Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Characterisation
2.3. Tablet Manufacture
2.4. Tablet Characterisation
2.5. Dissolution Testing
2.6. LIG-Microcrystalline Cellulose Antioxidant Activity
2.7. Statistical Analysis
3. Results
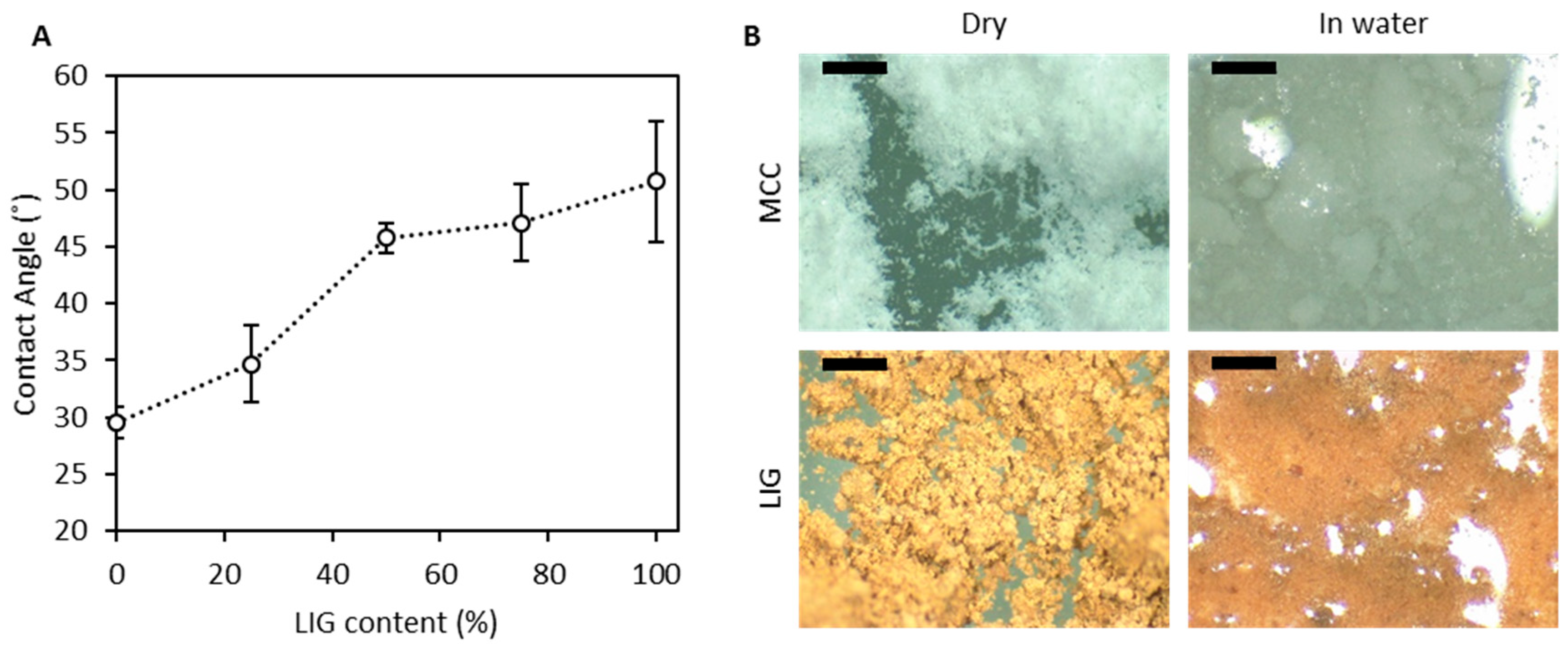
3.1. Powder Characterisation
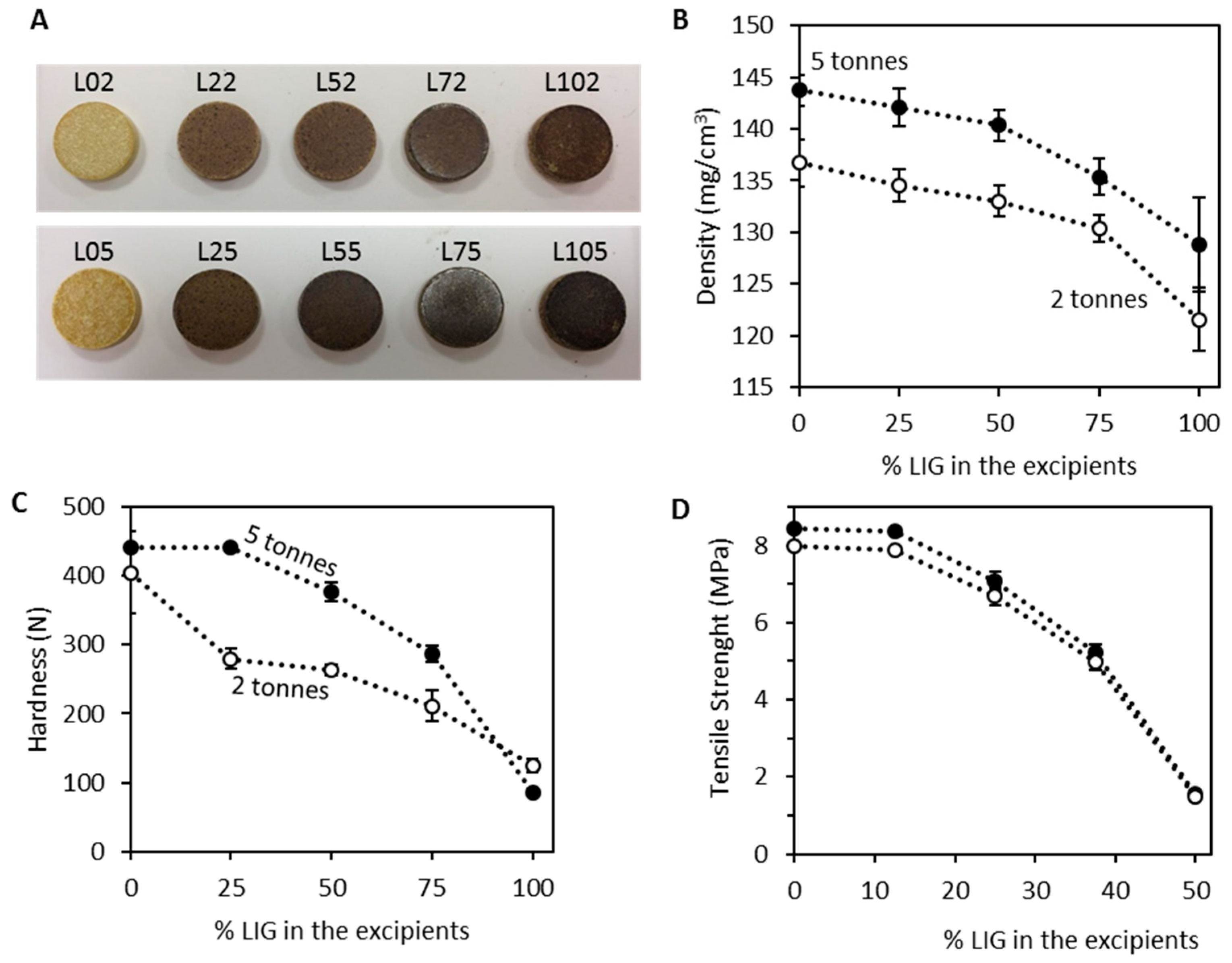
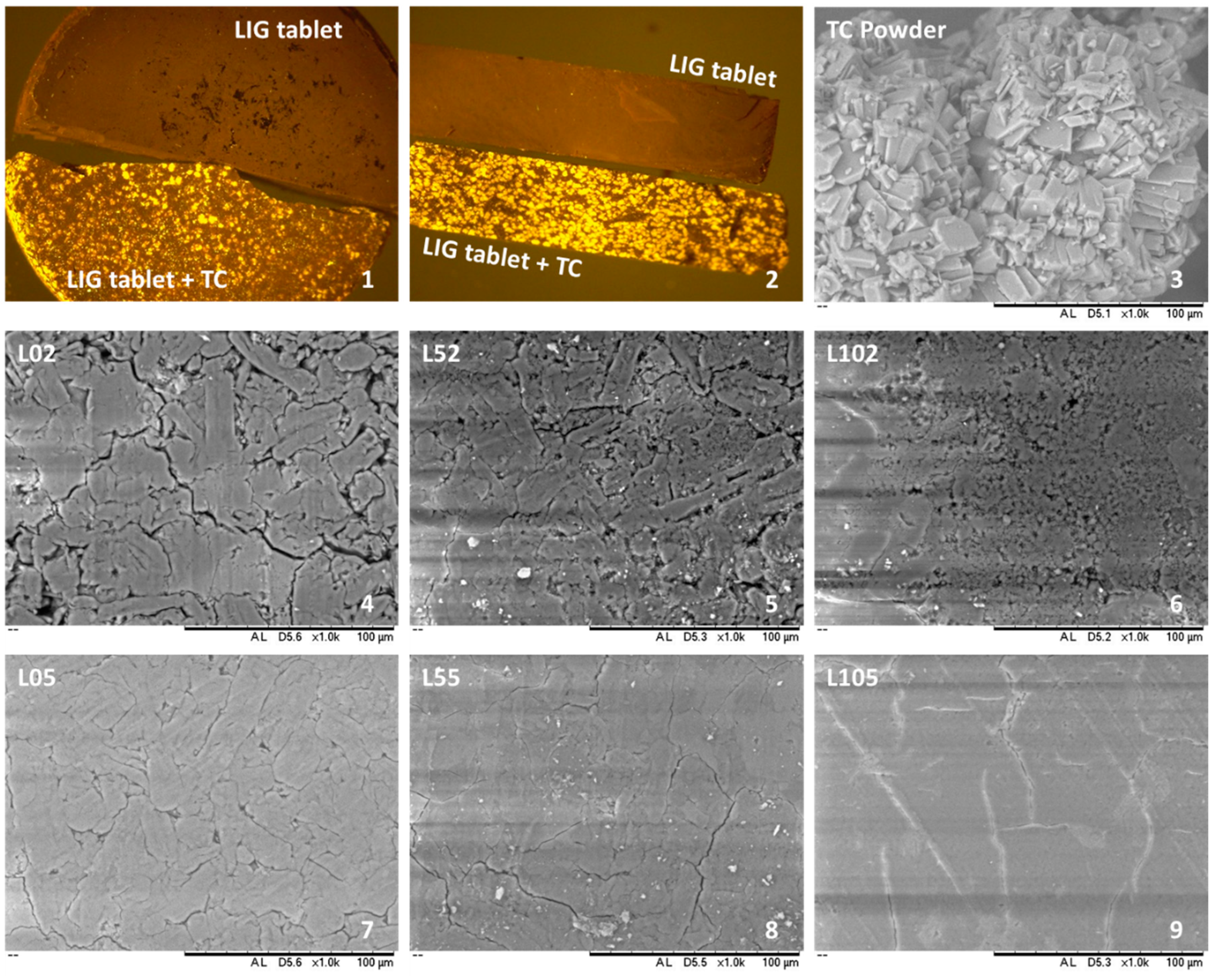
3.2. Tablet Morphology and Characterisation
3.3. Tetracycline Release from LIG/MCC Tablets
3.4. Antioxidant Capabilities of LIG and MCC Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mirani, A.G.; Patankar, S.P.; Borole, V.S.; Pawar, A.S.; Kadam, V.J. Direct Compression High Functionality Excipient using Coprocessing Technique: A Brief Review. Curr. Drug Deliv. 2011, 8, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.R.; Ibrahim, M.I.; Al-Haddad, M.S. The Influence of Consumers’ Preferences and Perceptions of Oral Solid Dosage Forms on their Treatment. Int. J. Clin. Pharm. 2012, 34, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline Cellulose, a Direct Compression Binder in a Quality by Design Environment—A Review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, I.I.; Martini, L.G.; Thomson, C.M. An Overview of the Different Excipients Useful for the Direct Compression of Tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric Plant-Derived Excipients in Drug Delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Larrañeta, E.; Martínez-Ohárriz, C.; Vélaz, I.; Zornoza, A.; Machín, R.; Isasi, J.R. In Vitro Release from Reverse Poloxamine/A-Cyclodextrin Matrices: Modelling and Comparison of Dissolution Profiles. J. Pharm. Sci. 2014, 103, 197–206. [Google Scholar] [CrossRef]
- Hsein, H.; Garrait, G.; Tamani, F.; Beyssac, E.; Hoffart, V. Denatured Whey Protein Powder as a New Matrix Excipient: Design and Evaluation of Mucoadhesive Tablets for Sustained Drug Release Applications. Pharm. Res. 2017, 34, 365–377. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Pandele, A.M.; Comanici, F.E.; Carp, C.A.; Miculescu, F.; Voicu, S.I.; Thakur, V.K.; Serban, B.C. Synthesis and Characterization of Cellulose Acetate-Hydroxyapatite Micro and Nano Composites Membranes for Water Purification and Biomedical Applications. Vacuum 2017, 146, 599–605. [Google Scholar] [CrossRef]
- Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Savy, D.; Mazzei, P.; Drosos, M.; Cozzolino, V.; Lama, L.; Piccolo, A. Molecular Characterization of Extracts from Biorefinery Wastes and Evaluation of their Plant Biostimulation. ACS Sustain. Chem. Eng. 2017, 5, 9023–9031. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Turner, M.K. Pharmaceuticals from Agriculture: Manufacture of Discovery? Ind. Crops Prod. 1992, 1, 125–131. [Google Scholar] [CrossRef]
- Daudt, R.M.; Külkamp-Guerreiro, I.C.; Cladera-Olivera, F.; Thys, R.C.S.; Marczak, L.D.F. Determination of Properties of Pinhão Starch: Analysis of its Applicability as Pharmaceutical Excipient. Ind. Crops Prod. 2014, 52, 420–429. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mazumdar, B. Feasibility and Characterization of Gummy Exudate of Cochlospermum Religiosum as Pharmaceutical Excipient. Ind. Crops Prod. 2013, 50, 776–786. [Google Scholar] [CrossRef]
- Wroblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pozoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- marketsandmarkets.com. Pharmaceutical Excipients Market by Type (Organic Chemical (Carbohydrate, Petrochemical), Inorganic Chemical), Functionality (Filler, Coating, Disintegrant, Binder), Formulation (Tablet, Capsule, Topical, Parenteral)—Global Forecast to 2023. Research and Markets, 19 April 2018. [Google Scholar]
- Penkina, A.; Antikainen, O.; Hakola, M.; Vuorinen, S.; Repo, T.; Yliruusi, J.; Veski, P.; Kogermann, K.; Heinamaki, J. Direct Compression of Cellulose and Lignin Isolated by a New Catalytic Treatment. AAPS PharmSciTech 2013, 14, 1129–1136. [Google Scholar] [CrossRef]
- Saha, S.; Shahiwala, A.F. Multifunctional Coprocessed Excipients for Improved Tabletting Performance. Expert Opin. Drug Deliv. 2009, 6, 197–208. [Google Scholar] [CrossRef]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.M.; Jameel, H. Lignin Structural Variation in Hardwood Species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef]
- Gellerstedt, G. Softwood Kraft Lignin: Raw Material for the Future. Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar] [CrossRef]
- Costa, S.; Rugiero, I.; Larenas Uria, C.; Pedrini, P.; Tamburini, E. Lignin Degradation Efficiency of Chemical Pre-Treatments on Banana Rachis Destined to Bioethanol Production. Biomolecules 2018, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Green Hydrogels from Lignin: A Review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Espinosa, E.; Savy, D.; Rosal, A.; Rodríguez, A. Biorefinery Process Combining Specel® Process and Selective Lignin Precipitation using Mineral Acids. Bioresources 2016, 11, 7061–7077. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Qian, Y.; Xiao, Y.; Du, S.; Qiu, X. Synergistic Antioxidant Performance of Lignin and Quercetin Mixtures. ACS Sustain. Chem. Eng. 2017, 5, 8424–8428. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tarrés, Q.; Delgado-Aguilar, M.; Rodríguez, A.; Espinach, F.X.; Mutjé, P. Approaching a New Generation of Fiberboards Taking Advantage of Self Lignin as Green Adhesive. Int. J. Biol. Macromol. 2018, 108, 927–935. [Google Scholar] [CrossRef]
- Greco, L.; Ullo, S.; Rigano, L.; Fontana, M.; Berardesca, E. Evaluation of the Soothing and Protective Properties of a Lignin Hydrolyzate. Cosmetics 2019, 6, 38. [Google Scholar] [CrossRef]
- Oliviero, M.; Verdolotti, L.; Di Maio, E.; Aurilia, M.; Iannace, S. Effect of Supramolecular Structures on Thermoplastic Zein-Lignin Bionanocomposites. J. Agric. Food Chem. 2011, 59, 10062–10070. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Danby, A.M.; Ramanathan, A.; Chaudhari, R.V.; Subramaniam, B. Zirconium-Incorporated Mesoporous Silicates show Remarkable Lignin Depolymerization Activity. ACS Sustain. Chem. Eng. 2017, 5, 7155–7164. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a Base Material for Materials Applications: Chemistry, Application and Economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Rencoret, J.; Prinsen, P.; Gutierrez, A.; Martinez, A.T.; Del Rio, J.C. Isolation and Structural Characterization of the Milled Wood Lignin, Dioxane Lignin, and Cellulolytic Lignin Preparations from Brewer’s Spent Grain. J. Agric. Food Chem. 2015, 63, 603–613. [Google Scholar] [CrossRef]
- Kai, D.; Low, Z.W.; Liow, S.; Abdul Karim, A.; Ye, H.; Jin, G.; Li, K.; Loh, X.J. Development of Lignin Supramolecular Hydrogels with Mechanically Responsive and Self-Healing Properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larraneta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Jiang, L.; Wong, H.Z.; Li, Z.; Zhang, Z.; Loh, X.J. Sustainable and Antioxidant Lignin-Polyester Copolymers and Nanofibers for Potential Healthcare Applications. ACS Sustain. Chem. Eng. 2017, 5, 6016–6025. [Google Scholar] [CrossRef]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Mishra, Y.K.; Thakur, V.K. Progress in Lignin Hydrogels and Nanocomposites for Water Purification: Future Perspectives. Vacuum 2017, 146, 342–355. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Peresin, M.S.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A.S. Lignin-Based Hydrogels with “super-Swelling” Capacities for Dye Removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domíguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In Vitro Evaluation of Biodegradable Lignin-Based Nanoparticles for Drug Delivery and Enhanced Antiproliferation Effect in Cancer Cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Sánchez, R.; Díaz-Carrasco, P.; Espinosa, E.; García-Domínguez, M.T.; Rodríguez, A. Isolation and Characterization of Lignins from Wheat Straw: Application as Binder in Lithium Batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline Cellulose, Lactose and Lignin Blends: Process Mapping of Dry Granulation Via Roll Compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Iqbal, J.; Shirazian, S.; Walker, G.M.; Collins, M.N. Effect of Lignin on the Release Rate of Acetylsalicylic Acid Tablets. Int. J. Biol. Macromol. 2019, 124, 354–359. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.S. Aqueous Acetone Fractionation of Kraft, Organosolv and Soda Lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Ahuja, S.; Scypinski, S. Handbook of Modern Pharmaceutical Analysis; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Khomane, K.S.; More, P.K.; Raghavendra, G.; Bansal, A.K. Molecular Understanding of the Compaction Behavior of Indomethacin Polymorphs. Mol. Pharm. 2013, 10, 631–639. [Google Scholar] [CrossRef]
- Nordstrom, J.; Klevan, I.; Alderborn, G. A Particle Rearrangement Index Based on the Kawakita Powder Compression Equation. J. Pharm. Sci. 2009, 98, 1053–1063. [Google Scholar] [CrossRef]
- Newton, J.M.; Rowley, G.; Fell, J.T.; Peacock, D.G.; Ridgway, K. Computer Analysis of the Relation between Tablet Strength and Compaction Pressure. J. Pharm. Pharmacol. 1971, 23, 195S–201S. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Wells, T.; Le, R.K.; Sadeghifar, F.; Yuan, J.S.; Ragauskas, A.J. Fractionation of Organosolv Lignin using Acetone:Water and Properties of the obtained Fractions. ACS Sustain. Chem. Eng. 2017, 5, 580–587. [Google Scholar] [CrossRef]
- Santl, M.; Ilic, I.; Vrecer, F.; Baumgartner, S. A Compressibility and Compactibility Study of Real Tableting Mixtures: The Effect of Granule Particle Size. Acta Pharm. 2012, 62, 325–340. [Google Scholar] [CrossRef]
- Rojas, J.; Lopez, A.; Guisao, S.; Ortiz, C. Evaluation of several Microcrystalline Celluloses obtained from Agricultural by-Products. J. Adv. Pharm. Technol. Res. 2011, 2, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; de la Luz Reus-Medina, M.; Yang, D. Preparation, Characterization, and Tabletting Properties of a New Cellulose-Based Pharmaceutical Aid. Int. J. Pharm. 2002, 235, 129–140. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative Evaluation of Flow for Pharmaceutical Powders and Granules. AAPS PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef]
- Ardizzone, S.; Dioguardi, F.S.; Mussini, T.; Mussini, P.R.; Rondinini, S.; Vercelli, B.; Vertova, A. Microcrystalline Cellulose Powders: Structure, Surface Features and Water Sorption Capability. Cellulose 1999, 6, 57–69. [Google Scholar] [CrossRef]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Comparison of Traditional and Novel Tableting Excipients: Physical and Compaction Properties. Pharm. Dev. Technol. 2012, 17, 649–653. [Google Scholar] [CrossRef]
- Asnaashari, S.; Khoei, N.S.; Zarrintan, M.H.; Adibkia, K.; Javadzadeh, Y. Preparation and Evaluation of Novel Metronidazole Sustained Release and Floating Matrix Tablets. Pharm. Dev. Technol. 2011, 16, 400–407. [Google Scholar] [CrossRef]
- Kitazawa, S.; Johno, I.; Ito, Y.; Teramura, S.; Okado, J. Effects of Hardness on the Disintegration Time and the Dissolution Rate of Uncoated Caffeine Tablets. J. Pharm. Pharmacol. 1975, 27, 765–770. [Google Scholar] [CrossRef]
- Notley, S.M.; Norgren, M. Surface Energy and Wettability of Spin-Coated Thin Films of Lignin Isolated from Wood. Langmuir 2010, 26, 5484–5490. [Google Scholar] [CrossRef]
- Tagami, A.; Gioia, C.; Lauberts, M.; Budnyak, T.; Moriana, R.; Lindström, M.E.; Sevastyanova, O. Solvent Fractionation of Softwood and Hardwood Kraft Lignins for More Efficient Uses: Compositional, Structural, Thermal, Antioxidant and Adsorption Properties. Ind. Crops Prod. 2019, 129, 123–134. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Wang, G.; Sui, W.; Tao, Z. Enhancing the Solubility and Antioxidant Activity of High-Molecular-Weight Lignin by Moderate Depolymerization Via in Situ Ethanol/Acid Catalysis. Ind. Crops Prod. 2019, 128, 177–185. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, X.; Ma, G.; Qian, Y.; Qiu, X.; Ruan, T. Improving Antioxidant Activity of Lignin by Hydrogenolysis. Ind. Crops Prod. 2018, 125, 228–235. [Google Scholar] [CrossRef]
- Kalasz, H.; Antal, I. Drug Excipients. Curr. Med. Chem. 2006, 13, 2535–2563. [Google Scholar] [CrossRef]
- Vinardell, P.M.; Mitjans, M. Lignins and their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [PubMed]
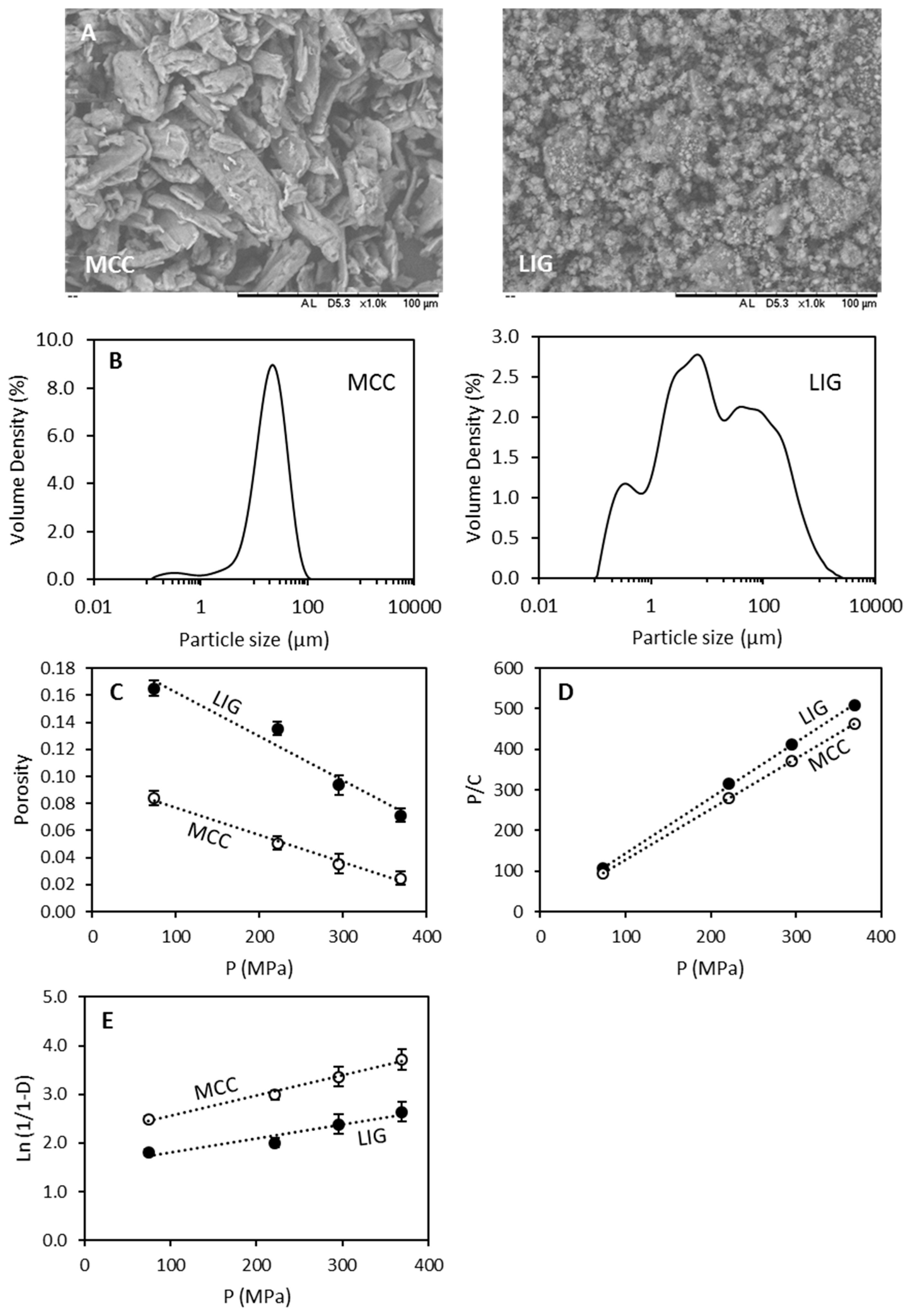


| Material | Bulk Density (g/mL) | Tapped Density (g/mL) | Hausner Ratio | Carr Index | BET Specific Surface Area (m2/g) | Pore Size (Å) | Porosity |
|---|---|---|---|---|---|---|---|
| MCC | 0.306 ± 0.002 | 0.348 ± 0.008 | 1.13 ± 0.02 | 12 ± 2 | 1.53 | 294 | 0.80 ± 0.02 |
| LIG | 0.354 ± 0.005 | 0.405 ± 0.005 | 1.14 ± 0.03 | 12 ± 2 | 5.45 | 238 | 0.74 ± 0.01 |
| Kawakita | Heckel | |||
|---|---|---|---|---|
| a | 1/b (MPa) | Pγ (MPa) | Da | |
| MCC | 0.80 ± 0.01 | 2.3 ± 0.4 | 242 ± 32 | 0.88 ± 0.01 |
| LIG | 0.73 ± 0.01 | 7.9 ± 1.0 | 361 ± 77 | 0.78 ± 0.03 |
| Formulation | Compression (Tonnes/MPa) | Excipient Composition (%) | Thickness (mm) | Mass Uniformity (%) | |
|---|---|---|---|---|---|
| LIG | MCC | ||||
| L02 | 2.0/147.8 | 0.0 | 100.0 | 2.70 ± 0.02 | 0.5 ± 0.7 |
| L22 | 25.0 | 75.0 | 2.74 ± 0.01 | 0.7 ± 0.4 | |
| L52 | 50.0 | 50.0 | 2.76 ± 0.01 | 1.1 ± 0.4 | |
| L72 | 75.0 | 25.0 | 2.82 ± 0.01 | 1.2 ± 0.3 | |
| L102 | 100.0 | 0.0 | 2.85 ± 0.02 | 6.5 ± 1.3 | |
| L05 | 5.0/369.4 | 0.0 | 100.0 | 2.56 ± 0.01 | 0.8 ± 0.3 |
| L25 | 25.0 | 75.0 | 2.58 ± 0.02 | 1.1 ± 0.3 | |
| L55 | 50.0 | 50.0 | 2.61 ± 0.01 | 1.3 ± 0.3 | |
| L75 | 75.0 | 25.0 | 2.69 ± 0.01 | 1.9 ± 0.6 | |
| L105 | 100.0 | 0.0 | 2.72 ± 0.01 | 5.6 ± 2.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Robles, J.; Stewart, S.A.; Rendl, A.; González, Z.; Donnelly, R.F.; Larrañeta, E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules 2019, 9, 423. https://doi.org/10.3390/biom9090423
Domínguez-Robles J, Stewart SA, Rendl A, González Z, Donnelly RF, Larrañeta E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules. 2019; 9(9):423. https://doi.org/10.3390/biom9090423
Chicago/Turabian StyleDomínguez-Robles, Juan, Sarah A. Stewart, Andreas Rendl, Zoilo González, Ryan F. Donnelly, and Eneko Larrañeta. 2019. "Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression" Biomolecules 9, no. 9: 423. https://doi.org/10.3390/biom9090423
APA StyleDomínguez-Robles, J., Stewart, S. A., Rendl, A., González, Z., Donnelly, R. F., & Larrañeta, E. (2019). Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules, 9(9), 423. https://doi.org/10.3390/biom9090423