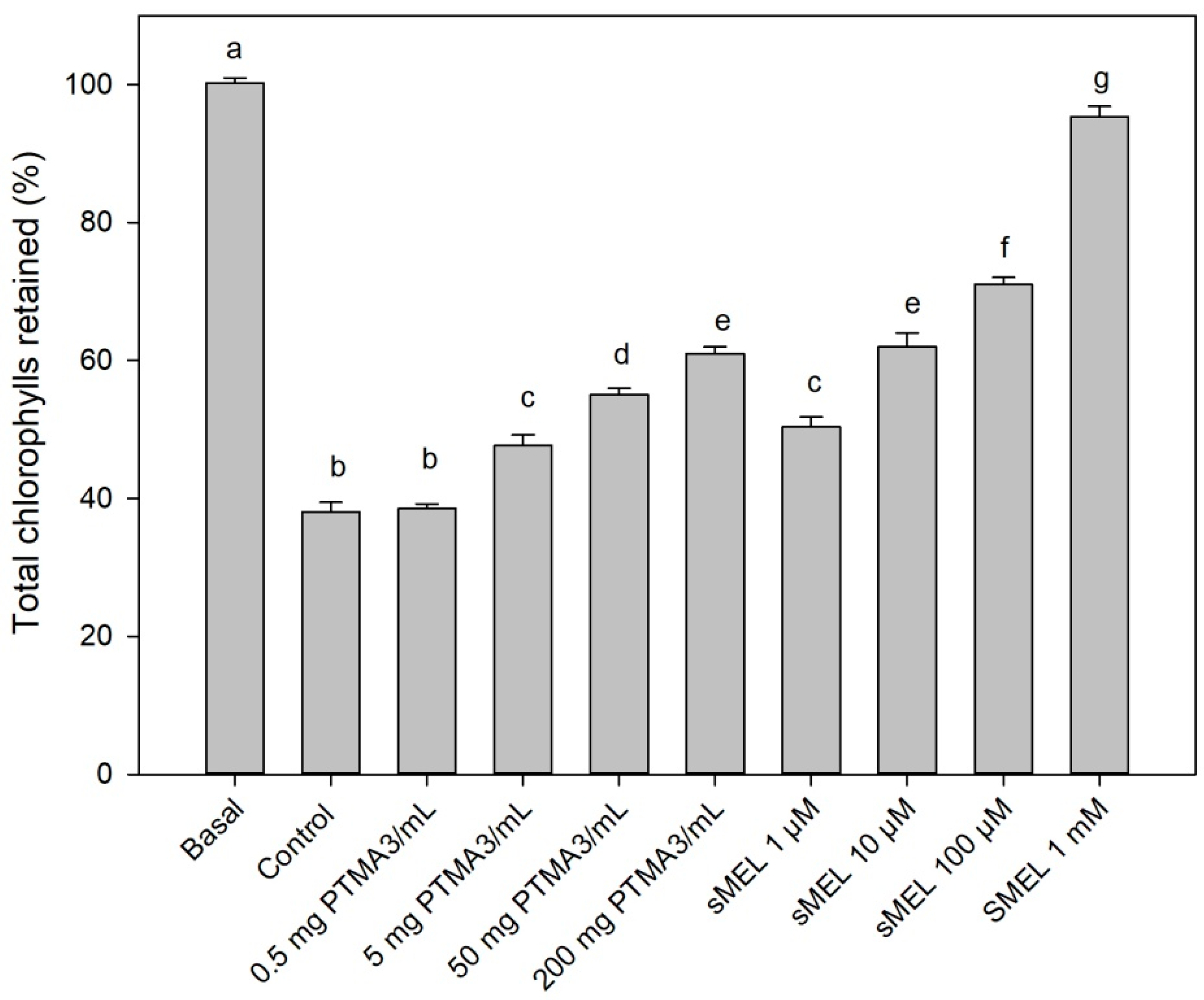
2.6. Biological Assay by Dark-Induced Senescence of Leaves
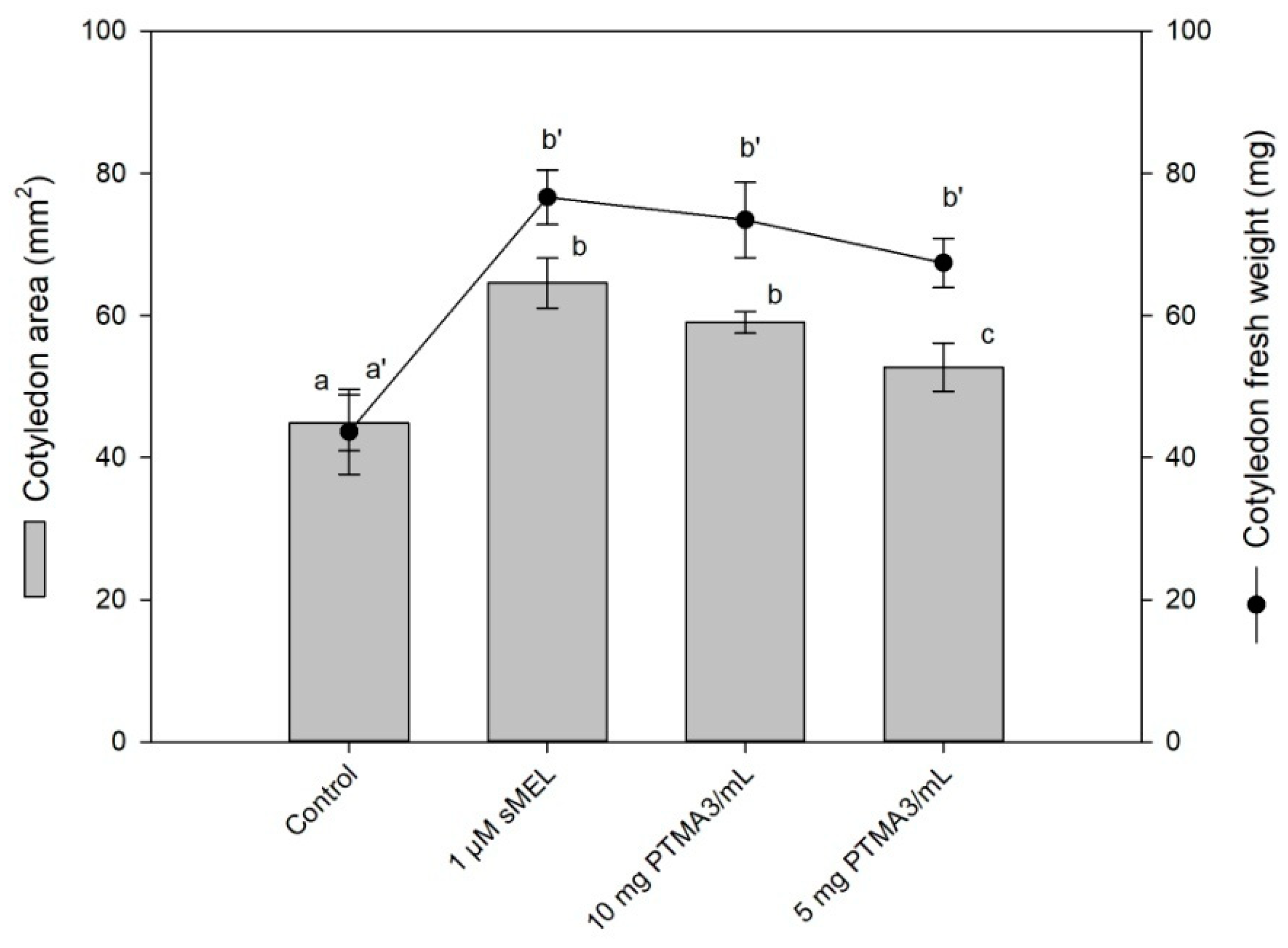
2.7. Biological Assay by Cotyledon Growth in Darkness
2.8. Statistical Analysis
3. Results and Discussion
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | ascorbic acid |
| COMT | caffeoyl-O-methyl transferase |
| DW | dry weight |
| ET | ethylene |
| FW | fresh weight |
| GAs | gibberellins |
| GSH | glutathione |
| IAA | indole-3-acetic acid |
| JA | jasmonic acid |
| NOS-like | nitric oxide synthase like-activity |
| NR | nitrate reductase |
| ORS | oleoresin |
| PMTR1 | phytomelatonin receptor |
| PTMA | phytomelatonin-rich extract Agro |
| RBOH | NADPH oxidase-dependent H2O2 production |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SAR | systemic acquired resistance |
| SHB | selected herbs |
| sMEL | synthetic melatonin |
| SNAT | serotonin N-acetyltransferase |
| T5H | tryptophan 5-hydroxylase |
| TDC | tryptophan decarboxylase |
References
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in higher plant determined by radioimmunoassay and liquid chromatography-mass spectrometry. Biol. Rhythm Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B. Phytomelatonin: Discovery, content, and role in plants. Adv. Bot. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin: Searching for plants with high levels as a natural source of nutraceu-ticals. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, F.R.S., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2015; Volume 46, pp. 519–545. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013, 138, 1212–1214. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth conditions determine different melatonin levels in Lupinus albus L. J. Pineal Res. 2013, 55, 149–155. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomol. 2019, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.; Sheteiwy, M.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef]
- Buttar, Z.; Wu, S.; Arnao, M.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Ruiz, J.H. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant. Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agron. 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. The multi-regulatory properties of melatonin in plants. In Neurotransmitters in Plants; Roshchina, V.V., Ramakrishna, A., Eds.; Taylor & Francis-CRC: New York, NY, USA, 2018; pp. 71–101. [Google Scholar]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 2019, 82, 25–34. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant. Sci. 2019, 10, 1388. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Role of melatonin to enhance phytoremediation capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant. Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant. Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Shahid, S.; Shakoor, A.; Sohail, H.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Prot. Pept. Sci. 2021, 22, 1–17. [Google Scholar]
- Menhas, S.; Yang, X.; Hayat, K.; Aftab, T.; Bundschuh, J.; Arnao, M.B.; Zhou, Y.; Zhou, P. Exogenous melatonin enhances Cd tolerance and phytoremediation efficiency by ameliorating Cd-induced stress in oilseed crops: A review. J. Plant. Growth Regul. 2021. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.-X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Li, D.-X.; Zhang, J.-R.; Shan, C.; Rengel, Z.; Song, Z.-B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, e12505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Sun, C.; Laborda, P.; He, Y.; Zhao, Y.; Li, Z.; Liu, F. Melatonin treatments reduce the pathogenicity and inhibit the growth of Xanthomonas oryzae pv. oryzicola. Plant. Pathol. 2018, 68, 288–296. [Google Scholar] [CrossRef]
- Chen, X.; Sun, C.; Laborda, P.; Zhao, Y.; Palmer, I.; Fu, Z.Q.; Qiu, J.; Liu, F. Melatonin treatment inhibits the growth of Xan-thomonas oryzae pv. oryzae. Front. Microbiol. 2018, 9, 2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehela, Y.; Killiny, N. Infection with phytopathogenic bacterium inhibits melatonin biosynthesis, decreases longevity of its vector, and suppresses the free radical-defense. J. Pineal Res. 2018, 65, e12511. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, Y.; Zeng, H.; Liu, G.; He, C.; Shi, H. RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J. Pineal Res. 2018, 64, e12454. [Google Scholar] [CrossRef]
- Hu, W.; Tie, W.; Ou, W.; Yan, Y.; Kong, H.; Zuo, J.; Ding, X.; Ding, Z.; Liu, Y.; Wu, C.; et al. Crosstalk between calcium and melatonin affects postharvest physiological deterioration and quality loss in cassava. Postharvest Biol. Technol. 2018, 140, 42–49. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Chang, Y.; Lin, D.; Reiter, R.J.; He, C.; Shi, H. Melatonin biosynthesis enzymes recruit WRKY transcription factors to regulate melatonin accumulation and transcriptional activity on W-box in cassava. J. Pineal Res. 2018, 65, e12487. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.; Wang, Q. Exogenous application of melatonin improves plant resistance to virus infection. Plant. Pathol. 2019, 68, 1287–1295. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Li, J.; Feng, C.; Cui, Z.; Zhao, L.; Wang, Q. Exogenous application of melatonin improves eradication of apple stem grooving virus from the infected in vitro shoots by shoot tip culture. Plant. Pathol. 2019, 68, 997–1006. [Google Scholar] [CrossRef]
- Lu, R.; Liu, Z.; Shao, Y.; Sun, F.; Zhang, Y.; Cui, J.; Zhou, Y.; Shen, W.; Zhou, T. Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol. J. 2019, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. The potential of phytomelatonin as a nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Ruiz, J.H. Phytomelatonin, natural melatonin from plants as a novel dietary supplement: Sources, activities and world market. J. Funct. Foods 2018, 48, 37–42. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Phyto-melatonin: A natural substance from plants with interesting nutraceutical properties. In Nutraceuticals: Prospects, Sources and Role in Health and Disease; Motohashi, N., Ed.; NOVA Science Publ.: New York, NY, USA, 2017; pp. 123–157. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant pro-tector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2006. [Google Scholar]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. Methods to measure the antioxidant activity in plant material. A comparative discussion. Free. Radic. Res. 1999, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. Hydrophilic and lipophilic antioxidant activity contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Assessment of different sample processing procedures applied to the determination of mel-atonin in plants. Phytochem. Anal. 2009, 20, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.H.; Arnao, M.B. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J. Agric. Food Chem. 2008, 56, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Lichtenhaler, K.; Wellburn, A. Determinations of total carotenoids and chlorophylls a and b in leaf extracts in different solvents. Biochem. Soc. Trans. 2019, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant. Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)/ trolox-equivalent antioxidant capacity) radical scavenging mixed-mode assay. In Measurement of Antioxidant Activity & Capacity. Recent Trends and Applica-tions; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons: Oxford, UK, 2018; pp. 117–139. [Google Scholar]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef]
- Ruiz, J.H.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]

| Components (in %, as Fresh Material) | SHB | PTMA1 | PTMA2 | PTMA3 |
|---|---|---|---|---|
| Moisture | 89.61 ± 4.36 | 25.50 ± 2.28 | 11.61 ± 0.89 | 10.22 ± 0.81 |
| Ash | 0.37 ± 0.02 | 2.18 ± 0.12 | Trace | Trace |
| Crude proteins | 3.50 ± 0.12 | 28.12 ± 2.11 | 5.72 ± 0.20 | 6.12 ± 0.40 |
| Crude fat | 0.55 ± 0.04 | 10.23 ± 1.09 | 54.84 ± 3.14 | 57.36 ± 3.82 |
| Dietary fiber | 2.34 ± 0.08 | 16.92 ± 1.58 | 0.97 ± 0.06 | 1.15 ± 0.07 |
| NFEM* (~carbohydrates) | 3.63 ± 0.22 | 17.05 ± 1.15 | 26.86 ± 0.41 | 25.15 ± 0.37 |
| Material | Phytomelatonin Content |
|---|---|
| SHB | 0.35 ± 0.02 µg/g DW |
| PTMA1 | 11.7 ± 0.9 µg/g ORS |
| PTMA2 | 35.0 ± 2.9 µg/g ORS |
| PTMA3 | 50.2 ± 3.7 µg/g ORS |
| Parameter | SHB | PTMA1 | PTMA2 | PTMA3 |
|---|---|---|---|---|
| Total phenolic content (TPC) (eq. gallic acid/g) | 243.1 ± 21.2 nmoles/g DW | 13.6 ± 1.1 µmoles/g ORS | 261.5 ± 21.7 µmoles/g ORS | 288.1 ± 23.0 µmoles/g ORS |
| Total flavonoid content (TFC) (eq. quercetin/g) | 56.5 ± 3.9 nmoles/g DW | 4.4 ± 0.3 µmoles/g ORS | 90.4 ± 8.1 µmoles/g ORS | 95.3 ± 7.4 µmoles/g ORS |
| Hydrophilic antioxidant activity (HAA) (eq. ascorbic acid/g) | 172.7 ± 9.6 nmoles/g DW | 9.3 ± 0.9 µmoles/g ORS | 152.6 ± 12.5 µmoles/g ORS | 162.7 ± 13.7 µmoles/g ORS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. A Phytomelatonin-Rich Extract Obtained from Selected Herbs with Application as Plant Growth Regulator. Plants 2021, 10, 2143. https://doi.org/10.3390/plants10102143
Hernández-Ruiz J, Cano A, Arnao MB. A Phytomelatonin-Rich Extract Obtained from Selected Herbs with Application as Plant Growth Regulator. Plants. 2021; 10(10):2143. https://doi.org/10.3390/plants10102143
Chicago/Turabian StyleHernández-Ruiz, Josefa, Antonio Cano, and Marino B. Arnao. 2021. "A Phytomelatonin-Rich Extract Obtained from Selected Herbs with Application as Plant Growth Regulator" Plants 10, no. 10: 2143. https://doi.org/10.3390/plants10102143
APA StyleHernández-Ruiz, J., Cano, A., & Arnao, M. B. (2021). A Phytomelatonin-Rich Extract Obtained from Selected Herbs with Application as Plant Growth Regulator. Plants, 10(10), 2143. https://doi.org/10.3390/plants10102143








