Interactions of Mycotoxin Alternariol with Cyclodextrins and Its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Spectroscopic Studies
2.3. Modeling Studies
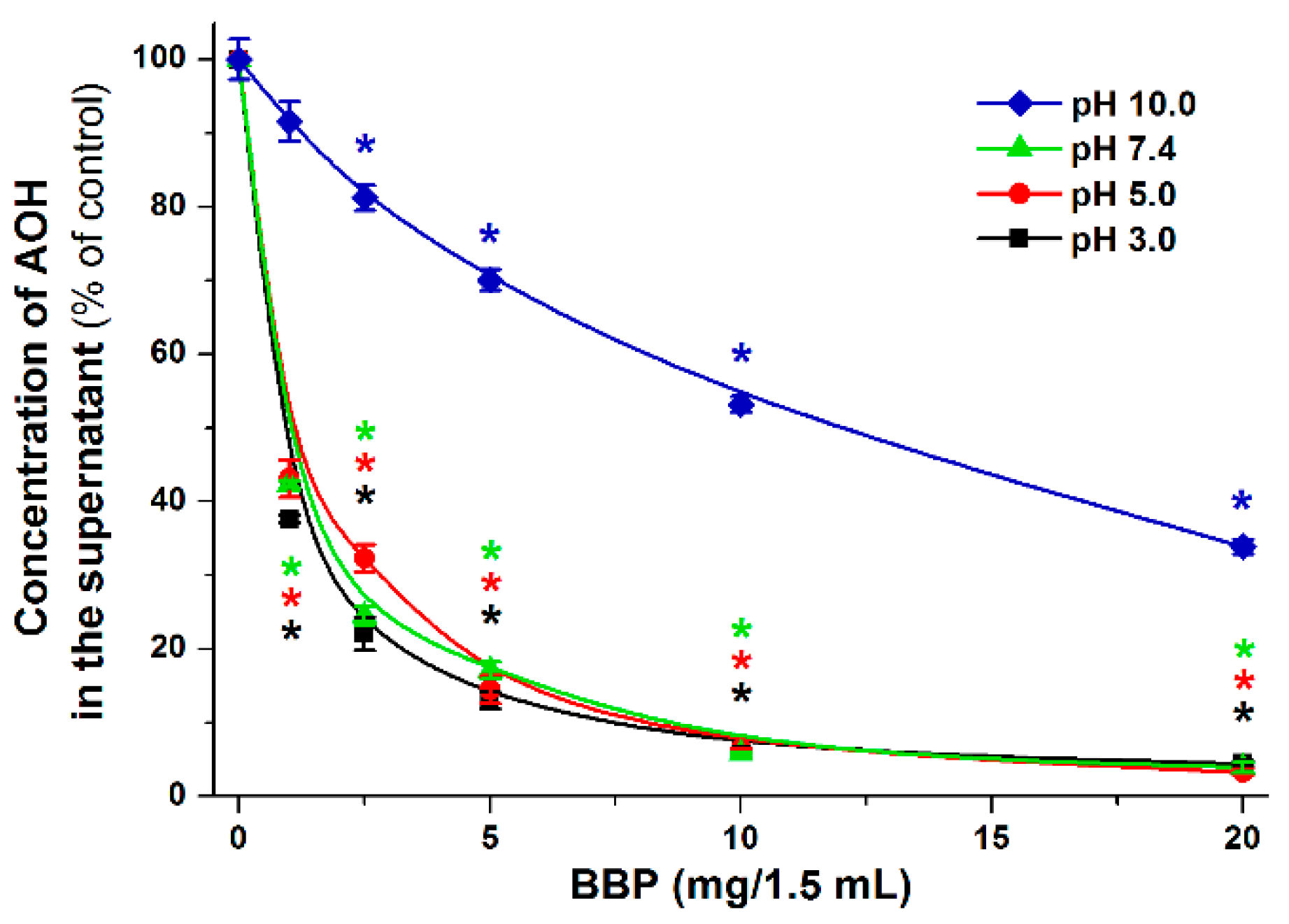
2.4. Extraction of AOH from Aqueous Solution with Insoluble β-Cyclodextrin Bead Polymer
2.5. HPLC Analyses
2.6. Comparison of the Interaction of Alternariol with β-Cyclodextrin, Soluble β-Cyclodextrin Polymer, and Insoluble β-Cyclodextrin Bead Polymer at pH 3
2.7. Statistical Analyses
3. Results
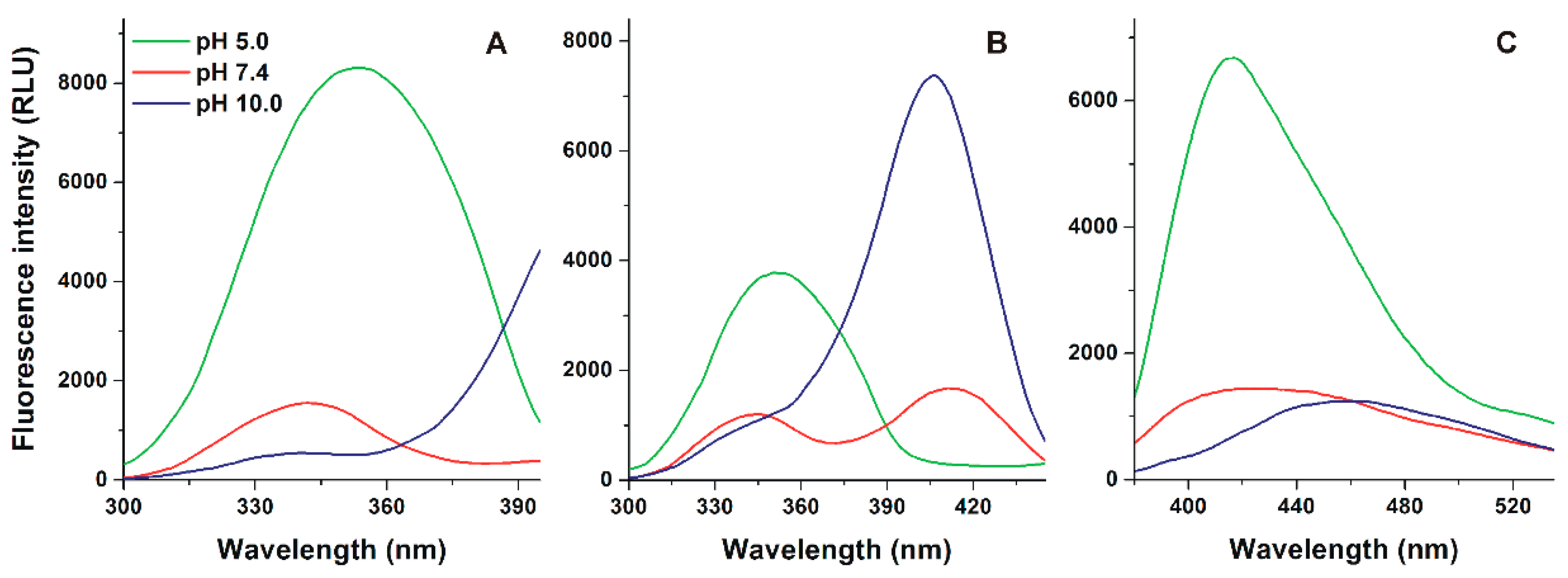
3.1. Effect of the Environmental pH on the Fluorescence Spectrum of Alternariol
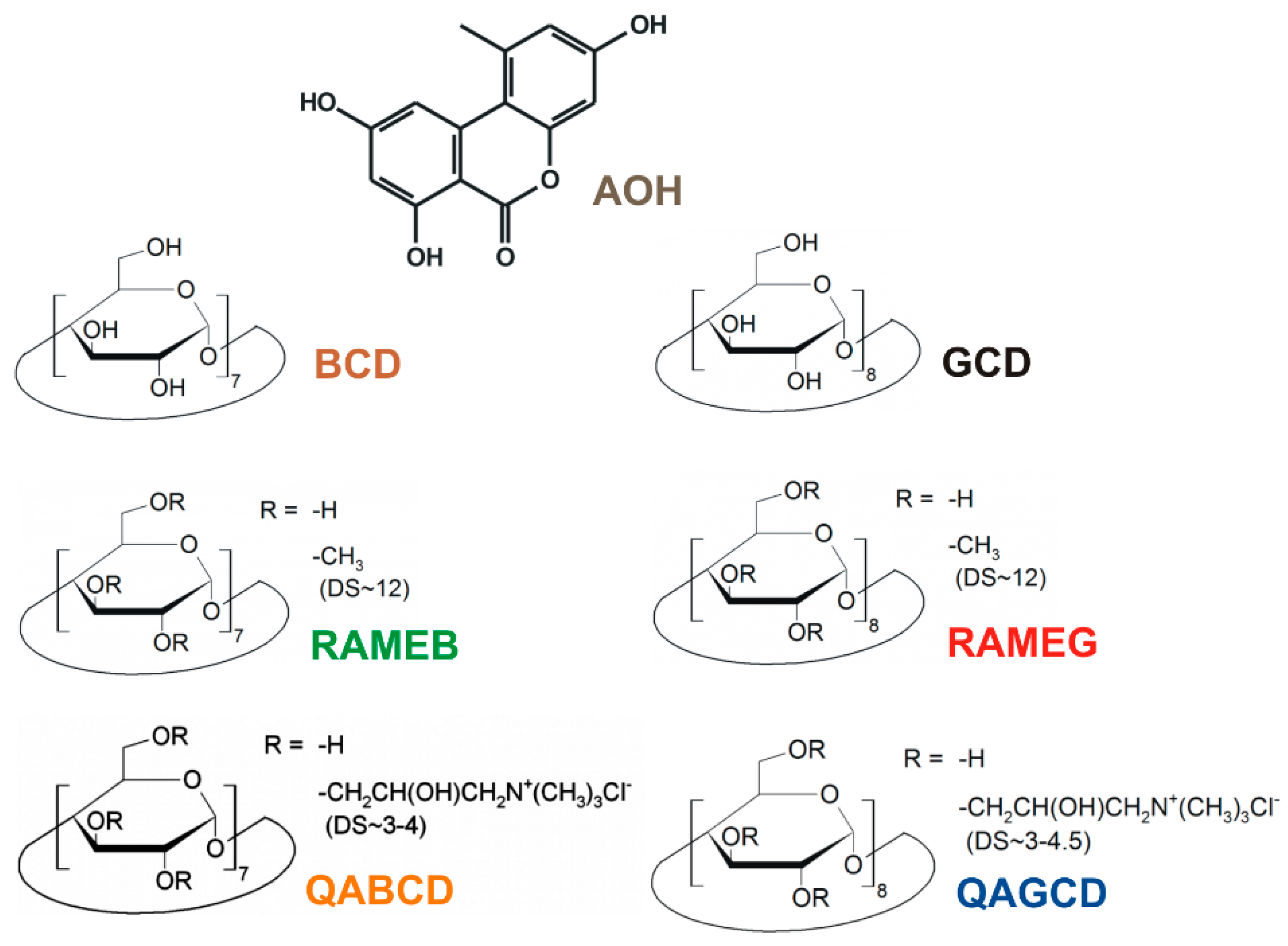
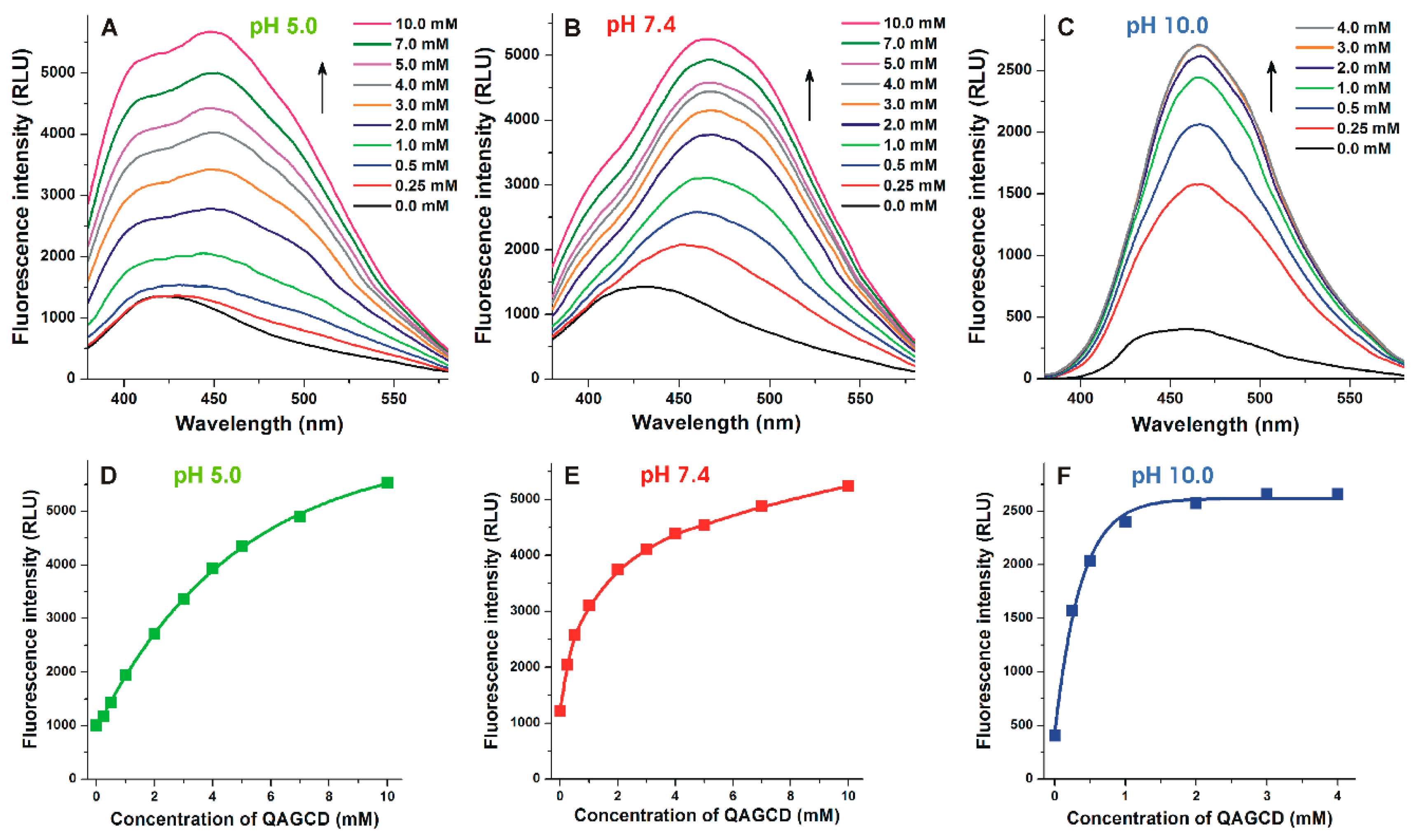
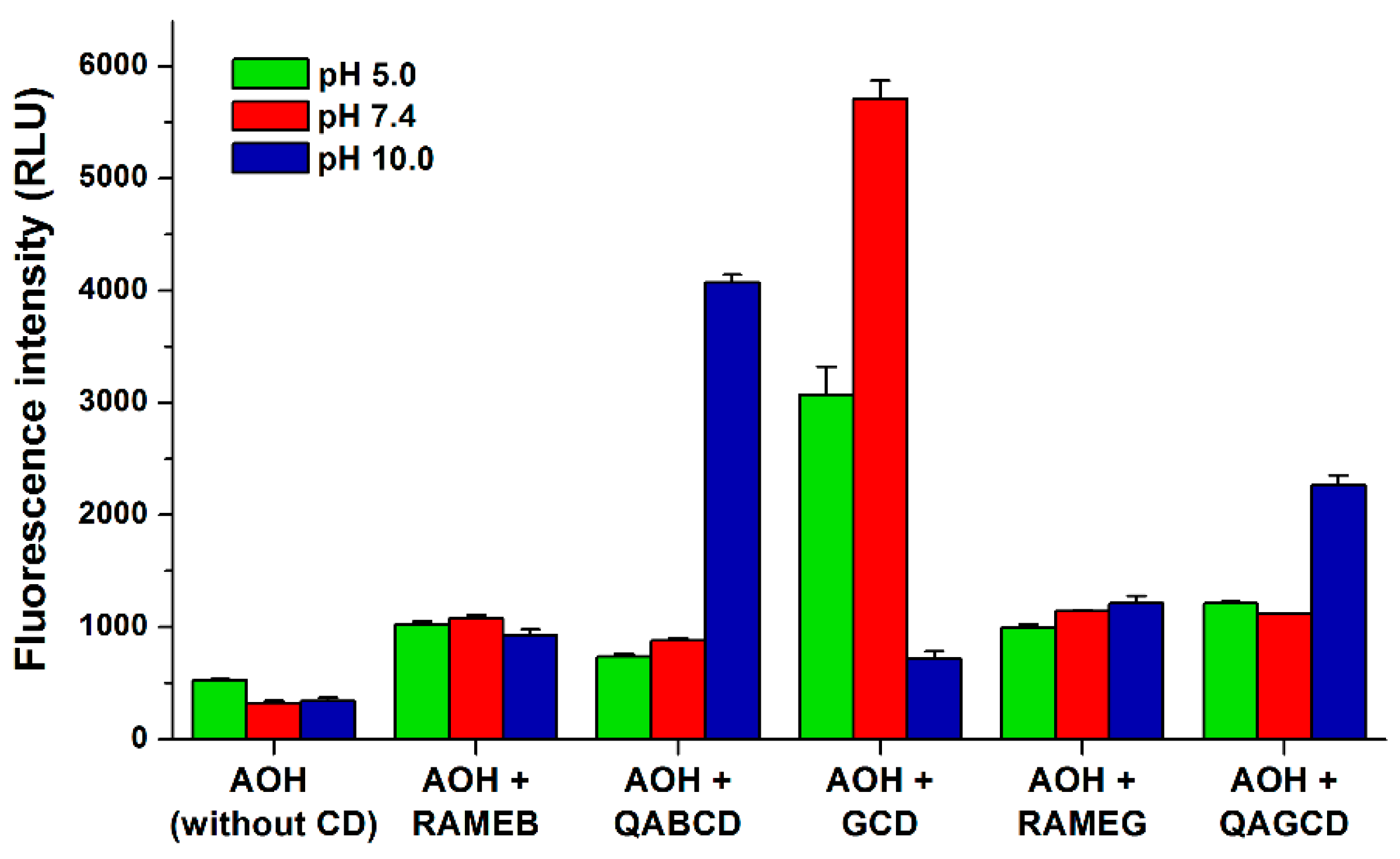
3.2. Effects of Cyclodextrins on the Fluorescence Spectrum of Alternariol
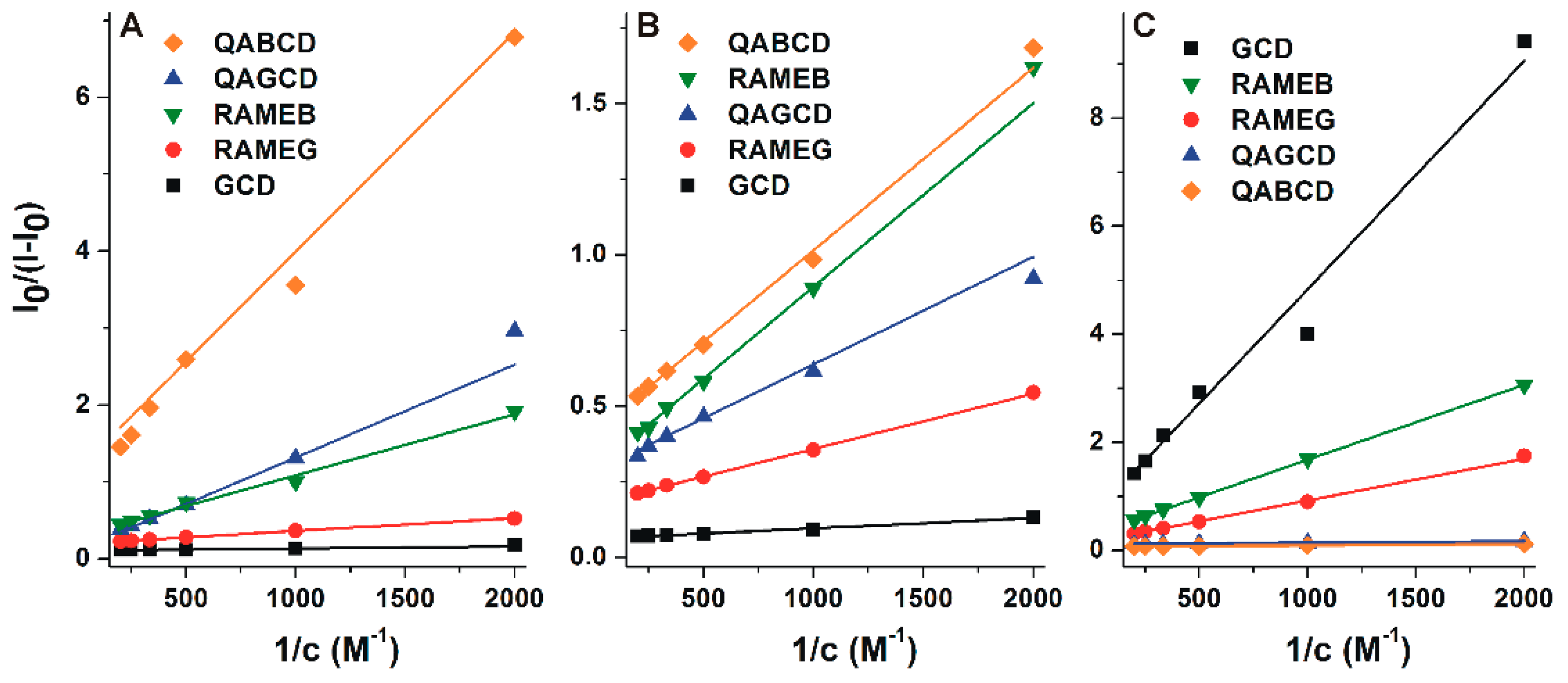
3.3. Binding Constants of Alternariol-Cyclodextrin Complexes
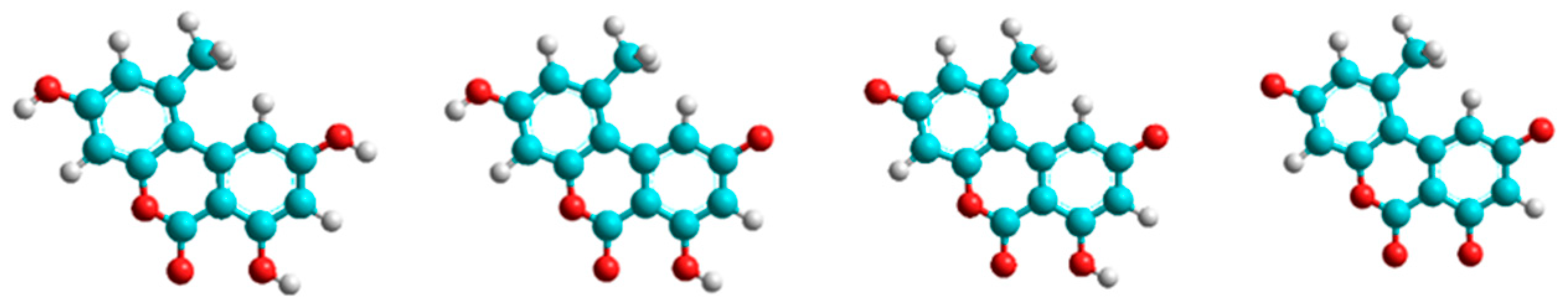
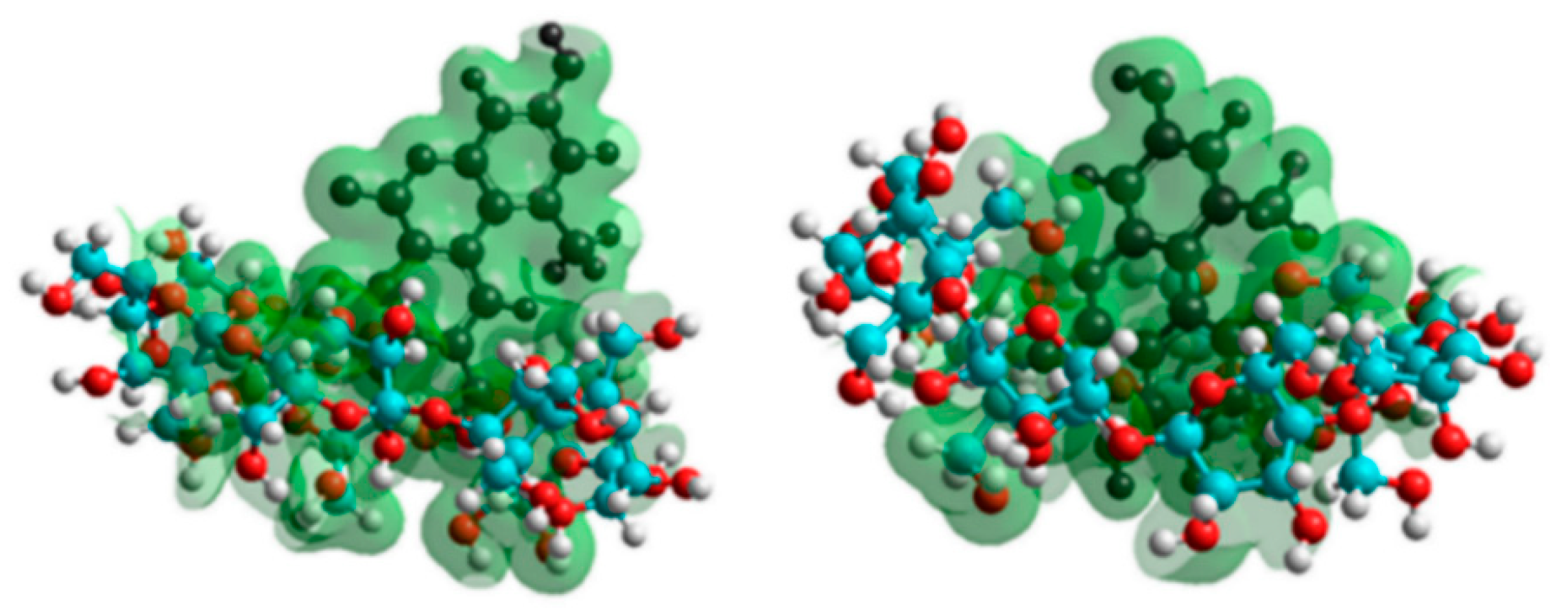
3.4. Modeling Studies
3.5. Extraction of Alternariol from Aqueous Solution by Insoluble β-Cyclodextrin Bead Polymer
3.6. Comparison of the Interactions of Alternariol with Native β-Cyclodextrin, Soluble β-Cyclodextrin Polymer, and Insoluble β-Cyclodextrin Bead Polymer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arcella, D.; Eskola, M.; Ruiz, J.A.G. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar]
- Scott, P.M.; Lawrence, G.A.; Lau, B.P.Y. Analysis of wines, grape juices and cranberry juices for Alternaria toxins. Mycotoxin Res. 2006, 22, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Stratakou, I.; Van Der Fels-Klerx, H.J. Mycotoxins in grapes and wine in Europe: Occurrence, factors affecting the occurrence and related toxicological effects. World Mycotoxin J. 2010, 3, 283–300. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J. Food Qual. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Patriarca, A. Alternaria in food products. Curr. Opin. Food Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- EFSA. European Food Safety Authority: Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Čolović, R.; Pezo, L.; Orčić, D.; Vukmirović, Đ.; Mastilović, J. Possibility of Alternaria toxins reduction by extrusion processing of whole wheat flour. Food Chem. 2016, 213, 784–790. [Google Scholar] [CrossRef]
- Delgado, T.; Gómez-Cordovés, C.; Scott, P. Determination of alternariol and alternariol methyl ether in apple juice using solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. A 1996, 731, 109–114. [Google Scholar] [CrossRef]
- Fan, C.; Cao, X.; Liu, M.; Wang, W. Determination of Alternaria mycotoxins in wine and juice using ionic liquid modified countercurrent chromatography as a pretreatment method followed by high-performance liquid chromatography. J. Chromatogr. A 2016, 1436, 133–140. [Google Scholar] [CrossRef]
- Fente, C.A.; Jaimez, J.; Vázquez, B.I.; Franco, C.M.; Cepeda, A. Determination of alternariol in tomato paste using solid phase extraction and high-performance liquid chromatography with fluorescence detection†. Anal. 1998, 123, 2277–2280. [Google Scholar] [CrossRef]
- Asam, S.; Konitzer, K.; Rychlik, M. Precise determination of the Alternaria mycotoxins alternariol and alternariol monomethyl ether in cereal, fruit and vegetable products using stable isotope dilution assays. Mycotoxin Res. 2011, 27, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Dellafiora, L.; Dall’Asta, C.; Cruciani, G.; Pethő, G.; Poór, M. Interaction of Mycotoxin Alternariol with Serum Albumin. Int. J. Mol. Sci. 2019, 20, 2352. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Zand, A.; Szente, L.; Lemli, B.; Kunsági-Máté, S. Interaction of α- and β-zearalenols with β-cyclodextrins. Molecules 2017, 22, 1910. [Google Scholar] [CrossRef] [PubMed]
- Bekers, O.; Uijtendaal, E.V.; Beijnen, J.H.; Bult, A.; Underberg, W.J.M. Cyclodextrins in the Pharmaceutical Field. Drug Dev. Ind. Pharm. 1991, 17, 1503–1549. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Buschmann, H.-J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar]
- Cravotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as Food Additives and in Food Processing. Curr. Nutr. Food Sci. 2006, 2, 343–350. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.O.K.; Tan, Y.T.F.; Peh, K.-K. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asian J. Pharm. Sci. 2016, 11, 536–546. [Google Scholar] [CrossRef]
- Khaoulani, S.; Chaker, H.; Cadet, C.; Bychkov, E.; Cherif, L.; Bengueddach, A.; Fourmentin, S. Wastewater treatment by cyclodextrin polymers and noble metal/mesoporous TiO2 photocatalysts. C. R. Chim. 2015, 18, 23–31. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Senhadji, O.; Milon, N.; Benamor, M.; Lahiani-Skiba, M. Inclusion and removal of pharmaceutical residues from aqueous solution using water-insoluble cyclodextrin polymers. Chem. Eng. Res. Des. 2015, 97, 145–158. [Google Scholar] [CrossRef]
- Maragos, C.M.; Appell, M.; Lippolis, V.; Visconti, A.; Catucci, L.; Pascale, M. Use of cyclodextrins as modifiers of fluorescence in the detection of mycotoxins. Food Add. Cont. 2008, 25, 164–171. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J. Cyclodextrins in analytical chemistry: Host-guest type molecular recognition. Anal. Chem. 2013, 85, 8024–8030. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Xu, H. Interactions of aflatoxin B1 and related secondary metabolites with native cyclodextrins and their potential utilization. Food Control 2018, 94, 102–107. [Google Scholar] [CrossRef]
- Poór, M.; Matisz, G.; Kunsági-Máté, S.; Derdák, D.; Szente, L.; Lemli, B. Fluorescence spectroscopic investigation of the interaction of citrinin with native and chemically modified cyclodextrins. J. Lumin. 2016, 172, 23–28. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Szente, L.; Matisz, G.; Secenji, G.; Czibulya, Z.; Kőszegi, T. Interaction of ochratoxin A with quaternary ammonium beta-cyclodextrin. Food Chem. 2015, 172, 143–149. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Faccini, A.; Galaverna, G.; Corradini, R.; Dossena, A.; Marchelli, R. Complexation of the mycotoxin zearalenone with β-cyclodextrin: Study of the interaction and first promising applications. Mycotoxin Res. 2008, 24, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, G.; Dall’Asta, C.; Corradini, R.; Dossena, A.; Marchelli, R. Cyclodextrins as selectors for mycotoxin recognition. World Mycotoxin J. 2008, 1, 397–406. [Google Scholar] [CrossRef]
- Maragos, C.M. Recent advances in the development of novel materials for mycotoxin analysis. Anal. Bioanal. Chem. 2009, 395, 1205–1213. [Google Scholar] [CrossRef]
- Appell, M.; Jackson, M.A. Synthesis and evaluation of cyclodextrin-based polymers for patulin extraction from aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 117–122. [Google Scholar] [CrossRef]
- Shirasawa, T.; Ueda, M.; Appell, M.; Goto, T. Use of cyclodextrin-based polymer for patulin analysis in apple juice. Mycotoxins 2013, 63, 1–8. [Google Scholar] [CrossRef]
- Appell, M.; Jackson, M.A. Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer. Toxins 2012, 4, 98–109. [Google Scholar] [CrossRef]
- Poór, M.; Faisal, Z.; Zand, A.; Bencsik, T.; Lemli, B.; Kunsági-Máté, S.; Szente, L. Removal of Zearalenone and Zearalenols from Aqueous Solutions Using Insoluble Beta-Cyclodextrin Bead Polymer. Toxins 2018, 10, 216. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Durán-Merás, I.; Muñoz de la Peña, A.; Salinas, F.; Rodríguez Cáceres, I. Spectrofluorimetric determination of nalidixic acid based on host–guest complexation with γ-cyclodextrin. Analyst 1994, 119, 1215–1219. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Complexation of resveratrol by native and modified cyclodextrins: Determination of complexation constant by enzymatic, solubility and fluorimetric assays. Food Chem. 2008, 111, 262–267. [Google Scholar] [CrossRef]
- Csepregi, R.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Kőszegi, T.; Németi, B.; Poór, M. Complex Formation of Resorufin and Resazurin with Β-Cyclodextrins: Can Cyclodextrins Interfere with a Resazurin Cell Viability Assay? Molecules 2018, 23, 382. [Google Scholar] [CrossRef] [PubMed]
- Faisal, Z.; Kunsági-Máté, S.; Lemli, B.; Szente, L.; Bergmann, D.; Humpf, H.-U.; Poór, M. Interaction of dihydrocitrinone with native and chemically modified cyclodextrins. Molecules 2019, 24, 1328. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-S.; Tseng, Y.J.; Appell, M. Quantum chemical investigation of the detection properties of alternariol and alternariol monomethyl ether. Struct. Chem. 2019. Article in Press. [Google Scholar] [CrossRef]
- Molina, P.G.; Zón, M.A.; Fernandez, H. Determination of the acid dissociation constants for some mycotoxins of the Alternaria alternata genus. Can. J. Chem. 1998, 76, 576–582. [Google Scholar]
- Sali, N.; Csepregi, R.; Kőszegi, T.; Kunsági-Máté, S.; Szente, L.; Poór, M. Complex formation of flavonoids fisetin and geraldol with β-cyclodextrins. J. Lumin. 2018, 194, 82–90. [Google Scholar] [CrossRef]
- Cozzini, P.; Ingletto, G.; Singh, R.; Dall’Asta, C. Mycotoxin Detection Plays “Cops and Robbers”: Cyclodextrin Chemosensors as Specialized Police? Int. J. Mol. Sci. 2008, 9, 2474–2494. [Google Scholar] [CrossRef]
- Verrone, R.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; Lippolis, V.; Pascale, M. Effect of β-cyclodextrin on spectroscopic properties of ochratoxin A in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 475–479. [Google Scholar] [CrossRef]
- Dobretsov, G.E.; Syrejschikova, T.I.; Smolina, N.V. On mechanisms of fluorescence quenching by water. Biophysics 2014, 59, 183–188. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Buvári-Barcza, Á.; Rohonczy, J. pH-dependent complex formation of amino acids with β-cyclodextrin and quaternary ammonium β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 199–210. [Google Scholar] [CrossRef]
- Bom, A.; Bradley, M.; Cameron, K.; Clark, J.K.; van Egmond, J.; Feilden, H.; MacLean, E.J.; Muir, A.W.; Palin, R.; Rees, D.C.; et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. Engl. 2002, 41, 265–270. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; Marcone, M.F. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of Ochratoxin A in Red Wines by Means of Adsorption Treatments with Commercial Fining Agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Faisal, Z.; Fliszár-Nyúl, E.; Dellafiora, L.; Galaverna, G.; Dall’Asta, C.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Cyclodextrins Can Entrap Zearalenone-14-Glucoside: Interaction of the Masked Mycotoxin with Cyclodextrins and Cyclodextrin Bead Polymer. Biomolecules 2019, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Odlaug, T.E.; Pflug, I.J. Thermal Destruction of Clostridium botulinum Spores Suspended in Tomato Juice in Aluminum Thermal Death Time Tubes. Appl. Environ. Microbiol. 1977, 34, 23–29. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Sali, N.; Kőszegi, T.; Szente, L.; Peles-Lemli, B. Interactions of zearalenone with native and chemically modified cyclodextrins and their potential utilization. J. Photochem. Photobiol. B 2015, 151, 63–68. [Google Scholar] [CrossRef]
- Harada, A.; Furue, M.; Nozakura, S. Cyclodextrin-containing polymers. 2. Cooperative effects in catalysis and binding. Macromolecules 1976, 9, 705–710. [Google Scholar] [CrossRef]
- Harada, A.; Furue, M.; Nozakura, S. Inclusion of aromatic compounds by a β-cyclodextrin-epichlorohydrin polymer. Polym. J. 1981, 13, 777–781. [Google Scholar] [CrossRef]
- Szemán, J.; Ueda, H.; Szejtli, J.; Fenyvesi, E.; Machida, Y.; Nagai, T. Complexation of several drugs with water-soluble cyclodextrin polymer. Chem. Pharm. Bull. 1987, 35, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.; Kajihara, T.; Miwa, K.; Iijima, T. Catalytic hydrolysis of phenyl esters in cyclodextrin/poly(allylamine) systems. Makromol. Chem. 1991, 192, 2357–2369. [Google Scholar] [CrossRef]



| Mycotoxin-CD Complex | logK (±SEM) | ||
|---|---|---|---|
| pH 5.0 | pH 7.4 | pH 10.0 | |
| AOH-BCD | 2.23 ± 0.01 | 2.52 ± 0.01 | 2.22 ± 0.03 |
| AOH-RAMEB | 2.50 ± 0.02 | 2.64 ± 0.01 | 2.11 ± 0.03 |
| AOH-QABCD | 2.38 ± 0.03 | 2.85 ± 0.04 | 3.40 ± 0.01 |
| AOH-GCD | 3.18 ± 0.06 | 3.21 ± 0.01 | 2.17 ± 0.03 |
| AOH-RAMEG | 2.96 ± 0.03 | 3.03 ± 0.03 | 2.23 ± 0.03 |
| AOH-QAGCD | 2.11 ± 0.03 | 2.97 ± 0.01 | 3.58 ± 0.03 |
| Host Molecule | Host Simulated as | LogK Values of AOH and Its Deprotonated Forms | |||
|---|---|---|---|---|---|
| AOH (Nonionic) | AOH (Monoanionic) | AOH (Dianionic) | AOH (Trianionic) | ||
| BCD | 0 BCD | 2.61 | 2.51 | 2.46 | 2.42 |
| RAMEB | −1 BCD | 2.52 | 2.41 | 2.32 | 2.14 |
| QABCD | +1 BCD | 2.48 | 2.81 | 2.98 | 3.40 |
| GCD | 0 GCD | 3.17 | 3.11 | 3.04 | 2.61 |
| RAMEG | −1 GCD | 3.11 | 2.87 | 2.72 | 2.46 |
| QAGCD | +1 GCD | 2.29 | 2.88 | 3.12 | 3.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Interactions of Mycotoxin Alternariol with Cyclodextrins and Its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer. Biomolecules 2019, 9, 428. https://doi.org/10.3390/biom9090428
Fliszár-Nyúl E, Lemli B, Kunsági-Máté S, Szente L, Poór M. Interactions of Mycotoxin Alternariol with Cyclodextrins and Its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer. Biomolecules. 2019; 9(9):428. https://doi.org/10.3390/biom9090428
Chicago/Turabian StyleFliszár-Nyúl, Eszter, Beáta Lemli, Sándor Kunsági-Máté, Lajos Szente, and Miklós Poór. 2019. "Interactions of Mycotoxin Alternariol with Cyclodextrins and Its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer" Biomolecules 9, no. 9: 428. https://doi.org/10.3390/biom9090428
APA StyleFliszár-Nyúl, E., Lemli, B., Kunsági-Máté, S., Szente, L., & Poór, M. (2019). Interactions of Mycotoxin Alternariol with Cyclodextrins and Its Removal from Aqueous Solution by Beta-Cyclodextrin Bead Polymer. Biomolecules, 9(9), 428. https://doi.org/10.3390/biom9090428