Design of N-Terminal Derivatives from a Novel Dermaseptin Exhibiting Broad-Spectrum Antimicrobial Activity against Isolates from Cystic Fibrosis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Skin Secretion of South American Tree-Frog, Phyllomedusa Coelestis
2.2. Identification of DM-PC Precursor-Encoding cDNA from Skin Secretion
2.3. Isolation of the Putative Mature Peptide from Skin Secretion
2.4. Peptide Synthesis
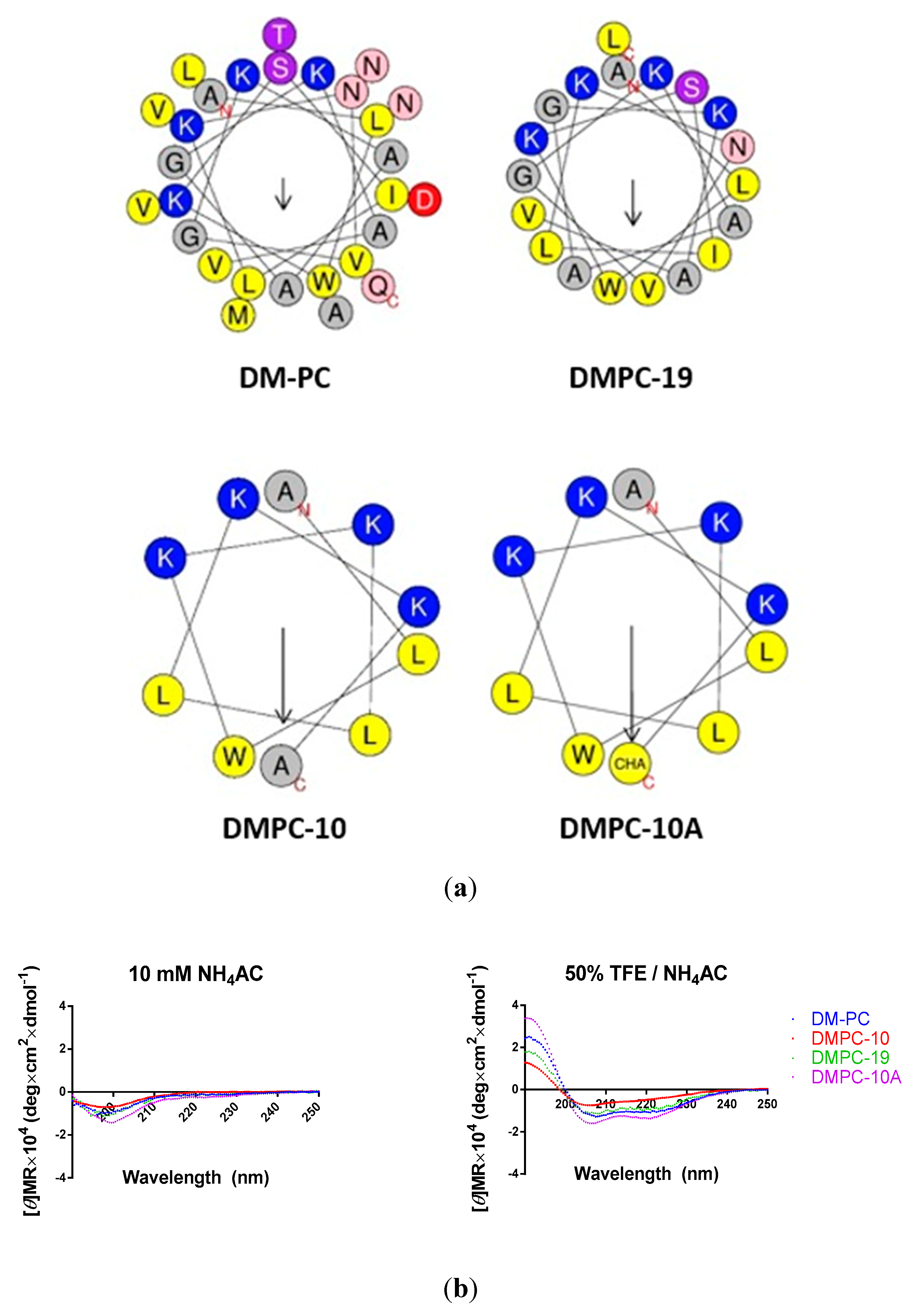
2.5. Secondary Structure Analysis of Synthetic Peptide
2.6. Antimicrobial Assays
2.7. Time Killing Assay
2.8. Membrane Permeability Kinetic Assay
2.9. Minimal Biofilm Inhibitory Concentration (MBIC) and Minimal Biofilm Eradication Concentration (MBEC) Assays
2.10. Haemolysis Assay
3. Results
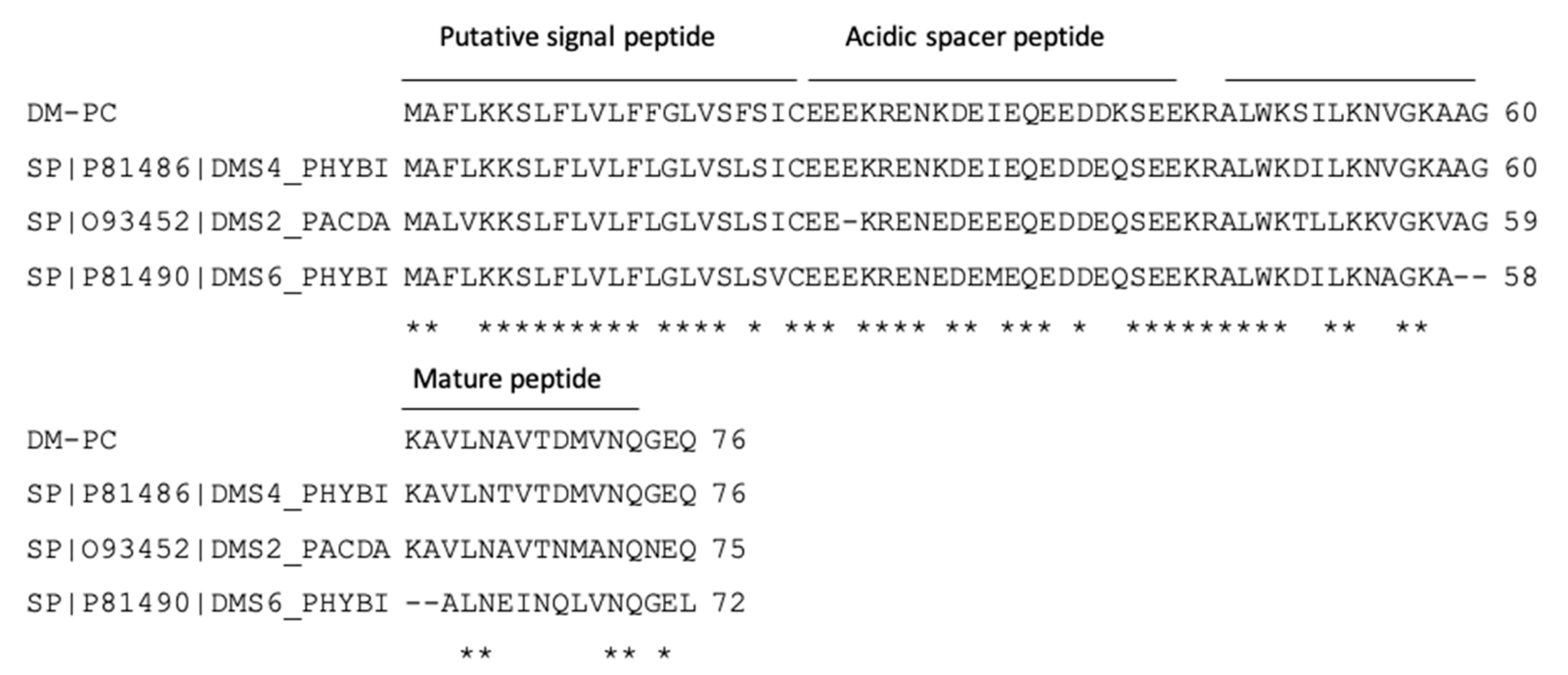
3.1. Identification and Characterisation of DM-PC from the Skin Secretion
3.2. Peptide Design
3.3. Antimicrobial Activities of DM-PC and the Derivatives
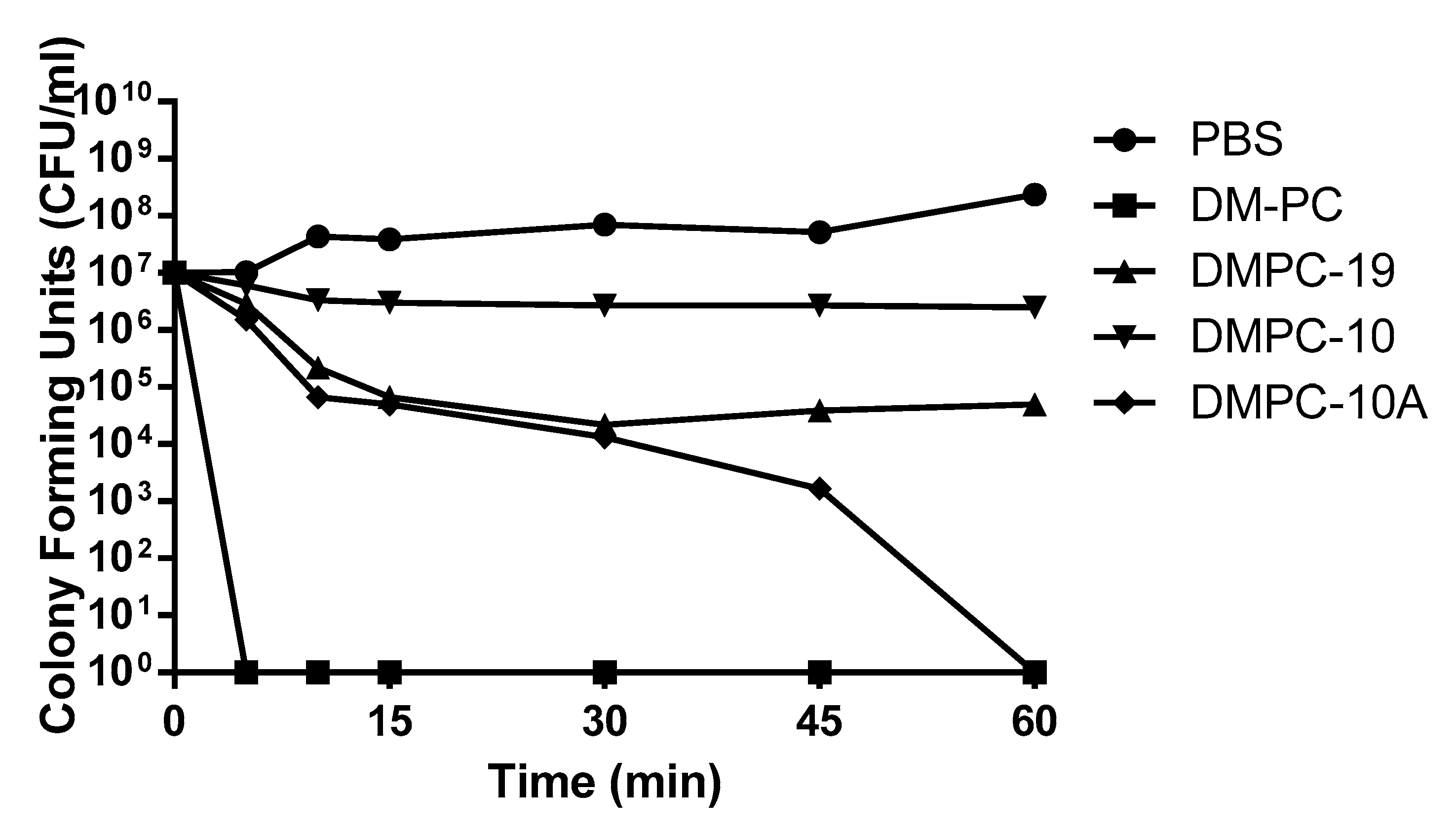
3.4. Killing Kinetics Against P. aeruginosa
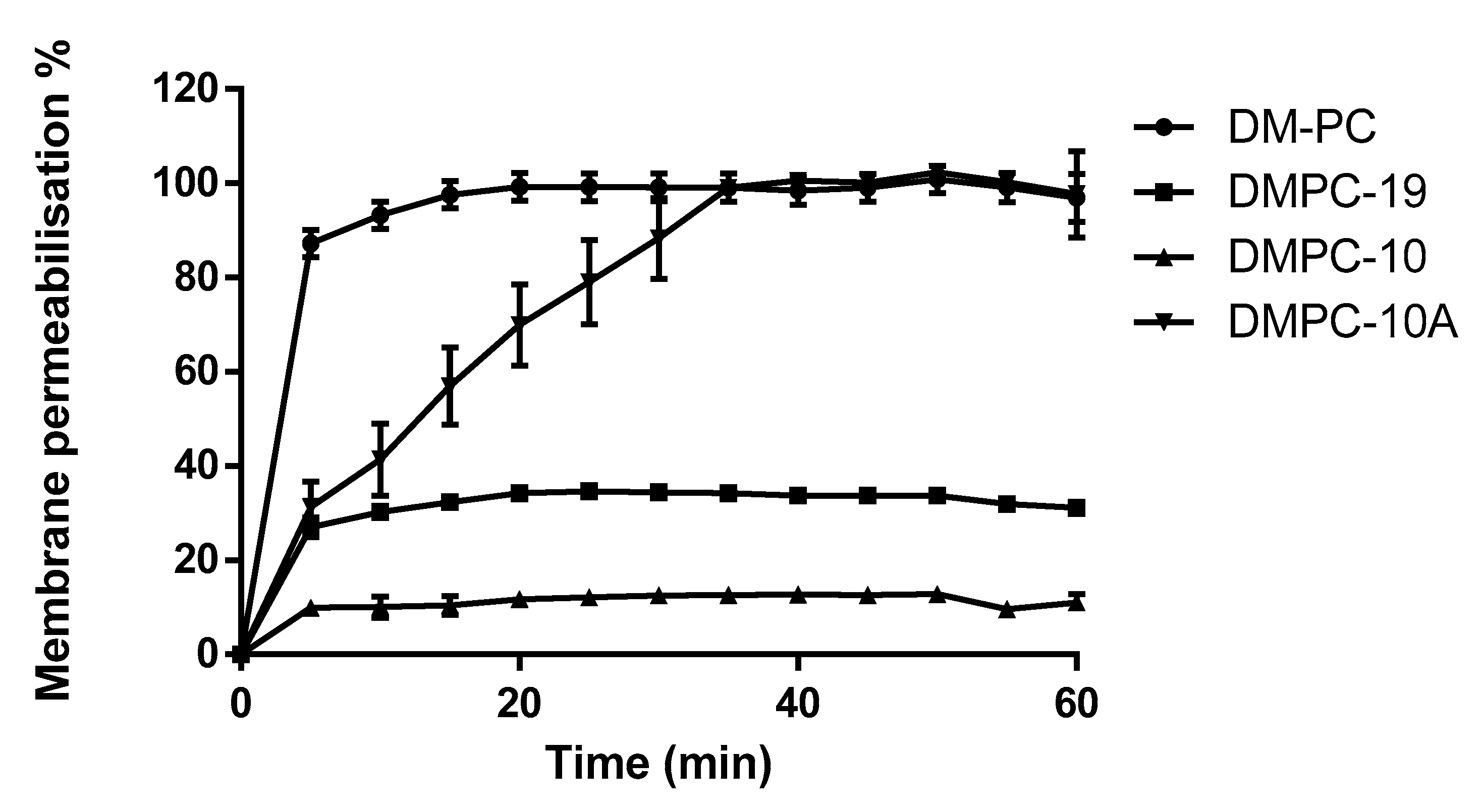
3.5. Membrane Permeability Kinetics on P. aeruginosa
3.6. Anti-Biofilm Activity of DM-PC and Its Derivatives
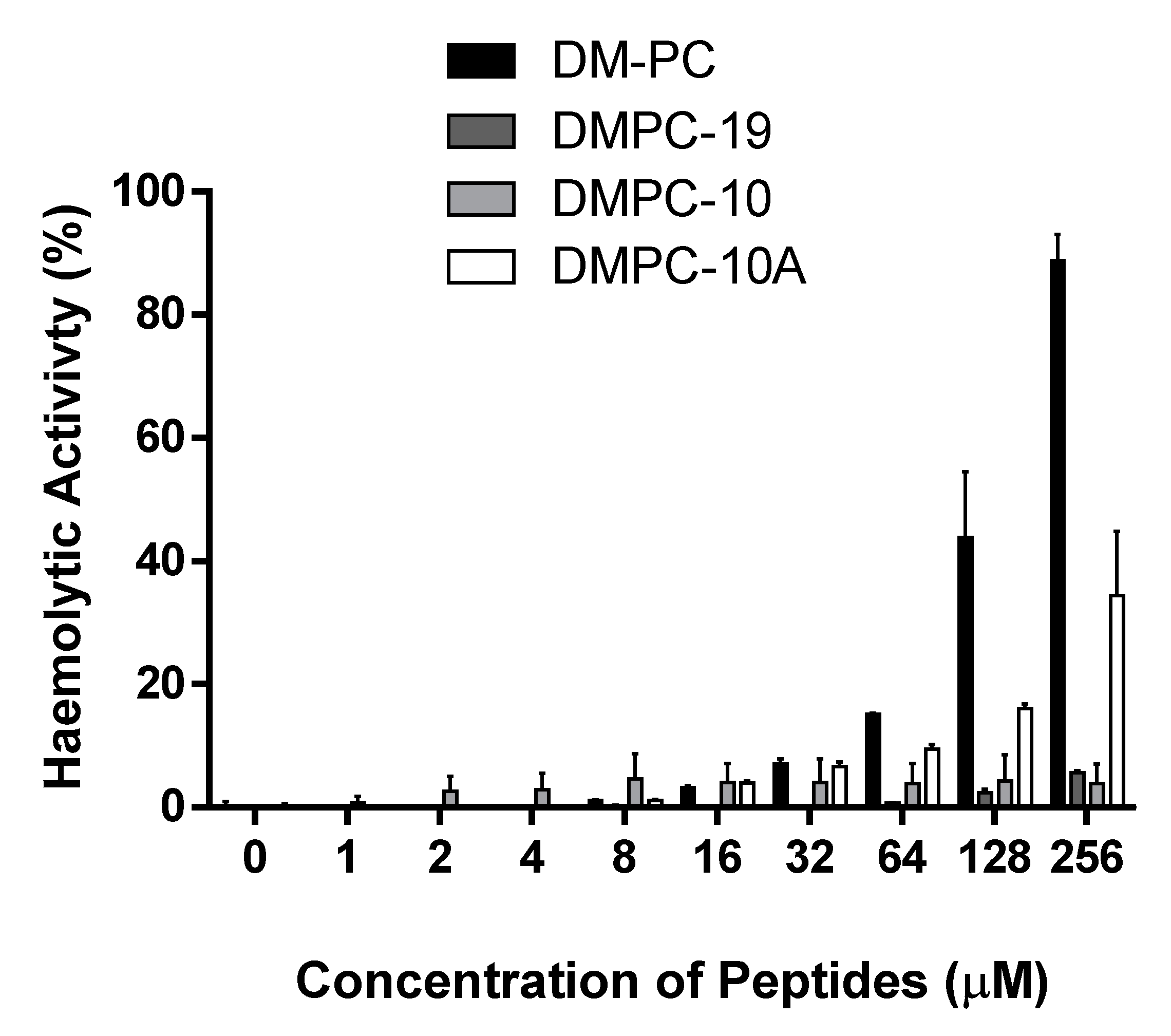
3.7. Haemolytic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebenhan, T.; Gheysens, O.; Kruger, H.G.; Zeevaart, J.R.; Sathekge, M.M. Antimicrobial peptides: Their role as infection-selective tracers for molecular imaging. Biomed. Res. Int. 2014, 2014, 867381. [Google Scholar] [CrossRef]
- Cao, H.; Ke, T.; Liu, R.; Yu, J.; Dong, C.; Cheng, M.; Huang, J.; Liu, S. Identification of a Novel Proline-Rich Antimicrobial Peptide from Brassica napus. PLoS ONE 2015, 10, e0137414. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Vineeth Kumar, T.V.; Sanil, G. A Review of the Mechanism of Action of Amphibian Antimicrobial Peptides Focusing on Peptide-Membrane Interaction and Membrane Curvature. Curr. Protein. Pept. Sci. 2017, 18, 1263–1272. [Google Scholar] [PubMed]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Zanjani, N.T.; Miranda-Saksena, M.; Cunningham, A.L.; Dehghani, F. Antimicrobial Peptides of Marine Crustaceans: The Potential and Challenges of Developing Therapeutic Agents. Curr. Med. Chem. 2018, 25, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.; O’Brien, S.; Brockhurst, M.A. Pseudomonas aeruginosa Evolutionary Adaptation and Diversification in Cystic Fibrosis Chronic Lung Infections. Trends Microbiol. 2016, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.S.; Egan, M.; Kazmierczak, B.I. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 2007, 19, 83–88. [Google Scholar] [CrossRef]
- Forde, E.; Schutte, A.; Reeves, E.; Greene, C.; Humphreys, H.; Mall, M.; Fitzgerald-Hughes, D.; Devocelle, M. Differential In Vitro and In Vivo Toxicities of Antimicrobial Peptide Prodrugs for Potential Use in Cystic Fibrosis. Antimicrob. Agents Chemother. 2016, 60, 2813–2821. [Google Scholar] [CrossRef]
- Yuan, Y.; Zai, Y.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Shaw, C.; Chen, T. A novel membrane-disruptive antimicrobial peptide from frog skin secretion against cystic fibrosis isolates and evaluation of anti-MRSA effect using Galleria mellonella model. Biochim. Biophys. Acta. Gen. Subj. 2019, 1863, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; El Amri, C. The dermaseptin superfamily: A gene-based combinatorial library of antimicrobial peptides. Biochim. Biophys. Acta. 2009, 1788, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Castiglione-Morelli, M.A.; Cristinziano, P.; Pepe, A.; Temussi, P.A. Conformation-activity relationship of a novel peptide antibiotic: Structural characterization of dermaseptin DS 01 in media that mimic the membrane environment. Biopolymers 2005, 80, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Van Zoggel, H.; Carpentier, G.; Dos Santos, C.; Hamma-Kourbali, Y.; Courty, J.; Amiche, M.; Delbe, J. Antitumor and angiostatic activities of the antimicrobial peptide dermaseptin B2. PLoS ONE 2012, 7, e44351. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Feder, R.; Gaidukov, L.; Carmeli, Y.; Mor, A. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 2002, 46, 689–694. [Google Scholar] [CrossRef]
- Krugliak, M.; Feder, R.; Zolotarev, V.Y.; Gaidukov, L.; Dagan, A.; Ginsburg, H.; Mor, A. Antimalarial activities of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 2000, 44, 2442–2451. [Google Scholar] [CrossRef]
- Irazazabal, L.N.; Porto, W.F.; Ribeiro, S.M.; Casale, S.; Humblot, V.; Ladram, A.; Franco, O.L. Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim. Biophys. Acta. 2016, 1858, 2699–2708. [Google Scholar] [CrossRef]
- Tyler, M.J.; Stone, D.J.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods. 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Zhou, M.; Duan, J.; Bininda-Emonds, O.R.P.; Chen, T.; et al. Targeted Modification of a Novel Amphibian Antimicrobial Peptide from Phyllomedusa tarsius to Enhance Its Activity against MRSA and Microbial Biofilm. Front. Microbiol. 2017, 8, 628. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. Antimicrobial Potential of Carvacrol against Uropathogenic Escherichia coli via Membrane Disruption, Depolarization, and Reactive Oxygen Species Generation. Front. Microbiol 2017, 8, 2421. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Dermaseptin-PH: A Novel Peptide with Antimicrobial and Anticancer Activities from the Skin Secretion of the South American Orange-Legged Leaf Frog, Pithecopus (Phyllomedusa) hypochondrialis. Molecules 2017, 22, 1805. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef] [PubMed]
- Kustanovich, I.; Shalev, D.E.; Mikhlin, M.; Gaidukov, L.; Mor, A. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 2002, 277, 16941–16951. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2014, 69, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Parente, J.; Harris, S.M.; Woods, D.E.; Hancock, R.E.W.; Falla, T.J. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 2005, 49, 2921–2927. [Google Scholar] [CrossRef]
- Shang, D.; Meng, X.; Zhang, D.; Kou, Z. Antibacterial activity of chensinin-1b, a peptide with a random coil conformation, against multiple-drug-resistant Pseudomonas aeruginosa. Biochem. Pharmacol. 2017, 143, 65–78. [Google Scholar] [CrossRef]
- Van Der Does, A.M.; Amatngalim, G.D.; Keijser, B.; Hiemstra, P.S.; Villenave, R. Contribution of Host Defence Proteins and Peptides to Host-Microbiota Interactions in Chronic Inflammatory Lung Diseases. Vaccines (Basel) 2018, 6, 49. [Google Scholar] [CrossRef]
- Chen, H.; Wubbolts, R.W.; Haagsman, H.P.; Veldhuizen, E.J.A. Inhibition and Eradication of Pseudomonas aeruginosa Biofilms by Host Defence Peptides. Sci. Rep. 2018, 8, 10446. [Google Scholar] [CrossRef]
- Nagarajan, D.; Nagarajan, T.; Roy, N.; Kulkarni, O.; Ravichandran, S.; Mishra, M.; Chakravortty, D.; Chandra, N. Computational antimicrobial peptide design and evaluation against multidrug-resistant clinical isolates of bacteria. J. Biol. Chem. 2018, 293, 3492–3509. [Google Scholar] [CrossRef]
- Fields, F.R.; Carothers, K.E.; Balsara, R.D.; Ploplis, V.A.; Castellino, F.J.; Lee, S.W. Rational design of syn-safencin, a novel linear antimicrobial peptide derived from the circular bacteriocin safencin AS-48. J. Antibiot. (Tokyo) 2018, 71, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Jeong, J.-H.; Kim, Y. Design and Engineering of Antimicrobial Peptides Based on LPcin-YK3, an Antimicrobial Peptide Derivative from Bovine Milk. J. Microbiol. Biotechnol. 2018, 28, 381–390. [Google Scholar] [CrossRef]
- Xu, H.; Tie, K.; Zhang, Y.; Feng, X.; Cao, Y.; Han, W. Design, expression, and characterization of the hybrid antimicrobial peptide T-catesbeianin-1 based on FyuA. J. Pept. Sci. 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, X.; Chen, X.; Huang, L.; Xi, X.; Ma, C.; Zhou, M.; Wang, L.; Chen, T. Evaluating the Bioactivity of a Novel Antimicrobial and Anticancer Peptide, Dermaseptin-PS4(Der-PS4), from the Skin Secretion of Phyllomedusa sauvagii. Molecules 2019, 24, 2974. [Google Scholar] [CrossRef]
- Feder, R.; Dagan, A.; Mor, A. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 2000, 275, 4230–4238. [Google Scholar] [CrossRef]
- Efron, L.; Dagan, A.; Gaidukov, L.; Ginsburg, H.; Mor, A. Direct interaction of dermaseptin S4 aminoheptanoyl derivative with intraerythrocytic malaria parasite leading to increased specific antiparasitic activity in culture. J. Biol. Chem. 2002, 277, 24067–24072. [Google Scholar] [CrossRef]
- Gaidukov, L.; Fish, A.; Mor, A. Analysis of membrane-binding properties of dermaseptin analogues: Relationships between binding and cytotoxicity. Biochemistry 2003, 42, 12866–12874. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, N.A.; Kennan, A.J. Coiled-coil formation governed by unnatural hydrophobic core side chains. J. Am. Chem. Soc. 2001, 123, 11081–11082. [Google Scholar] [CrossRef] [PubMed]



| Peptide | Primary Sequence | Net Charge |
|---|---|---|
| DM-PC | ALWKSILKNVGKAAGKAVLNAVTDMVNQ-NH2 | 4 |
| DMPC-19 | ALWKSILKNVGKAAGKAVL-NH2 | 5 |
| DMPC-10 | ALWKKLLKKA-NH2 | 5 |
| DMPC-10A | ALWKKLLKK-Cha-NH2 | 5 |
| Sources | Strains | MICs/MBCs (µM) | |||
|---|---|---|---|---|---|
| DM-PC | DMPC-19 | DMPC-10 | DMPC-10A | ||
| Reference strains | S. aureus NCTC 10788 | 2/4 | 2/8 | 64/128 | 4/8 |
| E. coli NCTC 10418 | 4/16 | 2/16 | 8/32 | 8/8 | |
| C. albicans NCYC 1467 | 2/8 | 2/16 | 64/128 | 4/8 | |
| MRSA ATCC 12493 | 2/4 | 8/64 | >256/>256 | 8/16 | |
| E. faecalis NCTC 12697 | 32/32 | 256/>256 | >256/>256 | 64/64 | |
| K. pneumoniae ATCC 43816 | 8/64 | 32/128 | >256/>256 | 4/64 | |
| P. aeruginosa ATCC 27853 | 4/8 | 4/16 | 32/128 | 4/4 | |
| CF isolated strains | P. aeruginosa B004 V2S2 B | 4/16 | 4/16 | 32/>256 | 4/8 |
| MRSA B038 V1S1 A | 4/4 | 16/16 | >256/>256 | 8/32 | |
| MRSA B042 V2E1 A | 4/4 | 8/16 | 256/>256 | 4/16 | |
| Concentration of Mg2+ (mM) | MICs (µM) | |||
|---|---|---|---|---|
| DM-PC | DMPC-19 | DMPC-10 | DMPC-10A | |
| 0 | 4 | 4 | 32 | 4 |
| 2 | 16 | 64 | 256 | 16 |
| 5 | 64 | 256 | >256 | 32 |
| 10 | >256 | >256 | >256 | 256 |
| MBIC/MBEC | DM-PC | DMPC-19 | DMPC-10 | DMPC-10A |
|---|---|---|---|---|
| MRSA B038 V1S1 A | 8/64 | 16/256 | >256/>256 | 16/256 |
| MRSA B042 V2E1 A | 4/32 | 16/256 | >256/>256 | 4/128 |
| P. aeruginosa ATCC 27853 | 16/32 | 64/128 | >256/>256 | 8/256 |
| P. aeruginosa B004 V2S2 B | 16/64 | 64/128 | >256/>256 | 8/256 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, Y.; Wang, H.; Xi, X.; Ma, C.; Liu, Y.; Zhou, M.; Du, Q.; Burrows, J.F.; Wei, M.; Chen, T.; et al. Design of N-Terminal Derivatives from a Novel Dermaseptin Exhibiting Broad-Spectrum Antimicrobial Activity against Isolates from Cystic Fibrosis Patients. Biomolecules 2019, 9, 646. https://doi.org/10.3390/biom9110646
Ying Y, Wang H, Xi X, Ma C, Liu Y, Zhou M, Du Q, Burrows JF, Wei M, Chen T, et al. Design of N-Terminal Derivatives from a Novel Dermaseptin Exhibiting Broad-Spectrum Antimicrobial Activity against Isolates from Cystic Fibrosis Patients. Biomolecules. 2019; 9(11):646. https://doi.org/10.3390/biom9110646
Chicago/Turabian StyleYing, Yuan, Hui Wang, Xinping Xi, Chengbang Ma, Yue Liu, Mei Zhou, Qiang Du, James F. Burrows, Minjie Wei, Tianbao Chen, and et al. 2019. "Design of N-Terminal Derivatives from a Novel Dermaseptin Exhibiting Broad-Spectrum Antimicrobial Activity against Isolates from Cystic Fibrosis Patients" Biomolecules 9, no. 11: 646. https://doi.org/10.3390/biom9110646
APA StyleYing, Y., Wang, H., Xi, X., Ma, C., Liu, Y., Zhou, M., Du, Q., Burrows, J. F., Wei, M., Chen, T., & Wang, L. (2019). Design of N-Terminal Derivatives from a Novel Dermaseptin Exhibiting Broad-Spectrum Antimicrobial Activity against Isolates from Cystic Fibrosis Patients. Biomolecules, 9(11), 646. https://doi.org/10.3390/biom9110646






