The Possible Role of Neurofilament Light Chain as a Serum Biomarker in Anorexia Nervosa: Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. NfL Assay
2.3. ELISA Protocol for Anti-Hypothalamus Autoantibodies Detection
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
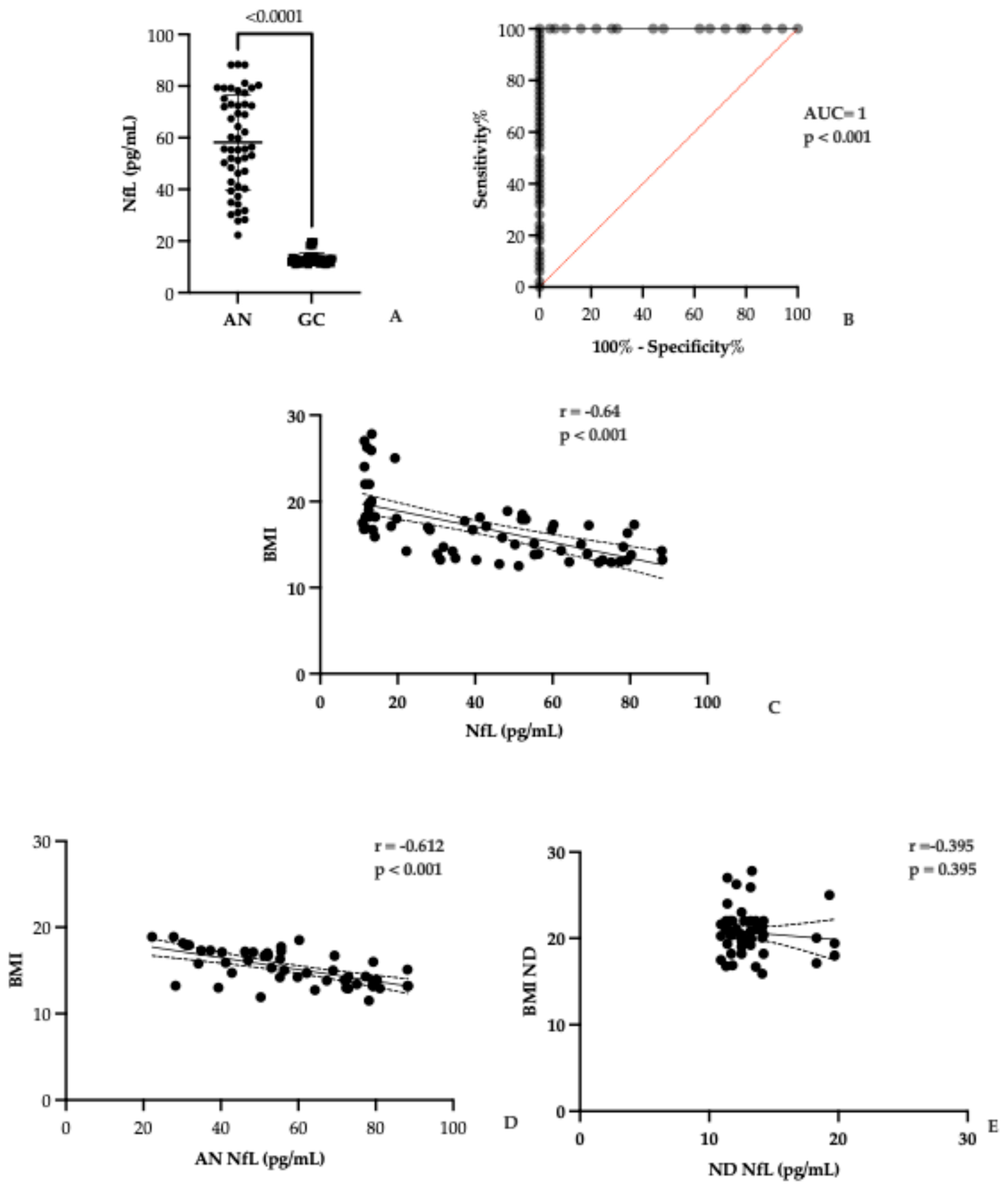
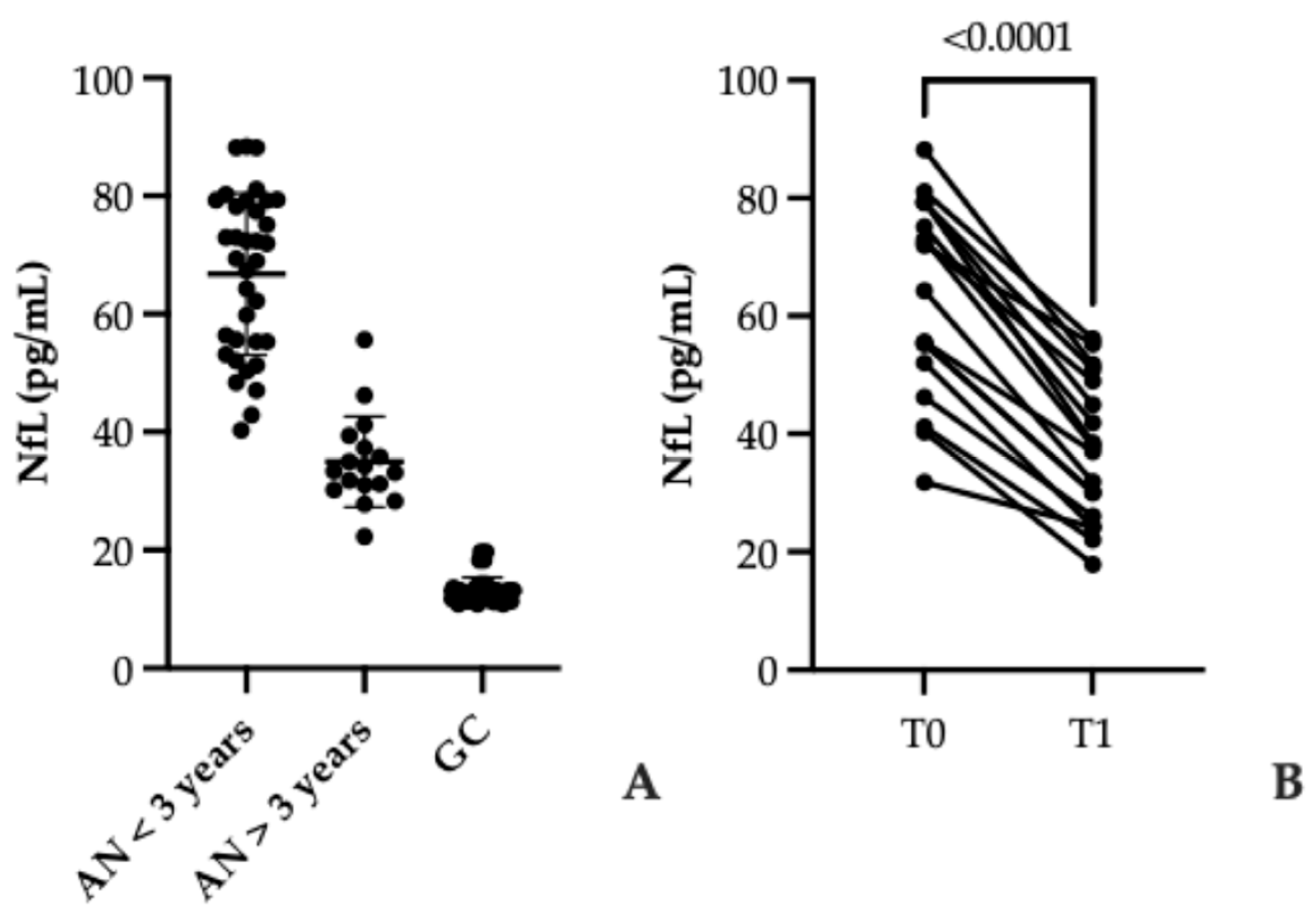
3.2. Serum NfL Levels in AN and Controls
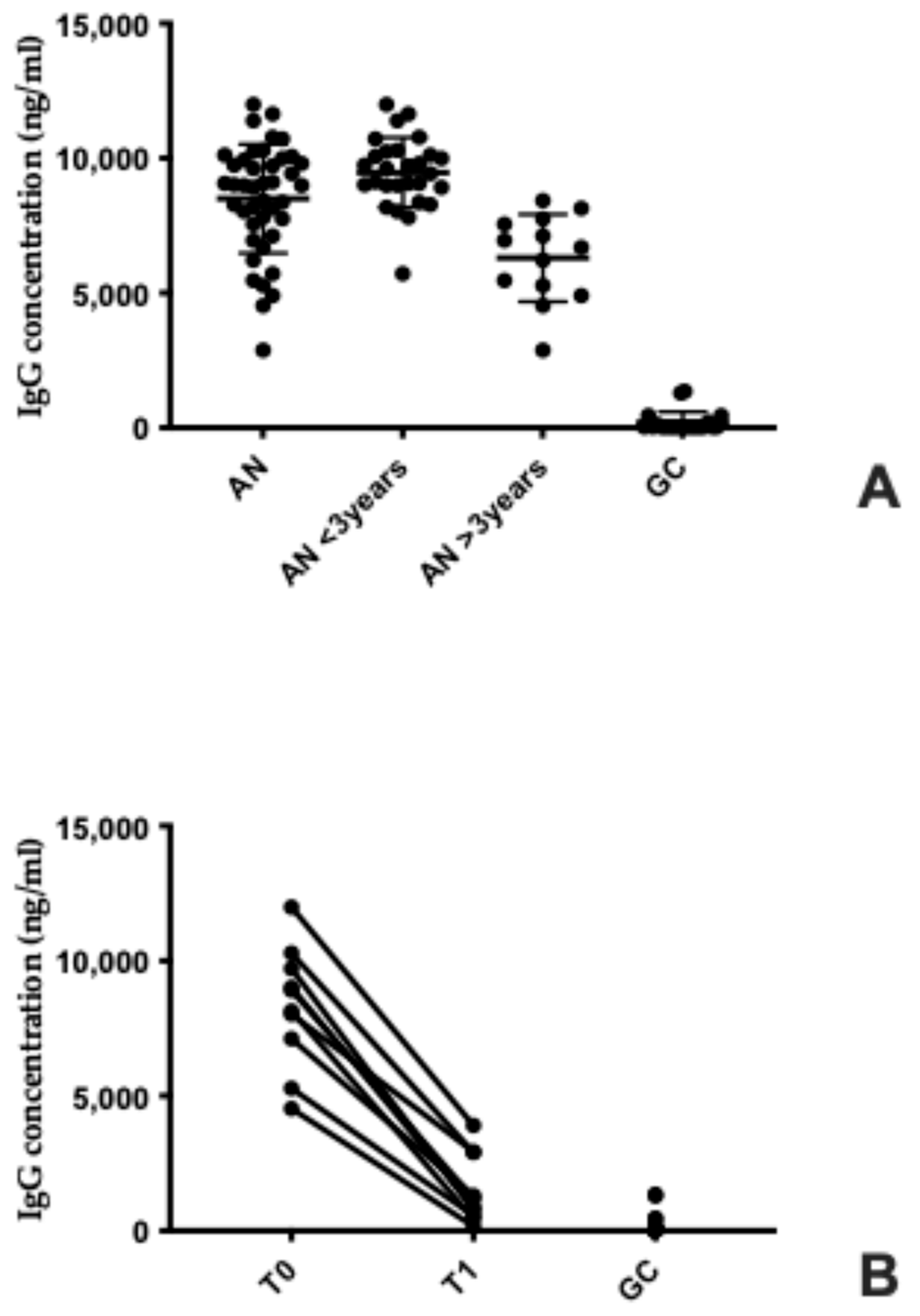
3.3. Elevated Serum Anti-Hypothalamus Autoantibodies in Anorexia Nervosa: Association with Disease Duration and Nutritional Recovery
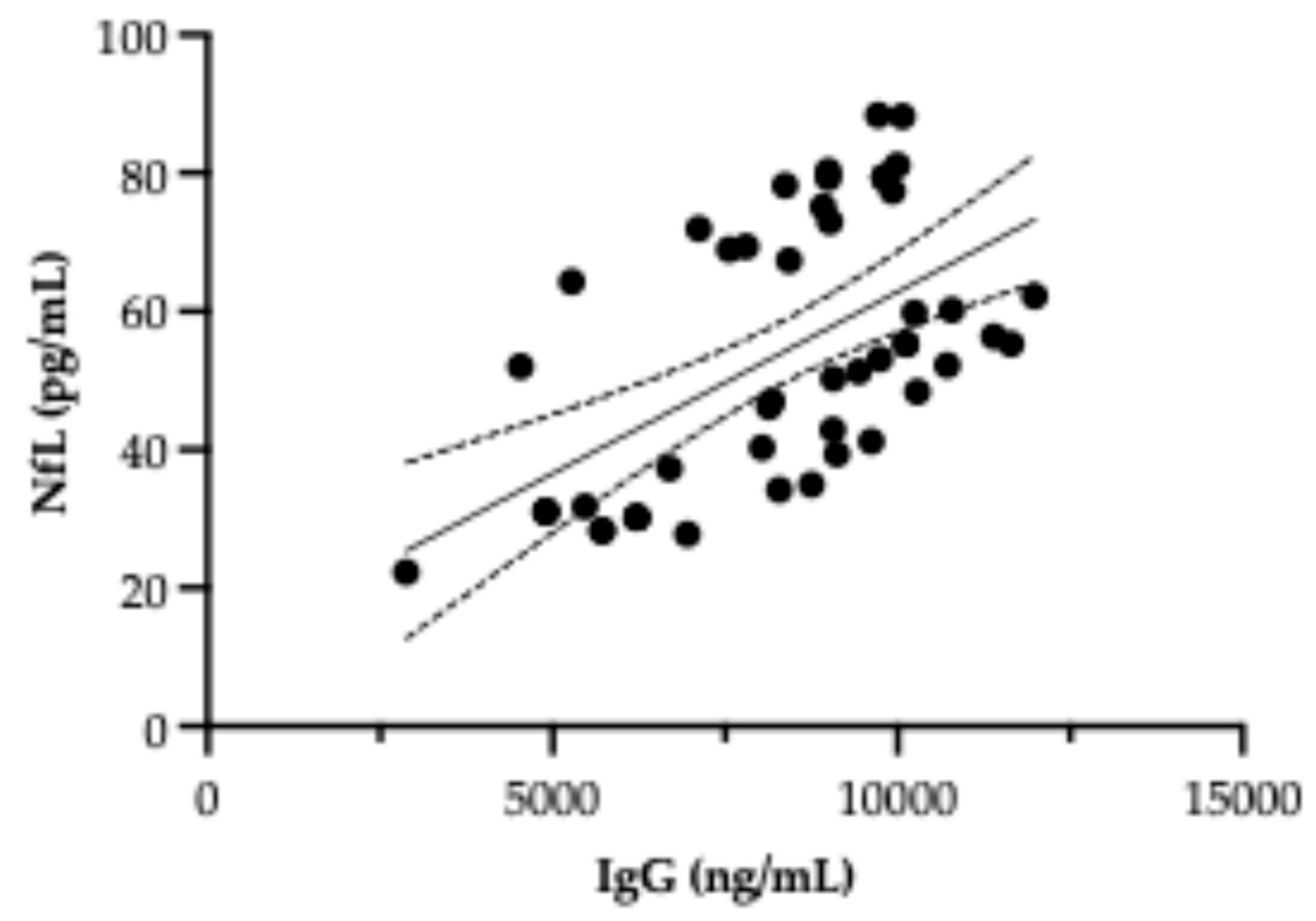
3.4. Investigation of a Possible Correlation Between NfL and Autoantibody Levels
4. Discussion
4.1. NfL as a Marker of Neuronal Injury in AN
4.2. Specificity and Limitations of Serum NfL
4.3. Autoantibodies and Hypothalamic Dysfunction
4.4. Clinical Implications
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westmoreland, P.; Krantz, M.J.; Mehler, P.S. Medical complications of anorexia nervosa and bulimia. Am. J. Med. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Fricke, C.; Voderholzer, U. Hormonal aspects of eating disorders. In Eating Disorders; Robinson, P., Wade, T., Herpertz-Dahlmann, B., Fernandez-Aranda, F., Treasure, J., Wonderlich, S., Eds.; Springer: Cham, Switzerland, 2024; pp. 497–510. [Google Scholar] [CrossRef]
- King, J.A.; Frank, G.K.W.; Thompson, P.M.; Ehrlich, S. Structural neuroimaging of anorexia nervosa: Future directions in the quest for mechanisms underlying dynamic alterations. Biol. Psychiatry 2018, 83, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Gong, J.; Tang, G.; Qiu, S.; Chen, P.; Chen, G.; Wang, J.; Huang, L.; Wang, Y. Structural and functional brain alterations in anorexia nervosa: A multimodal meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2021, 42, 5154–5169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seitz, J.; Bühren, K.; von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa: A meta-analysis and qualitative review. Z. Für Kinder Und Jugendpsychiatrie Und Psychother. 2014, 42, 7–18. [Google Scholar] [CrossRef]
- Bernardoni, F.; King, J.A.; Geisler, D.; Stein, E.; Jaite, C.; Nätsch, D.; Tam, F.I.; Boehm, I.; Seidel, M.; Roessner, V.; et al. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. NeuroImage 2016, 130, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.; Bernardoni, F.; Batury, V.-L.; Bahnsen, K.; Larivière, S.; Abbate-Daga, G.; Andres-Perpiña, S.; Bang, L.; Bischoff-Grethe, A.; Brooks, S.J.; et al. Brain structure in acutely underweight and partially weight-restored individuals with anorexia nervosa: A coordinated analysis by the ENIGMA Eating Disorders Working Group. Biol. Psychiatry 2022, 92, 730–738. [Google Scholar] [CrossRef]
- Micali, N.; Miletta, M.C.; Clemmensen, C.; Pappaianni, E.; Lazeyras, F.; Cuenoud, B.; Sandi, C. Providing alternative fuel for the brain in anorexia nervosa: A review of the literature on ketones and their effects on metabolism and the brain. Transl. Psychiatry. 2025, 15, 412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahnsen, K.; Wronski, M.L.; Keeler, J.L.; King, J.A.; Preusker, Q.; Kolb, T.; Weidner, K.; Roessner, V.; Bernardoni, F.; Ehrlich, S. Differential longitudinal changes of hippocampal subfields in patients with anorexia nervosa. Psychiatry Clin. Neurosci. 2024, 78, 186–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Specht, H.E.; Mannig, N.; Belheouane, M.; Andreani, N.A.; Tenbrock, K.; Biemann, R.; Borucki, K.; Dahmen, B.; Dempfle, A.; Baines, J.F.; et al. Lower serum levels of IL-1β and IL-6 cytokines in adolescents with anorexia nervosa and their association with gut microbiota in a longitudinal study. Front. Psychiatry 2022, 13, 920665. [Google Scholar] [CrossRef]
- Brown, R.F.; Bartrop, R.; Birmingham, C.L. Immunological disturbance and infectious disease in anorexia nervosa: A review. Acta Neuropsychiatr. 2008, 20, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.; Mehler, P.S. Anorexia nervosa and the immune system—A narrative review. J. Clin. Med. 2019, 8, 1915. [Google Scholar] [CrossRef]
- Maunder, K.; Molloy, E.; Jenkins, E.; Hayden, J.; Adamis, D.; McNicholas, F. Anorexia nervosa in vivo cytokine production: A systematic review. Psychoneuroendocrinology 2023, 158, 106390. [Google Scholar] [CrossRef]
- Słotwińska, S.M.; Słotwiński, R. Immune disorders in anorexia. Cent. Eur. J. Immunol. 2017, 42, 294–300. [Google Scholar] [CrossRef]
- Amerio, A.; Martino, E.; Strangio, A.; Aguglia, A.; Escelsior, A.; Conio, B.; Sukkar, S.G.; Saverino, D. Autoantibodies, oxidative stress, and nutritional state in anorexia nervosa. Antibodies 2025, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Amerio, A.; Escelsior, A.; Martino, E.; Strangio, A.; Giacomini, C.; Montagna, E.; Aguglia, A.; Bellomo, M.; Sukkar, S.G.; Saverino, D. Dysfunction of Inflammatory Pathways and Their Relationship with Anti-Hypothalamic Autoantibodies in Patients with Anorexia Nervosa. Nutrients 2023, 15, 2199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dani, C.; Tarchi, L.; Cassioli, E.; Rossi, E.; Merola, G.P.; Ficola, A.; Cordasco, V.Z.; Ricca, V.; Castellini, G. A transdiagnostic and diagnostic-specific approach on inflammatory biomarkers in eating disorders: A meta-analysis and systematic review. Psychiatry Res. 2024, 340, 116115. [Google Scholar] [CrossRef]
- Doose, M.; Tsui, J.; Steinberg, M.B.; Xing, C.Y.; Lin, Y.; Cantor, J.C.; Hong, C.-C.; Demissie, K.; Bandera, E.V. Patterns of chronic disease management and health outcomes in a population-based cohort of Black women with breast cancer. Cancer Causes Control 2021, 32, 157–168. [Google Scholar] [CrossRef]
- Hellerhoff, I.; King, J.A.; Tam, F.I.; Pauligk, S.; Seidel, M.; Geisler, D.; Bahnsen, K.; Kretschmann, N.; Akgün, K.; Roessner, V.; et al. Differential longitudinal changes of neuronal and glial damage markers in anorexia nervosa after partial weight restoration. Transl. Psychiatry 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Bergquist, M. The DOs and DON’Ts in social norms: A descriptive don’t-norm increases conformity. J. Theor. Soc. Psychol. 2019, 3, 158–166. [Google Scholar] [CrossRef]
- Wentz, L.M.; Kashi, D.S.; Oliver, S.J.; Roberts, R.; Carswell, A.T.; Tang, J.C.Y.; Harrison, S.E. Vitamin D and the hepatitis B vaccine response: A prospective cohort study and a randomized, placebo-controlled oral vitamin D3 and simulated sunlight intervention. Eur. J. Nutr. 2021, 60, 475–491. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Ehrlich, A.H. The Dominant Animal: Human Evolution and the Environment; Island Press: Washington, DC, USA, 2008. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [Google Scholar] [CrossRef] [PubMed]
- Quanterix Corporation. Advances in Ultra-Sensitive Biomarker Detection. 2017. Available online: https://www.quanterix.com (accessed on 1 July 2025).
- Salama, A.; Khalil, W.; Al-Zaky, M.; Abdallah, S.; Kandil, N.; Shaker, A.; Hasanein, M.; Luciani, G.B.; Azzazy, H.M.E. MicroRNA-208a: A good diagnostic marker and a predictor of no-reflow in STEMI patients undergoing primary percutaneous coronary intervention. J. Cardiovasc. Transl. Res. 2020, 13, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Ayrignac, X.; Le Bars, E.; Duhamel, G.; Maleska, M.; Hirtz, C.; Mura, T.; Carra-Dalliere, C.; Charif, M.; Menjot de Champfleur, N.; Labauge, P.; et al. Serum neurofilament light chain levels as a marker of disease activity in multiple sclerosis. J. Neurol. 2020, 267, 282–290. [Google Scholar]
- Dimitrova, L.I.; Dean, S.L.; Schlumpf, Y.R.; Vissia, E.M.; Nijenhuis, E.R.S.; Chatzi, V.; Jäncke, L.; Veltman, D.J.; Chalavi, S.; Reinders, A.A.T.S. A neurostructural biomarker of dissociative amnesia: A hippocampal study in dissociative identity disorder. Psychol. Med. 2021, 53, 805–813. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zhang, Y.; Zhou, G.; Chen, B.; Zeng, Z.; Chen, M.; Zhang, S. A novel model based on serum biomarkers to predict primary non-response to infliximab in Crohn’s disease. Front. Immunol. 2021, 12, 646673. [Google Scholar] [CrossRef]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef]
- Bavato, F.; Barro, C.; Schnider, L.K.; Simrén, J.; Zetterberg, H.; Seifritz, E.; Quednow, B.B. Introducing neurofilament light chain measure in psychiatry: Current evidence, opportunities, and pitfalls. Mol. Psychiatry 2024, 29, 2543–2559. [Google Scholar] [CrossRef]
- Hohm, H.L.; Schuster, R.; Buciu, V.B.; Serban, D.M.; Ciurescu, S.; Cornea, A.; Sharma, A.; Nistor, D.; Kundnani, N.R. Glial Cytokine and Metabolic Networks in Progressive Multiple Sclerosis: From Pathophysiology to Biomarkers and Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 8817. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Escelsior, A.; Cogorno, L.; Sukkar, S.G.; Amerio, A.; Donini, L.M.; Bellomo, M.; Iervasi, E.; Amore, M.; Saverino, D. Anti-hypothalamus autoantibodies in anorexia nervosa: A possible new mechanism in neuro-physiological derangement? Eat Weight Disord. 2022, 27, 2481–2496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stephens, M.A. Tests based on EDF statistics. In Goodness-of-Fit Techniques; D’Agostino, R.B., Stephens, M.A., Eds.; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Cheo, D.; Romero, D.; Kabir, Z.; Padmanabhan, N.; Joe, J.; Lynch, C.; Sandsmark, D.; Everett, A.; Bembea, M.; Diaz-Arrastia, R.; et al. Highly Sensitive Assay for Neurofilament Light in Serum and Plasma. Meso Scale Discovery. 2021. Available online: https://www.mesoscale.com/~/media/files/scientific%20poster/highly-sensitive-assay-neurofilament-light-serum-plasma-tbi-2021.pdf?la=en (accessed on 1 July 2025).
- Zimmermann, A.; Rupprecht, H.; Lang, S.; Wienecke, R.; Henschke, H.S.; Dickert, K.; Schuster, K.; Staffeld, A.; Berger, C.; Dück, A.; et al. Increased Serum Neurofilament Light Chain Concentration Associated With Microglial Morphology Changes in Chronically-Starved Mice. Int. J. Eat Disord. 2025, 58, 1130–1143. [Google Scholar] [CrossRef]
- Varhaug, K.N.; Torkildsen, Ø.; Myhr, K.-M.; Vedeler, C.A. Neurofilament light chain as a biomarker in multiple sclerosis. Front. Neurol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Jung, Y.; Damoiseaux, J.S. The potential of blood neurofilament light as a marker of neurodegeneration for Alzheimer’s disease. Brain 2024, 147, 12–25. [Google Scholar] [CrossRef]
- Turner, M.R.; Thompson, A.G.; Teunissen, C.E. Blood level of neurofilament light chain as a biomarker for neurological disorders. BMJ Med. 2025, 4, e000958. [Google Scholar] [CrossRef]
- Cavaletti, G.; Pizzamiglio, C.; Man, A.; Engber, T.M.; Comi, C.; Wilbraham, D. Studies to Assess the Utility of Serum Neurofilament Light Chain as a Biomarker in Chemotherapy-Induced Peripheral Neuropathy. Cancers 2023, 15, 4216. [Google Scholar] [CrossRef]
- Azcue, N.; Tijero-Merino, B.; Acera, M.; Pérez-Garay, R.; Fernández-Valle, T.; Ayo-Mentxakatorre, N.; Ruiz-López, M.; Lafuente, J.V.; Gómez Esteban, J.C.; Del Pino, R. Plasma Neurofilament Light Chain: A Potential Biomarker for Neurological Dysfunction in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2024, 12, 1539. [Google Scholar] [CrossRef]
- Giovannini, G.; Bedin, R.; Ferraro, D.; Vaudano, A.E.; Mandrioli, J.; Meletti, S. Serum neurofilament light as biomarker of seizure-related neuronal injury in status epilepticus. Epilepsia 2021, 62, 2943–2954. [Google Scholar] [CrossRef]
- Fiorillo, A.; Gallego, J.J.; Casanova-Ferrer, F.; Urios, A.; Ballester, M.-P.; San Miguel, T.; Megías, J.; Kosenko, E.; Tosca, J.; Rios, M.-P.; et al. Neurofilament Light Chain Protein in Plasma and Extracellular Vesicles Is Associated with Minimal Hepatic Encephalopathy and Responses to Rifaximin Treatment in Cirrhotic Patients. Int. J. Mol. Sci. 2023, 24, 14727. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, A.; Ostovan, V.R.; Ghezel, M.A.; Kavari, K.; Kardeh, S.; Tabrizi, R. Neurofilament light chain as a promising biomarker for depression diagnosis: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 617. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef]
- Pafiti, A.; Krashias, G.; Tzartos, J.; Tzartos, S.; Stergiou, C.; Gaglia, E.; Smoleski, I.; Christodoulou, C.; Pantzaris, M.; Lambrianides, A. A Comparison of Two Analytical Approaches for the Quantification of Neurofilament Light Chain, a Biomarker of Axonal Damage in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 10787. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Westwater, M.L.; Furlong, L.; Ziauddeen, H.; Nilsson, I.A.K.; Millischer, V.; Karrenbauer, V.D.; Juréus, A.; Salehi, A.M.; Norring, C.; von Hausswolff-Juhlin., Y.; et al. Blood neurofilament light chain levels are elevated in individuals with anorexia nervosa. Transl. Psychiatr 2021, 11, 1–9. [Google Scholar]
- Himmerich, H.; Mirzaei, K. Body Image, Nutrition, and Mental Health. Nutrients 2024, 16, 1106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Anorexia Nervosa | Control Group | p-Value | |
|---|---|---|---|
| Age (years) | 20.8 ± 9.7 | 27.1 ± 8.61 | 0.45 |
| Body Mass Index (kg/m2) | 16.1 ± 2.0 | 22.3 ± 1.5 | <0.001 |
| Serum markers | |||
| IgG autoantibody to hypothalamic cells (ng/mL) | 8501 ± 2243 | 207 ± 392 | <0.001 |
| NfL (pg/mL) | 58.18 ± 18.44 | 13.39 ± 2.22 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amerio, A.; Martino, E.; Strangio, A.; Aguglia, A.; Conio, B.; Sukkar, S.G.; Saverino, D. The Possible Role of Neurofilament Light Chain as a Serum Biomarker in Anorexia Nervosa: Clinical Implications. Biomolecules 2025, 15, 1644. https://doi.org/10.3390/biom15121644
Amerio A, Martino E, Strangio A, Aguglia A, Conio B, Sukkar SG, Saverino D. The Possible Role of Neurofilament Light Chain as a Serum Biomarker in Anorexia Nervosa: Clinical Implications. Biomolecules. 2025; 15(12):1644. https://doi.org/10.3390/biom15121644
Chicago/Turabian StyleAmerio, Andrea, Eleonora Martino, Antonella Strangio, Andrea Aguglia, Benedetta Conio, Samir Giuseppe Sukkar, and Daniele Saverino. 2025. "The Possible Role of Neurofilament Light Chain as a Serum Biomarker in Anorexia Nervosa: Clinical Implications" Biomolecules 15, no. 12: 1644. https://doi.org/10.3390/biom15121644
APA StyleAmerio, A., Martino, E., Strangio, A., Aguglia, A., Conio, B., Sukkar, S. G., & Saverino, D. (2025). The Possible Role of Neurofilament Light Chain as a Serum Biomarker in Anorexia Nervosa: Clinical Implications. Biomolecules, 15(12), 1644. https://doi.org/10.3390/biom15121644










