N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Blood Sampling
3. N-glycan Determination in Plasma
3.1. Glycan Release and Labelling in Plasma
3.2. Hydrophilic Interaction High Performance Liquid Chromatography (HILIC)
4. N-glycan Determination in IgG
4.1. Glycan Release and Labelling in IgG
4.2. Ultraperformance Liquid Chromatography (UPLC) Analysis of IgG Glycans
5. Statistical Analysis
6. Results
6.1. N-glycans in Plasma
6.2. N-glycans Attached to IgG
6.3. GlycoAge Test
6.4. N-glycans and PTSD Severity
7. Discussion
7.1. N-glycans in Plasma
7.2. N-glycans Attached to IgG
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Dursa, E.K.; Reinhard, M.J.; Barth, S.K.; Schneiderman, A.I. Prevalence of a positive screen for PTSD among OEF/OIF and OEF/OIF-era veterans in a large population-based cohort. J. Trauma Stress 2014, 27, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Komar, Z.; Vukusic, H. Post-traumatic stress disorder in Croatia war veterans: Prevalence and psycho-social characteristics. In New Insight in Post-Traumatic Stress Disorder (PTSD); Dekaris, D., Sabioncello, A., Eds.; Croatian Academy of Sciences and Arts: Zagreb, Croatia, 1999; pp. 42–44. [Google Scholar]
- Britvic, D.; Anticevic, V.; Kaliterna, M.; Lusic, L.; Beg, A.; Brajevic-Gizdic, I.; Kudric, M.; Stupalo, Z.; Krolo, V.; Pivac, N. Comorbidities with Posttraumatic Stress Disorder (PTSD) among combat veterans: 15 years postwar analysis. Int. J. Clin. Health Psychol. 2015, 15, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Gautam, A.; Hammamieh, R.; Jett, M.; Wolkowitz, O.M. Metabolism, Metabolomics, and Inflammation in Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 866–875. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, A.; Cohen, B.E.; Seal, K.H.; Bertenthal, D.; Margaretten, M.; Nishimi, K.; Neylan, T.C. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol. Psychiatry 2015, 77, 365–374. [Google Scholar] [CrossRef]
- Speer, K.; Upton, D.; Semple, S.; McKune, A. Systemic low-grade inflammation in post-traumatic stress disorder: A systematic review. J. Inflamm. Res. 2018, 11, 111–121. [Google Scholar] [CrossRef]
- McFarlane, A.C. Post-traumatic stress disorder is a systemic illness, not a mental disorder: Is Cartesian dualism dead? Med. J. Aust. 2017, 206, 248–249. [Google Scholar] [CrossRef]
- Domingo-Fernandez, D.; Provost, A.; Kodamullil, A.T.; Marin-Llao, J.; Lasseter, H.; Diaz, K.; Daskalakis, N.P.; Lancashire, L.; Hofmann-Apitius, M.; Haas, M. PTSD Biomarker Database: Deep dive metadatabase for PTSD biomarkers, visualizations and analysis tools. Database 2019. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef]
- Pucic, M.; Knezevic, A.; Vidic, J.; Adamczyk, B.; Novokmet, M.; Polasek, O.; Gornik, O.; Supraha-Goreta, S.; Wormald, M.R.; Redzic, I.; et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell Proteom. 2011, 10, M111-010090. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, V.; Dewaele, S.; Libert, C.; Engelborghs, S.; De Deyn, P.P.; Toussaint, O.; Debacq-Chainiaux, F.; Poulain, M.; Glupczynski, Y.; Franceschi, C.; et al. Serum N-glycan profile shift during human ageing. Exp. Gerontol. 2010, 45, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Stanta, J.L.; Saldova, R.; Struwe, W.B.; Byrne, J.C.; Leweke, F.M.; Rothermund, M.; Rahmoune, H.; Levin, Y.; Guest, P.C.; Bahn, S.; et al. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J. Proteome Res. 2010, 9, 4476–4489. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Stambuk, J.; Razdorov, G.; Pucic-Bakovic, M.; Martins-de-Souza, D.; Lauc, G.; Turck, C.W. Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response. Sci. Rep. 2018, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Pivac, N.; Knezevic, A.; Gornik, O.; Pucic, M.; Igl, W.; Peeters, H.; Crepel, A.; Steyaert, J.; Novokmet, M.; Redzic, I.; et al. Human Plasma Glycome in Attention-Deficit Hyperactivity Disorder and Autism Spectrum Disorders. Mol. Cell Proteom. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, S.L.; Yang, H.; Lyutvinskiy, Y.; Rutishauser, D.; Herukka, S.K.; Soininen, H.; Zubarev, R.A. Blood plasma IgG Fc glycans are significantly altered in Alzheimer’s disease and progressive mild cognitive impairment. J. Alzheimers Dis. 2014, 38, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villanueva, M.; Morath, J.; Vanhooren, V.; Elbert, T.; Kolassa, S.; Libert, C.; Burkle, A.; Kolassa, I.T. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl. Psychiatry 2013, 3, e320. [Google Scholar] [CrossRef]
- Lauc, G.; Dabelic; Dumic, J.; Flogel, M. Stressin and natural killer cell activity in professional soldiers. Ann. N. Y. Acad. Sci. 1998, 851, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Nie, H.; Sun, X.; Sun, W.; Qu, Y.; Liu, X.; Yao, Y.; Liang, X.; Chen, C.C.; Li, Y. Human serum N-glycan profiles are age and sex dependent. Age Ageing 2011, 40, 568–575. [Google Scholar] [CrossRef]
- Gebrehiwot, A.G.; Melka, D.S.; Kassaye, Y.M.; Rehan, I.F.; Rangappa, S.; Hinou, H.; Kamiyama, T.; Nishimura, S.I. Healthy human serum N-glycan profiling reveals the influence of ethnic variation on the identified cancer-relevant glycan biomarkers. PLoS ONE 2018, 13, e0209515. [Google Scholar] [CrossRef]
- Weathers, F.W.; Keane, T.M.; Davidson, J.R. Clinician-administered PTSD scale: A review of the first ten years of research. Depress. Anxiety 2001, 13, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Akmacic, I.T.; Ugrina, I.; Stambuk, J.; Gudelj, I.; Vuckovic, F.; Lauc, G.; Pucic-Bakovic, M. High-throughput glycomics: Optimization of sample preparation. Biochem. Mosc. 2015, 80, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Shehni, A.A.; Haakensen, V.D.; Steinfeld, I.; Hilliard, M.; Kifer, I.; Helland, A.; Yakhini, Z.; Borresen-Dale, A.L.; Rudd, P.M. Association of N-Glycosylation with Breast Carcinoma and Systemic Features Using High-Resolution Quantitative UPLC. J. Proteome Res. 2014, 13, 2314–2327. [Google Scholar] [CrossRef]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Knezevic, A.; Gornik, O.; Polasek, O.; Pucic, M.; Redzic, I.; Novokmet, M.; Rudd, P.M.; Wright, A.F.; Campbell, H.; Rudan, I.; et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 2010, 20, 959–969. [Google Scholar] [CrossRef]
- Perkovic, M.N.; Bakovic, M.P.; Kristic, J.; Novokmet, M.; Huffman, J.E.; Vitart, V.; Hayward, C.; Rudand, I.; Wilson, J.F.; Campbell, H.; et al. The association between galactosylation of immunoglobulin G and body mass index. Prog. Neuropsychopharmacol. Biol. Psych. 2014, 48, 20–25. [Google Scholar] [CrossRef]
- Konjevod, M.; Tudor, L.; Strac, D.S.; Erjavec, G.N.; Barbas, C.; Zarkovic, N.; Perkovic, M.N.; Uzun, S.; Kozumplik, O.; Lauc, G.; et al. Metabolomic and glycomic findings in posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psych. 2019, 88, 181–193. [Google Scholar] [CrossRef]
- Kristic, J.; Vuckovic, F.; Menni, C.; Klaric, L.; Keser, T.; Beceheli, I.; Pucic-Bakovic, M.; Novokmet, M.; Mangino, M.; Thaqi, K.; et al. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 779–789. [Google Scholar] [CrossRef]
- Clerc, F.; Novokmet, M.; Dotz, V.; Reiding, K.R.; de Haan, N.; Kammeijer, G.S.M.; Dalebout, H.; Bladergroen, M.R.; Vukovic, F.; Rapp, E.; et al. Plasma N-Glycan Signatures Are Associated With Features of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Baciarello, M.; Ugrina, I.; De Gregori, M.; Napolioni, V.; Ingelmo, P.M.; Bugada, D.; De Gregori, S.; Derek, L.; Pucic-Bakovic, M.; et al. Changes in total plasma and serum N-glycome composition and patient-controlled analgesia after major abdominal surgery. Sci Rep. 2016, 6, 31234. [Google Scholar] [CrossRef] [PubMed]
- Pivac, N.; Kozaric-Kovacic, D. Pharmacotherapy of treatment-resistant combat-related posttraumatic stress disorder with psychotic features. Croat. Med. J. 2006, 47, 440–451. [Google Scholar] [PubMed]
- Brinkman-van der Linden, E.C.; de Haan, P.F.; Havenaar, E.C.; van Dijk, W. Inflammation-induced expression of sialyl LewisX is not restricted to alpha1-acid glycoprotein but also occurs to a lesser extent on alpha1-antichymotrypsin and haptoglobin. Glycoconjugate J. 1998, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef]
- Talabnin, K.; Talabnin, C.; Ishihara, M.; Azadi, P. Increased expression of the high-mannose M6N2 and NeuAc3H3N3M3N2F tri-antennary N-glycans in cholangiocarcinoma. Oncol. Lett. 2018, 15, 1030–1036. [Google Scholar] [CrossRef]
- Bhat, G.; Hothpet, V.R.; Lin, M.F.; Cheng, P.W. Shifted Golgi targeting of glycosyltransferases and alpha-mannosidase IA from giantin to GM130-GRASP65 results in formation of high mannose N-glycans in aggressive prostate cancer cells. Biochim. Biophys Acta Gen. Subj. 2017, 1861, 2891–2901. [Google Scholar] [CrossRef]
- De Leoz, M.L.; Young, L.J.; An, H.J.; Kronewitter, S.R.; Kim, J.; Miyamoto, S.; Borowsky, A.D.; Chew, H.K.; Lebrilla, C.B. High-mannose glycans are elevated during breast cancer progression. Mol. Cell Proteom. 2011, 10, M110-002717. [Google Scholar] [CrossRef]
- Tucholski, J.; Simmons, M.S.; Pinner, A.L.; Haroutunian, V.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr. Res. 2013, 146, 177–183. [Google Scholar] [CrossRef]
- Brunngraber, E.G.; Webster, J.C. Changes in glycoprotein carbohydrate content in the aging human brain. Neurochem. Res. 1986, 11, 579–588. [Google Scholar] [CrossRef]
- Masuda, K.; Kubota, T.; Kaneko, E.; Iida, S.; Wakitani, M.; Kobayashi-Natsume, Y.; Kubota, A.; Shitara, K.; Nakamura, K. Enhanced binding affinity for FcgammaRIIIa of fucose-negative antibody is sufficient to induce maximal antibody-dependent cellular cytotoxicity. Mol. Immunol. 2007, 44, 3122–3131. [Google Scholar] [CrossRef] [PubMed]
| Plasma N-glycan Peak | Discovery Cohort (N = 233) | Replication Cohort (N = 330) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N = 73) | PTSD (N = 160) | Statistics | Control (N = 171) | PTSD (N = 139) | Statistics | |||||||||||
| Median | 25th | 75th | Median | 25th | 75th | MW U | p | Median | 25th | 75th | Median | 25th | 75th | MW U | p | |
| GP1 | 4.615 | 3.885 | 5.514 | 4.161 | 3.392 | 5.103 | 4810.0 | 0.062 | 5.457 | 4.454 | 6.559 | 5.895 | 4.568 | 6.843 | 11094.0 | 0.524 |
| GP2 | 1.994 | 1.740 | 2.267 | 1.970 | 1.726 | 2.261 | 5630.0 | 0.777 | 2.168 | 1.911 | 2.449 | 2.237 | 1.901 | 2.510 | 11476.0 | 0.719 |
| GP3 | 0.084 | 0.071 | 0.101 | 0.089 | 0.068 | 0.109 | 5448.0 | 0.515 | 0.103 | 0.083 | 0.125 | 0.106 | 0.087 | 0.132 | 11490.0 | 0.719 |
| GP4 | 3.759 | 3.232 | 4.411 | 3.234 | 2.867 | 3.711 | 3783.0 | <0.001 | 3.770 | 3.178 | 4.426 | 3.666 | 3.183 | 4.308 | 11793.0 | 0.908 |
| GP5 | 2.062 | 1.658 | 2.394 | 1.684 | 1.394 | 1.971 | 3475.0 | <0.001 | 2.085 | 1.645 | 2.570 | 1.961 | 1.617 | 2.393 | 10989.0 | 0.484 |
| GP6 | 1.212 | 1.048 | 1.387 | 1.157 | 1.025 | 1.341 | 5085.0 | 0.182 | 1.336 | 1.162 | 1.503 | 1.336 | 1.131 | 1.506 | 11633.0 | 0.810 |
| GP7 | 1.023 | 0.960 | 1.124 | 0.979 | 0.902 | 1.144 | 5235.0 | 0.304 | 1.059 | 0.945 | 1.180 | 0.991 | 0.885 | 1.110 | 9285.0 | 0.014 |
| GP8 | 1.046 | 0.993 | 1.134 | 1.088 | 0.974 | 1.189 | 5260.0 | 0.310 | 1.047 | 0.964 | 1.173 | 1.113 | 1.017 | 1.246 | 9453.0 | 0.016 |
| GP9 | 0.098 | 0.087 | 0.112 | 0.099 | 0.083 | 0.113 | 5827.0 | 0.986 | 0.111 | 0.095 | 0.127 | 0.109 | 0.092 | 0.126 | 11156.0 | 0.545 |
| GP10 | 3.345 | 2.983 | 4.145 | 2.809 | 2.313 | 3.501 | 3820.0 | <0.001 | 3.157 | 2.529 | 3.822 | 3.064 | 2.511 | 3.573 | 11242.0 | 0.612 |
| GP11 | 0.701 | 0.641 | 0.880 | 0.701 | 0.590 | 0.840 | 5516.0 | 0.603 | 0.820 | 0.710 | 0.939 | 0.757 | 0.654 | 0.894 | 9913.0 | 0.043 |
| GP12 | 0.998 | 0.939 | 1.062 | 1.022 | 0.905 | 1.105 | 5414.0 | 0.481 | 1.005 | 0.927 | 1.089 | 0.972 | 0.885 | 1.070 | 10629.0 | 0.278 |
| GP13 | 0.710 | 0.627 | 0.875 | 0.655 | 0.559 | 0.761 | 4210.0 | 0.002 | 0.801 | 0.683 | 0.943 | 0.775 | 0.626 | 0.933 | 11038.0 | 0.489 |
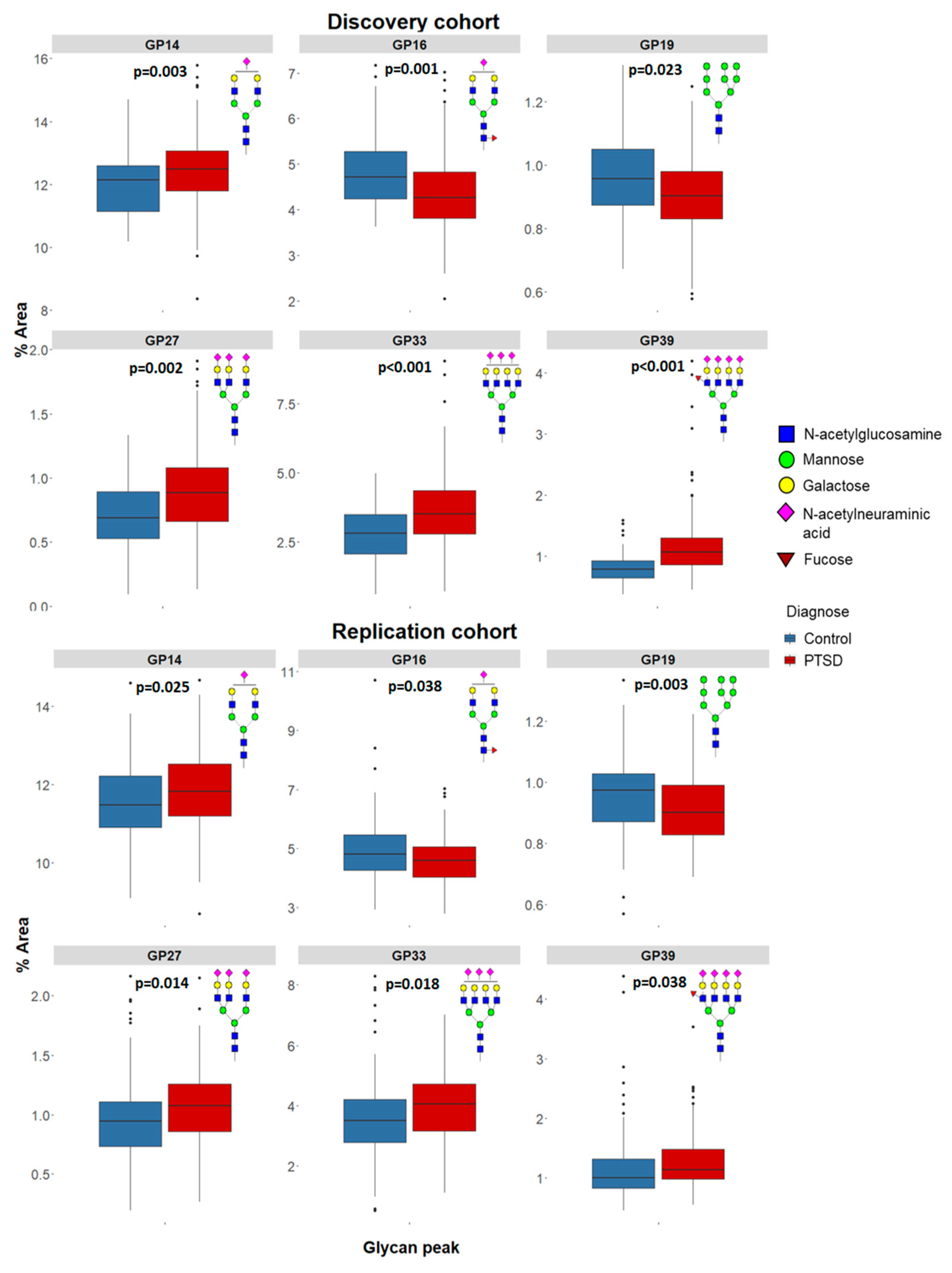
| GP14 | 12.136 | 11.145 | 12.590 | 12.476 | 11.790 | 13.067 | 4261.0 | 0.003 | 11.478 | 10.904 | 12.237 | 11.823 | 11.177 | 12.529 | 9652.0 | 0.025 |
| GP15 | 0.396 | 0.349 | 0.451 | 0.443 | 0.377 | 0.509 | 4278.0 | 0.003 | 0.518 | 0.448 | 0.582 | 0.474 | 0.427 | 0.546 | 9919.0 | 0.043 |
| GP16 | 4.716 | 4.240 | 5.276 | 4.262 | 3.813 | 4.843 | 4080.0 | 0.001 | 4.807 | 4.236 | 5.467 | 4.594 | 4.028 | 5.060 | 9813.0 | 0.038 |
| GP17 | 1.652 | 1.480 | 1.977 | 1.779 | 1.410 | 2.272 | 5254.0 | 0.310 | 2.063 | 1.763 | 2.404 | 1.844 | 1.598 | 2.224 | 9375.0 | 0.014 |
| GP18 | 3.714 | 3.174 | 4.006 | 3.363 | 3.073 | 3.765 | 4873.0 | 0.082 | 3.158 | 2.865 | 3.495 | 3.144 | 2.900 | 3.619 | 11353.0 | 0.712 |
| GP19 | 0.957 | 0.873 | 1.051 | 0.902 | 0.829 | 0.982 | 4627.0 | 0.023 | 0.974 | 0.869 | 1.030 | 0.902 | 0.828 | 0.991 | 8778.0 | 0.003 |
| GP20 | 29.128 | 27.579 | 30.740 | 29.152 | 27.525 | 30.769 | 5657.0 | 0.802 | 26.692 | 24.870 | 27.838 | 26.487 | 24.682 | 28.160 | 11505.0 | 0.719 |
| GP21 | 0.510 | 0.466 | 0.544 | 0.513 | 0.468 | 0.545 | 5391.0 | 0.463 | 0.616 | 0.560 | 0.664 | 0.612 | 0.557 | 0.662 | 11575.0 | 0.771 |
| GP22 | 4.243 | 3.813 | 4.699 | 3.859 | 3.422 | 4.258 | 4089.0 | 0.001 | 4.001 | 3.607 | 4.529 | 3.936 | 3.459 | 4.572 | 10881.0 | 0.403 |
| GP23 | 2.009 | 1.585 | 2.420 | 2.029 | 1.545 | 2.372 | 5687.0 | 0.833 | 2.281 | 1.930 | 2.751 | 2.163 | 1.805 | 2.632 | 10772.0 | 0.360 |
| GP24 | 1.383 | 1.240 | 1.725 | 1.532 | 1.232 | 1.814 | 5061.0 | 0.171 | 1.432 | 1.130 | 1.693 | 1.442 | 1.191 | 1.739 | 11457.0 | 0.719 |
| GP25 | 0.292 | 0.269 | 0.316 | 0.294 | 0.270 | 0.319 | 5717.0 | 0.839 | 0.274 | 0.252 | 0.299 | 0.279 | 0.247 | 0.305 | 11409.0 | 0.719 |
| GP26 | 1.434 | 1.340 | 1.731 | 1.621 | 1.402 | 1.836 | 4590.0 | 0.020 | 1.534 | 1.322 | 1.720 | 1.499 | 1.275 | 1.683 | 10634.0 | 0.278 |
| GP27 | 0.690 | 0.528 | 0.891 | 0.884 | 0.662 | 1.084 | 4160.0 | 0.002 | 0.946 | 0.734 | 1.117 | 1.075 | 0.853 | 1.263 | 9374.0 | 0.014 |
| GP28 | 0.683 | 0.589 | 0.767 | 0.672 | 0.551 | 0.795 | 5831.0 | 0.986 | 0.636 | 0.520 | 0.736 | 0.604 | 0.508 | 0.739 | 11157.0 | 0.545 |
| GP29 | 0.201 | 0.170 | 0.231 | 0.184 | 0.158 | 0.220 | 4975.0 | 0.127 | 0.181 | 0.154 | 0.201 | 0.172 | 0.155 | 0.203 | 11454.0 | 0.719 |
| GP30 | 4.684 | 4.279 | 5.584 | 5.210 | 4.170 | 5.869 | 5029.0 | 0.156 | 4.826 | 3.944 | 5.490 | 4.602 | 3.892 | 5.347 | 10852.0 | 0.397 |
| GP31 | 0.425 | 0.358 | 0.523 | 0.430 | 0.339 | 0.525 | 5703.0 | 0.838 | 0.433 | 0.350 | 0.531 | 0.395 | 0.320 | 0.478 | 9931.0 | 0.043 |
| GP32 | 1.503 | 1.199 | 1.730 | 1.774 | 1.468 | 2.045 | 3733.0 | <0.001 | 1.598 | 1.344 | 1.846 | 1.594 | 1.315 | 1.894 | 11696.0 | 0.848 |
| GP33 | 2.807 | 2.083 | 3.490 | 3.523 | 2.781 | 4.361 | 3883.0 | <0.001 | 3.504 | 2.766 | 4.213 | 4.049 | 3.165 | 4.728 | 9528.0 | 0.018 |
| GP34 | 0.347 | 0.310 | 0.409 | 0.395 | 0.342 | 0.445 | 4210.0 | 0.002 | 0.385 | 0.340 | 0.454 | 0.384 | 0.347 | 0.434 | 11482.0 | 0.719 |
| GP35 | 0.352 | 0.261 | 0.460 | 0.424 | 0.335 | 0.546 | 4287.0 | 0.003 | 0.417 | 0.346 | 0.518 | 0.454 | 0.369 | 0.566 | 10481.0 | 0.211 |
| GP36 | 0.488 | 0.429 | 0.549 | 0.603 | 0.547 | 0.690 | 2393.0 | <0.001 | 0.557 | 0.485 | 0.627 | 0.593 | 0.515 | 0.651 | 10436.0 | 0.200 |
| GP37 | 0.412 | 0.349 | 0.473 | 0.465 | 0.371 | 0.568 | 4533.0 | 0.015 | 0.452 | 0.385 | 0.553 | 0.462 | 0.389 | 0.546 | 11712.0 | 0.848 |
| GP38 | 0.890 | 0.737 | 1.013 | 1.067 | 0.936 | 1.258 | 3029.0 | <0.001 | 1.038 | 0.923 | 1.222 | 1.098 | 0.985 | 1.234 | 10786.0 | 0.360 |
| GP39 | 0.783 | 0.645 | 0.920 | 1.062 | 0.856 | 1.300 | 2931.0 | <0.001 | 1.000 | 0.826 | 1.318 | 1.133 | 0.974 | 1.481 | 9822.0 | 0.038 |
| GlycoAge | 0.112 | 0.017 | 0.238 | 0.163 | 0.040 | 0.295 | 5126.0 | 0.208 | 0.219 | 0.128 | 0.384 | 0.256 | 0.145 | 0.394 | 11021.0 | 0.489 |
| IgG N-glycan Peak | Discovery cohort (N = 233) | Replication cohort (N = 330) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N = 73) | PTSD (N = 160) | Statistics | Control (N = 171) | PTSD (N = 139) | Statistics | |||||||||||
| Median | 25th | 75th | Median | 25th | 75th | MW U | p | Median | 25th | 75th | Median | 25th | 75th | MW U | p | |
| GP1 | 0.085 | 0.070 | 0.111 | 0.089 | 0.072 | 0.110 | 5668.0 | 0.889 | 0.097 | 0.080 | 0.114 | 0.100 | 0.081 | 0.124 | 11164.0 | 0.778 |
| GP2 | 0.536 | 0.368 | 0.719 | 0.642 | 0.399 | 0.924 | 4887.0 | 0.170 | 0.739 | 0.515 | 1.001 | 0.710 | 0.493 | 1.111 | 11671.0 | 0.987 |
| GP3 | 0.104 | 0.088 | 0.116 | 0.108 | 0.091 | 0.125 | 5009.0 | 0.288 | 0.123 | 0.111 | 0.141 | 0.125 | 0.110 | 0.140 | 11811.0 | 0.987 |
| GP4 | 21.850 | 17.974 | 25.144 | 22.733 | 19.818 | 27.247 | 4905.0 | 0.218 | 24.042 | 21.203 | 28.427 | 25.366 | 21.501 | 29.433 | 10892.0 | 0.778 |
| GP5 | 0.163 | 0.150 | 0.178 | 0.157 | 0.142 | 0.174 | 5214.0 | 0.218 | 0.184 | 0.157 | 0.209 | 0.183 | 0.153 | 0.214 | 11700.0 | 0.894 |
| GP6 | 5.340 | 4.262 | 6.201 | 6.167 | 5.056 | 7.334 | 4046.0 | 0.012 | 6.036 | 4.952 | 7.274 | 6.253 | 5.229 | 7.436 | 11067.0 | 0.778 |
| GP7 | 0.365 | 0.289 | 0.450 | 0.402 | 0.274 | 0.531 | 5495.0 | 0.551 | 0.452 | 0.333 | 0.562 | 0.442 | 0.354 | 0.597 | 11456.0 | 0.833 |
| GP8 | 18.936 | 17.695 | 19.801 | 18.551 | 17.409 | 19.584 | 5297.0 | 0.428 | 18.179 | 16.802 | 19.415 | 18.129 | 17.002 | 19.232 | 11641.0 | 0.833 |
| GP9 | 9.594 | 8.789 | 10.605 | 9.142 | 8.198 | 10.011 | 4672.0 | 0.061 | 9.593 | 8.564 | 10.422 | 9.241 | 8.488 | 10.050 | 10592.0 | 0.778 |
| GP10 | 4.910 | 4.291 | 5.618 | 5.172 | 4.574 | 6.129 | 4688.0 | 0.067 | 5.083 | 4.405 | 5.728 | 5.134 | 4.342 | 6.037 | 11427.0 | 0.833 |
| GP11 | 0.670 | 0.578 | 0.732 | 0.708 | 0.638 | 0.821 | 4489.0 | 0.061 | 0.683 | 0.612 | 0.756 | 0.678 | 0.593 | 0.765 | 11853.0 | 0.987 |
| GP12 | 0.684 | 0.544 | 0.895 | 0.643 | 0.466 | 0.928 | 5521.0 | 0.730 | 0.607 | 0.456 | 0.923 | 0.615 | 0.477 | 0.966 | 11571.0 | 0.833 |
| GP13 | 0.264 | 0.230 | 0.298 | 0.258 | 0.229 | 0.293 | 5688.0 | 0.885 | 0.261 | 0.228 | 0.297 | 0.257 | 0.225 | 0.300 | 11484.0 | 0.833 |
| GP14 | 13.171 | 11.025 | 15.405 | 11.864 | 9.800 | 13.913 | 4284.0 | 0.051 | 11.689 | 8.929 | 13.593 | 11.015 | 8.708 | 12.681 | 11005.0 | 0.778 |
| GP15 | 1.762 | 1.595 | 1.919 | 1.736 | 1.482 | 1.960 | 5570.0 | 0.885 | 1.651 | 1.445 | 1.861 | 1.639 | 1.438 | 1.849 | 11831.0 | 0.987 |
| GP16 | 3.126 | 2.837 | 3.437 | 3.086 | 2.768 | 3.393 | 5539.0 | 0.430 | 3.086 | 2.838 | 3.429 | 3.071 | 2.739 | 3.406 | 11222.0 | 0.778 |
| GP17 | 0.973 | 0.917 | 1.088 | 0.967 | 0.881 | 1.110 | 5638.0 | 0.885 | 1.008 | 0.878 | 1.125 | 0.986 | 0.882 | 1.119 | 11677.0 | 0.833 |
| GP18 | 9.359 | 7.857 | 10.641 | 8.559 | 6.916 | 9.896 | 4443.0 | 0.061 | 7.824 | 6.701 | 9.282 | 7.705 | 6.738 | 8.886 | 11115.0 | 0.778 |
| GP19 | 1.992 | 1.749 | 2.206 | 2.010 | 1.752 | 2.314 | 5461.0 | 0.612 | 1.966 | 1.762 | 2.259 | 1.952 | 1.602 | 2.207 | 10479.0 | 0.373 |
| GP20 | 0.419 | 0.373 | 0.471 | 0.428 | 0.380 | 0.472 | 5837.0 | 0.885 | 0.404 | 0.362 | 0.460 | 0.398 | 0.353 | 0.455 | 11154.0 | 0.778 |
| GP21 | 0.851 | 0.783 | 0.942 | 0.875 | 0.792 | 0.972 | 5459.0 | 0.430 | 0.908 | 0.834 | 1.003 | 0.874 | 0.802 | 0.958 | 9792.0 | 0.174 |
| GP22 | 0.165 | 0.132 | 0.201 | 0.173 | 0.143 | 0.210 | 5273.0 | 0.327 | 0.148 | 0.122 | 0.182 | 0.146 | 0.120 | 0.178 | 11278.0 | 0.778 |
| GP23 | 1.790 | 1.508 | 2.226 | 1.587 | 1.340 | 1.888 | 4307.0 | 0.025 | 1.615 | 1.426 | 2.035 | 1.623 | 1.303 | 1.847 | 10358.0 | 0.373 |
| GP24 | 1.816 | 1.637 | 2.070 | 1.939 | 1.669 | 2.253 | 5011.0 | 0.259 | 1.960 | 1.735 | 2.229 | 1.865 | 1.559 | 2.241 | 10417.0 | 0.373 |
| Glycoage | 0.211 | 0.053 | 0.335 | 0.283 | 0.155 | 0.446 | 4735.0 | 0.067 | 0.313 | 0.207 | 0.521 | 0.354 | 0.245 | 0.520 | 11271.0 | 0.778 |
| Plasma N-Glycan Peak | Discovery Cohort (N = 233) | Replication Cohort (N = 330) | ||||||
|---|---|---|---|---|---|---|---|---|
| rho | p | MW U | p | rho | p | MW U | p | |
| GP1 | 0.035 | 0.791 | 875.0 | 0.984 | 0.076 | 0.991 | 612.0 | 0.921 |
| GP2 | 0.031 | 0.801 | 885.0 | 0.984 | 0.009 | 0.991 | 644.0 | 0.921 |
| GP3 | 0.126 | 0.364 | 804.0 | 0.899 | 0.078 | 0.991 | 562.0 | 0.921 |
| GP4 | 0.139 | 0.330 | 821.0 | 0.899 | 0.003 | 0.991 | 615.0 | 0.921 |
| GP5 | 0.149 | 0.330 | 815.0 | 0.899 | 0.034 | 0.991 | 576.0 | 0.921 |
| GP6 | 0.104 | 0.388 | 741.0 | 0.829 | 0.076 | 0.991 | 683.0 | 0.969 |
| GP7 | 0.095 | 0.395 | 753.0 | 0.829 | 0.119 | 0.991 | 687.0 | 0.969 |
| GP8 | 0.026 | 0.826 | 693.0 | 0.829 | 0.009 | 0.991 | 649.0 | 0.921 |
| GP9 | 0.111 | 0.388 | 816.0 | 0.899 | 0.037 | 0.991 | 668.0 | 0.969 |
| GP10 | 0.110 | 0.388 | 877.0 | 0.984 | 0.017 | 0.991 | 635.0 | 0.921 |
| GP11 | 0.055 | 0.735 | 686.0 | 0.829 | 0.060 | 0.991 | 699.0 | 0.969 |
| GP12 | 0.013 | 0.904 | 680.0 | 0.829 | 0.020 | 0.991 | 648.0 | 0.921 |
| GP13 | 0.110 | 0.388 | 839.0 | 0.969 | 0.020 | 0.991 | 508.0 | 0.921 |
| GP14 | 0.047 | 0.773 | 678.0 | 0.829 | 0.005 | 0.991 | 570.0 | 0.921 |
| GP15 | 0.100 | 0.388 | 817.0 | 0.899 | 0.014 | 0.991 | 525.0 | 0.921 |
| GP16 | 0.100 | 0.388 | 770.0 | 0.829 | 0.012 | 0.991 | 544.0 | 0.921 |
| GP17 | 0.044 | 0.784 | 735.0 | 0.829 | 0.018 | 0.991 | 641.0 | 0.921 |
| GP18 | 0.041 | 0.791 | 745.0 | 0.829 | 0.001 | 0.991 | 685.0 | 0.969 |
| GP19 | 0.148 | 0.330 | 602.0 | 0.829 | 0.204 | 0.312 | 593.0 | 0.921 |
| GP20 | 0.118 | 0.388 | 798.0 | 0.899 | 0.004 | 0.991 | 605.0 | 0.921 |
| GP21 | 0.034 | 0.791 | 881.0 | 0.984 | 0.092 | 0.991 | 625.0 | 0.921 |
| GP22 | 0.095 | 0.395 | 845.0 | 0.976 | 0.029 | 0.991 | 605.0 | 0.921 |
| GP23 | 0.012 | 0.904 | 850.0 | 0.977 | 0.097 | 0.991 | 514.0 | 0.921 |
| GP24 | 0.137 | 0.330 | 823.0 | 0.899 | 0.025 | 0.991 | 696.0 | 0.969 |
| GP25 | 0.034 | 0.791 | 635.0 | 0.829 | 0.205 | 0.312 | 505.0 | 0.921 |
| GP26 | 0.048 | 0.773 | 685.0 | 0.829 | 0.070 | 0.991 | 586.0 | 0.921 |
| GP27 | 0.136 | 0.330 | 725.0 | 0.829 | 0.064 | 0.991 | 452.0 | 0.921 |
| GP28 | 0.170 | 0.330 | 736.0 | 0.829 | 0.012 | 0.991 | 676.0 | 0.969 |
| GP29 | 0.009 | 0.914 | 770.0 | 0.829 | 0.058 | 0.991 | 672.0 | 0.969 |
| GP30 | 0.133 | 0.330 | 621.0 | 0.829 | 0.018 | 0.991 | 693.0 | 0.969 |
| GP31 | 0.158 | 0.330 | 668.0 | 0.829 | 0.117 | 0.991 | 641.0 | 0.921 |
| GP32 | 0.104 | 0.388 | 872.0 | 0.984 | 0.008 | 0.991 | 602.0 | 0.921 |
| GP33 | 0.146 | 0.330 | 760.0 | 0.829 | 0.011 | 0.991 | 508.0 | 0.921 |
| GP34 | 0.017 | 0.901 | 752.0 | 0.829 | 0.134 | 0.991 | 590.0 | 0.921 |
| GP35 | 0.106 | 0.388 | 773.0 | 0.829 | 0.038 | 0.991 | 515.0 | 0.921 |
| GP36 | 0.136 | 0.330 | 867.0 | 0.984 | 0.093 | 0.991 | 580.0 | 0.921 |
| GP37 | 0.056 | 0.735 | 696.0 | 0.829 | 0.002 | 0.991 | 614.0 | 0.921 |
| GP38 | 0.074 | 0.575 | 772.0 | 0.829 | 0.028 | 0.991 | 695.0 | 0.969 |
| GP39 | 0.151 | 0.330 | 806.0 | 0.899 | 0.025 | 0.991 | 628.0 | 0.921 |
| GlycoAge | 0.036 | 0.791 | 875.0 | 0.984 | 0.013 | 0.991 | 689.0 | 0.969 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudor, L.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Konjevod, M.; Svob Strac, D.; Uzun, S.; Kozumplik, O.; Jovanovic, T.; Lauc, G.; Pivac, N. N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder. Biomolecules 2019, 9, 834. https://doi.org/10.3390/biom9120834
Tudor L, Nedic Erjavec G, Nikolac Perkovic M, Konjevod M, Svob Strac D, Uzun S, Kozumplik O, Jovanovic T, Lauc G, Pivac N. N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder. Biomolecules. 2019; 9(12):834. https://doi.org/10.3390/biom9120834
Chicago/Turabian StyleTudor, Lucija, Gordana Nedic Erjavec, Matea Nikolac Perkovic, Marcela Konjevod, Dubravka Svob Strac, Suzana Uzun, Oliver Kozumplik, Tanja Jovanovic, Gordan Lauc, and Nela Pivac. 2019. "N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder" Biomolecules 9, no. 12: 834. https://doi.org/10.3390/biom9120834
APA StyleTudor, L., Nedic Erjavec, G., Nikolac Perkovic, M., Konjevod, M., Svob Strac, D., Uzun, S., Kozumplik, O., Jovanovic, T., Lauc, G., & Pivac, N. (2019). N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder. Biomolecules, 9(12), 834. https://doi.org/10.3390/biom9120834






