Emerging Role of Calycosin in Inflammatory Diseases: Molecular Mechanisms and Potential Therapeutic Applications
Abstract
1. Introduction
2. Emerging Roles and Underlying Molecular Mechanisms of Calycosin in Inflammatory Lesions and Diseases
2.1. Cardiovascular Diseases
2.1.1. Atherosclerosis
2.1.2. Heart Failure
2.1.3. Myocardial Infarction
2.1.4. Hypertension
2.2. Joint Diseases
2.2.1. Osteoarthritis
2.2.2. Gouty Arthritis
2.2.3. Rheumatoid Arthritis
2.3. Digestive System Diseases
2.3.1. Acute Pancreatitis
2.3.2. Acute Liver Failure
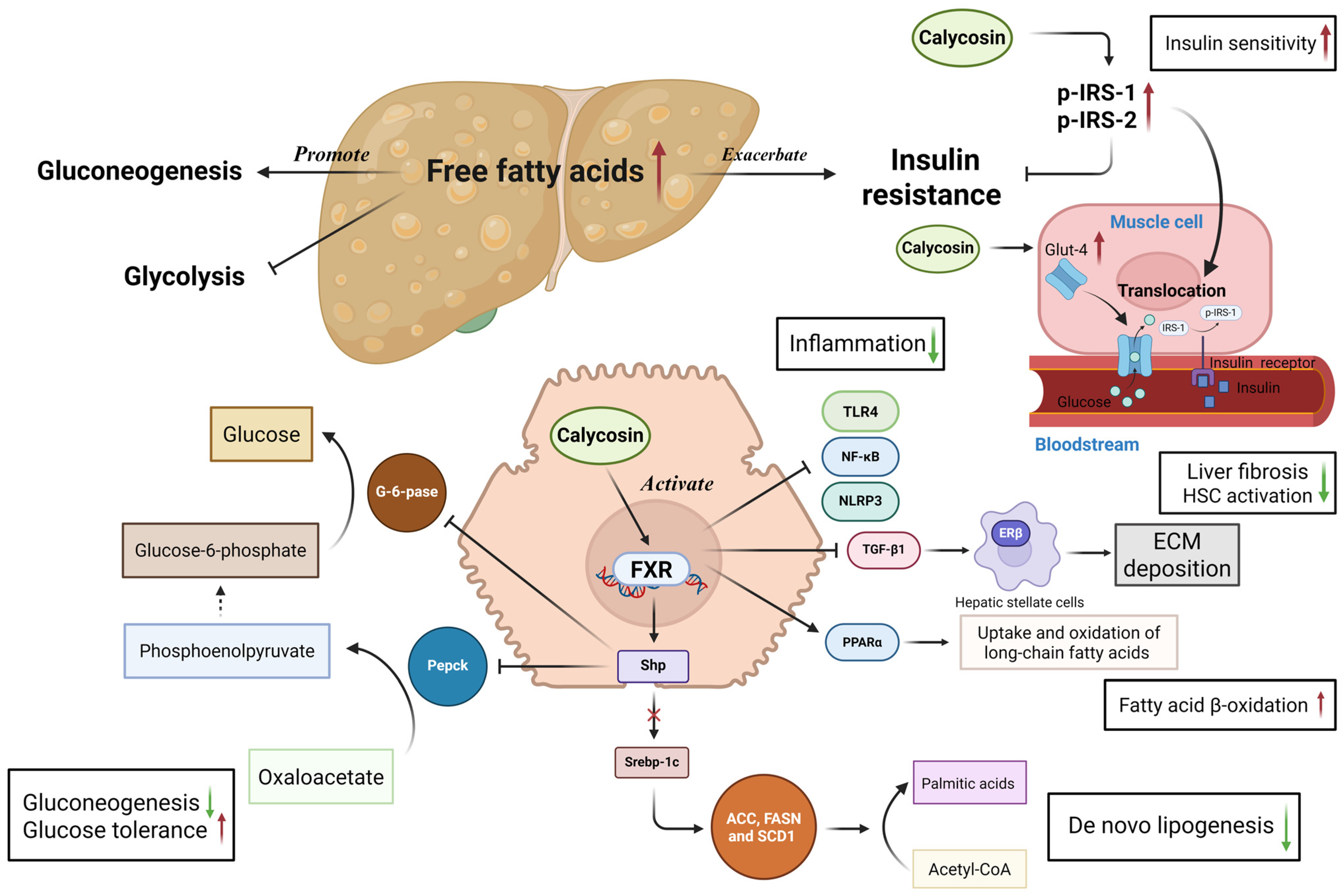
2.3.3. Non-Alcoholic Fatty Liver Disease
2.3.4. Diabetes Mellitus
2.3.5. Colitis
2.4. Urinary System Diseases
2.4.1. Diabetes-Induced Renal Inflammation
2.4.2. Renal Ischemia/Reperfusion Injury
2.4.3. Chronic Prostatitis
2.5. Nervous System Diseases
2.5.1. Intracerebral Hemorrhage Induced Brain Damage
2.5.2. Cerebral Ischemia Injury
2.6. Skin Diseases
2.6.1. Allergic Dermatitis
2.6.2. Atopic Dermatitis
2.7. Infectious Diseases
2.7.1. Sepsis-Induced Acute Lung Injury
2.7.2. Bacterial, Viral, and Parasitic Infections
2.8. Tumor and Cancer
2.8.1. Apoptosis Induction
2.8.2. Migration and Invasion Inhibition
2.8.3. Proliferation and Growth Inhibition
3. Application Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AGEs | Advanced glycation end products |
| Akt | Serine/threonine kinase |
| ALDH2 | Aldehyde dehydrogenase 2 |
| AMPK | Adenosine 5′-monophosphate (AMP)-activated protein kinase |
| ApoE−/− | Apolipoprotein E gene-deficient |
| ARE | Antioxidant response element |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| BATF | Basic leucine zipper ATF-like transcription factor |
| BDNF | Brain-derived neurotrophic factor |
| BKCa | Large-conductance calcium activated potassium channels |
| BMDMs | Bone marrow-derived macrophages |
| CA | Calycosin |
| CD31 | Cluster of differentiation 31 |
| CDK | Cyclin-dependent kinase |
| CM | Conditioned media |
| Col-2 | Type II collagen |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer |
| CREB | cAMP-response element binding protein |
| CXCL10 | Chemokine C-X-C chemokine ligand 10 |
| DARPP-32 | Dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa |
| db/+ | C57BL/KsJ-Lep |
| db/db | C57BL/KsJ |
| Drp1 | Dynamin-related protein 1 |
| DSS | Dextran sulfate sodium |
| ECT2 | Epithelial cell transforming sequence 2 |
| EGFR | Epidermal growth factor receptor |
| EGR1 | Early growth response 1 |
| EMT | Epithelial-mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ERα/β | Estrogen receptor α/β |
| ERβ | Estrogen receptor β |
| EWSAT1 | Ewing sarcoma-associated transcript 1 |
| FoxO1 | Forkhead box O1 |
| Foxp3 | Forkhead box P3 |
| FXR | Farnesoid X receptor |
| G-6-pase | Glucose-6-phosphatase |
| Glut-1 | Glucose transporter-1 |
| Glut-4 | Glucose transporter-4 |
| GPD1L | Glycerol-3-phosphate dehydrogenase 1 like |
| GPR30 | G-protein coupled estrogen receptor 30 |
| GSH | Glutathione |
| HIF-1α | Hypoxia-inducible factor-1α |
| HMGB1 | High mobility group protein 1 |
| HO-1 | Heme oxygenase-1 |
| HOTAIR | HOX transcript antisense RNA |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IKK | IκB kinase |
| IL | Interleukin |
| IL-6R | Interleukin-6 receptor |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| KLF2 | Krüppel-like factor 2 |
| lncRNA | Long non-coding RNA |
| LPS | Lipopolysaccharide |
| LTB4DH | Leukotriene B4 12-hydroxydehydrogenase |
| MAPK | Mitogen-activated protein kinases |
| MCR-1 | Mobilized colistin resistance-1 |
| MDA | Malondialdehyde |
| miR | MicroRNA |
| MLKL | Mixed lineage kinase domain-like protein |
| MMP-9 | Matrix metalloproteinase-9 |
| MMPs | Matrix metalloproteinases |
| MNNG | N-methyl-N′-nitro-N-nitrosoguanidine |
| MPO | Myeloperoxidase |
| MSU | Monosodium urate |
| mTOR | Mammalian target of rapamycin |
| MyD88 | Myeloid differentiation factor 88 |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NBR1 | Neighbor of BRCA1 gene 1 |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | NOD-like receptor 3 |
| NO | Nitric oxide |
| NQO1 | NAD(P)H dehydrogenase quinone 1 |
| NRF1 | Nuclear respiratory factor-1 |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| Pepck | Phosphoenolpyruvate carboxykinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| PI3K | Phosphatidylinositol three kinase |
| PKC-α | Protein kinase C-α |
| PLGC | Precancerous lesions of gastric carcinoma |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PTEN | Phosphatase and tensin homolog |
| RA | Radix astragali |
| Rab27B | Member RAS oncogene family |
| RASD1 | Ras-related protein 1 |
| RNF38 | Ring finger protein 38 |
| ROC | Receptor-operated calcium channel |
| ROCK2 | Rho-associated coiled-coil-containing protein kinase 2 |
| ROS | Reactive oxygen species |
| S1P | Sphingosine1-phosphate |
| S1PR1 | Sphingosine-1-phosphate receptor 1 |
| SESN2 | Sestrin2 |
| Shp | Small heterodimer partner |
| SHP-1 | SH2-containing protein tyrosine phosphatase 1 |
| Sirt1 | Sirtuin 1 |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| SOD | Superoxide dismutase |
| Sox-9 | SRY-Box Transcription Factor 9 |
| ST2 | Growth stimulation expressed gene 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF | Transforming growth factor |
| TGFBR1 | Transforming growth factor-beta receptor 1 |
| Th | T helper |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor α |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| Treg | Regulatory T |
| TrkB | Tropomyosin-related kinase B |
| TRPC6 | Transient receptor potential canonical 6 |
| VEGF | Vascular endothelial growth factor |
| VOC | Voltage-operated calcium channel |
| α-SMA | α-smooth muscle actin |
References
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-Inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Chan, J.Y.; Cheng, L.; Lau, C.P.; Han, S.Q.; Leung, P.C.; Fung, K.P.; Lau, C.B. Isolation of anti-inflammatory fractions and compounds from the root of Astragalus membranaceus. Phytother. Res. 2013, 27, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF-kappaB). Planta Medica 2003, 69, 973–983. [Google Scholar] [CrossRef]
- Zeng, X.; Deng, X.; Ni, Y.; Bi, H.; Jiang, M.; Wang, D.; Dong, P.; Xiao, Y.; Jiang, M. LPS inhibits TRIM65 expression in macrophages and C57BL/6J mouse by activating the ERK1/2 signaling pathway. Exp. Ther. Med. 2023, 25, 188. [Google Scholar] [CrossRef]
- An, Y.; Ni, Y.; Xu, Z.; Shi, S.; He, J.; Liu, Y.; Deng, K.Y.; Fu, M.; Jiang, M.; Xin, H.B. TRIM59 expression is regulated by Sp1 and Nrf1 in LPS-activated macrophages through JNK signaling pathway. Cell Signal. 2020, 67, 109522. [Google Scholar] [CrossRef]
- Ke, J.; Li, M.T.; Xu, S.; Ma, J.; Liu, M.Y.; Han, Y. Advances for pharmacological activities of Polygonum cuspidatum—A review. Pharm. Biol. 2023, 61, 177–188. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and Pharmacological Research in Agrimonia eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Li, K.; Cao, Y.X.; Jiao, S.M.; Du, G.H.; Du, Y.G.; Qin, X.M. Structural Characterization and Immune Activity Screening of Polysaccharides with Different Molecular Weights from Astragali Radix. Front. Pharmacol. 2020, 11, 582091. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hasiqiqige; Huan, Y.; Wang, X.; Tao, M.; Jiang, T.; Xie, H.; Jisiguleng, W.; Xing, W.; Zhu, Z.; et al. Calycosin ameliorates spinal cord injury by targeting Hsp90 to inhibit oxidative stress and apoptosis of nerve cells. J. Chem. Neuroanat. 2023, 127, 102190. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.F.; Wei, W.S.; Zhang, J.; Li, Z.Z. The cardiovascular protective effect and mechanism of calycosin and its derivatives. Chin. J. Nat. Med. 2020, 18, 907–915. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.J.; Chen, T.; Zhao, D. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm. Biol. 2014, 52, 1217–1222. [Google Scholar] [CrossRef]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Bugger, H.; Zirlik, A. Anti-Inflammatory Strategies in Atherosclerosis. Hämostaseologie 2021, 41, 433–442. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, H.Q.; Hao, Y.M.; Ren, Z.; Qu, S.L.; Liu, L.S.; Wei, D.H.; Tang, Z.H.; Zhang, J.F.; Jiang, Z.S. Macrophage polarization in atherosclerosis. Clin. Chim. Acta 2020, 501, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bi, H.; Jiang, M.; Chen, Y.; Jiang, M. An update on the role of TRIM/NLRP3 signaling pathway in atherosclerosis. Biomed. Pharmacother. 2023, 160, 114321. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C. Inflammation Resolution: Implications for Atherosclerosis. Circ. Res. 2022, 130, 130–148. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Ma, C.; Wu, H.; Yang, G.; Xiang, J.; Feng, K.; Zhang, J.; Hua, Y.; Kang, L.; Fan, G.; Yang, S. Calycosin ameliorates atherosclerosis by enhancing autophagy via regulating the interaction between KLF2 and MLKL in apolipoprotein E gene-deleted mice. Br. J. Pharmacol. 2021, 179, 252–269. [Google Scholar] [CrossRef]
- Han, F.; Li, K.; Pan, R.; Xu, W.; Han, X.; Hou, N.; Sun, X. Calycosin directly improves perivascular adipose tissue dysfunction by upregulating the adiponectin/AMPK/eNOS pathway in obese mice. Food Funct. 2018, 9, 2409–2415. [Google Scholar] [CrossRef]
- Tong, W.; Leng, L.; Wang, Y.; Guo, J.; Owusu, F.B.; Zhang, Y.; Wang, F.; Li, R.; Li, Y.; Chang, Y.; et al. Buyang huanwu decoction inhibits diabetes-accelerated atherosclerosis via reduction of AMPK-Drp1-mitochondrial fission axis. J. Ethnopharmacol. 2023, 312, 116432. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Jiang, W.; Wang, Z. Molecular Mechanism of Calycosin Inhibited Vascular Calcification. Nutrients 2023, 16, 99. [Google Scholar] [CrossRef]
- Baman, J.R.; Ahmad, F.S. Heart Failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef]
- Wang, X.; Guo, D.; Li, W.; Zhang, Q.; Jiang, Y.; Wang, Q.; Li, C.; Qiu, Q.; Wang, Y. Danshen (Salvia miltiorrhiza) restricts MD2/TLR4-MyD88 complex formation and signalling in acute myocardial infarction-induced heart failure. J. Cell. Mol. Med. 2020, 24, 10677–10692. [Google Scholar] [CrossRef]
- Bacmeister, L.; Schwarzl, M.; Warnke, S.; Stoffers, B.; Blankenberg, S.; Westermann, D.; Lindner, D. Inflammation and fibrosis in murine models of heart failure. Basic. Res. Cardiol. 2019, 114, 19. [Google Scholar] [CrossRef]
- Rhee, A.J.; Lavine, K.J. New Approaches to Target Inflammation in Heart Failure: Harnessing Insights from Studies of Immune Cell Diversity. Annu. Rev. Physiol. 2020, 82, 1–20. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhang, Y.; Sun, Q.; Cao, J.; Tan, N.; Yang, S.; Lu, L.; Zhang, Q.; Wei, P.; et al. Calycosin as a Novel PI3K Activator Reduces Inflammation and Fibrosis in Heart Failure Through AKT–IKK/STAT3 Axis. Front. Pharmacol. 2022, 13, 828061. [Google Scholar] [CrossRef]
- Li, Y.; Xia, J.; Jiang, N.; Xian, Y.; Ju, H.; Wei, Y.; Zhang, X. Corin protects H2O2-induced apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes. Biomed. Pharmacother. 2018, 97, 594–599. [Google Scholar] [CrossRef]
- Chen, G.; Xu, H.; Xu, T.; Ding, W.; Zhang, G.; Hua, Y.; Wu, Y.; Han, X.; Xie, L.; Liu, B.; et al. Calycosin reduces myocardial fibrosis and improves cardiac function in post-myocardial infarction mice by suppressing TGFBR1 signaling pathways. Phytomedicine 2022, 104, 154277. [Google Scholar] [CrossRef]
- Lu, X.; Lu, L.; Gao, L.; Wang, Y.; Wang, W. Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomed. Pharmacother. 2021, 137, 111375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, C.; Jiao, H.C.; Zhang, Q.; Jiang, Y.H.; Cui, J.; Liu, Y.; Jiang, Y.H.; Zhang, J.; Yang, M.Q.; et al. Calycosin Alleviates Doxorubicin-Induced Cardiotoxicity and Pyroptosis by Inhibiting NLRP3 Inflammasome Activation. Oxidative Med. Cell. Longev. 2022, 2022, 1733834. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Tao, L.; Zhang, S.; Gao, H.; Zhang, Y.; Sun, J.; Song, Y.; Qu, X. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother. Res. 2020, 34, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Natural compounds against cytotoxic drug-induced cardiotoxicity: A review on the involvement of PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22683. [Google Scholar] [CrossRef]
- Qi, X.M.; Qiao, Y.B.; Zhang, Y.L.; Wang, A.C.; Ren, J.H.; Wei, H.Z.; Li, Q.S. PGC-1α/NRF1-dependent cardiac mitochondrial biogenesis: A druggable pathway of calycosin against triptolide cardiotoxicity. Food Chem. Toxicol. 2023, 171, 113513. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, A. Telomeres, oxidative stress, and myocardial infarction. Eur. Heart J. 2017, 38, 3105–3107. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.J.; Chen, G.H.; Deng, S.H.; Zeng, K.F.; Lin, K.L.; Deng, B.; Zhang, S.W.; Tan, Z.B.; Xu, Y.C.; Chen, S.; et al. Calycosin protects against oxidative stress-induced cardiomyocyte apoptosis by activating aldehyde dehydrogenase 2. Phytother. Res. 2023, 37, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Liu, W.; Liu, N.; Fu, X.; Kwan, H.; Liu, S.; Liu, B.; Zhang, S.; Yu, Z.; et al. Calycosin inhibits oxidative stress-induced cardiomyocyte apoptosis via activating estrogen receptor-α/β. Bioorganic Med. Chem. Lett. 2016, 26, 181–185. [Google Scholar] [CrossRef]
- Junqing, G.; Tao, C.; Huigen, J.; Zongjun, L.; Deqiang, Z. Effect of calycosin on left ventricular ejection fraction and angiogenesis in rat models with myocardial infarction. J. Tradit. Chin. Med. 2015, 35, 160–167. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, J.; Tse, H.F.; Le, X.C.; Rong, J. Plant Natural Products Calycosin and Gallic Acid Synergistically Attenuate Neutrophil Infiltration and Subsequent Injury in Isoproterenol-Induced Myocardial Infarction: A Possible Role for Leukotriene B4 12-Hydroxydehydrogenase? Oxidative Med. Cell. Longev. 2015, 2015, 434052. [Google Scholar] [CrossRef]
- Yokomizo, T.; Izumi, T.; Shimizu, T. Leukotriene B4: Metabolism and signal transduction. Arch. Biochem. Biophys. 2001, 385, 231–241. [Google Scholar] [CrossRef]
- Yan, J.; Guo, J.; Wang, Y.; Xing, X.; Zhang, X.; Zhang, G.; Dong, Z. Acute myocardial infarction therapy using calycosin and tanshinone co-loaded mitochondria targeted lipid-polymer hybrid nano-system: Preparation, characterization, and anti myocardial infarction activity assessment. Biomed. Pharmacother. 2022, 155, 113650. [Google Scholar] [CrossRef]
- Yan, J.; Guo, J.; Wang, Y.; Xing, X.; Zhang, X.; Zhang, G.; Dong, Z. Acute myocardial infarction therapy using calycosin and tanshinone co-loaded; mitochondrion-targeted tetrapeptide and cyclic arginyl-glycyl-aspartic acid peptide co-modified lipid-polymer hybrid nano-system: Preparation, characterization, and anti myocardial infarction activity assessment. Drug Deliv. 2022, 29, 2815–2823. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Elliott, W.J. Systemic hypertension. Curr. Probl. Cardiol. 2007, 32, 201–259. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, Y.Y.; Cheng, J.; Zhao, Y.Y. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacol. Sin. 2006, 27, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Vong, C.T.; Leung, G.P.; Seto, S.W.; Kwan, Y.W.; Lee, S.M.; Hoi, M.P. Calycosin and Formononetin Induce Endothelium-Dependent Vasodilation by the Activation of Large-Conductance Ca2+-Activated K+ Channels (BKCa). Evid.-Based Complement. Altern. Med. 2016, 2016, 5272531. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, W.; Zhong, X.; Xu, J.; Huang, H.; Zheng, X.; Zhang, J.; Yang, S.; Shang, P.; Tang, Q.; et al. Arctigenin prevents the progression of osteoarthritis by targeting PI3K/Akt/NF-κB axis: In vitro and in vivo studies. J. Cell. Mol. Med. 2020, 24, 4183–4193. [Google Scholar] [CrossRef]
- Jiang, R.H.; Xu, J.J.; Zhu, D.C.; Li, J.F.; Zhang, C.X.; Lin, N.; Gao, W.Y. Glycyrrhizin inhibits osteoarthritis development through suppressing the PI3K/AKT/NF-κB signaling pathway in vivo and in vitro. Food Funct. 2020, 11, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ni, B.; Mao, Z.; Xi, Y.; Chu, X.; Zhang, R.; Ma, X.; You, H. NOV/CCN3 induces cartilage protection by inhibiting PI3K/AKT/mTOR pathway. J. Cell. Mol. Med. 2019, 23, 7525–7534. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jie, L.; Wu, P.; Zhang, N.; Mao, J.; Wang, P.; Yin, S. Calycosin mitigates chondrocyte inflammation and apoptosis by inhibiting the PI3K/AKT and NF-κB pathways. J. Ethnopharmacol. 2022, 297, 115536. [Google Scholar] [CrossRef]
- Guo, X.; Pan, X.; Wu, J.; Li, Y.; Nie, N. Calycosin prevents IL-1β-induced articular chondrocyte damage in osteoarthritis through regulating the PI3K/AKT/FoxO1 pathway. In Vitro Cell. Dev. Biol.-Anim. 2022, 58, 491–502. [Google Scholar] [CrossRef]
- Su, H.; Yan, Q.; Du, W.; Hu, E.; Yang, Z.; Zhang, W.; Li, Y.; Tang, T.; Zhao, S.; Wang, Y. Calycosin ameliorates osteoarthritis by regulating the imbalance between chondrocyte synthesis and catabolism. BMC Complement. Med. Ther. 2024, 24, 48. [Google Scholar] [CrossRef]
- Wilson, L.; Saseen, J.J. Gouty Arthritis: A Review of Acute Management and Prevention. Pharmacotherapy 2016, 36, 906–922. [Google Scholar] [CrossRef]
- Keller, S.F.; Mandell, B.F. Management and Cure of Gouty Arthritis. Med. Clin. N. Am. 2021, 105, 297–310. [Google Scholar] [CrossRef]
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, D.; Xiang, L.; Xie, B.; Wang, B.; Li, Y.; Liu, X. Calycosin represses AIM2 inflammasome-mediated inflammation and pyroptosis to attenuate monosodium urate-induced gouty arthritis through NF-κB and p62-Keap1 pathways. Drug Dev. Res. 2022, 83, 1654–1672. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Zhao, Y.; Xiao, J.; Lu, Y.; Shi, Q.; Wang, Y.; Wang, H.; Liang, Q. Polyphyllin I Ameliorates Collagen-Induced Arthritis by Suppressing the Inflammation Response in Macrophages Through the NF-κB Pathway. Front. Immunol. 2018, 9, 2091. [Google Scholar] [CrossRef]
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Huang, Q.; Chen, J.; Wang, M.; Pan, H.; Wang, R.; Zhou, H.; Zhou, Z.; Liu, J.; Yang, F.; et al. Calycosin suppresses expression of pro-inflammatory cytokines via the activation of p62/Nrf2-linked heme oxygenase 1 in rheumatoid arthritis synovial fibroblasts. Pharmacol. Res. 2016, 113, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Han, H.; Yang, C.; Hou, T.; Jiao, D.; Wang, T.; Zhao, Y.; Wang, Y.; Xu, H.; et al. Calycosin ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages via the JNK and NF-κB pathway. J. Funct. Foods 2023, 110, 105843. [Google Scholar] [CrossRef]
- Lee, W.Y.; Chen, H.Y.; Chen, K.C.; Chen, C.Y. Treatment of rheumatoid arthritis with traditional chinese medicine. Biomed. Res. Int. 2014, 2014, 528018. [Google Scholar] [CrossRef]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef]
- Ma, R.; Yuan, F.; Wang, S.; Liu, Y.; Fan, T.; Wang, F. Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-κB signal pathways in mice. Biomed. Pharmacother. 2018, 105, 599–605. [Google Scholar] [CrossRef]
- Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Samuel, D.; Simpson, K.J.; Yaron, I.; Bernardi, M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, B.; Zaldana, F.; Liu, C. The Pathology of Acute Liver Failure. Clin. Liver Dis. 2018, 22, 257–268. [Google Scholar] [CrossRef]
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef]
- Chen, X.; Meng, Q.; Wang, C.; Liu, Q.; Sun, H.; Huo, X.; Sun, P.; Yang, X.; Peng, J.; Liu, K. Protective effects of calycosin against CCl4-induced liver injury with activation of FXR and STAT3 in mice. Pharm. Res. 2015, 32, 538–548. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A. Non-alcoholic fatty liver disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef]
- Adorini, L.; Trauner, M. FXR agonists in NASH treatment. J. Hepatol. 2023, 79, 1317–1331. [Google Scholar] [CrossRef]
- Duan, X.; Meng, Q.; Wang, C.; Liu, Z.; Sun, H.; Huo, X.; Sun, P.; Ma, X.; Peng, J.; Liu, K. Effects of calycosin against high-fat diet-induced nonalcoholic fatty liver disease in mice. J. Gastroenterol. Hepatol. 2018, 33, 533–542. [Google Scholar] [CrossRef]
- Duan, X.; Meng, Q.; Wang, C.; Liu, Z.; Liu, Q.; Sun, H.; Sun, P.; Yang, X.; Huo, X.; Peng, J.; et al. Calycosin attenuates triglyceride accumulation and hepatic fibrosis in murine model of non-alcoholic steatohepatitis via activating farnesoid X receptor. Phytomedicine 2017, 25, 83–92. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Zhu, G.; Sun, C.; Wang, J. Hepatoprotective effect and possible mechanism of phytoestrogen calycosin on carbon tetrachloride-induced liver fibrosis in mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 189–204. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Z.L.; Zhao, Q.; Zhao, Z.M.; Liu, C.H. A combination of astragaloside I, levistilide A and calycosin exerts anti-liver fibrosis effects in vitro and in vivo. Acta Pharmacol. Sin. 2018, 39, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Liu, J.; Zhang, M.; Wang, Y.; Zhu, G.; Wang, J. Inhibition effect of phytoestrogen calycosin on TGF-β(1)-induced hepatic stellate cell activation, proliferation, and migration via estrogen receptor β. Can. J. Physiol. Pharmacol. 2018, 96, 1268–1275. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhou, J.; Fang, H.; Wang, J. Overexpression of estrogen receptor β inhibits cellular functions of human hepatic stellate cells and promotes the anti-fibrosis effect of calycosin via inhibiting STAT3 phosphorylation. BMC Pharmacol. Toxicol. 2022, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, N.; Liu, H.; Huang, N.; Sun, X.; Zhang, G. Bioinformatic and biochemical findings disclosed anti-hepatic steatosis mechanism of calycosin. Bioorg. Chem. 2020, 100, 103914. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Waddell, T.; Banerjee, R.; Guess, N. Nutrition and Nonalcoholic Fatty Liver Disease: Current Perspectives. Gastroenterol. Clin. N. Am. 2020, 49, 63–94. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011, 34 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef]
- Brody, H. Diabetes. Nature 2012, 485, S1. [Google Scholar] [CrossRef]
- Thornalley, P.J. Dietary AGEs and ALEs and risk to human health by their interaction with the receptor for advanced glycation endproducts (RAGE)—An introduction. Mol. Nutr. Food Res. 2007, 51, 1107–1110. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, J.; Zhao, Y.; He, B.; Zheng, Z.; Chu, G.; Zhu, Q. Calycosin Rebalances Advanced Glycation End Products-Induced Glucose Uptake Dysfunction of Hepatocyte In Vitro. Am. J. Chin. Med. 2015, 43, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Xiong, J.; Wang, S.S.; Tang, D.; Wang, R.S.; Zhu, Q. Calycosin entered HUVECs and ameliorated AGEs-promoted cell apoptosis via the Bcl-2 pathway. J. Nat. Med. 2014, 68, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Roy, A.; Jung, H.A.; Jung, H.J.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory activities of Pueraria lobata root and its constituents. J. Ethnopharmacol. 2016, 194, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, B.; Li, Y.; Yu, S.; Wang, Y. The isoflavonoid calycosin inhibits inflammation and enhances beta cell function in gestational diabetes mellitus by suppressing RNF38 expression. Immunopharmacol. Immunotoxicol. 2020, 42, 366–372. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Thoreson, R.; Cullen, J.J. Pathophysiology of inflammatory bowel disease: An overview. Surg. Clin. N. Am. 2007, 87, 575–585. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Chao, L.; Zheng, P.; Xia, L.; Yong, Y.; Lu, G.; Tang, F.; Zhao, Z. Calycosin attenuates dextran sulfate sodium (DSS)-induced experimental colitis. Iran. J. Basic. Med. Sci. 2017, 20, 1056–1062. [Google Scholar] [CrossRef]
- Liu, J.; Deng, T.; Wang, Y.; Zhang, M.; Zhu, G.; Fang, H.; Wang, J. Calycosin Inhibits Intestinal Fibrosis on CCD-18Co Cells via Modulating Transforming Growth Factor-β/Smad Signaling Pathway. Pharmacology 2019, 104, 81–89. [Google Scholar] [CrossRef]
- Podolsky, D.K. Inflammatory bowel disease (1). N. Engl. J. Med. 1991, 325, 928–937. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Natarajan, R. Diabetic nephropathy--emerging epigenetic mechanisms. Nat. Rev. Nephrol. 2014, 10, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shi, X.; Zhao, M.; Ma, S.; Zhang, Y. Pharmacological potential of Astragali Radix for the treatment of kidney diseases. Phytomedicine 2024, 123, 155196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-y.; Tan, R.-z.; Zhang, X.-q.; Yu, Y.; Yu, C. Calycosin Ameliorates Diabetes-Induced Renal Inflammation via the NF-κB Pathway In Vitro and In Vivo. Med. Sci. Monit. 2019, 25, 1671–1678. [Google Scholar] [CrossRef]
- Huang, D.; Shen, P.; Wang, C.; Gao, J.; Ye, C.; Wu, F. Calycosin plays a protective role in diabetic kidney disease through the regulation of ferroptosis. Pharm. Biol. 2022, 60, 990–996. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Said, E.; Atef, H.; Zaitone, S.A. Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: Effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem.-Biol. Interact. 2020, 315, 108897. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Y.; Wu, Z.; Song, X.; Luo, J.; Yang, H.; Chen, X.; Liu, X. Effects of Huangqi Liuyi Decoction in the Treatment of Diabetic Nephropathy and Tissue Distribution Difference of Its Six Active Constituents Between Normal and Diabetic Nephropathy Mouse Models. Front. Pharmacol. 2022, 13, 934720. [Google Scholar] [CrossRef]
- Huang, C.; Xue, L.F.; Hu, B.; Liu, H.H.; Huang, S.B.; Khan, S.; Meng, Y. Calycosin-loaded nanoliposomes as potential nanoplatforms for treatment of diabetic nephropathy through regulation of mitochondrial respiratory function. J. Nanobiotechnol. 2021, 19, 178. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Câmara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Ko, D.S. Reducing Ischemia-Reperfusion Injury in Renal Transplantation. J. Urol. 2015, 194, 1531–1532. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef]
- Zhang, N.; Guan, C.; Liu, Z.; Li, C.; Yang, C.; Xu, L.; Niu, M.; Zhao, L.; Zhou, B.; Che, L.; et al. Calycosin attenuates renal ischemia/reperfusion injury by suppressing NF-κB mediated inflammation via PPARγ/EGR1 pathway. Front. Pharmacol. 2022, 13, 970616. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Luo, H.; Xu, H.; Qian, B.; Zou, X.; Zhang, G.; Zeng, F.; Zou, J. Preclinical models and evaluation criteria of prostatitis. Front. Immunol. 2023, 14, 1183895. [Google Scholar] [CrossRef] [PubMed]
- Pirola, G.M.; Verdacchi, T.; Rosadi, S.; Annino, F.; De Angelis, M. Chronic prostatitis: Current treatment options. Res. Rep. Urol. 2019, 11, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, L.; Liu, Z.; Xu, X.; Zhang, H.; Mao, P.; Li, M. Calycosin protects against chronic prostatitis in rats via inhibition of the p38MAPK/NF-κB pathway. Open Med. 2023, 18, 20230770. [Google Scholar] [CrossRef]
- Hostettler, I.C.; Seiffge, D.J.; Werring, D.J. Intracerebral hemorrhage: An update on diagnosis and treatment. Expert. Rev. Neurother. 2019, 19, 679–694. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- Chen, C.; Cui, J.; Ji, X.; Yao, L. Neuroprotective functions of calycosin against intracerebral hemorrhage-induced oxidative stress and neuroinflammation. Future Med. Chem. 2020, 12, 583–592. [Google Scholar] [CrossRef]
- Xu, S.; Huang, P.; Yang, J.; Du, H.; Wan, H.; He, Y. Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway. Phytomedicine 2023, 115, 154845. [Google Scholar] [CrossRef]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, A.; Zhao, Z.; Ren, B.; Gao, Z.; Fang, D.; Hei, B.; Sun, J.; Bao, X.; Ma, L.; et al. Epidemiology and future trend predictions of ischemic stroke based on the global burden of disease study 1990–2021. Commun. Med. 2025, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.; Lu, H.; Wang, W.; Cai, L.; Chen, J.; Wang, Y. Calycosin attenuates the inflammatory damage of microglia induced by oxygen and glucose deprivation through the HMGB1/TLR4/NF-κB signaling pathway. Acta Biochim. Biophys. Sin. 2023, 55, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Z.; Yan, M.; Zhang, Q.; Jiang, T.; Xue, J. Calycosin decreases cerebral ischemia/reperfusion injury by suppressing ACSL4-dependent ferroptosis. Arch. Biochem. Biophys. 2023, 734, 109488. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Q.; Zhang, X.; Lu, H.; Chen, J. Neuroprotective Mechanisms of Calycosin Against Focal Cerebral Ischemia and Reperfusion Injury in Rats. Cell. Physiol. Biochem. 2018, 45, 537–546. [Google Scholar] [CrossRef]
- Hsu, C.C.; Kuo, T.W.; Liu, W.P.; Chang, C.P.; Lin, H.J. Calycosin Preserves BDNF/TrkB Signaling and Reduces Post-Stroke Neurological Injury After Cerebral Ischemia by Reducing Accumulation of Hypertrophic and TNF-α-Containing Microglia in Rats. J. Neuroimmune Pharmacol. 2020, 15, 326–339. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Li, Z.; Wang, W.; Tian, J.; Chen, J. Downregulated RASD1 and upregulated miR-375 are involved in protective effects of calycosin on cerebral ischemia/reperfusion rats. J. Neurol. Sci. 2014, 339, 144–148. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Zeng, K.; Nie, C.; Huang, J.; Zhu, L.; Pei, D.; Zhang, Y. Study on the action mechanism of Buyang Huanwu Decoction against ischemic stroke based on S1P/S1PR1/PI3K/Akt signaling pathway. J. Ethnopharmacol. 2023, 312, 116471. [Google Scholar] [CrossRef]
- Guo, C.; Tong, L.; Xi, M.; Yang, H.; Dong, H.; Wen, A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 2012, 144, 768–774. [Google Scholar] [CrossRef]
- Guo, C.; Ma, Y.; Ma, S.; Mu, F.; Deng, J.; Duan, J.; Xiong, L.; Yin, Y.; Wang, Y.; Xi, M.; et al. The Role of TRPC6 in the Neuroprotection of Calycosin Against Cerebral Ischemic Injury. Sci. Rep. 2017, 7, 3039. [Google Scholar] [CrossRef]
- Zhang, W.W.; Xu, F.; Wang, D.; Ye, J.; Cai, S.Q. Buyang Huanwu Decoction ameliorates ischemic stroke by modulating multiple targets with multiple components: In vitro evidences. Chin. J. Nat. Med. 2018, 16, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Day, C.H.; Kuo, C.H.; Wang, T.F.; Ho, T.J.; Lai, P.F.; Chen, R.J.; Yao, C.H.; Viswanadha, V.P.; Kuo, W.W.; et al. Calycosin alleviates H2O2-induced astrocyte injury by restricting oxidative stress through the Akt/Nrf2/HO-1 signaling pathway. Environ. Toxicol. 2022, 37, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, X.; Wang, X.; Wei, P.; Li, L.; Wu, P.; Hong, M. Calycosin alleviates allergic contact dermatitis by repairing epithelial tight junctions via down-regulating HIF-1α. J. Cell. Mol. Med. 2018, 22, 4507–4521. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; DeLeo, V.A. Allergic Contact Dermatitis. JAMA Dermatol. 2021, 157, 364. [Google Scholar] [CrossRef]
- Shen, D.; Xie, X.; Zhu, Z.; Yu, X.; Liu, H.; Wang, H.; Fan, H.; Wang, D.; Jiang, G.; Hong, M. Screening active components from Yu-ping-feng-san for regulating initiative key factors in allergic sensitization. PLoS ONE 2014, 9, e107279. [Google Scholar] [CrossRef]
- Yuan, W.Y.; Li, L.Q.; Chen, Y.Y.; Zhou, Y.J.; Bao, K.F.; Zheng, J.; Hua, Y.Q.; Jiang, G.R.; Hong, M. Frontline Science: Two flavonoid compounds attenuate allergic asthma by regulating epithelial barrier via G protein-coupled estrogen receptor: Probing a possible target for allergic inflammation. J. Leukoc. Biol. 2020, 108, 59–71. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Wang, X.; Wang, C.; Bao, K.; Ji, L.; Jiang, G.; Hong, M. Calycosin Suppresses Epithelial Derived Initiative Key Factors and Maintains Epithelial Barrier in Allergic Inflammation via TLR4 Mediated NF-κB Pathway. Cell. Physiol. Biochem. 2017, 44, 1106–1119. [Google Scholar] [CrossRef]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Deng, G.; Tian, N.; Wang, H.; Zhao, H.; Kuai, L.; Luo, Y.; Gao, C.; Ding, X.; Li, B.; et al. Calycosin enhances Treg differentiation for alleviating skin inflammation in atopic dermatitis. J. Ethnopharmacol. 2024, 326, 117883. [Google Scholar] [CrossRef] [PubMed]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, Y.M. HMGB1: LPS Delivery Vehicle for Caspase-11-Mediated Pyroptosis. Immunity 2018, 49, 582–584. [Google Scholar] [CrossRef]
- Chen, G.; Hou, Y.; Li, X.; Pan, R.; Zhao, D. Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and NLRP3 inflammasome. Int. Immunopharmacol. 2021, 96, 107623. [Google Scholar] [CrossRef]
- Zhu, C.J.; Yang, W.G.; Li, D.J.; Song, Y.D.; Chen, S.Y.; Wang, Q.F.; Liu, Y.N.; Zhang, Y.; Cheng, B.; Wu, Z.W.; et al. Calycosin attenuates severe acute pancreatitis-associated acute lung injury by curtailing high mobility group box 1-induced inflammation. World J. Gastroenterol. 2021, 27, 7669–7686. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, Y.; Sun, Y.; Hong, X.; Tang, Y.; Yu, J.; Hu, H.; Ma, W.; Qin, K.; Bao, R. Calycosin Alleviates Sepsis-Induced Acute Lung Injury via the Inhibition of Mitochondrial ROS-Mediated Inflammasome Activation. Front. Pharmacol. 2021, 12, 690549. [Google Scholar] [CrossRef]
- Yao, J.; Cheng, M.; Yang, F. Calycosin Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Mice through the miR-375-3p/ROCK2 Axis. J. Investig. Surg. 2023, 36, 2211166. [Google Scholar] [CrossRef]
- Bednash, J.S.; Mallampalli, R.K. Regulation of inflammasomes by ubiquitination. Cell. Mol. Immunol. 2016, 13, 722–728. [Google Scholar] [CrossRef]
- van Tong, H.; Brindley, P.J.; Meyer, C.G.; Velavan, T.P. Parasite Infection, Carcinogenesis and Human Malignancy. EBioMedicine 2017, 15, 12–23. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Deng, X.; Lv, X.; Wang, J. Calycosin enhances the bactericidal efficacy of polymyxin B by inhibiting MCR-1 in vitro. J. Appl. Microbiol. 2020, 129, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J.; Kong, L.; Nurahmat, M.; Chen, M.; Luo, Q.; Li, B.; Wu, X.; Dong, J. BuShenYiQi Formula strengthens Th1 response and suppresses Th2-Th17 responses in RSV-induced asthma exacerbated mice. J. Ethnopharmacol. 2014, 154, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Pien, F.D.; Pien, B.C. Angiostrongylus cantonensis eosinophilic meningitis. Int. J. Infect. Dis. 1999, 3, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-Y.; Chen, K.-M.; Kuo, W.-W.; Lai, S.-C.; Ho, T.-J.; Lai, P.-T.; Huang, C.-Y.; Wang, T.-F. Calycosin attenuates Angiostrongylus cantonensis-induced parasitic meningitis through modulation of HO-1 and NF-κB activation. Parasitology 2022, 150, 311–320. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 2019, 19, 197–214. [Google Scholar] [CrossRef]
- El-Kott, A.F.; Al-Kahtani, M.A.; Shati, A.A. Calycosin induces apoptosis in adenocarcinoma HT29 cells by inducing cytotoxic autophagy mediated by SIRT1/AMPK-induced inhibition of Akt/mTOR. Clin. Exp. Pharmacol. Physiol. 2019, 46, 944–954. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Ren, Q.; Tian, J.; Chen, J. Calycosin induces apoptosis in colorectal cancer cells, through modulating the ERβ/MiR-95 and IGF-1R, PI3K/Akt signaling pathways. Gene 2016, 591, 123–128. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, X.; Li, X.; Wu, Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015, 6, 3091–3097. [Google Scholar] [CrossRef]
- Chen, J.; Lin, C.; Yong, W.; Ye, Y.; Huang, Z. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell. Physiol. Biochem. 2015, 35, 722–728. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Z.W.; Yuan, B.M.; Bao, Y.G. Calycosin induces apoptosis in osteosarcoma cell line via ERβ-mediated PI3K/Akt signaling pathways. Mol. Med. Rep. 2020, 21, 2349–2356. [Google Scholar] [CrossRef]
- Sun, H.; Yin, M.; Qian, W.; Yin, H. Calycosin, a Phytoestrogen Isoflavone, Induces Apoptosis of Estrogen Receptor-Positive MG-63 Osteosarcoma Cells via the Phosphatidylinositol 3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway. Med. Sci. Monit. 2018, 24, 6178–6186. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Ma, G.; Li, X.; Shi, Q.; Li, X.; Zhou, X.; Tang, Y.; Xie, Z.; Liao, S.; Qin, Y.; et al. Clinical case report of patients with osteosarcoma and anticancer benefit of calycosin against human osteosarcoma cells. J. Cell. Biochem. 2019, 120, 10697–10706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Q.H.; Liu, C.L.; Lin, L. Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by activating caspases and Bcl-2 family proteins. Tumor Biol. 2015, 36, 5333–5339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.Q.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Zhao, Y.; Sun, G.; Fu, Z.R.; Zhang, Q.; et al. Calycosin Induces Gastric Cancer Cell Apoptosis via the ROS-Mediated MAPK/STAT3/NF-κB Pathway. OncoTargets Ther. 2021, 14, 2505–2517. [Google Scholar] [CrossRef]
- Qu, N.; Qu, J.; Huang, N.; Zhang, K.; Ye, T.; Shi, J.; Chen, B.; Kan, C.; Zhang, J.; Han, F.; et al. Calycosin induces autophagy and apoptosis via Sestrin2/AMPK/mTOR in human papillary thyroid cancer cells. Front. Pharmacol. 2022, 13, 1056687. [Google Scholar] [CrossRef]
- Liu, Y.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Zuo, W.B.; Sun, G.; Fu, Z.R.; et al. Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol. In Vitro 2021, 70, 105052. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, M.; Wang, J.; Yang, F.; Yang, P.; Liu, Y.; Chen, Z.; Zheng, Y. Calycosin inhibits breast cancer cell migration and invasion by suppressing EMT via BATF/TGF-β1. Aging-Us 2021, 13, 16009–16023. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Feng, C.; Wu, G.; Ye, Y.; Tian, J. Calycosin Inhibits the Migration and Invasion of Human Breast Cancer Cells by Down-Regulation of Foxp3 Expression. Cell. Physiol. Biochem. 2017, 44, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Niu, M.; Qin, J.; Wang, Y.; Tian, J. Inactivation of Rab27B-dependent signaling pathway by calycosin inhibits migration and invasion of ER-negative breast cancer cells. Gene 2019, 709, 48–55. [Google Scholar] [CrossRef]
- Qiu, R.; Li, X.; Qin, K.; Chen, X.; Wang, R.; Dai, Y.; Deng, L.; Ye, Y. Antimetastatic effects of calycosin on osteosarcoma and the underlying mechanism. Biofactors 2019, 45, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.H.; Ou-yang, J.; Xing, Y.; Li, D.Y.; Liu, R.E.; Xu, R.X. Calycosin inhibits migration and invasion through modulation of transforming growth factor beta-mediated mesenchymal properties in U87 and U251 cells. Drug Des. Dev. Ther. 2016, 10, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, D.; Li, M.; Liao, J.; He, L.; Chen, L.; Xu, R.; Zhang, M. Calycosin (CA) inhibits proliferation, migration and invasion by suppression of CXCL10 signaling pathway in glioma. Aging-Us 2024, 16, 4191–4203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, G.; Peng, L.; Tian, J.; Zhang, H. Calycosin inhibits viability, induces apoptosis, and suppresses invasion of cervical cancer cells by upregulating tumor suppressor miR-375. Arch. Biochem. Biophys. 2020, 691, 108478. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zhang, X.; Ren, Q.; Li, R.; Huang, Y.; Lu, H.; Chen, J. Calycosin inhibits the in vitro and in vivo growth of breast cancer cells through WDR7-7-GPR30 Signaling. J. Exp. Clin. Cancer Res. 2017, 36, 153. [Google Scholar] [CrossRef]
- Cheng, X.D.; Gu, J.F.; Yuan, J.R.; Feng, L.; Jia, X.B. Suppression of A549 cell proliferation and metastasis by calycosin via inhibition of the PKC-α/ERK1/2 pathway: An in vitro investigation. Mol. Med. Rep. 2015, 12, 7992–8002. [Google Scholar] [CrossRef]
- Kong, L.; Li, X.; Wang, H.; He, G.; Tang, A. Calycosin inhibits nasopharyngeal carcinoma cells by influencing EWSAT1 expression to regulate the TRAF6-related pathways. Biomed. Pharmacother. 2018, 106, 342–348. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Li, Y.; Kang, X.; Zhang, S. Gastro-Protective Effects of Calycosin Against Precancerous Lesions of Gastric Carcinoma in Rats. Drug Des. Dev. Ther. 2020, 14, 2207–2219. [Google Scholar] [CrossRef]
- Zhang, Z.; Auyeung, K.K.; Sze, S.C.; Zhang, S.; Yung, K.K.; Ko, J.K. The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: A “TGF-β paradox”. Phytomedicine 2020, 68, 153177. [Google Scholar] [CrossRef]
- Deng, M.; Chen, H.; Long, J.; Song, J.; Xie, L.; Li, X. Calycosin: A Review of its Pharmacological Effects and Application Prospects. Expert. Rev. Anti-Infect. Ther. 2021, 19, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zheng, Y.; Yang, Y.; Sui, Y.; Wen, Z. Pharmaceutical Values of Calycosin: One Type of Flavonoid Isolated from Astragalus. Evid.-Based Complement. Altern. Med. 2021, 2021, 9952578. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.; Li, N.; Lau, K.M.; Lee, P.S.; Yan, L.; Xu, M.L.; Lam, C.T.; Kong, A.Y.; Lin, H.Q.; Dong, T.T.; et al. Calycosin orchestrates the functions of Danggui Buxue Tang, a Chinese herbal decoction composing of Astragali Radix and Angelica Sinensis Radix: An evaluation by using calycosin-knock out herbal extract. J. Ethnopharmacol. 2015, 168, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Lee, Y.M.; Koh, Y.S.; Kim, Y.H. Flavonoids from Astragalus membranaceus and their inhibitory effects on LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Arch. Pharmacal Res. 2014, 37, 186–192. [Google Scholar] [CrossRef]
- Sohel, M.; Zahra Shova, F.T.; Shuvo, S.; Mahjabin, T.; Mojnu Mia, M.; Halder, D.; Islam, H.; Roman Mogal, M.; Biswas, P.; Saha, H.R.; et al. Unveiling the potential anti-cancer activity of calycosin against multivarious cancers with molecular insights: A promising frontier in cancer research. Cancer Med. 2024, 13, e6924. [Google Scholar] [CrossRef]
- Zhang, G.; Ou, R.; Li, F.; Wu, J.; Zheng, L.; Tong, Y.; Liu, Y.; Liu, Z.; Lu, L. Regulation of drug-metabolizing enzymes and efflux transporters by Astragali radix decoction and its main bioactive compounds: Implication for clinical drug-drug interactions. J. Ethnopharmacol. 2016, 180, 104–113. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Yu, S.L.; Wu, M.L.; Li, P.H.; Chen, Y.C.; Xie, J.; Xu, X.Y.; Ma, D.B.; Feng, Y.; Shen, J.G.; Lin, X. Calycosin synergizes with methotrexate in the treatment of Sjögren’s disease by targeting BATF in T follicular helper cells. Acta Pharmacol. Sin. 2025, 46, 1990–2005. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef]
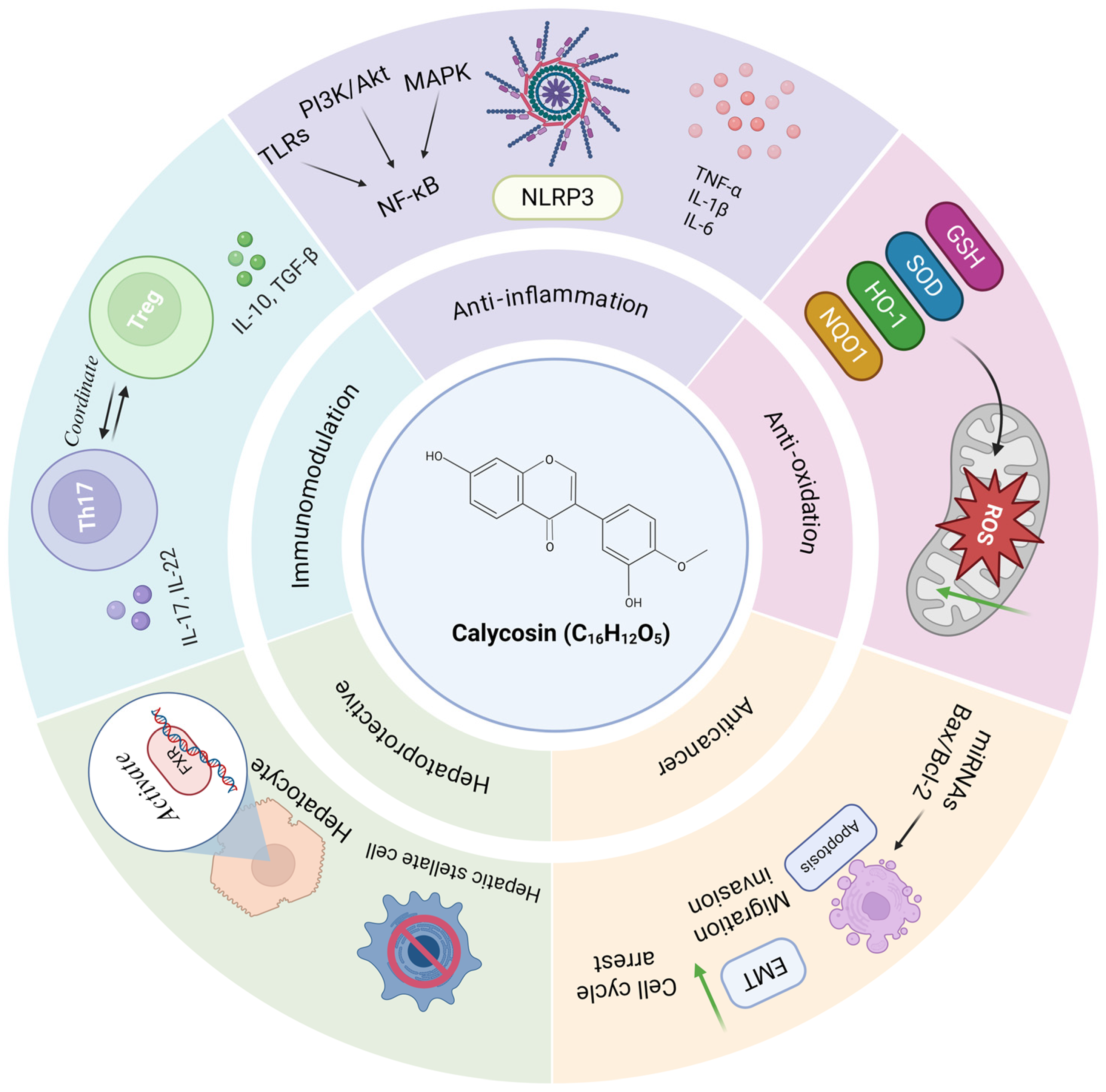
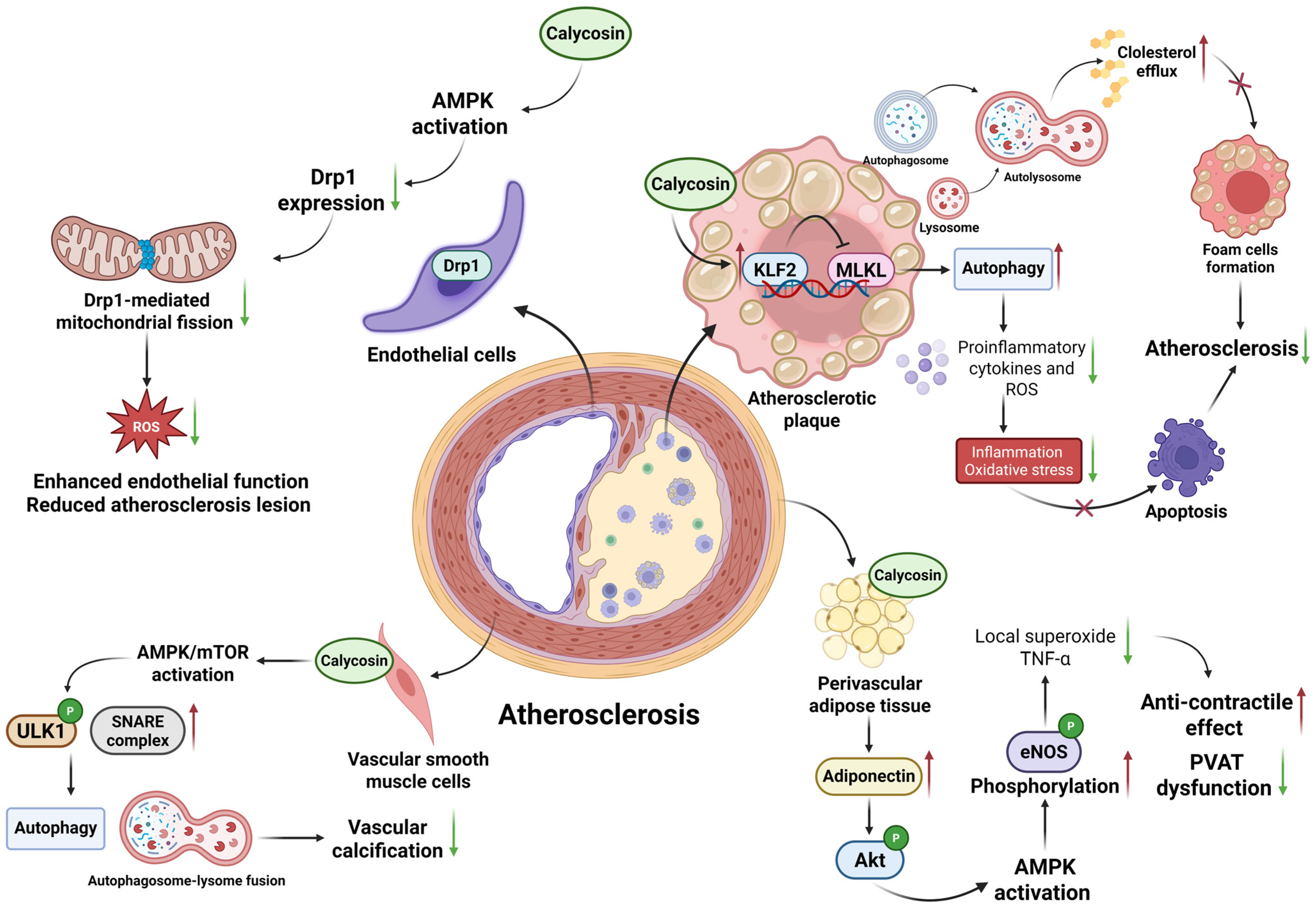
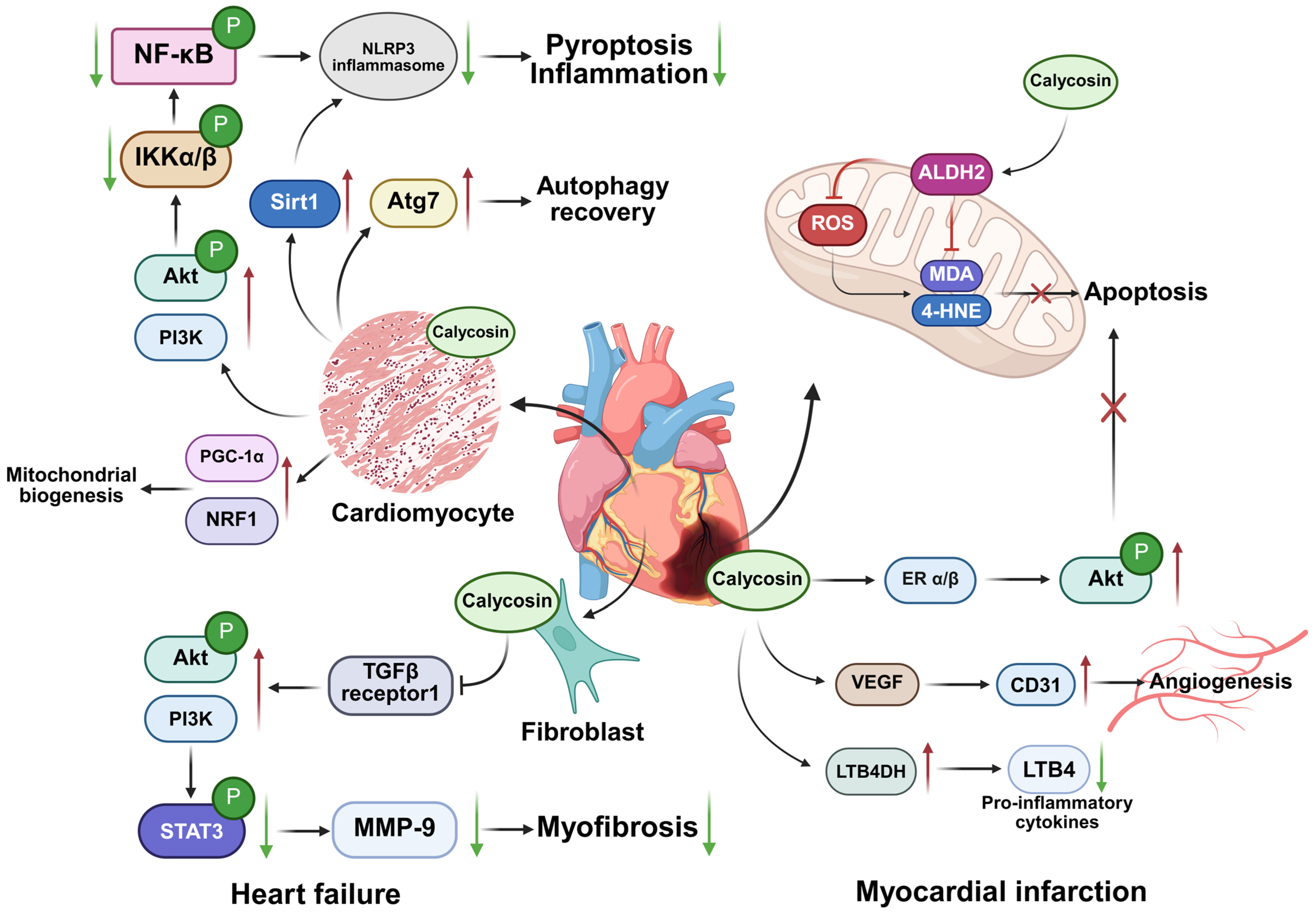
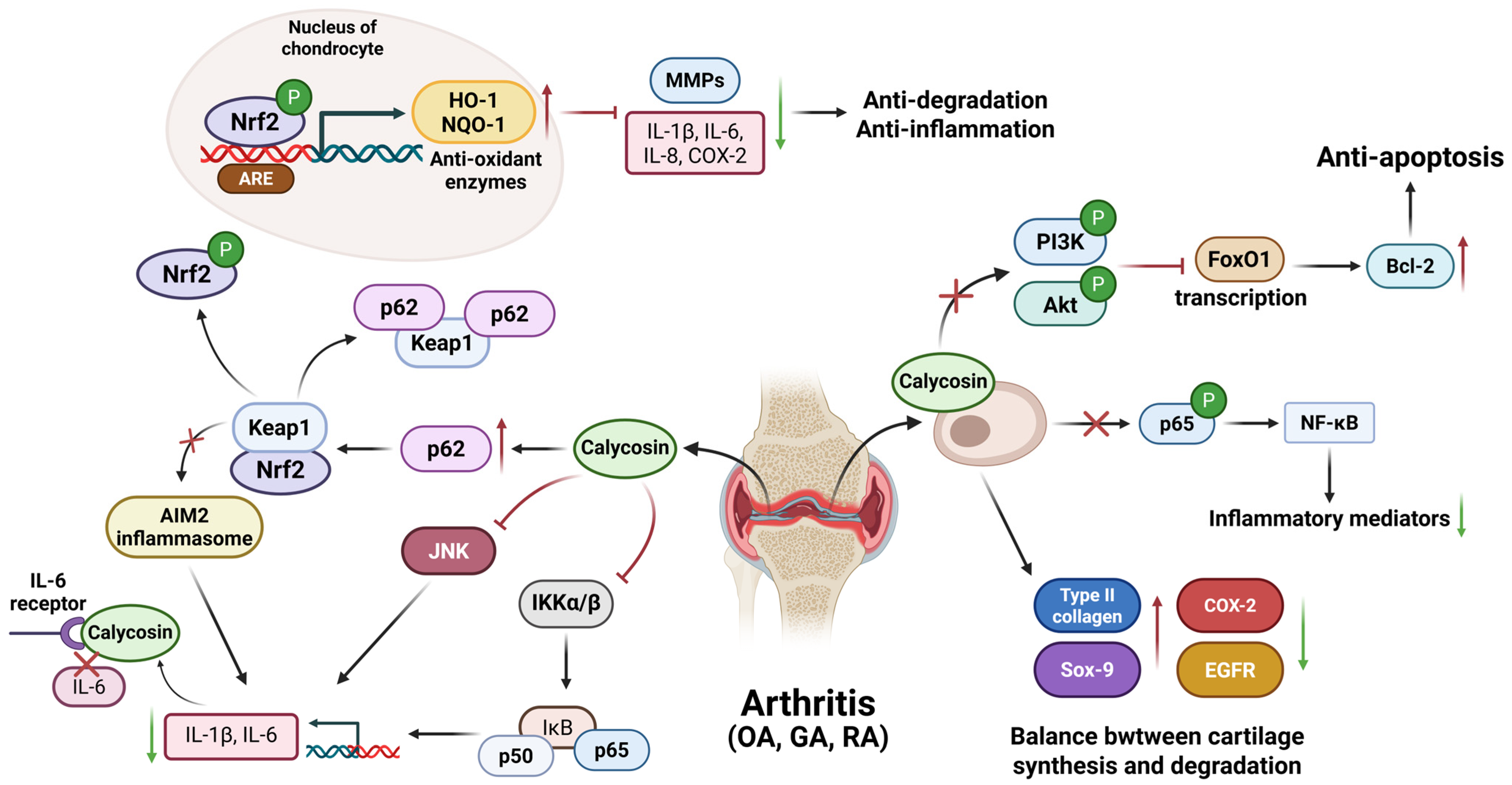
| Inflammatory Diseases | Cell Lines or Animal Models | Dosage | Specific Mechanisms | Application | References |
|---|---|---|---|---|---|
| Atherosclerosis | ApoE-deficient mice | 60 mg/kg/day | Up-regulate expression of KLF2 and inhibit expression of MLKL, decrease inflammatory cascade reactions | In vivo | [26] |
| Male C57BL/6 mice | 50 mg/kg/day | Increase adiponectin level, activate AMPK phosphorylation and promote eNOS to product NO | In vivo | [27] | |
| Rat thoracic aortic smooth muscle cell line A7r5 | 5–20 µM | Activate AMPK/mTOR pathway to induce autophagy and SNARE complex-mediated autophagosome-lysosome fusion | In vitro | [29] | |
| Heart failure | TGFβ-induced cardiac fibroblast model | 5 µM | Decrease α-SMA expression and reduce p-STAT3 and MMP-9 levels | In vitro | [35] |
| Male C57/BL6 mice | 50 mg/kg/day | Inhibit TGFBR1 pathway and down-regulate intracellular signal transducers Smad2/3 | In vivo | [36] | |
| Left anterior descending (LAD) artery ligation-induced heart failure rat model | 5 µM | Increase expression of PI3K and phosphorylated Akt | In vivo | [34] | |
| Adult zebrafish doxorubicin-induced cardiotoxicity model | 5 µmol/L | Increase the expression of Atg7 to promote autophagy recovery | In vivo | [37] | |
| Doxorubicin stimulated H9c2 cells | 0–20 μM | Inhibit NLRP3 inflammasome-induced pyroptosis | In vitro | [38] | |
| 50–200 μM | Up-regulate Sirt 1 expression but down-regulate NLRP3 expression | In vitro | [39] | ||
| Triptolide-induced cardiotoxicity H9c2 cardiomyocytes | 100 μM | Promote formation of PGC-1α/NRF1 complex | In vitro | [41] | |
| Myocardial infarction | Isoproterenol-induced myocardial infarction mice model | 40 mg/kg/day | Induce LTB4DH expression and reduce neutrophil infiltration | In vivo | [48] |
| Neonatal rat cardiomyocytes and C57BL/6 mouse myocardial infarction model | 2.5–10 µM 10–20 mg/kg/day | Increase ALDH2 activity, down-regulate Bax expression and up-regulate Bcl-2 expression | In vitro and vivo | [45] | |
| H9C2 myocardial cells | 5–20 µM | Increase ERα/β expression and enhance PI3K/Akt phosphorylation | In vitro | [46] | |
| Adult male Sprague Dawley rats | 4 mg/kg | Activate VEGF expression and increase CD31 expression to promote angiogenesis | In vivo | [47] | |
| Hypertension | Isolated male Sprague Dawley rat thoracic aortic rings | 30 μmol/L | Inhibit VOC and ROC, respectively, in KCl and phenylephrine induced contraction | In vivo | [54] |
| Human umbilical vein endothelial cells | 1–100 µM | Activate endothelial NOS/neural NOS-dependent NO production and BKCa, enhance endothelium hyperpolarization | In vitro | [55] | |
| Osteoarthritis | IL-1β treated chondrocytes | 100–400 µM | Inhibit PI3K/AKT and NF-κB pathway | In vitro | [64] |
| Human primary chondrocytes stimulated with IL-1β | 1–10 µM | Improve cartilage formation via FoxO1 | In vitro | [65] | |
| Anterior cruciate ligament transection mouse model Chondrocytes ADTC5 cells | 50 mg/kg 16–64 μM | Increase cartilage synthesis biomarkers Col-2 and Sox-9, decrease COX-2 and EGFR, improve balance of cartilage synthesis and degradation | In vitro and vivo | [66] | |
| Gouty arthritis | Mouse model induced by MSU Peripheral blood mono-nuclear cells (PBMCs) and THP-1 cells | 50 mg/kg 10 μM | Inhibit activation of the NF-κB pathway and modulate p62-Keap 1 pathway | In vitro and vivo | [70] |
| Rheumatoid arthritis | Rheumatoid arthritis synovial fibroblasts | 10–100 μM | Down-regulate expression of proinflammatory cytokines by activating p62/Nrf2-linked heme oxygenase 1 | In vitro | [73] |
| Collagen-induced arthritis mouse model | 1 mg/kg | Inhibit activation of inflammatory pathway modulators JNK, IKKα/β, and p65 | In vivo | [74] | |
| None | None | Bind to and inhibit IL-6R with high affinity | None | [75] | |
| Acute pancreatitis | Balb/C mice models | 20 or 50 mg/kg | Inhibit expression of NF-κB/p65 and phosphorylate IκBα and p38 MAPK | In vivo | [80] |
| Decrease MPO level and increase SOD activity | |||||
| Acute liver failure | LPS-induced human liver epithelial cell line (L02) cells CCl4-induced C57BL/6 mice liver injury | 12.5–50 mg/kg | Increase FXR target gene FoxM1B and SHP expression and STAT3 phosphorylation | In vivo | [85] |
| Non-alcoholic fatty liver disease | C57BL/6J male mice fed with high fat diet-induced NAFLD model | 50 mg/kg | Facilitate FXR activation, regulate Shp/Pepck/G-6-pase pathway, improve glucose and lipid metabolism, and glucose transport | In vivo | [91] |
| NASH mice model | 12.5–50 mg/kg | Increase fatty acid β-oxidation, mitigate liver fibrosis | In vivo | [92] | |
| Carbon tetrachlorid-induced liver fibrosis mice HSC-T6 cells LX-2 cells | 20–80 mg/kg 25–200 μM 25–200 μM | Increase ERβ expression and activate subsequent JAK2-STAT3 to inhibit hepatic stellate cells activation and collagen deposition | In vivo and vitro | [93,94,95,96] | |
| Diabetes mellitus | Normal rat hepatocyte cell line (BRL-3A) cultured by high glucose | 1 × 10−7 M | Reverse Glut-1 expression, reduce AGE receptor expression and directly bind to AGEs | In vitro | [102] |
| IC50 = 6.84 ± 1.58 µM | Inhibit α-glucosidase, inhibit glucose uptake into blood | In vitro | [104] | ||
| Human umbilical vein endothelial cells | 10−4 M | Inhibit AGEs-RAGE ligation and downstream NF-κB inflammatory pathway and increase Bcl-2 expression, decreasing Bad/Bax expression | In vitro | [103] | |
| Pregnant db/+ diabetic mice | 15 and 30 mg/kg | Suppress RNF38 expression, inhibit STAT3 activation, and increase SHP-1 expression | In vivo | [105] | |
| Colitis | Dextran sulfate sodium-induced colitis mice | 25 and 50 mg/kg | Inhibit NF-κB and phosphorylation of IKKα/β, IκBα and p65 | In vivo | [109] |
| Increase concentration of GSH and SOD to alleviate abnormal redox reactions | |||||
| Human intestinal fibroblasts (CCD-18Co) cells | 12.5–50 μmol/L | Inhibit TGF-β/Smad pathway | In vitro | [110] | |
| Diabetes-induced renal inflammation | The db/db mice and mouse tubular epithelial cells | 10 mg/(kg·d) 10 μM | Inhibit phosphorylation of IκBα and NF-κB p65 | In vivo and vitro | [115] |
| Mouse tubular epithelial cells | 5–80 μM | Inhibit ferroptosis iron-dependent cell death | In vitro | [116] | |
| High fat diet-fed/STZ injected rats | 5 mg/kg | Inhibit IL-33/ST2 axis, substantially activate NF-κB inflammatory pathway and TGF-β/Smad pathway, and activate Nrf2/ARE pathway | In vivo | [117] | |
| Renal ischemia/reperfusion injury | Mice with renal ischemia/reperfusion injury | 5–20 mg/kg | Up-regulate expression of PPARγ and suppress EGR1 | In vivo | [123] |
| Chronic prostatitis | Rats with chronic prostatitis | 10–30 mg/kg | Inhibit activation of p38MAPK/NF-κB signaling pathway | In vivo | [126] |
| Intracerebral hemorrhage-induced brain damage | Collagenase type VII-induced intracerebral hemorrhage mouse model | 50 mg/kg | Block activation of NLRP3 inflammasome and classical NF-κB pathway | In vivo | [130] |
| Cerebral ischemia injury | Oxygen-glucose deprivation/reoxygenation (OGD/R) model of rat microglia | 1–4 μM | Decrease HMGB1/TLR4/NF-κB signaling pathway | In vitro | [134] |
| Rat middle cerebral artery occlusion/reperfusion (MCAO/R) model and PC12 cells | 30 mg/kg 60 μM | Inhibit autophagy via STAT3/FOXO3a signaling pathway and ACSL4 dependent ferroptosis | In vivo and vitro | [131,135] | |
| Middle cerebral artery occlusion (MCAO) rats | 5–20 mg/kg | Increase expression of autophagy-related protein p62 and NBR1 and anti-apoptotic Bcl-2, decrease TNF-α expression | In vivo | [136] | |
| 5–20 mg/kg | Reduce RASD1 expression, up-regulate ER-α, miR-375 and Bcl-2 | In vivo | [138] | ||
| 0.44 mg/kg | Activate S1P/S1PR1/PI3K/Akt pathway | In vivo | [139] | ||
| 30 mg/kg | Increase BDNF and TrkB expression in brain to switch the TNF-α containing microglia from the activated state to the resting state | In vivo | [137] | ||
| 5–20 mg/kg | Inhibit calpain activation and increase TRPC6 and CREB expression | In vivo | [141] | ||
| Rat brain astrocytes | 0–100 μM | Activate Akt, promote phosphorylation of Nrf2 and downstream HO-1 and SOD activation to limit H2O2-induced ROS production | In vitro | [143] | |
| Allergic dermatitis | Balb/C mice and HaCaT cell | 2–50 mg/kg 10 μmol/L | Down-regulate expression of HIF-1α to repair epithelial tight junctions | In vivo and vitro | [144,146] |
| House dust mite (HDM)-induced allergic asthma mouse model and TNF-α and Poly (I:C) co-stimulated human bronchial epithelial cell line | 10 mg/kg 10 μM | Increase occludin expression, improve E-cadherin distribution, and inhibit TSLP production | In vivo and vitro | [147] | |
| Atopic dermatitis | Initial stage of AD model and HaCaT cells | 0.4–10 mg/kg 0.1–10 μM | Inhibit TLR4 mediated NF-κB signaling pathway | In vivo and vitro | [151] |
| Calcipotriol-induced mouse model HaCaT cells | 1–5 mg/mL 2–10 μM | Promote Treg cells differentiation but inhibit Th17 cells | In vivo and vitro | [154] | |
| Sepsis-induced acute lung injury | The cecal ligation and puncture (CLP)-treated young rats | 50 mg/kg | Inhibit the HMGB1/MyD88/NF-κB pathway and activate NLRP3 inflammasome | In vivo | [158] |
| 12.5–50 mg/kg | inhibit mitochondrial ROS mediated inflammasome activation | In vivo | [160] | ||
| LPS-induced MLE-12 cells | 3.75–50 μg/ml | Up-regulate miR-375-3p expression and silence ROCK2 to attenuate inflammation and increase cell viability | In vitro | [161] | |
| Bacterial, viral, and parasitic infections | Mcr-1-positive bacterial strains | 32 µg/ml | Inhibit MCR-1 activity and restore the anti-bacterial activity of polymyxin B | In vitro | [164] |
| Respiratory Syncytial Virus (RSV)-induced asthma-exacerbated BALB/c mice | 0.174 mg/g | Enhance Th1 response and inhibit Th2/Th17 responses to up-regulate interferon-γ expression | In vivo | [165] | |
| A. cantonensis-induced angiostrongyliasis BALB/c mice | 30 mg/kg | Facilitate antioxidant HO-1 expression and inhibit NF-κB pathway activation | In vivo | [167] |
| Category | Cancer Cell Lines or Animal Models | Specific Mechanisms | Dosage | References |
|---|---|---|---|---|
| Apoptosis induction | Human colorectal (HT29) carcinoma cells | Activate Sirt1 and AMPK, inhibit Akt/mTOR signaling pathway | 50 μM | [169] |
| Human colorectal cancer cell lines SW480 | Up-regulate ERβ, decrease IGF-1R and Akt, down-regulate miR-95 | 10–80 μM | [170] | |
| HCT-116 CRC cells | Increase ERβ expression, decrease miR-17, and up-regulate PTEN | 10–100 μM | [171] | |
| Human breast cancer cell lines MCF-7 | Deactivate HOTAIR/p-Akt signaling pathway | 20–100 μM | [172] | |
| Human osteosarcoma MG-63 cells | Increase ERβ expression, inhibit activation of PI3K/Akt pathway | 100 μM | [173] | |
| ER-positive MG-63 human osteosarcoma cells | Increase protein expression of PI3K/Akt/mTOR pathway | 25–100 μM | [174] | |
| Human osteosarcoma cell line 143B | Inhibit miR-223 expression, decrease NF-κB/p65 and IκBα | 60–180 μM | [175] | |
| Human ovarian carcinoma SKOV3 cells | Up-regulate ratio of Bax/Bcl-2 and increase expression of caspase protein | 25–100 μM | [176] | |
| Gastric cancer cells AGS | Increase ROS level and MAPK/STAT3/NF-κB pathway | 47 μM | [177] | |
| Human papillary thyroid (B-CPAP) cancer cells | Activate SESN2 and up-regulate p-AMPK, inhibit p-mTOR | 100 μM | [178] | |
| HepG2 hepatocellular carcinoma cells | Down-regulate Bcl-2, up-regulate Bax and caspase-3 | 1–100 μM | [179] | |
| Migration and invasion inhibition | Human breast cancer cell lines T47D and MCF-7 | Suppress BATF/TGFβ1 signaling pathway | 100–400 μM | [180] |
| Human breast cancer cell lines T47D and MCF-7 | Decrease Foxp3 expression and down-regulate VEGF and MMP-9 | 50–150 μM | [181] | |
| ER- breast cancer cell line MDA-MB-231 | Reduce Rab27B expression and β-catenin-induced VEGF | 150 μM | [182] | |
| Human cell line of 143B and BALB/c nude mice | Suppress IκBα/ECT2 expression and reduce IL-6 and MMPs | 60–180 μmol/L 30–120 mg/kg | [183] | |
| Human U87 and U251 cell lines | Down-regulate TGFβ and inhibit activation of EMT and MMP | 0–200 μM | [184] | |
| Human U87 and U251 cell lines | Down-regulate inflammatory chemokine CXCL10 | 100–400 μM | [185] | |
| Human cervical cancer cell lines SiHa and CaSki | Decrease tumor suppressor miR-375 | 30–50 μM | [186] | |
| Proliferation and growth inhibition | MDA-MB-468 and SKBR3 cell lines | Up-regulate lncRNA WDR7-7 and decrease GPR30 level | 4–16 μM | [187] |
| LUAD cells A549 | Suppress PKC-α/ERK1/2 pathway and decrease MMP expression | 20–40 μM | [188] | |
| Nasopharyngeal carcinoma cell lines CNE1 and CNE2 | Decrease expression of lncRNA EWSAT1 and TRAF6 | 8–50 μM | [189] | |
| MNNG-induced PLGC rats | Down-regulate the levels of NF-κB, DARPP-32, and STAT3 | 40 and 80 mg/kg | [190] | |
| PANC1 and PaCa-2 cell lines | TGF-β induced the activation of the CDK inhibitor p21Waf1/Cip1 | 50–100 μM | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Ye, Y.; Hu, Y.; Jiang, M. Emerging Role of Calycosin in Inflammatory Diseases: Molecular Mechanisms and Potential Therapeutic Applications. Biomolecules 2025, 15, 1643. https://doi.org/10.3390/biom15121643
Liu T, Ye Y, Hu Y, Jiang M. Emerging Role of Calycosin in Inflammatory Diseases: Molecular Mechanisms and Potential Therapeutic Applications. Biomolecules. 2025; 15(12):1643. https://doi.org/10.3390/biom15121643
Chicago/Turabian StyleLiu, Tongzhan, Yifei Ye, Yu Hu, and Meixiu Jiang. 2025. "Emerging Role of Calycosin in Inflammatory Diseases: Molecular Mechanisms and Potential Therapeutic Applications" Biomolecules 15, no. 12: 1643. https://doi.org/10.3390/biom15121643
APA StyleLiu, T., Ye, Y., Hu, Y., & Jiang, M. (2025). Emerging Role of Calycosin in Inflammatory Diseases: Molecular Mechanisms and Potential Therapeutic Applications. Biomolecules, 15(12), 1643. https://doi.org/10.3390/biom15121643









