Chemokines in Pregnancy
Abstract
1. Introduction
2. The General Chemokine System
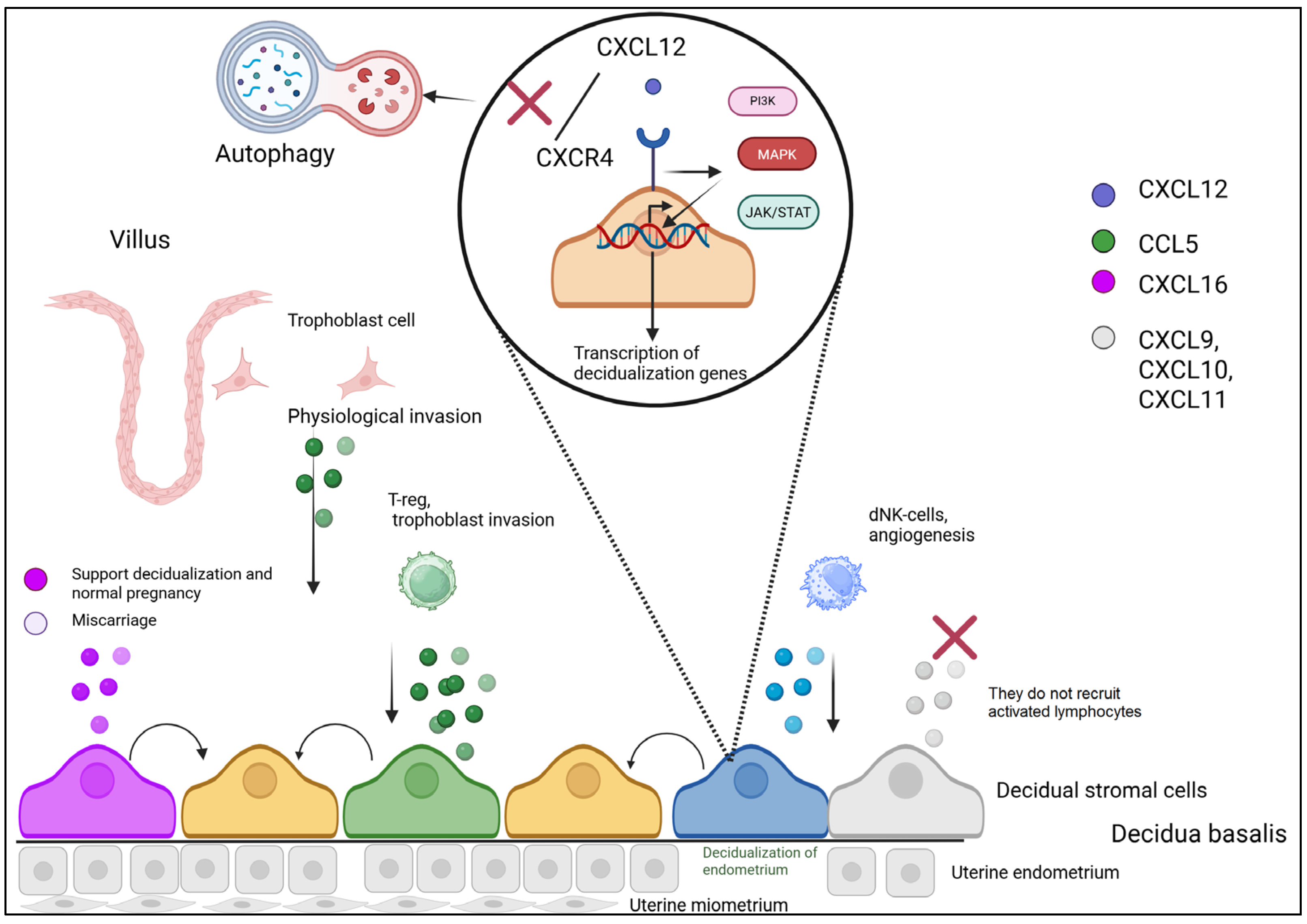
3. Chemokines in the Decidualization Process
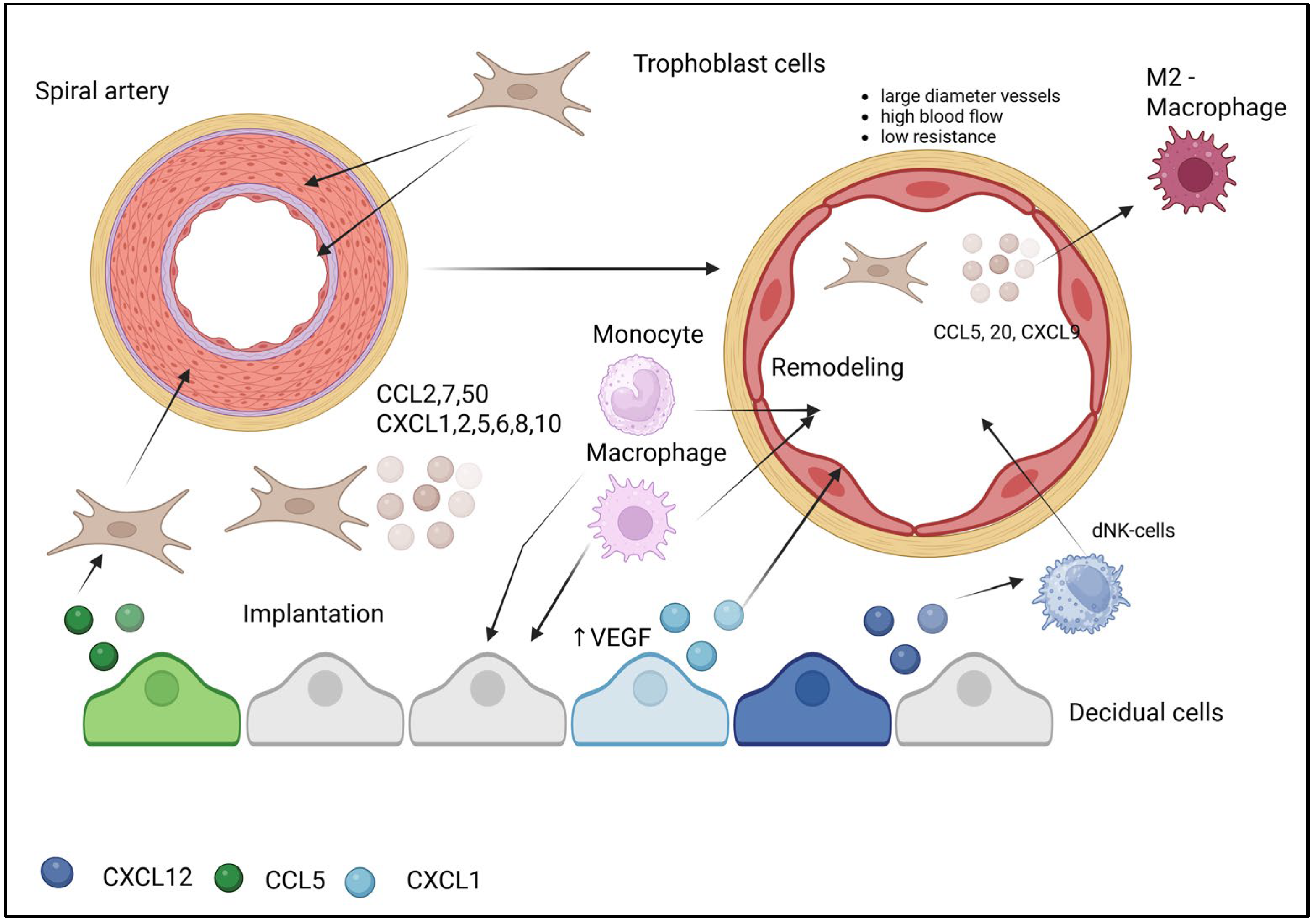
4. Chemokine-Mediated Mechanisms in Uterine Spiral Artery Remodeling
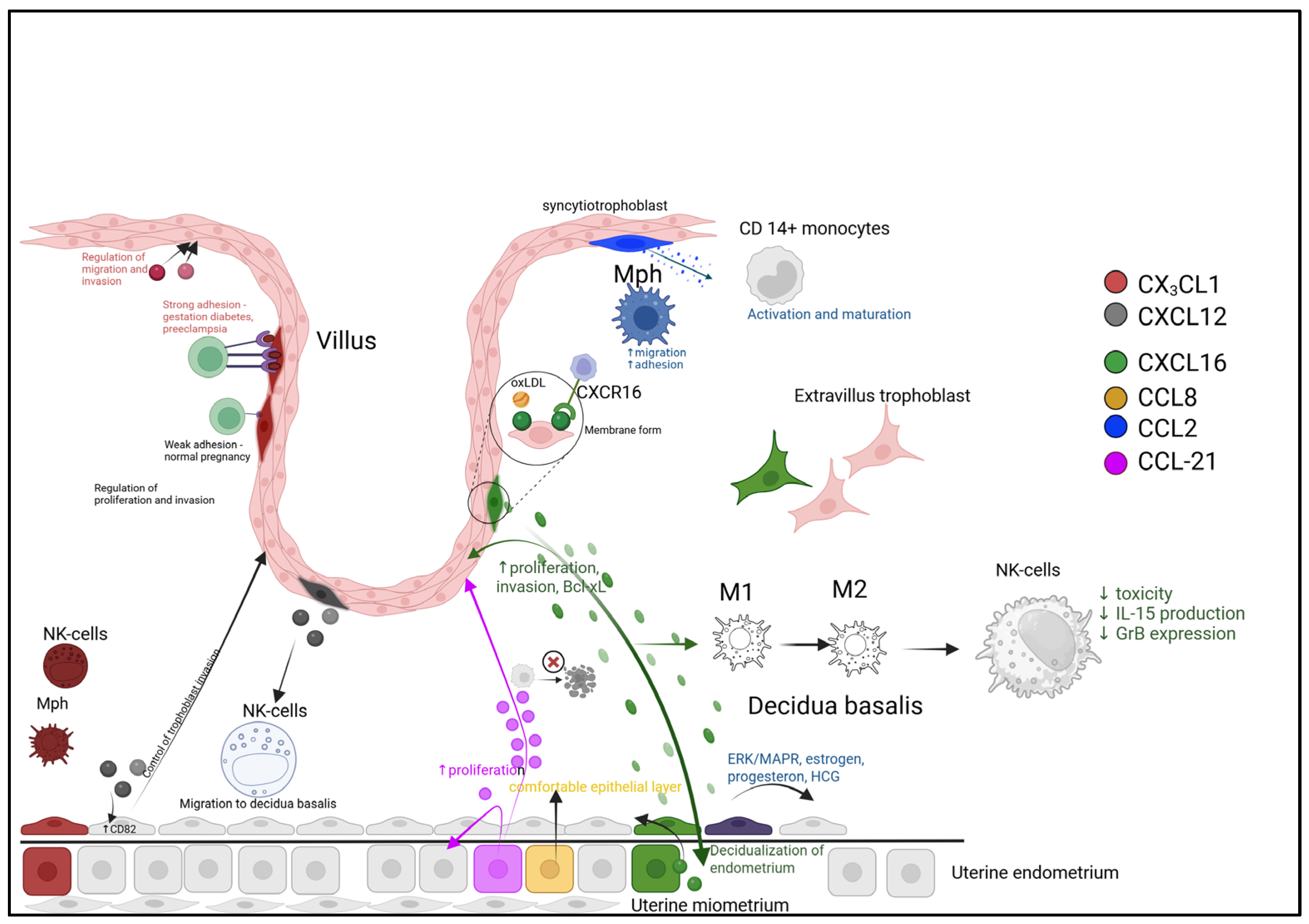
5. Chemokines in Implantation and Placentation During Pregnancy
6. Chemokine Regulation of Implantation and Pregnancy Maintenance
- Cellular Recruitment and Education. Trophoblast-derived CXCL16 acts via CXCR6 to recruit maternal T-cells and monocytes, shaping the decidual leukocyte composition. The CCL21/CCR7 axis promotes trophoblast and endometrial cell proliferation while suppressing stress-induced apoptosis, thereby enhancing tissue viability.
- Macrophage Polarization Balance. The local microenvironment, influenced by factors like oxidized LDL (oxLDL), drives macrophage differentiation. A balance between pro-inflammatory (M1, potentially modulated by CCL2) and regenerative (M2, modulated by CCL18/CCL22) phenotypes is crucial for immune tolerance and tissue repair.
- Spatial and Dynamic Regulation. Chemokines are strategically localized, with CCL8 enriched at the implantation zone to support epithelial reorganization, and CCL21 at the trophectoderm interface. Furthermore, ligand-induced receptor internalization (e.g., CCR1/CCR5) provides a dynamic feedback mechanism to attenuate signaling, while persistently membrane-bound receptors (e.g., CXCR3/CXCR4) may sustain chemotactic gradients.
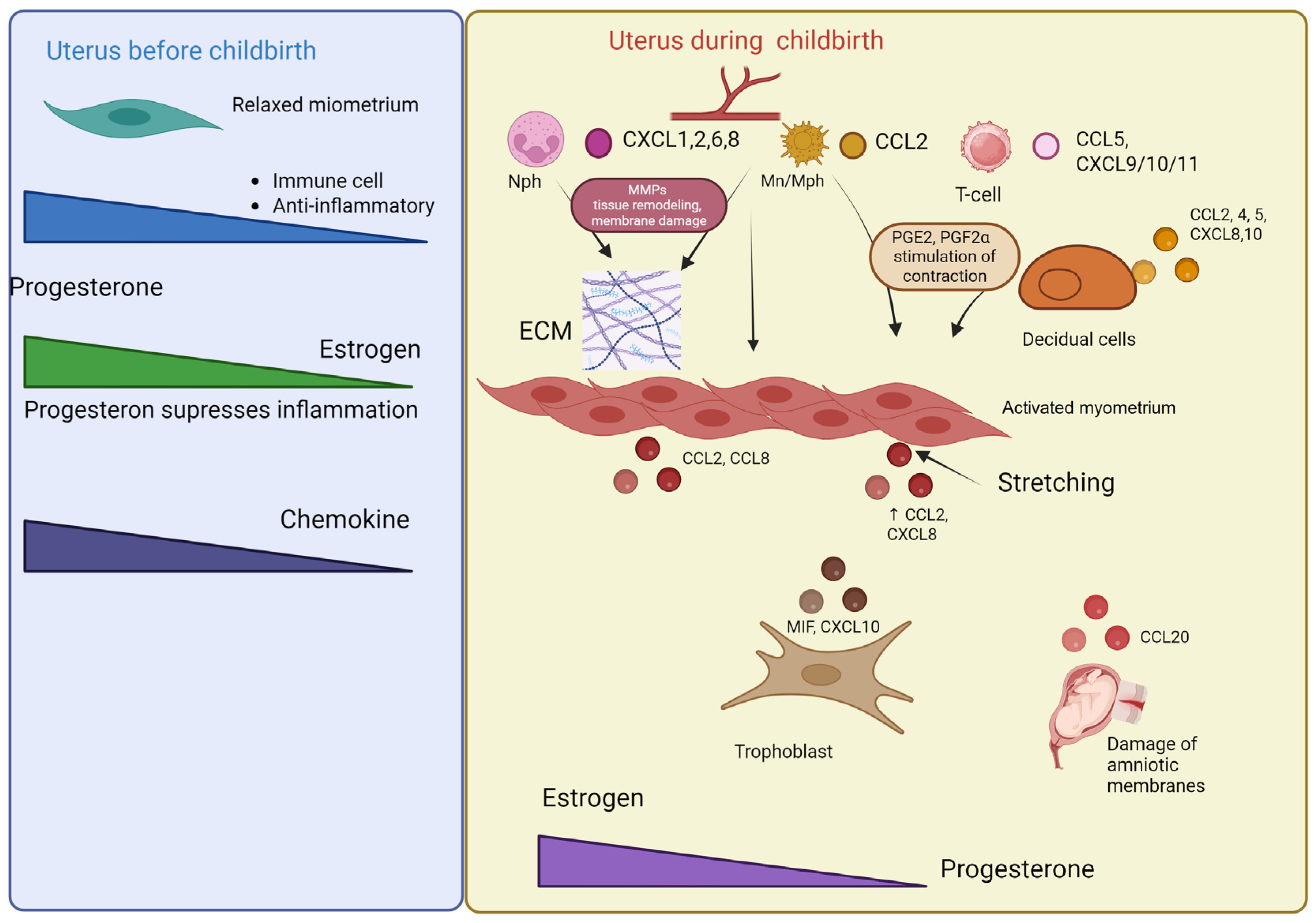
7. Chemokines in the Regulation of Labor
8. Preterm Birth: Chemokine-Mediated Mechanisms and Pathophysiology
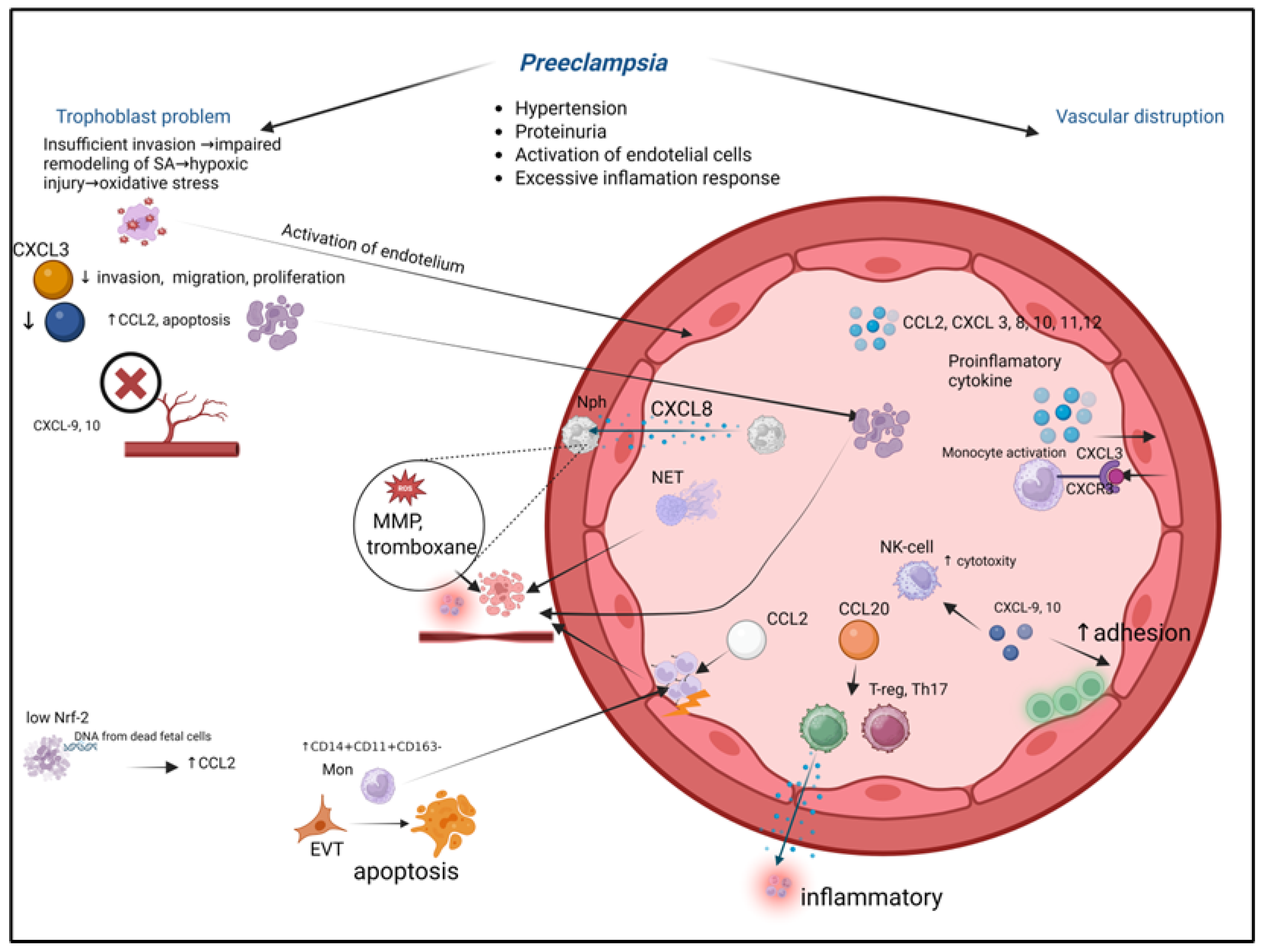
9. Chemokines in Pregnancy Pathologies: Preeclampsia
- Placental Insult. Shallow extravillous trophoblast (EVT) invasion fails to adequately remodel maternal spiral arteries, causing placental hypoxia and oxidative stress.
- Chemokine Storm. The stressed placenta releases a surge of pro-inflammatory chemokines, including CCL2, CXCL8, and CXCL10.
- Immune Activation and Vascular Dysfunction. These placental chemokines recruit and activate maternal neutrophils and monocytes into the intervillous space and systemic circulation. Activated immune cells exacerbate local damage, induce endothelial dysfunction, and contribute to a systemic inflammatory response.
- Systemic Consequences. The widespread endothelial activation manifests clinically as hypertension and proteinuria. Furthermore, the altered chemokine milieu disrupts the delicate immune tolerance at the maternal-fetal interface, perpetuating the pathological cycle. This figure integrates how localized placental pathology, mediated by chemokine networks, escalates into the multisystemic maternal syndrome of preeclampsia.
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Delgado, M.G.; Lennon-Duménil, A.M. How cell migration helps immune sentinels. Front. Cell Dev. Biol. 2022, 10, 932472. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 12, 738–752. [Google Scholar] [CrossRef]
- Wang, J.; Knaut, H. Chemokine signaling in development and disease. Development 2014, 141, 4199–4205. [Google Scholar] [CrossRef]
- Xu, H.; Lin, S.; Zhou, Z.; Li, D.; Zhang, X.; Yu, M.; Zhao, R.; Wang, Y.; Qian, J.; Li, X.; et al. New genetic and epigenetic insights into the chemokine system: The latest discoveries aiding progression toward precision medicine. Cell Mol. Immunol. 2023, 20, 739–776. [Google Scholar] [CrossRef]
- Tang, P.; Wang, J.M. Chemokines: The past, the present and the future. Cell Mol. Immunol. 2018, 15, 295–298. [Google Scholar] [CrossRef]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacol. Rev. 2014, 66, 1–79. [Google Scholar] [CrossRef]
- Comerford, I.; McColl, S.R. Atypical chemokine receptors in the immune system. Nat. Rev. Immunol. 2024, 24, 753–769. [Google Scholar] [CrossRef]
- Blanchet, X.; Weber, C.; von Hundelshausen, P. Chemokine Heteromers and Their Impact on Cellular Function-A Conceptual Framework. Int. J. Mol. Sci. 2023, 24, 10925. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Wiens, M.; Müller, I.M.; Schröder, H.C. The chemokine networks in sponges: Potential roles in morphogenesis, immunity and stem cell formation. Prog. Mol. Subcell. Biol. 2004, 34, 103–143. [Google Scholar]
- Shakola, F.; Suri, P.; Ruggiu, M. Splicing Regulation of Pro-Inflammatory Cytokines and Chemokines: At the Interface of the Neuroendocrine and Immune Systems. Biomolecules 2015, 5, 2073–2100. [Google Scholar] [CrossRef] [PubMed]
- Colobran, R.; Pujol-Borrell, R.; Armengol, M.P.; Juan, M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin. Exp. Immunol. 2007, 148, 208–217. [Google Scholar] [CrossRef] [PubMed]
- García-Velasco, J.A.; Arici, A. Chemokines and human reproduction. Fertil. Steril. 1999, 71, 983–993. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gomez, T.; Dominguez, F.; Quiñonero, A.; Diaz-Gimeno, P.; Kapidzic, M.; Gormley, M.; Ona, K.; Padilla-Iserte, P.; McMaster, M.; Genbacev, O.; et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. USA 2017, 114, E8468–E8477. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef]
- Ruiz-Magaña, M.J.; Llorca, T.; Martinez-Aguilar, R.; Abadia-Molina, A.C.; Ruiz-Ruiz, C.; Olivares, E.G. Stromal cells of the endometrium and decidua: In search of a name and an identity†. Biol. Reprod. 2022, 107, 1166–1176. [Google Scholar] [CrossRef]
- Llorca, T.; Ruiz-Magaña, M.J.; Martinez-Aguilar, R.; García-Valdeavero, O.M.; Rodríguez-Doña, L.; Abadia-Molina, A.C.; Ruiz-Ruiz, C.; Olivares, E.G. Decidualized human decidual stromal cells inhibit chemotaxis of activated T cells: A potential mechanism of maternal-fetal immune tolerance. Front. Immunol. 2023, 14, 1223539. [Google Scholar] [CrossRef]
- Nancy, P.; Tagliani, E.; Tay, C.S.; Asp, P.; Levy, D.E.; Erlebacher, A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012, 336, 1317–1321. [Google Scholar] [CrossRef]
- Lei, G.Q.; Wu, Z.Y.; Jiang, W.B.; Luo, J.; Xu, H.; Luo, S.F.; Peng, Z.Y.; Wang, W.; Chen, M.; Yu, L.L. Effect of CXCL12/CXCR4 on migration of decidua-derived mesenchymal stem cells from pregnancies with preeclampsia. Am. J. Reprod. Immunol. 2019, 82, e13180. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Qi, T.; Gao, R.; Xie, H.; Ruan, L.; He, J.; Li, F.; Liu, T.; Xu, H.; et al. Benzo(a)pyrene promotes autophagy to impair endometrial decidualization via inhibiting CXCL12/CXCR4 axis. Chem. Biol. Interact. 2025, 405, 111288. [Google Scholar] [CrossRef]
- Mei, J.; Yan, Y.; Li, S.Y.; Zhou, W.J.; Zhang, Q.; Li, M.Q.; Sun, H.X. CXCL16/CXCR6 interaction promotes endometrial decidualization via the PI3K/AKT pathway. Reproduction 2019, 157, 273–282. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Williams, P.J.; Lash, G.E. Immune cells in the placental bed. Int. J. Dev. Biol. 2010, 54, 281–294. [Google Scholar] [CrossRef]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Feng, X.; Lash, G.E. Unraveling the mysteries of spiral artery remodeling. Placenta 2023, 141, 51–56. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Pepe, G.J. Regulation of Uterine Spiral Artery Remodeling: A Review. Reprod. Sci. 2020, 27, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, X.; Ye, S.; Li, W.; Yang, Q.; Li, Y.X.; Wang, Y.; Wang, Y.L. Immune-regulatory properties of endovascular extravillous trophoblast cells in human placenta. Placenta. 2024, 145, 107–116. [Google Scholar] [CrossRef]
- Wallace, A.E.; Cartwright, J.E.; Begum, R.; Laing, K.; Thilaganathan, B.; Whitley, G.S. Trophoblast-induced changes in C-x-C motif chemokine 10 expression contribute to vascular smooth muscle cell dedifferentiation during spiral artery remodeling. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e93–e101. [Google Scholar] [CrossRef] [PubMed]
- Varberg, K.M.; Soares, M.J. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021, 113, 48–56. [Google Scholar] [CrossRef]
- Sato, Y. Endovascular trophoblast and spiral artery remodeling. Mol. Cell Endocrinol. 2020, 503, 110699. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.H.; Dunk, C.E.; Lye, S.J.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J. Immunol. 2017, 198, 4115–4128. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Zhao, Y.; Fang, C.; Lian, Y.; Gou, W.; Han, T.; Zhu, X. Insights into the mechanism of CXCL12-mediated signaling in trophoblast functions and placental angiogenesis. Acta Biochim. Biophys. Sin. 2015, 47, 663–672. [Google Scholar] [CrossRef]
- Lash, G.E.; Ernerudh, J. Decidual cytokines and pregnancy complications: Focus on spontaneous miscarriage. J. Reprod. Immunol. 2015, 108, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Windsperger, K.; Dekan, S.; Pils, S.; Golletz, C.; Kunihs, V.; Fiala, C.; Kristiansen, G.; Knöfler, M.; Pollheimer, J. Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Hum. Reprod. 2017, 32, 1208–1217. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knöfler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Ma, C.; Liu, G.; Liu, W.; Xu, W.; Li, H.; Piao, S.; Sui, Y.; Feng, W. CXCL1 stimulates decidual angiogenesis via the VEGF-A pathway during the first trimester of pregnancy. Mol. Cell Biochem. 2021, 476, 2989–2998. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Wu, G.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bayless, K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol. Hum. Reprod. 2010, 16, 135–152. [Google Scholar] [CrossRef]
- Griebel, C.P.; Halvorsen, J.; Golemon, T.B.; Day, A.A. Management of spontaneous abortion. Am. Fam. Physician 2005, 72, 1243–1250. [Google Scholar] [PubMed]
- van den Brûle, F.; Berndt, S.; Simon, N.; Coulon, C.; Le Goarant, J.; Munaut, C.; Noël, A.; Frankenne, F.; Foidart, J.M. Trophoblast invasion and placentation: Molecular mechanisms and regulation. Chem. Immunol. Allergy 2005, 88, 163–180. [Google Scholar]
- Hannan, N.J.; Jones, R.L.; Critchley, H.O.; Kovacs, G.J.; Rogers, P.A.; Affandi, B.; Salamonsen, L.A. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J. Clin. Endocrinol. Metab. 2004, 89, 6119–6129. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, P.; Yang, L.; Chen, Y.; Yan, J.; Duan, E.; Qiao, J. Fractalkine is expressed in the human ovary and increases progesterone biosynthesis in human luteinised granulosa cells. Reprod. Biol. Endocrinol. 2011, 9, 95. [Google Scholar] [CrossRef]
- Cook, D.N.; Chen, S.C.; Sullivan, L.M.; Manfra, D.J.; Wiekowski, M.T.; Prosser, D.M.; Vassileva, G.; Lira, S.A. Generation and analysis of mice lacking the chemokine fractalkine. Mol. Cell Biol. 2001, 21, 3159–3165. [Google Scholar] [CrossRef]
- Kervancioglu Demirci, E.; Salamonsen, L.A.; Gauster, M. The role of CX3CL1 in fetal-maternal interaction during human gestation. Cell Adh. Migr. 2016, 10, 189–196. [Google Scholar] [CrossRef]
- Ao, D.; Li, D.J.; Li, M.Q. CXCL12 in normal and pathological pregnancies: A review. Am. J. Reprod. Immunol. 2020, 84, e13280. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.S.; Yoon, M.J.; Hong, S.H.; Ahn, J.; Cha, H.; Lee, D.; Ko, J.E.; Kwon, H.; Choi, D.H.; Lee, K.A.; et al. CXCL12 enhances pregnancy outcome via improvement of endometrial receptivity in mice. Sci. Rep. 2021, 11, 7397. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Tang, C.L.; Du, M.R.; Fan, D.X.; Zhao, H.B.; Xu, B.; Li, D.J. CXCL12 controls over-invasion of trophoblasts via upregulating CD82 expression in DSCs at maternal-fetal interface of human early pregnancy in a paracrine manner. Int. J. Clin. Exp. Pathol. 2011, 4, 276–286. [Google Scholar] [PubMed]
- Nishigaki, A.; Okada, H.; Okamoto, R.; Shimoi, K.; Miyashiro, H.; Yasuda, K.; Kanzaki, H. The concentration of human follicular fluid stromal cell-derived factor-1 is correlated with luteinization in follicles. Gynecol. Endocrinol. 2013, 29, 230–234. [Google Scholar] [CrossRef]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef]
- McIntosh, S.Z.; Quinn, K.E.; Ashley, R.L. CXCL12 May Drive Inflammatory Potential in the Ovine Corpus Luteum During Implantation. Reprod. Sci. 2022, 29, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.E.; Reynolds, L.P.; Grazul-Bilska, A.T.; Borowicz, P.P.; Ashley, R.L. Placental development during early pregnancy: Effects of embryo origin on expression of chemokine ligand twelve (CXCL12). Placenta 2016, 43, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Ren, L.; Sun, Y.; Cao, Y.; Xiong, Y.; Zhang, Y. Expression and Functional Analysis of CXCL12 and Its Receptors in Human Term Trophoblast Cells. Reprod. Sci. 2020, 27, 46–54. [Google Scholar] [CrossRef]
- Zheng, J.; Qu, D.; Wang, C.; Ding, L.; Zhou, W. Involvement of CXCL12/CXCR4 in the motility of human first-trimester endometrial epithelial cells through an autocrine mechanism by activating PI3K/AKT signaling. BMC Pregnancy Childbirth 2020, 20, 87. [Google Scholar] [CrossRef]
- Behfar, S.; Hassanshahi, G.; Nazari, A.; Khorramdelazad, H. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine 2018, 110, 226–231. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, J.L.; Chen, M.; Zheng, Z.M.; Li, M.Q.; Shao, J. CCL2: An important cytokine in normal and pathological pregnancies: A review. Front. Immunol. 2023, 13, 1053457. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; He, X.J.; Guo, P.F.; Du, M.R.; Shao, J.; Li, M.Q.; Li, D.J. The decidual stromal cells-secreted CCL2 induces and maintains decidual leukocytes into Th2 bias in human early pregnancy. Clin. Immunol. 2012, 145, 161–173. [Google Scholar] [CrossRef]
- Gibson, D.A.; Greaves, E.; Critchley, H.O.; Saunders, P.T. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum. Reprod. 2015, 30, 1290–1301. [Google Scholar] [CrossRef]
- Vafaei, H.; Faraji, S.; Ahmadi, M.; Tabei, S.M.B.; Fereidoni, S.; Shiravani, Z.; Hosseini, S.N.; Asadi, N.; Kasraeian, M.; Faraji, A.; et al. Alteration in IFN-γ and CCL2 serum levels at first trimester of pregnancy contribute to development of preeclampsia and fetal growth restriction. Taiwan. J. Obstet. Gynecol. 2023, 62, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Li, M.Q.; Zhu, X.Y.; Li, D.J. Immune status of decidual macrophages is dependent on the CCL2/CCR2/JAK2 pathway during early pregnancy. Am. J. Reprod. Immunol. 2021, 86, e13480. [Google Scholar] [CrossRef]
- Qin, X.Y.; Shen, H.H.; Zhang, X.Y.; Zhang, X.; Xie, F.; Wang, W.J.; Xiong, Y.; Mei, J.; Li, M.Q. Hypoxia-mediated chemotaxis and residence of macrophage in decidua by secreting VEGFA and CCL2 during normal pregnancy. Reproduction 2023, 165, 543–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, D.; Sun, J.; Zhao, L.; Wang, Q.; Shao, Q.; Kong, B.; Zhang, Y.; Qu, X. Human trophoblast cells induced MDSCs from peripheral blood CD14(+) myelomonocytic cells via elevated levels of CCL2. Cell Mol. Immunol. 2016, 13, 615–627. [Google Scholar] [CrossRef]
- Tok, A.; Seyithanoglu, M.; Ozer, A.; Erkayiran, U.; Karakucuk, S.; Celebi, A. The serum level of soluble CXCL16 is increased in preeclampsia and associated with hepatic/renal damage. J. Matern. Fetal Neonatal Med. 2021, 34, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.Y.; Du, M.R.; Wu, X.; Wang, M.Y.; Li, D.J. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum. Reprod. 2006, 21, 1083–1091. [Google Scholar] [CrossRef]
- Shi, J.W.; Yang, H.L.; Fan, D.X.; Yang, S.L.; Qiu, X.M.; Wang, Y.; Lai, Z.Z.; Ha, S.Y.; Ruan, L.Y.; Shen, H.H.; et al. The role of CXC chemokine ligand 16 in physiological and pathological pregnancies. Am. J. Reprod. Immunol. 2020, 83, e13223. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.Y.; Du, M.R.; Li, D.J. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J. Immunol. 2008, 180, 2367–2375. [Google Scholar] [CrossRef]
- Fan, D.X.; Zhou, W.J.; Jin, L.P.; Li, M.Q.; Xu, X.H.; Xu, C.J. Trophoblast-Derived CXCL16 Decreased Granzyme B Production of Decidual γδ T Cells and Promoted Bcl-xL Expression of Trophoblasts. Reprod. Sci. 2019, 26, 532–542. [Google Scholar] [CrossRef]
- Borroni, E.M.; Mantovani, A.; Locati, M.; Bonecchi, R. Chemokine receptors intracellular trafficking. Pharmacol. Ther. 2010, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Złotkowska, A.; Andronowska, A. Chemokines as the modulators of endometrial epithelial cells remodelling. Sci. Rep. 2019, 9, 12968, Erratum in Sci. Rep. 2019, 9, 14467. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Nagira, M.; Kitaura, M.; Imagawa, N.; Imai, T.; Yoshie, O. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem. 1998, 273, 7118–7122. [Google Scholar] [CrossRef]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Bae, H.; Lim, W.; Bazer, F.W.; Whang, K.Y.; Song, G. Mitigation of ER-stress and inflammation by chemokine (C-C motif) ligand 21 during early pregnancy. Dev. Comp. Immunol. 2019, 94, 73–84. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Devvanshi, H.; Kachhwaha, R.; Manhswita, A.; Bhatnagar, S.; Kshetrapal, P. Immunological Changes in Pregnancy and Prospects of Therapeutic Pla-Xosomes in Adverse Pregnancy Outcomes. Front. Pharmacol. 2022, 13, 895254. [Google Scholar] [CrossRef]
- Tagoma, A.; Haller-Kikkatalo, K.; Roos, K.; Oras, A.; Kirss, A.; Ilonen, J.; Uibo, R. Interleukin-7, T helper 1, and regulatory T-cell activity-related cytokines are increased during the second trimester of healthy pregnancy compared to non-pregnant women. Am. J. Reprod. Immunol. 2019, 82, e13188. [Google Scholar] [CrossRef]
- Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines. Int. J. Mol. Sci. 2023, 24, 8130. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, J.; Zhang, Y.; Liu, S.; Yang, J.; Yin, T. Regulation and Function of Chemokines at the Maternal-Fetal Interface. Front. Cell Dev. Biol. 2022, 10, 826053. [Google Scholar] [CrossRef]
- Baston-Büst, D.M.; Schanz, A.; Böddeker, S.J.; Altergot-Ahmad, O.; Krüssel, J.S.; Rein, D.; Hess, A.P. CXCL1 expression in human decidua in vitro is mediated via the MAPK signalling cascade. Cytokine 2013, 64, 79–85. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Lin, W.; Yao, J.; Jiang, X.; Shu, Q.; Mao, X.; Tu, J.; Liang, X.; Li, L. Extramedullary hematopoiesis contributes to enhanced erythropoiesis during pregnancy via TGF-β signaling. Front. Immunol. 2023, 14, 1295717. [Google Scholar] [CrossRef]
- Dunsmore, G.; Koleva, P.; Ghobakhloo, N.; Sutton, R.; Ambrosio, L.; Meng, X.; Hotte, N.; Nguyen, V.; Madsen, K.L.; Dieleman, L.A.; et al. Lower Abundance and Impaired Function of CD71+ Erythroid Cells in Inflammatory Bowel Disease Patients During Pregnancy. J. Crohns Colitis 2019, 13, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Peltier, M.R. Immunology of term and preterm labor. Reprod. Biol. Endocrinol. 2003, 1, 122. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Zhao, Y.; Li, J.; Xie, M.; Lu, Y.; Peng, Q.; Zhang, J.; Li, P.; Dai, L. Deciphering the Intercellular Communication Network of Peripartum Decidua that Orchestrates Delivery. Front. Cell Dev. Biol. 2021, 9, 770621. [Google Scholar] [CrossRef] [PubMed]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife 2019, 8, e52004. [Google Scholar] [CrossRef]
- Vikberg, S.; Lindau, R.; Solders, M.; Raffetseder, J.; Budhwar, S.; Ernerudh, J.; Tiblad, E.; Kaipe, H. Labour promotes systemic mobilisation of monocytes, T cell activation and local secretion of chemotactic factors in the intervillous space of the placenta. Front. Immunol. 2023, 14, 1129261. [Google Scholar] [CrossRef]
- Haddad, R.; Tromp, G.; Kuivaniemi, H.; Chaiworapongsa, T.; Kim, Y.M.; Mazor, M.; Romero, R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am. J. Obstet. Gynecol. 2006, 195, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pan, J.; Tian, X.; Dong, X.; Ju, W.; Wang, Y.; Zhong, N. Transcriptomics-determined chemokine-cytokine pathway presents a common pathogenic mechanism in pregnancy loss and spontaneous preterm birth. Am. J. Reprod. Immunol. 2021, 86, e13398. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Boros-Rausch, A.; Farine, T.; Adams Waldorf, K.M.; Dunk, C.; Lye, S.J. Decidual Inflammation Drives Chemokine-Mediated Immune Infiltration Contributing to Term Labor. J. Immunol. 2021, 207, 2015–2026. [Google Scholar] [CrossRef]
- Svenvik, M.; Jenmalm, M.C.; Brudin, L.; Raffetseder, J.; Hellberg, S.; Axelsson, D.; Lindell, G.; Blomberg, M.; Ernerudh, J. Chemokine and cytokine profiles in preterm and term labor, in preterm prelabor rupture of the membranes, and in normal pregnancy. J. Reprod. Immunol. 2024, 164, 104278. [Google Scholar] [CrossRef]
- Laudanski, P.; Lemancewicz, A.; Kuc, P.; Charkiewicz, K.; Ramotowska, B.; Kretowska, M.; Jasinska, E.; Raba, G.; Karwasik-Kajszczarek, K.; Kraczkowski, J.; et al. Chemokines profiling of patients with preterm birth. Mediat. Inflamm. 2014, 2014, 185758. [Google Scholar] [CrossRef]
- Baba, M.; Imai, T.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Hieshima, K.; Nomiyama, H.; Yoshie, O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 1997, 272, 14893–14898. [Google Scholar] [CrossRef]
- Soto, E.; Espinoza, J.; Nien, J.K.; Kusanovic, J.P.; Erez, O.; Richani, K.; Santolaya-Forgas, J.; Romero, R. Human beta-defensin-2: A natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J. Matern. Fetal Neonatal Med. 2007, 20, 15–22. [Google Scholar] [CrossRef]
- Hamill, N.; Romero, R.; Gotsch, F.; Kusanovic, J.P.; Edwin, S.; Erez, O.; Than, N.G.; Mittal, P.; Espinoza, J.; Friel, L.A.; et al. Exodus-1 (CCL20): Evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J. Perinat. Med. 2008, 36, 217–227. [Google Scholar] [CrossRef]
- Hamilton, S.A.; Tower, C.L.; Jones, R.L. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: Potential novel targets for preterm labour. PLoS ONE 2013, 8, e56946. [Google Scholar] [CrossRef]
- Young, A.; Thomson, A.J.; Ledingham, M.; Jordan, F.; Greer, I.A.; Norman, J.E. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol. Reprod. 2002, 66, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasingam, S.P.; Imami, N.; Johnson, M.R. Myometrial cytokines and their role in the onset of labour. J. Endocrinol. 2016, 231, R101–R119. [Google Scholar] [CrossRef]
- Shynlova, O.; Dorogin, A.; Li, Y.; Lye, S. Inhibition of infection-mediated preterm birth by administration of broad spectrum chemokine inhibitor in mice. J. Cell Mol. Med. 2014, 18, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Pease, J.E.; Sooranna, S.R.; Viney, J.M.; Nelson, S.M.; Myatt, L.; Bennett, P.R.; Johnson, M.R. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-κB activation. Endocrinology 2012, 153, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Estrada-Gutierrez, G.; Jimenez-Zamudio, L.; Vega-Sanchez, R.; Vadillo-Ortega, F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J. Reprod. Immunol. 2009, 80, 122–131. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Hernandez-Santiago, S.; Lobb, A.P.; Olson, D.M.; Vadillo-Ortega, F. Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod. Sci. 2013, 20, 276–284. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; StLouis, D.; Lehr, M.A.; Sanchez-Rodriguez, E.N.; Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell Mol. Immunol. 2014, 11, 571–581. [Google Scholar] [CrossRef]
- Shynlova, O.; Tsui, P.; Dorogin, A.; Lye, S.J. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J. Immunol. 2008, 181, 1470–1479. [Google Scholar] [CrossRef]
- Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Young, A.; Crawford, M.; Norman, J.E. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet. Gynecol. 2001, 97, 235–242. [Google Scholar]
- Jones, R.L.; Hannan, N.J.; Kaitu’u, T.J.; Zhang, J.; Salamonsen, L.A. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J. Clin. Endocrinol. Metab. 2004, 89, 6155–6167. [Google Scholar] [CrossRef]
- Mittal, P.; Romero, R.; Tarca, A.L.; Gonzalez, J.; Draghici, S.; Xu, Y.; Dong, Z.; Nhan-Chang, C.L.; Chaiworapongsa, T.; Lye, S.; et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J. Perinat. Med. 2010, 38, 617–643. [Google Scholar] [CrossRef]
- Wuyts, A.; Struyf, S.; Gijsbers, K.; Schutyser, E.; Put, W.; Conings, R.; Lenaerts, J.P.; Geboes, K.; Opdenakker, G.; Menten, P.; et al. The CXC chemokine GCP-2/CXCL6 is predominantly induced in mesenchymal cells by interleukin-1beta and is down-regulated by interferon-gamma: Comparison with interleukin-8/CXCL8. Lab. Investig. 2003, 83, 23–34. [Google Scholar] [CrossRef]
- Bradley, E.; Blencowe, H.; Moller, A.B.; Okwaraji, Y.B.; Sadler, F.; Gruending, A.; Moran, A.C.; Requejo, J.; Ohuma, E.O.; Lawn, J.E. Born too soon: Global epidemiology of preterm birth and drivers for change. Reprod. Health 2025, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The biological basis and prevention of preterm birth. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 13–22. [Google Scholar] [CrossRef]
- Vinketova, K.; Mourdjeva, M.; Oreshkova, T. Human Decidual Stromal Cells as a Component of the Implantation Niche and a Modulator of Maternal Immunity. J. Pregnancy 2016, 2016, 8689436. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Lee, Y.H.; Srikhajon, K.; Lye, S.J. Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reprod. Sci. 2013, 20, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.G.; Shynlova, O.; Wu, X.; Kibschull, M.; Wang, K.; Price, N.D.; Lye, S.J. MicroRNA-transcriptome networks in whole blood and monocytes of women undergoing preterm labour. J. Cell Mol. Med. 2019, 23, 6835–6845. [Google Scholar] [CrossRef]
- Shynlova, O.; Nadeem, L.; Zhang, J.; Dunk, C.; Lye, S. Myometrial activation: Novel concepts underlying labor. Placenta 2020, 92, 28–36. [Google Scholar] [CrossRef]
- Singh, N.; Herbert, B.; Sooranna, G.; Das, A.; Sooranna, S.R.; Yellon, S.M.; Johnson, M.R. Distinct preterm labor phenotypes have unique inflammatory signatures and contraction associated protein profiles†. Biol. Reprod. 2019, 101, 1031–1045. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Galaz, J.; Xu, Y.; Panaitescu, B.; Slutsky, R.; Motomura, K.; Gill, N.; Para, R.; Pacora, P.; et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am. J. Reprod. Immunol. 2020, 84. [Google Scholar] [CrossRef]
- Romero, R.; Grivel, J.C.; Tarca, A.L.; Chaemsaithong, P.; Xu, Z.; Fitzgerald, W.; Hassan, S.S.; Chaiworapongsa, T.; Margolis, L. Evidence of perturbations of the cytokine network in preterm labor. Am. J. Obstet. Gynecol. 2015, 213, 836.e1–836.e18. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Lin, Y.K.; Zhang, F.; Lei, W.J.; Pan, F.; Zhu, Y.N.; Lu, J.W.; Zhang, C.Y.; Zhou, Q.; Ying, H.; et al. Single cell transcriptomic analysis of human amnion identifies cell-specific signatures associated with membrane rupture and parturition. Cell Biosci. 2022, 12, 64. [Google Scholar] [CrossRef]
- Maymon, E.; Romero, R.; Bhatti, G.; Chaemsaithong, P.; Gomez-Lopez, N.; Panaitescu, B.; Chaiyasit, N.; Pacora, P.; Dong, Z.; Hassan, S.S.; et al. Chronic inflammatory lesions of the placenta are associated with an up-regulation of amniotic fluid CXCR3: A marker of allograft rejection. J. Perinat. Med. 2018, 46, 123–137. [Google Scholar] [CrossRef]
- Kahouadji, S.; Giguère, Y.; Lambert, S.; Forest, J.C.; Bernard, N.; Blanchon, L.; Marceau, G.; Durif, J.; Pereira, B.; Gallot, D.; et al. CX3CL1/Fractalkine as a biomarker for early pregnancy prediction of preterm premature rupture of membranes. Clin. Chem. Lab. Med. 2024, 62, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1775–1812, Erratum in Lancet 2017, 389, e1. [Google Scholar]
- Liu, X.; Dai, L.I.; Zhou, R. Association between preeclampsia and the CXC chemokine family (Review). Exp. Ther. Med. 2015, 9, 1572–1576. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A critical review of early-onset and late-onset preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef]
- Kim, Y.N.; Lee, D.S.; Jeong, D.H.; Sung, M.S.; Kim, K.T. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat. Diagn. 2009, 29, 464–470. [Google Scholar] [CrossRef]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Molvarec, A.; Szarka, A.; Walentin, S.; Beko, G.; Karádi, I.; Prohászka, Z.; Rigó, J., Jr. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod. Biol. Endocrinol. 2011, 9, 124. [Google Scholar] [CrossRef]
- Kauma, S.; Takacs, P.; Scordalakes, C.; Walsh, S.; Green, K.; Peng, T. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet. Gynecol. 2002, 100, 706–714. [Google Scholar]
- Schanz, A.; Winn, V.D.; Fisher, S.J.; Blumenstein, M.; Heiss, C.; Hess, A.P.; Kruessel, J.S.; Mcmaster, M.; North, R.A. Pre-eclampsia is associated with elevated CXCL12 levels in placental syncytiotrophoblasts and maternal blood. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 32–37. [Google Scholar] [CrossRef]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Wang, X.; Yip, K.C.; He, A.; Tang, J.; Liu, S.; Yan, R.; Zhang, Q.; Li, R. Plasma Olink Proteomics Identifies CCL20 as a Novel Predictive and Diagnostic Inflammatory Marker for Preeclampsia. J. Proteome Res. 2022, 21, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.T.; Seow, K.M.; Chen, K.H. The Pathophysiological, Genetic, and Hormonal Changes in Preeclampsia: A Systematic Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2024, 25, 4532. [Google Scholar] [CrossRef]
- Gui, S.; Ni, S.; Jia, J.; Gong, Y.; Gao, L.; Zhang, L.; Zhou, R. Inconformity of CXCL3 plasma level and placenta expression in preeclampsia and its effect on trophoblast viability and invasion. PLoS ONE 2014, 9, e114408. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Dai, L.; Cao, W.; Ye, L.; Gao, L.; Zhou, B.; Zhou, R. Effects of CXCL3 on migration, invasion, proliferation and tube formation of trophoblast cells. Placenta 2018, 66, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Q.; Liu, H.; Gao, Y.; Yang, X.; Ren, Z.; Gao, Y.; Xiao, L.; Zhong, M.; Yu, Y.; et al. Preeclampsia-Associated lncRNA INHBA-AS1 Regulates the Proliferation, Invasion, and Migration of Placental Trophoblast Cells. Mol. Ther. Nucleic Acids 2020, 22, 684–695. [Google Scholar] [CrossRef]
- Liao, L.; Liu, M.; Gao, Y.; Wei, X.; Yin, Y.; Gao, L.; Zhou, R. The long noncoding RNA TARID regulates the CXCL3/ERK/MAPK pathway in trophoblasts and is associated with preeclampsia. Reprod. Biol. Endocrinol. 2022, 20, 159. [Google Scholar] [CrossRef]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential roles of CXCL2 and CXCL3 and their receptors in regulating normal and asthmatic airway smooth muscle cell migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef]
- Qi, Y.L.; Li, Y.; Man, X.X.; Sui, H.Y.; Zhao, X.L.; Zhang, P.X.; Qu, X.S.; Zhang, H.; Wang, B.X.; Li, J.; et al. CXCL3 overexpression promotes the tumorigenic potential of uterine cervical cancer cells via the MAPK/ERK pathway. J. Cell Physiol. 2020, 235, 4756–4765. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Liu, X.; Liu, B.; Che, Y.; Han, R. Downregulation of miR-92a in Decidual Stromal Cells Suppresses Migration Ability of Trophoblasts by Promoting Macrophage Polarization. DNA Cell Biol. 2023, 42, 507–514. [Google Scholar] [CrossRef]
- Hu, X.; Huang, X.; Yin, T.; Chen, J.; Zhao, W.; Yu, M.; Liu, L.; Du, M. CX3CL1 (Fractalkine): An important cytokine in physiological and pathological pregnancies. J. Reprod. Immunol. 2024, 166, 104392. [Google Scholar] [CrossRef]
- Lekva, T.; Sugulle, M.; Moe, K.; Redman, C.; Dechend, R.; Staff, A.C. Multiplex Analysis of Circulating Maternal Cardiovascular Biomarkers Comparing Preeclampsia Subtypes. Hypertension 2020, 75, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Zhao, J.; Singla, R.K.; Shen, B. Pathophysiological impact of CXC and CX3CL1 chemokines in preeclampsia and gestational diabetes mellitus. Front. Cell Dev. Biol. 2023, 11, 1272536. [Google Scholar] [CrossRef]
- Szewczyk, G.; Pyzlak, M.; Pankiewicz, K.; Szczerba, E.; Stangret, A.; Szukiewicz, D.; Skoda, M.; Bierła, J.; Cukrowska, B.; Fijałkowska, A. The potential association between a new angiogenic marker fractalkine and a placental vascularization in preeclampsia. Arch. Gynecol. Obstet. 2021, 304, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Namkoong, S.; Kim, Y.M.; Kim, C.K.; Lee, H.; Ha, K.S.; Chung, H.T.; Kwon, Y.G.; Kim, Y.M. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2836–H2846. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, O.; Mollica Poeta, V.; Setten, E.; Massara, M.; Bonecchi, R. Corrigendum: ACKR2: An Atypical Chemokine Receptor Regulating Lymphatic Biology. Front. Immunol. 2017, 8, 520. [Google Scholar] [CrossRef]
- Bonecchi, R.; Garlanda, C.; Mantovani, A.; Riva, F. Cytokine decoy and scavenger receptors as key regulators of immunity and inflammation. Cytokine 2016, 87, 37–45. [Google Scholar] [CrossRef]
- Graham, G.J. D6/ACKR2. Front. Immunol. 2015, 6, 280. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Kriehuber, E.; Ponath, P.D.; Parent, D.; Qin, S.; Campbell, J.D.; Henderson, A.; Kerjaschki, D.; Maurer, D.; Graham, G.J.; et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am. J. Pathol. 2001, 158, 867–877. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Wylie, S.M.; Yang, J.; Landau, N.R.; Graham, G.J. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. J. Biol. Chem. 1997, 272, 32078–32083. [Google Scholar] [CrossRef]
- Yan, S.; Cui, S.; Zhang, L.; Yang, B.; Yuan, Y.; Lv, X.; Fu, H.; Li, Y.; Huang, C.; Wang, P. Expression of ACKR2 in placentas from different types of preeclampsia. Placenta 2020, 90, 121–127. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Placental stress and pre-eclampsia: A revised view. Placenta 2009, 30, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Salazar Garcia, M.D.; Mobley, Y.; Henson, J.; Davies, M.; Skariah, A.; Dambaeva, S.; Gilman-Sachs, A.; Beaman, K.; Lampley, C.; Kwak-Kim, J. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J. Reprod. Immunol. 2018, 125, 25–31. [Google Scholar] [CrossRef]
- Karimabad, M.N.; Mahmoodi, M.; Jafarzadeh, A.; Darehkordi, A.; Hajizadeh, M.R.; Khorramdelazad, H.; Sayadi, A.R.; Rahmani, F.; Hassanshahi, G. Evaluating of OCT-4 and NANOG was differentially regulated by a new derivative indole in leukemia cell line. Immunol. Lett. 2017, 190, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Hassanshahi, G.; Mofidifar, Z.; Soltani, B.; Karimabad, M.N. CXCL9/CXCL10 angiostasis CXC-chemokines in parallel with the CXCL12 as an angiogenesis CXC-chemokine are variously expressed in pre-eclamptic-women and their neonates. Pregnancy Hypertens. 2019, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Najmaddini, H.; Hassanshahi, G.; Ostadebrahimi, H.; Barkhordari, H.; Mashayekhi, H.; Nazari, M.; Moogooei, M.; Arababadi, Y.S.; Peighambari, F.; Karimabad, M.N. Overproduction of CXC chemokines CXCL1, CXCL9, CXCL10 and CXCL12 in β-thalassemia major or patients. Ann. Saudi Med. 2014, 34, 122–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevchenko, J.A.; Gizbrekht, A.A.; Sennikov, S.V. Chemokines in Pregnancy. Biomolecules 2025, 15, 1645. https://doi.org/10.3390/biom15121645
Shevchenko JA, Gizbrekht AA, Sennikov SV. Chemokines in Pregnancy. Biomolecules. 2025; 15(12):1645. https://doi.org/10.3390/biom15121645
Chicago/Turabian StyleShevchenko, Julia A., Alina A. Gizbrekht, and Sergey V. Sennikov. 2025. "Chemokines in Pregnancy" Biomolecules 15, no. 12: 1645. https://doi.org/10.3390/biom15121645
APA StyleShevchenko, J. A., Gizbrekht, A. A., & Sennikov, S. V. (2025). Chemokines in Pregnancy. Biomolecules, 15(12), 1645. https://doi.org/10.3390/biom15121645





