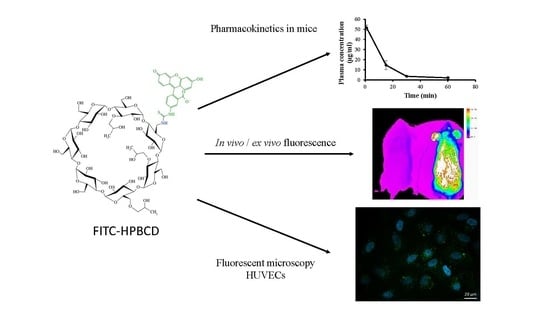
Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Intracellular FITC-HPBCD Accumulation in HUVEC Cells
2.3.1. Fluorescence Microscopy
2.3.2. Flow Cytometry
2.4. In Vivo Pharmacokinetic Investigation
2.5. In Vivo Fluorescence Imaging Experiments
2.6. Statistical Analysis
3. Results
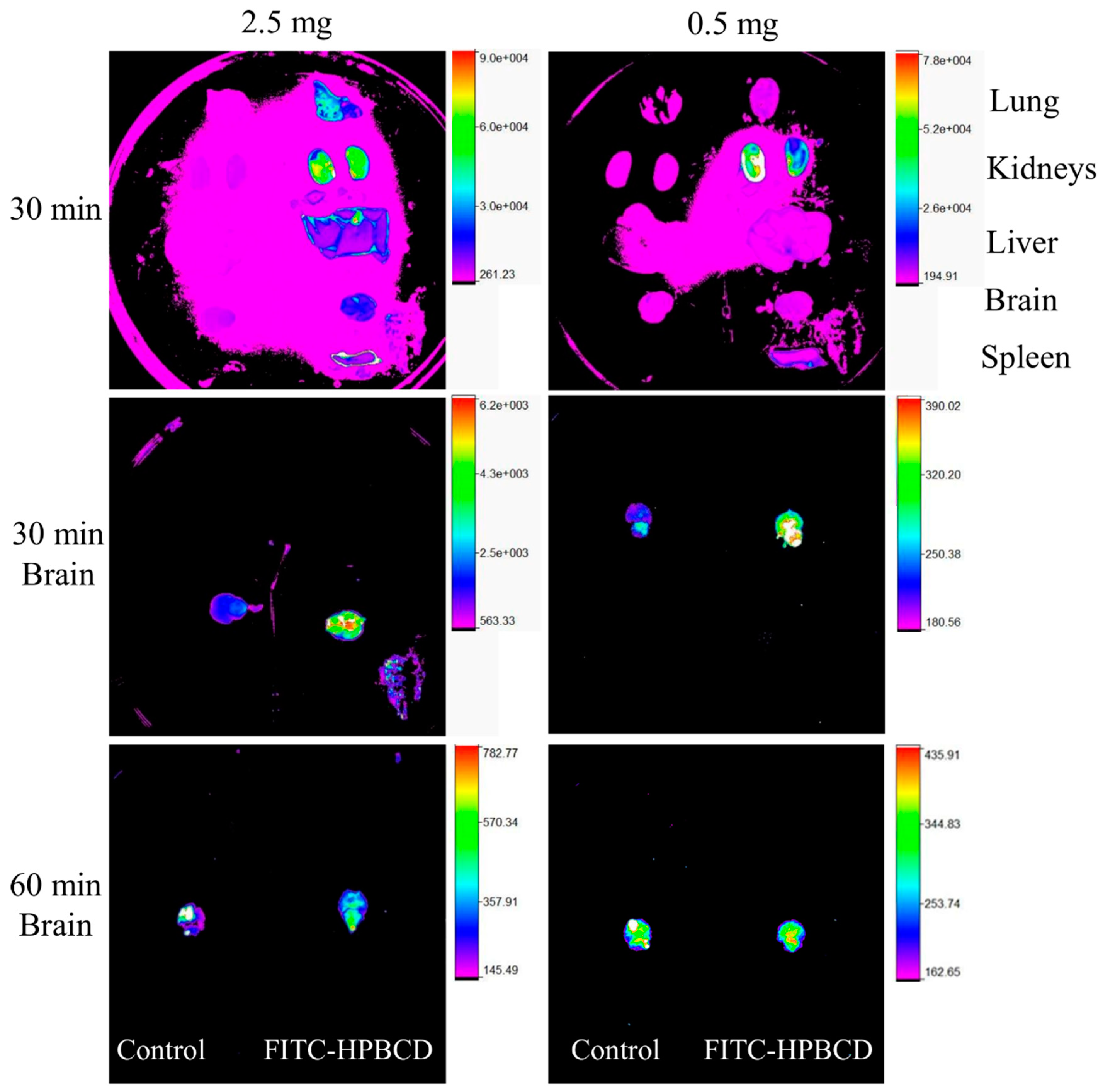
3.1. In Vivo Pharmacokinetic Investigation
3.2. In Vivo Fluorescence Imaging Experiments
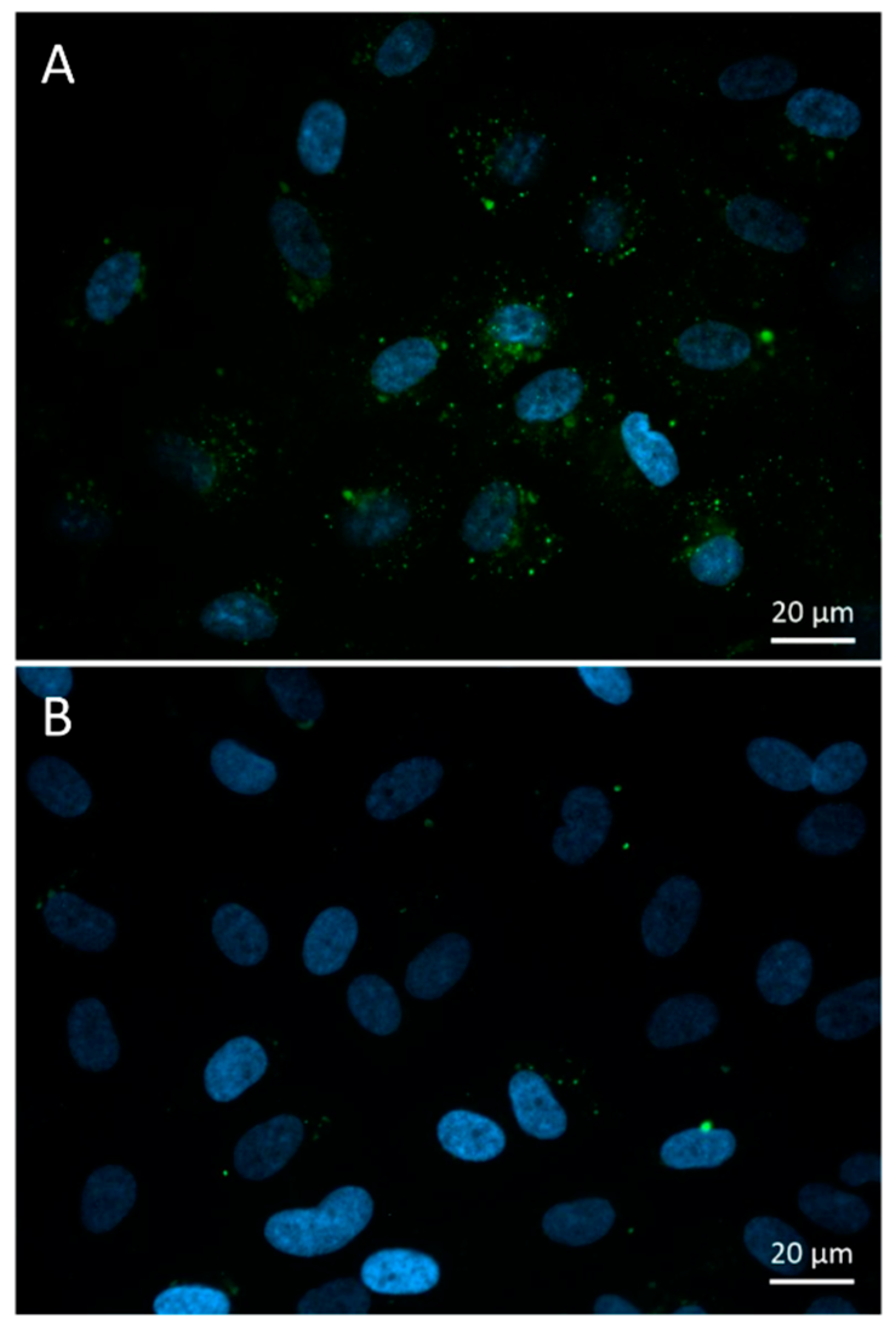
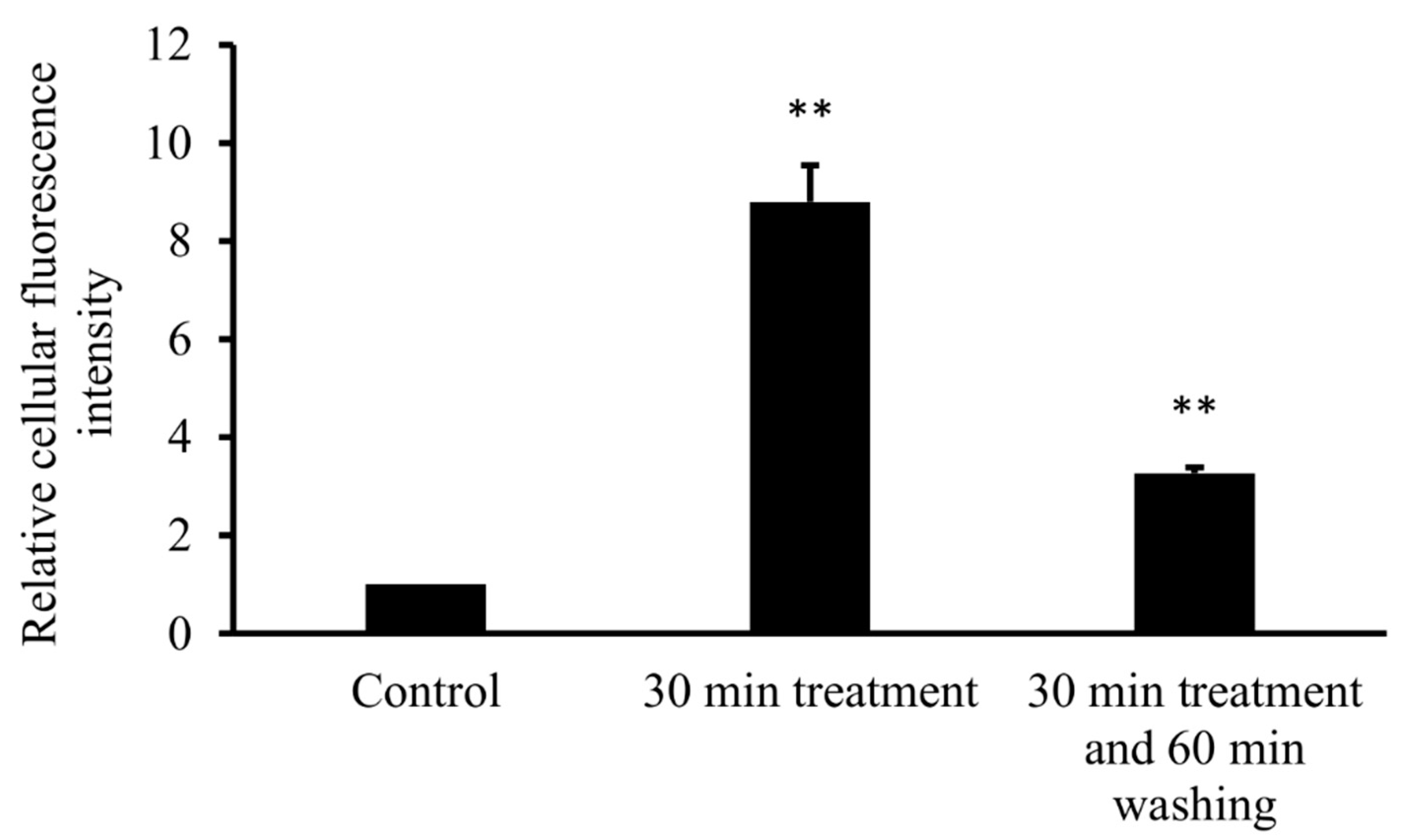
3.3. Intracellular FITC-HPBCD Accumulation in HUVEC Cells
3.3.1. Fluorescence Microscopy
3.3.2. Flow Cytometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Committee for Orphan Medicinal Products, European Medicines Agency. Public Summary of Opinion on Orphan Designation. EMA/COMP/546608/2011 Rev.1. 12 March 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2011/09/WC500112733.pdf (accessed on 18 March 2018).
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1–2 trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- FDA List of Inactive Pharmaceutical Ingredients. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=browseByLetter.page&Letter=H (accessed on 18 March 2018).
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-b-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. “Back to the future”: A new look at hydroxypropyl beta-cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Kantner, I.; Erben, R.G. Long-term parenteral administration of 2-hydroxypropyl-β-cyclodextrin causes bone loss. Toxicol. Pathol. 2012, 40, 742–750. [Google Scholar] [CrossRef]
- Chien, Y.H.; Shieh, Y.D.; Yang, C.Y.; Lee, N.C.; Hwu, W.L. Lung toxicity of hydroxypropyl-β-cyclodextrin infusion. Mol. Genet. Metab. 2013, 109, 231–232. [Google Scholar] [CrossRef]
- Crumling, M.A.; King, K.A.; Duncan, R.K. Cyclodextrins and iatrogenic hearing loss: new drugs with significant risk. Front. Cell. Neurosci. 2017, 11, 355. [Google Scholar] [CrossRef]
- Ward, S.; O’Donnell, P.; Fernandez, S.; Vite, C.H. 2-Hydroxypropyl-β-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-pick type C disease. Pediatr. Res. 2010, 68, 52–56. [Google Scholar] [CrossRef]
- Crumling, M.A.; Liu, L.; Thomas, P.V.; Benson, J.; Kanicki, A.; Kabara, L.; Hälsey, K.; Dolan, D.; Duncan, R.K. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-β-cyclodextrin. PLoS ONE 2012, 7, e53280. [Google Scholar] [CrossRef]
- Cronin, S.; Lin, A.; Thompson, K.; Hoenerhoff, M.; Duncan, R.K. Hearing loss and otopathology following systemic and intracerebroventricular delivery of 2-hydroxypropyl-beta-cyclodextrin. J. Assoc. Res. Otolaryngol. 2015, 16, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Réti-Nagy, K.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vámosi, G.; Váradi, J.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Róka, E.; et al. Endocytosis of fluorescent cyclodextrins by intestinal Caco—2 cells and its role in paclitaxel drug delivery. Int. J. Pharm. 2015, 496, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Réti-Nagy, K.; Bacsó, Z.; Gutay-Tóth, Z.; Malanga, M.; Fenyvesi, É.; Szente, L.; Váradi, J.; Ujhelyi, Z.; Fehér, P.; et al. Fluorescently labeled methyl-beta-cyclodextrin enters intestinal epithelial Caco-2 cells by fluid-phase endocytosis. PLoS ONE 2014, 9, e84856. [Google Scholar] [CrossRef] [PubMed]
- Plazzo, A.P.; Höfer, C.T.; Jicsinszky, L.; Fenyvesi, É.; Szente, L.; Schiller, J.; Herrmann, A.; Müller, P. Uptake of a fluorescent methyl-β-cyclodextrin via clathrin-dependent endocytosis. Chem. Phys. Lipids 2012, 165, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zheng, W.Z.; Diakur, J.; Wiebe, L.I. Confocal laser scanning microscopy (CLSM) based evidence for cell permeation by mono-4-(N-6-deoxy-6-amino-beta-cyclodextrin)-7-nitrobenzofuran (NBD-beta-CyD). Int. J. Pharm. 2011, 403, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Frijlink, H.W.; Visser, J.; Hefting, N.R.; Oosting, R.; Meijer, D.K.F.; Lerk, C.F. The pharmacokinetics of β-cyclodextrin and hydroxypropyl-β-cyclodextrin in the rat. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1990, 7, 1248–1252. [Google Scholar]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-hydroxypropyl-β-cyclodextrin in Niemann—Pick disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol. Pharm. Bull. 2015, 38, 844–851. [Google Scholar] [CrossRef]
- Hajdu, I.; Angyal, J.; Szikra, D.; Kertész, I.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vecsernyés, M.; Bácskay, I.; Váradi, J.; et al. Radiochemical synthesis and preclinical evaluation of 68Ga-labeled NODAGA-hydroxypropyl-beta-cyclodextrin (68Ga-NODAGA-HPBCD). Eur. J. Pharm. Sci. 2019, 128, 202–208. [Google Scholar] [CrossRef]
- Benkovics, G.; Malanga, M.; Fenyvesi, É. The ‘Visualized’ macrocycles: Chemistry and application of fluorophore tagged cyclodextrins. Int. J. Pharm. 2017, 531, 689–700. [Google Scholar] [CrossRef]
- Malanga, M.; Jicsinszky, L.; Fenyvesi, É. Rhodamine-labeled cyclodextrin derivatives. J. Drug Deliv. Sci. Technol. 2012, 22, 260–265. [Google Scholar] [CrossRef]
- Jeney, V.; Balla, J.; Yachie, A.; Varga, Z.; Vercellotti, G.M.; Eaton, J.W.; Balla, G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002, 100, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sidhu, R.; Fujiwara, H.; De Meulder, M.; De Vries, R.; Gong, Y.; Kao, M.; Porter, F.D.; Yanjanin, N.M.; Carillo-Carasco, N.; et al. Development and validation of sensitive LC-MS/MS assays for quantification of HP-β-CD in human plasma and CSF. J. Lipid Res. 2014, 55, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinfurter, S.; Pinto, P.C.; Pretze, M.; Kränzlin, B.; Pill, J.; Federica, R.; Perciaccante, R.; Ciana, L.D.; Masereeuw, R.; et al. Fluorescently labeled cyclodextrin derivatives as exogenous markers for real-time transcutaneous measurement of renal function. Bioconjug. Chem. 2016, 27, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Committee for Human Medicinal Products (CHMP), European Medicines Agency. Cyclodextrins Used as Excipients. EMA/CHMP/333892/2013. 9 October 2017. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/10/WC500235904.pdf (accessed on 18 March 2018).
- Chapman, M.E.; Hu, L.; Plato, C.F.; Kohan, D.E. Bioimpedance spectroscopy for the estimation of body fluid volumes in mice. Am. J. Physiol. Physiol. 2010, 299, F280–F283. [Google Scholar] [CrossRef] [PubMed]
- Pontikis, C.C.; Davidson, C.D.; Walkley, S.U.; Platt, F.M.; Begley, D.J. Cyclodextrin alleviates neuronal storage of cholesterol in Niemann-Pick C disease without evidence of detectable blood-brain barrier permeability. J. Inherit. Metab. Dis. 2013, 36, 491–498. [Google Scholar] [CrossRef]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konráosdóttir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef]
- Goon, P.K.Y.; Watson, T.; Shantsila, E.; Boos, C.J.; Lip, G.Y.H. Standardization of circulating endothelial cell enumeration by the use of human umbilical vein endothelial cells. J. Thromb. Haemost. 2007, 5, 870–872. [Google Scholar] [CrossRef]
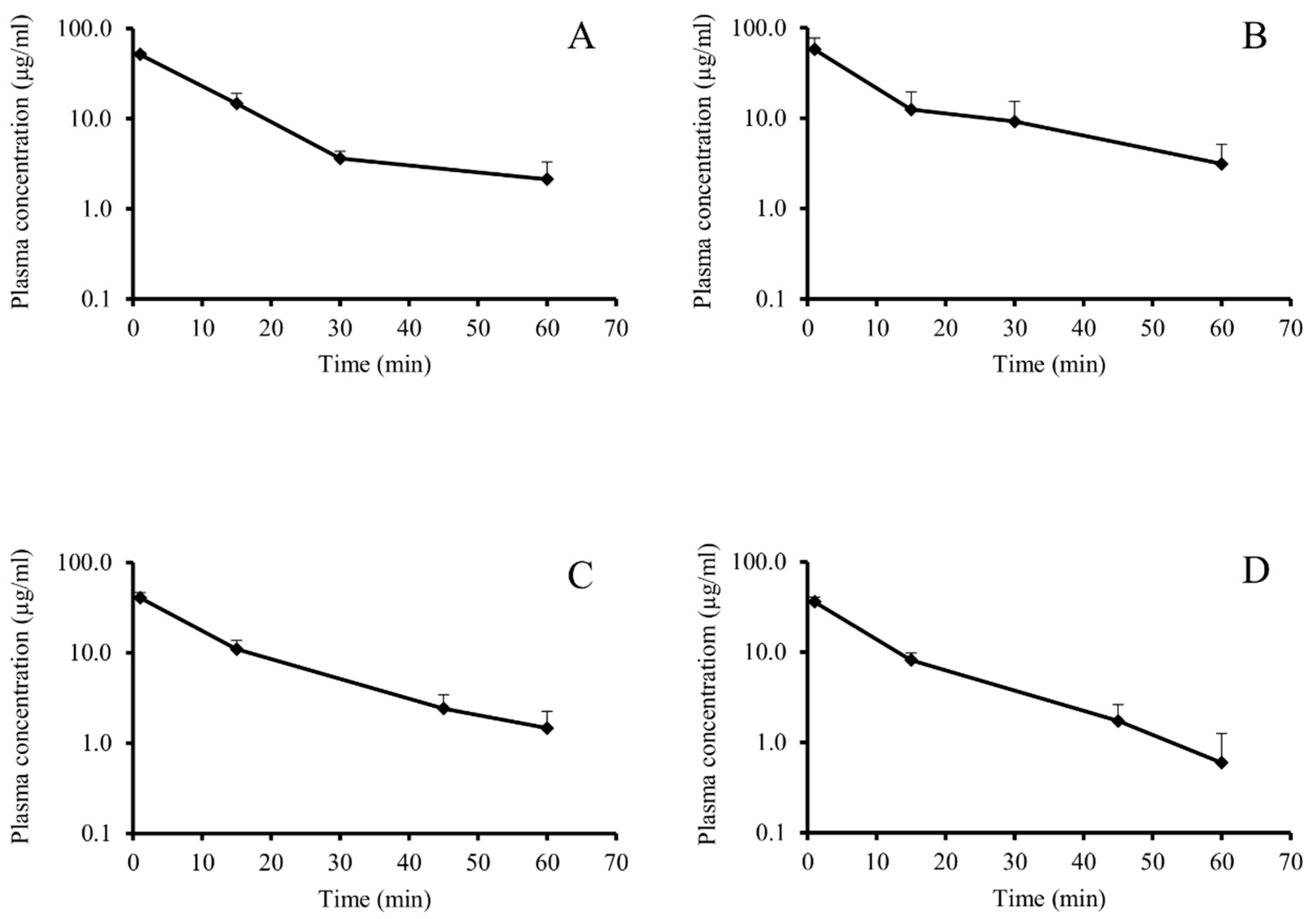
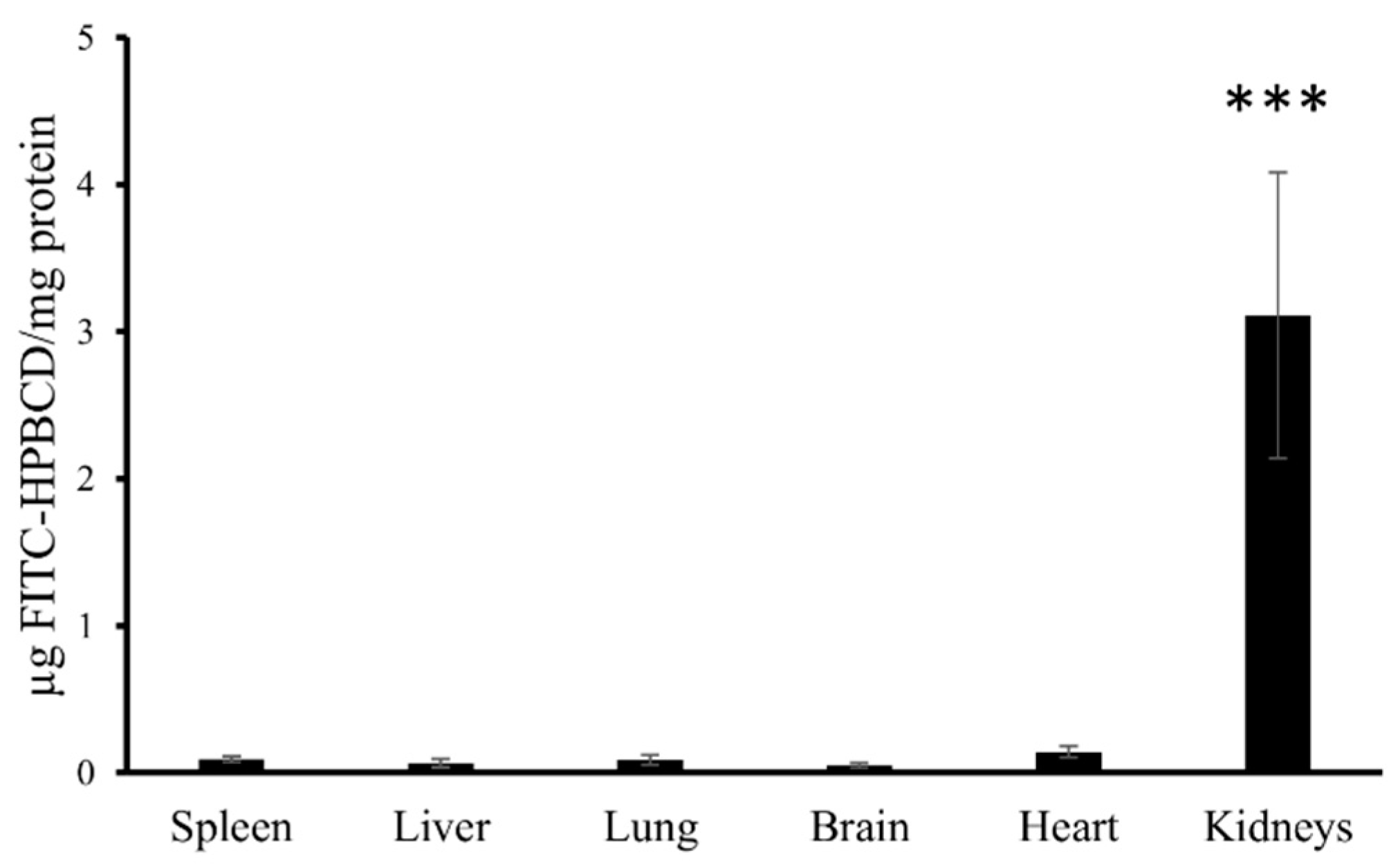
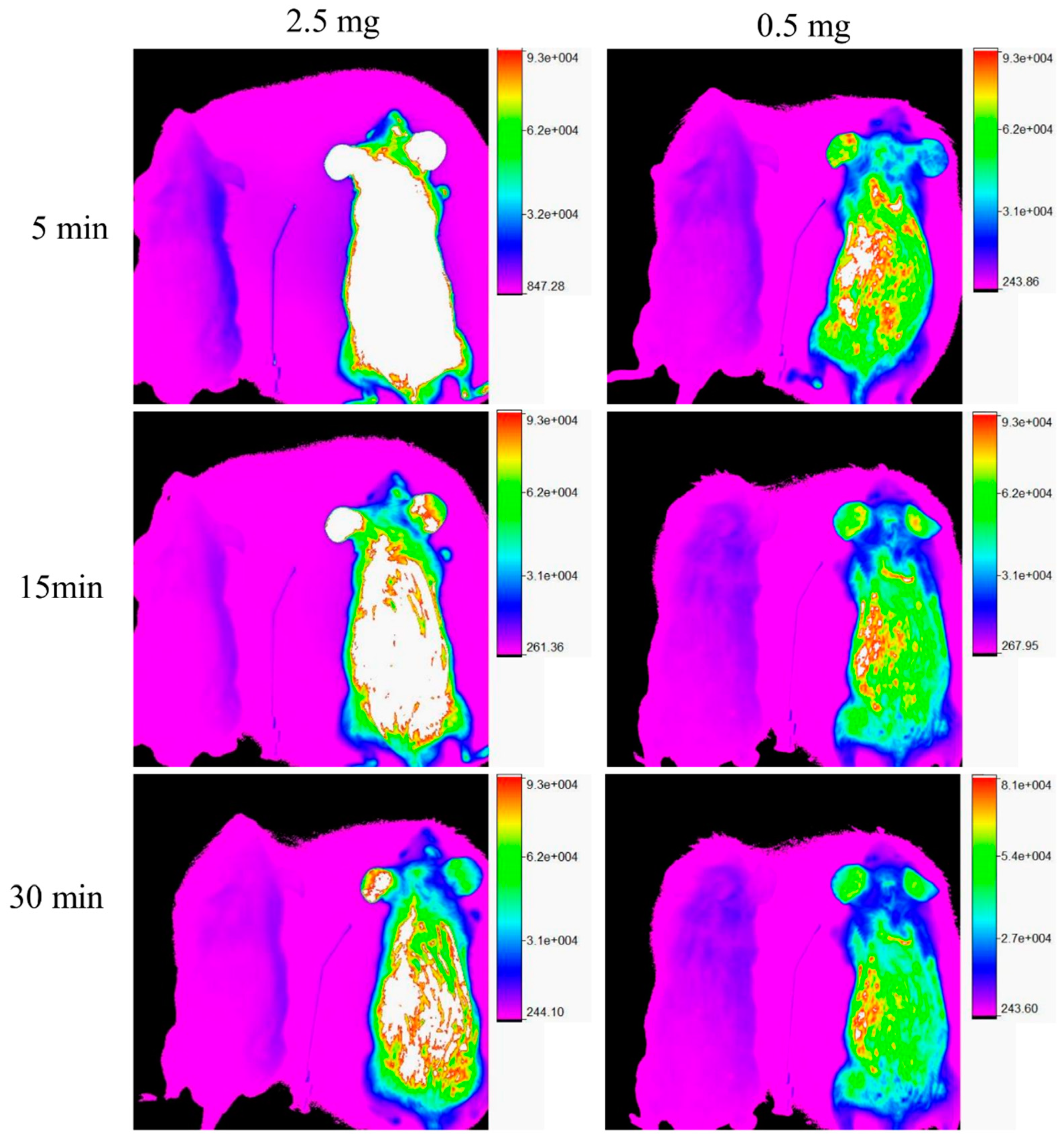
| Group F | Group NF | Group F | Group NF | ||
|---|---|---|---|---|---|
| Dose (µg) | 250 | 250 | Dose (µg) | 250 | 250 |
| t1/2 (1–15 min) | 7.82 ± 1.48 | 6.13 ± 0.78 | t1/2 (1–15 min) | 7.5 ± 1.13 | 6.50 ± 0.31 |
| t1/2 (15–30 min) | 7.54 ± 0.52 | 35.01 ± 10.15 | t1/2 (15–45 min) | 13.55 ± 1.45 | 13.36 ± 3.05 |
| t1/2 (30–60 min) | 122.3 ± 109.4 | 16.61 ± 7.12 | t1/2 (45–60 min) | 20.06 ± 3.89 | 8.99 ± 4.54 |
| ke (1–15 min) | 0.092 ± 0.023 | 0.115 ± 0.017 | ke (1–15 min) | 0.095 ± 0.017 | 0.107 ± 0.006 |
| ke (15–30 min) | 0.092 ± 0.007 | 0.022 ± 0.009 | ke (15–45 min) | 0.052 ± 0.007 | 0.054 ± 0.013 |
| ke (30–60 min) | 0.022 ± 0.029 | 0.049 ± 0.020 | ke (45–60 min) | 0.036 ± 0.008 | 0.095 ± 0.044 |
| kc (1–15 min) | 0.109 ± 0.026 | nd | kc (1–15 min) | 0.142 ± 0.033 | nd |
| Vd (mL) | 4.86 ± 0.21 | 4.70 ± 1.27 | Vd (mL) | 6.25 ± 0.77 | 7.01 ± 0.81 |
| CL (mL/min) | 0.11 ± 0.15 | 0.25 ± 0.15 | CL (mL/min) | 0.23 ± 0.08 | 0.69 ± 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Váradi, J.; Hermenean, A.; Gesztelyi, R.; Jeney, V.; Balogh, E.; Majoros, L.; Malanga, M.; Fenyvesi, É.; Szente, L.; Bácskay, I.; et al. Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin. Biomolecules 2019, 9, 509. https://doi.org/10.3390/biom9100509
Váradi J, Hermenean A, Gesztelyi R, Jeney V, Balogh E, Majoros L, Malanga M, Fenyvesi É, Szente L, Bácskay I, et al. Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin. Biomolecules. 2019; 9(10):509. https://doi.org/10.3390/biom9100509
Chicago/Turabian StyleVáradi, Judit, Anca Hermenean, Rudolf Gesztelyi, Viktória Jeney, Enikő Balogh, László Majoros, Milo Malanga, Éva Fenyvesi, Lajos Szente, Ildikó Bácskay, and et al. 2019. "Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin" Biomolecules 9, no. 10: 509. https://doi.org/10.3390/biom9100509
APA StyleVáradi, J., Hermenean, A., Gesztelyi, R., Jeney, V., Balogh, E., Majoros, L., Malanga, M., Fenyvesi, É., Szente, L., Bácskay, I., Vecsernyés, M., Fehér, P., Ujhelyi, Z., Vasvári, G., Árvai, I., Rusznyák, Á., Balta, C., Herman, H., & Fenyvesi, F. (2019). Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin. Biomolecules, 9(10), 509. https://doi.org/10.3390/biom9100509