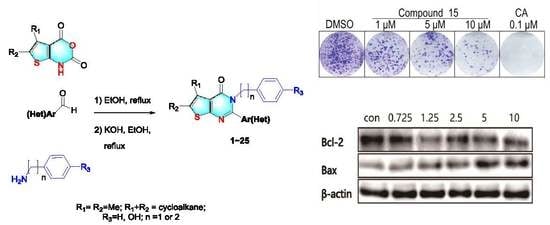
Synthesis of Novel Analogs of Thieno[2,3-d] Pyrimidin-4(3H)-ones as Selective Inhibitors of Cancer Cell Growth
Abstract
1. Introduction
2. Materials and Methods
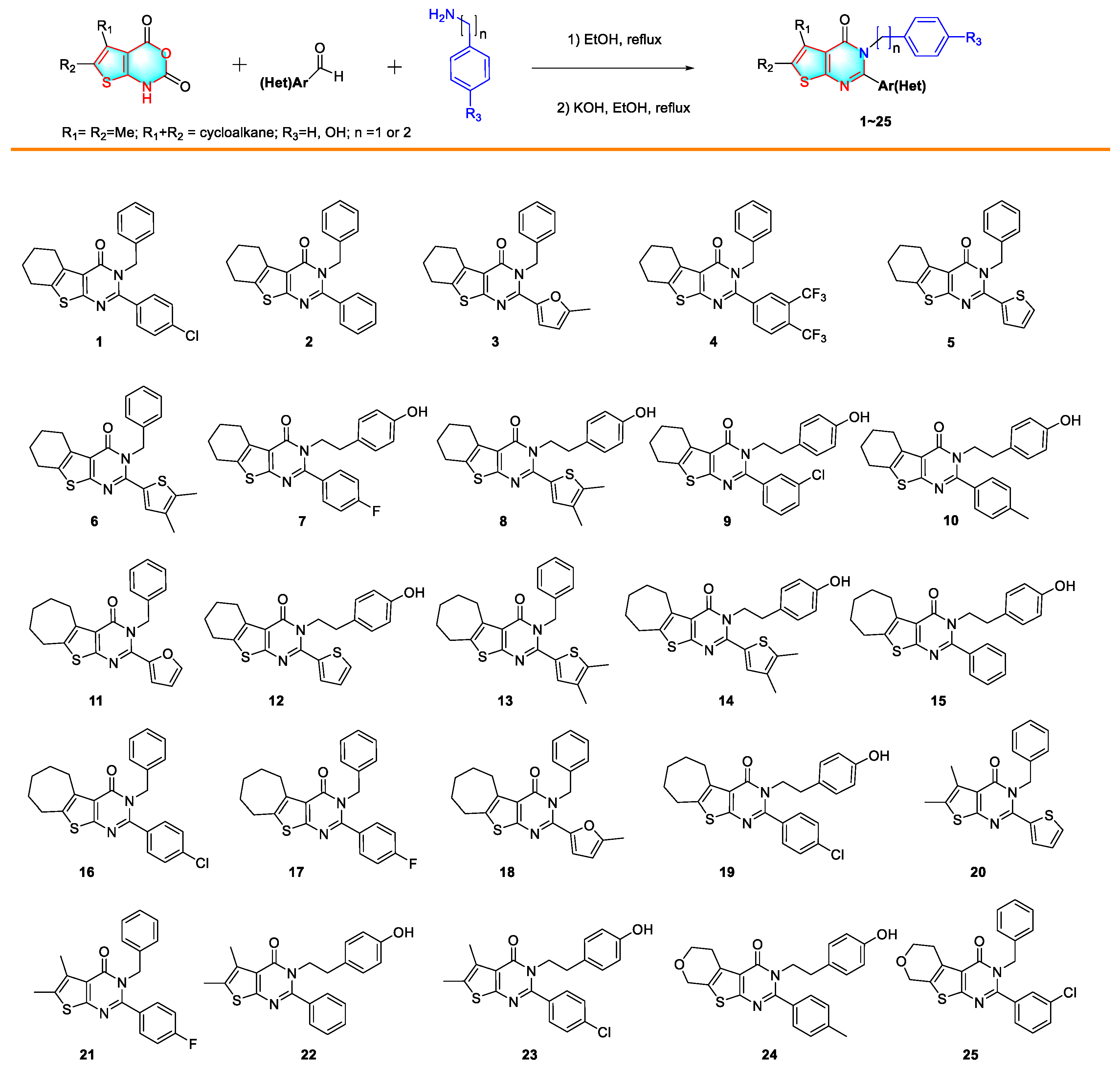
2.1. Synthesis of 2,3-Disubstituted Thieno[2,3-d] Pyrimidin-4(3H)-Ones
2.2. Cell Culture and Reagents
2.3. MTT (Methyl Thiazolyl Tetrazolium Bromide) Assay
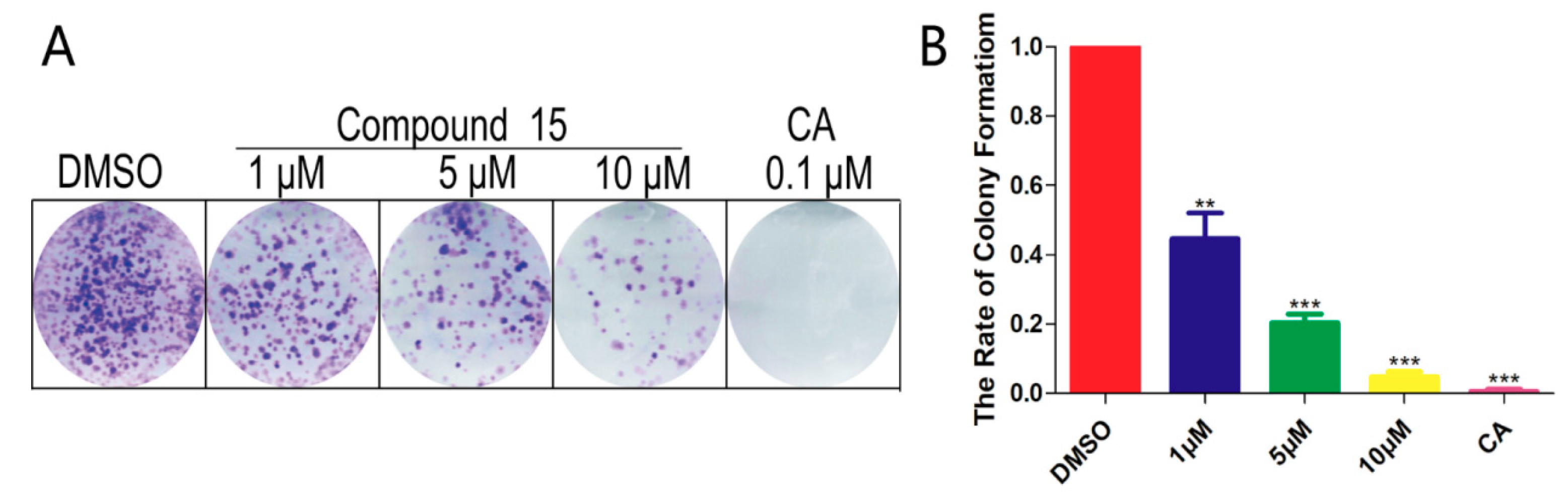
2.4. Colony Formation Assays
2.5. Hoechst 33258 Staining
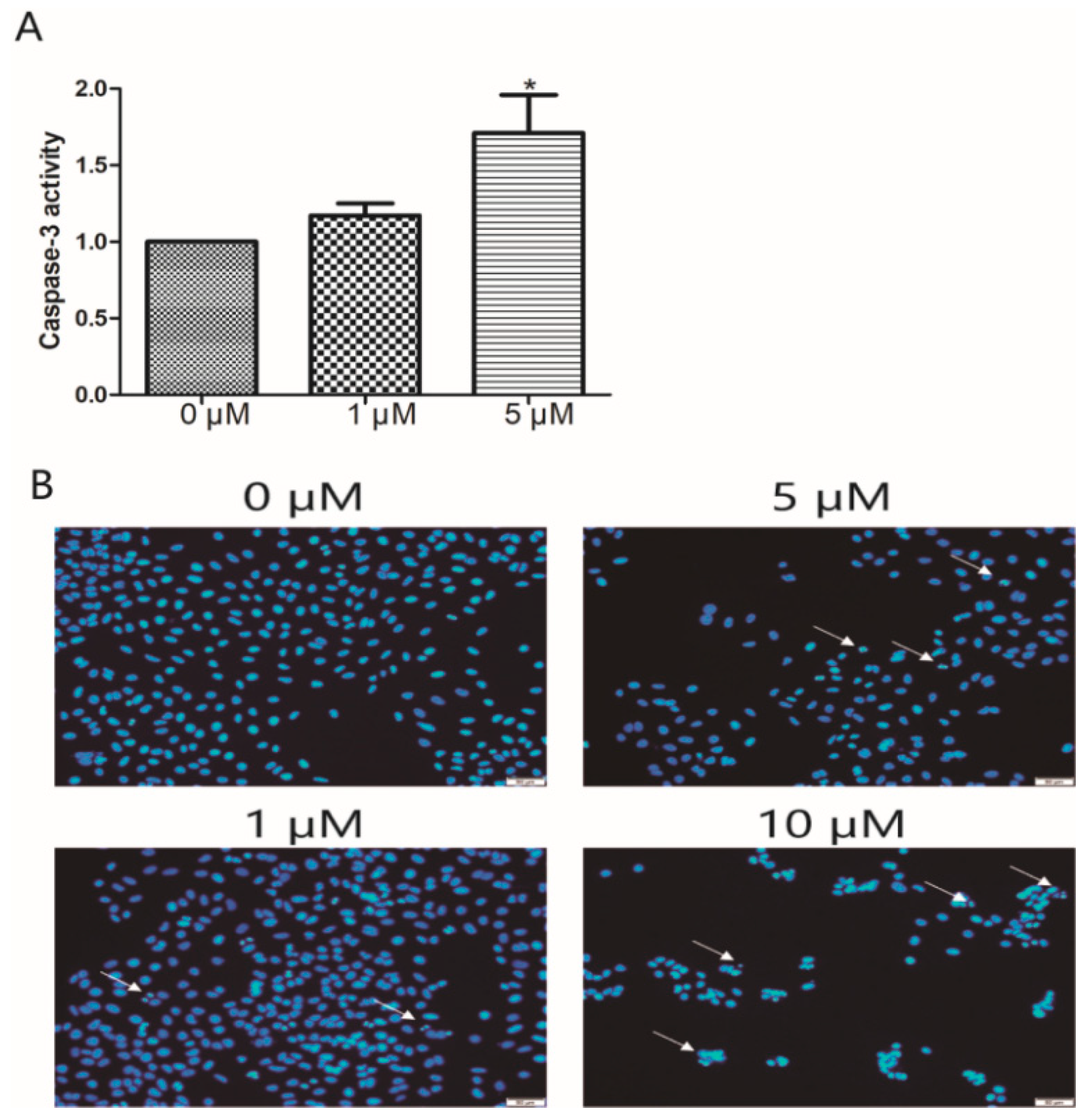
2.6. Caspase-3 Activity Assay
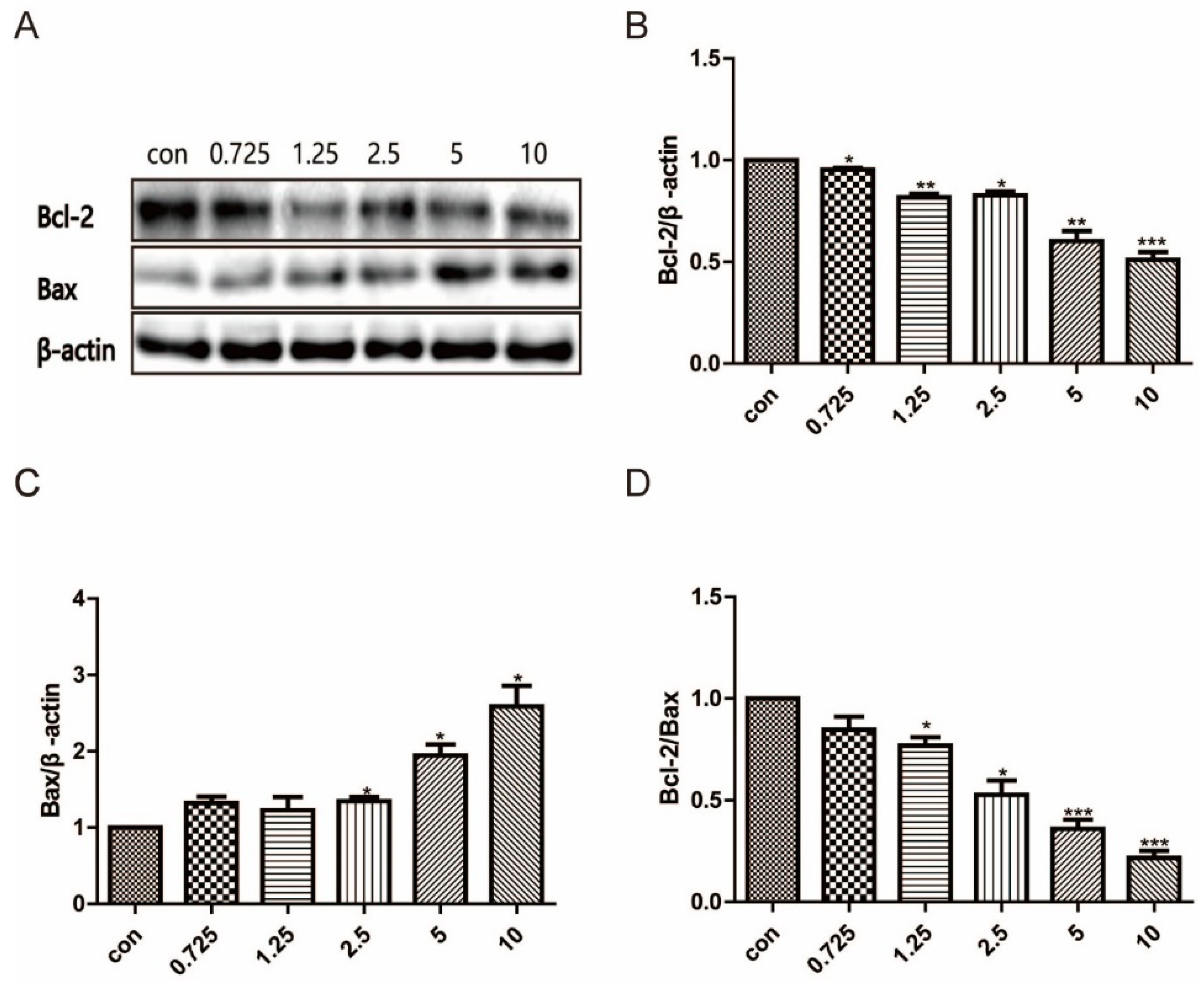
2.7. Western Blotting
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicities of Synthesized 2,3-Disubstituted Thieno[2,3-d] Pyrimidin-4(3H)-Ones
3.2. Cell Clone Formation Assay of Compounds 15
3.3. Apoptosis Analysis of Compound 15 by Hoechst 33258 Staining
3.4. Effects on Apoptosis-Related Proteins of Compound 15
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Ali, H.A.; Di, J.; Mei, W.; Zhang, Y.C.; Li, Y.; Du, Z.W.; Zhang, G.Z. Antitumor activity of lentivirus-mediated interleukin—12 gene modified dendritic cells in human lung cancer in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 611–616. [Google Scholar] [PubMed]
- Ettinger, D.S.; Akerley, W.; Bepler, G.; Blum, M.G.; Chang, A.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.; et al. Non-small cell lung cancer. J. Natl. Compr. Cancer Netw. 2010, 8, 740–801. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Popat, S.; Reinmuth, N.; De Ruysscher, D.; Kerr, K.M.; Peters, S. Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 27–39. [Google Scholar] [CrossRef]
- Das, D.; Preet, R.; Mohapatra, P.; Satapathy, S.R.; Kundu, C.N. 1,3-Bis(2-chloroethyl)-1-nitrosourea enhances the inhibitory effect of resveratrol on 5-fluorouracil sensitive/resistant colon cancer cells. World J. Gastroenterol. 2013, 19, 7374–7388. [Google Scholar] [CrossRef]
- Tong, C.W.S.; Wu, W.K.K.; Loong, H.H.F.; Cho, W.C.S.; To, K.K.W. Drug combination approach to overcome resistance to EGFR tyrosine kinase inhibitors in lung cancer. Cancer Lett. 2017, 405, 100–110. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.Y.; Elmuradov, B.; Pataer, A.; Aisa, H.A. Recent developments regarding the use of thieno[2,3-d]pyrimidin-4-one derivatives in medicinal chemistry, with a focus on their synthesis and anticancer properties. Eur. J. Med. Chem. 2015, 102, 552–573. [Google Scholar] [CrossRef]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.-H. Thieno[2,3-d]pyrimidine as a promising scaffold in medicinal chemistry: Recent advances. Bioorg. Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. The chemistry of thienopyridines. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: San Diego, CA, USA, 2007; Volume 93, pp. 117–178. [Google Scholar]
- Lilienkampf, A.; Karkola, S.; Alho-Richmond, S.; Koskimies, P.; Johansson, N.; Huhtinen, K.; Vihko, K.; Wahala, K. Synthesis and biological evaluation of 17 beta-hydroxysteroid dehydrogenase type 1 (17 beta-HSD1) inhibitors based on a thieno[2,3-d] pyrimidin-4(3H)-one core. J. Med. Chem. 2009, 52, 6660–6671. [Google Scholar] [CrossRef] [PubMed]
- Di Fruscia, P.; Zacharioudakis, E.; Liu, C.; Moniot, S.; Laohasinnarong, S.; Khongkow, M.; Harrison, I.F.; Koltsida, K.; Reynolds, C.R.; Schmidtkunz, K.; et al. The discovery of a highly selective 5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d] pyrimidin-4(3H)-one SIRT2 inhibitor that is neuroprotective in an in vitro parkinson’s disease model. Chem. Med. Chem. 2015, 10, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Guo, S.B.; Hu, Y.G.; Zeng, X.H.; Yang, G.Y. Synthesis and antitumor activity of some novel 5,6,7,8-tetrahydrobenzo-thieno[2,3-d]pyrimidin-4(3H)-one derivatives. Chin. J. Org. Chem. 2015, 35, 1075–1080. [Google Scholar] [CrossRef]
- Malothu, N.; Bhandaru, J.S.; Kulandaivelu, U.; Jojula, M.; Adidala, R.R.; Umadevi, K.R.; Dusthackeer, A.V.N.; Kaki, V.R.; Akkinepally, R.R. Synthesis, in vitro antimycobacterial evaluation and docking studies of some new 5,6,7,8-tetrahydropyrido[4′,3′:4,5]thieno[2,3-d]pyrimidin-4(3H)-one schiff bases. Bioorg. Med. Chem. Lett. 2016, 26, 836–840. [Google Scholar]
- Guo, P.; Xie, Z.X.; Zhang, H.; Zhang, Z.K.; Han, C.; Cheng, D.H.; Lin, D.; Zhang, Y.; Wang, X.B.; Guo, X.; et al. Design, synthesis, and biological evaluation of C-2 substituted 3H-thieno[2,3-d]pyrimidin-4-one derivatives as novel FGFR1 inhibitors. Med. Chem. 2017, 13, 753–760. [Google Scholar] [CrossRef]
- De Candia, M.; Altamura, C.; Denora, N.; Cellamare, S.; Nuzzolese, M.; De Vito, D.; Voskressensky, L.G.; Varlamov, A.V.; Altomare, C.D. Physicochemical properties and antimicrobial activity of new spirocyclic thieno[2,3-d]pyrimidin-4(3H)-one derivatives. Chem. Heterocycl. Compd. 2017, 53, 357–363. [Google Scholar] [CrossRef]
- Kurasawa, O.; Homma, M.; Oguro, Y.; Miyazaki, T.; Mori, K.; Uchiyama, N.; Iwai, K.; Ohashi, A.; Hara, H.; Yoshida, S.; et al. 2-Aminomethylthieno[3,2-d]pyrimidin-4(3H)-ones bearing 3-methylpyrazole hinge binding moiety: Highly potent, selective, and time-dependent inhibitors of Cdc7 kinase. Bioorg. Med. Chem. 2017, 25, 3658–3670. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, L.; Tao, J.; Pan, Z.; He, M.; Su, D.; He, G.; Jiang, Q. Design, synthesis and biological evaluation of 4-aniline-thieno[2,3-d]pyrimidine derivatives as MNK1 inhibitors against renal cell carcinoma and nasopharyngeal carcinoma. Bioorg. Med. Chem. 2019, 27, 2268–2279. [Google Scholar] [CrossRef]
- Hesse, S.; Perspicace, E.; Kirsch, G. Microwave-assisted synthesis of 2-aminothiophene-3-carboxylic acid derivatives, 3H-thieno[2,3-d]pyrimidin-4-one and 4-chlorothieno[2,3-d]pyrimidine. Tetrahedron Lett. 2007, 48, 5261–5264. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.F.; Song, G.W. Sequential one-pot synthesis of 5,6,8-trihydropyrano[3′,4′:4,5]thieno[2,3-d]pyrimidin-4(3H)-one. Chin. J. Org. Chem. 2012, 32, 1314–1319. [Google Scholar] [CrossRef]
- Hu, Y.G.; Zheng, A.H.; Li, G.J.; Dong, M.Z.; Ye, F.; Sun, F.; Liu, Z.Y.; Li, W. Efficient synthesis of new thieno[2,3-d] pyrimidin-4(3H)-one derivatives for evaluation as anticancer agents. J. Heterocycl. Chem. 2014, 51, E84–E88. [Google Scholar] [CrossRef]
- Sureja, D.K.; Vadalia, K.R. POCl3 catalyzed, one-step, solvent-free synthesis of some novel thieno[2,3-d]pyrimidin-4(3H)-one derivatives as antimicrobial agent. J. Saudi Chem. Soc. 2018, 22, 248–253. [Google Scholar] [CrossRef]
- Shi, T.D.; Kaneko, L.; Sandino, M.; Busse, R.; Zhang, M.; Mason, D.; Machulis, J.; Ambrose, A.J.; Zhang, D.D.; Chapman, E. One-step synthesis of thieno[2,3-d]pyrimidin-4(3H)-ones via a catalytic four-component reaction of ketones, ethyl cyanoacetate, S-8, and formamide. ACS Sustain. Chem. Eng. 2019, 7, 1524–1528. [Google Scholar] [CrossRef]
- Huang, G.; Kling, B.; Darras, F.H.; Heilmann, J.; Decker, M. Identification of a neuroprotective and selective butyrylcholinesterase inhibitor derived from the natural alkaloid evodiamine. Eur. J. Med. Chem. 2014, 81, 15–21. [Google Scholar] [CrossRef]
- Huang, G.; Roos, D.; Stadtmüller, P.; Decker, M. A simple heterocyclic fusion reaction and its application for expeditious syntheses of rutaecarpine and its analogs. Tetrahedron Lett. 2014, 55, 3607–3609. [Google Scholar] [CrossRef]
- Liu, F.; Hou, X.; Nie, L.F.; Bozorov, K.; Decker, M.; Huang, G. A convenient one-pot synthesis of 2,3-disubstituted thieno[2,3-d]pyrimidin-4(3H)-ones from 2H-thieno[2,3-d][1,3]oxazine-2,4(1H)-diones, aromatic aldehydes and amines. SynOpen 2018, 2, 0207–0212. [Google Scholar] [CrossRef]
- Yang, C.-R.; Peng, B.; Cao, S.-L.; Ren, T.-T.; Jiang, W.; Wang, F.-C.; Li, Y.-S.; Wang, G.; Li, Z.; Xu, S.; et al. Synthesis, cytotoxic evaluation and target identification of thieno[2,3-d]pyrimidine derivatives with a dithiocarbamate side chain at C2 position. Eur. J. Med. Chem. 2018, 154, 324–340. [Google Scholar] [CrossRef]
- Fouad, M.M.; El-Bendary, E.R.; suddek, G.M.; Shehata, I.A.; El-Kerdawy, M.M. Synthesis and in vitro antitumor evaluation of some new thiophenes and thieno[2,3-d] pyrimidine derivatives. Bioorg. Chem. 2018, 81, 587–598. [Google Scholar] [CrossRef]
- Mina, E.A.; Ehab, M.G.; Afaf, A.E.-M.; Farag, A.E.-T. Synthesis of novel thieno[2,3-d]pyrimidine derivatives and evaluation of their cytotoxicity and EGFR inhibitory activity. Anti Cancer Agents Med. Chem. 2018, 18, 747–756. [Google Scholar]
- He, S.; Dong, G.; Wang, Z.; Chen, W.; Huang, Y.; Li, Z.; Jiang, Y.; Liu, N.; Yao, J.; Miao, Z.; et al. Discovery of novel multiacting topoisomerase I/II and histone deacetylase inhibitors. ACS Med. Chem. Lett. 2015, 6, 239–243. [Google Scholar] [CrossRef]
- Hatok, J.; Racay, P. Bcl-2 family proteins: Master regulators of cell survival. Biomol. Concepts 2016, 7, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yenari, M.A.; Cheng, D.; Sapolsky, R.M.; Steinberg, G.K. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J. Neurochem. 2003, 85, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Setareh, M.; Ochs, R.L.; Lotz, M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997, 40, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Meng, L.; Sun, H.; Zhu, Y.; Liu, H. Cochinchina momordica seed suppresses proliferation and metastasis in human lung cancer cells by regulating multiple molecular targets. Am. J. Chin. Med. 2015, 43, 149–166. [Google Scholar] [CrossRef] [PubMed]
| Compound | Cell Lines | ||||
|---|---|---|---|---|---|
| HL-7702 | PC-3 | PC-9 | MCF-7 | A549 | |
| 1 | >100 | >100 | >100 | >100 | >100 |
| 2 | >100 | >100 | >100 | >100 | >100 |
| 3 | 55.17 ± 0.50 | 92.93 ± 2.29 | 86.09 ± 1.28 | >100 | 54.42 ± 1.83 |
| 4 | >100 | >100 | >100 | 24.67 ± 2.04 | 58.24 ± 0.65 |
| 5 | >100 | >100 | 80.97 ± 3.94 | 97.50 ± 0.13 | >100 |
| 6 | >100 | >100 | >100 | 59.86 ± 0.34 | 17.38 ± 0.88 |
| 7 | >100 | >100 | 44.76 ± 4.47 | 10.36 ± 1.32 | 6.86 ± 0.98 |
| 8 | >100 | 87.75 ± 0.63 | 92.97 ± 0.52 | >100 | 50.92 ± 0.07 |
| 9 | >100 | >100 | >100 | 30.70 ± 0.29 | 19.59 ± 0.33 |
| 10 | >100 | 43.43 ± 2.73 | 49.68 ± 2.27 | 27.47 ± 0.02 | 8.51 ± 0.16 |
| 11 | >100 | >100 | >100 | 65.63 ± 0.26 | 51.42 ± 0.46 |
| 12 | >100 | 21.95 ± 1.76 | 54.20 ± 2.06 | 36.39 ± 1.41 | 11.65 ± 0.12 |
| 13 | >100 | 92.52 ± 1.89 | >100 | 75.94 ± 0.17 | 49.86 ± 0.30 |
| 14 | 68.64 ± 0.26 | 84.68 ± 0.48 | 86.33 ± 0.22 | 46.09 ± 0.48 | 49.83 ± 0.68 |
| 15 | >100 | 47.51 ± 3.39 | >100 | 17.32 ± 0.20 | 0.94 ± 0.01 |
| 16 | >100 | >100 | >100 | >100 | 80.96 ± 1.04 |
| 17 | >100 | >100 | >100 | >100 | >100 |
| 18 | >100 | 92.03 ± 2.34 | 58.28 ± 0.18 | 96.36 ± 0.72 | 63.20 ± 0.03 |
| 19 | 19.16 ± 0.06 | 25.14 ± 0.37 | 26.71 ± 1.38 | 13.04 ± 0.11 | 6.26 ± 0.87 |
| 20 | >100 | >100 | >100 | >100 | 80.09 ± 0.83 |
| 21 | >100 | >100 | >100 | >100 | >100 |
| 22 | >100 | >100 | >100 | 21.89 ± 0.82 | >100 |
| 23 | >100 | >100 | >100 | 70.64 ± 0.59 | 46.14 ± 0.17 |
| 24 | >100 | >100 | >100 | >100 | 32.92 ± 1.84 |
| 25 | >100 | 94.21 ± 1.83 | >100 | >100 | 43.95 ± 0.71 |
| CAMPTOTHECIN | 0.11 ± 0.03 | 0.85 ± 0.01 | 0.09 ± 0.01 | 0.26 ± 0.05 | 0.10 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, F.; Hou, X.; Cao, J.; Dai, X.; Yu, J.; Huang, G. Synthesis of Novel Analogs of Thieno[2,3-d] Pyrimidin-4(3H)-ones as Selective Inhibitors of Cancer Cell Growth. Biomolecules 2019, 9, 631. https://doi.org/10.3390/biom9100631
Zhang S, Liu F, Hou X, Cao J, Dai X, Yu J, Huang G. Synthesis of Novel Analogs of Thieno[2,3-d] Pyrimidin-4(3H)-ones as Selective Inhibitors of Cancer Cell Growth. Biomolecules. 2019; 9(10):631. https://doi.org/10.3390/biom9100631
Chicago/Turabian StyleZhang, Sheng, Feize Liu, Xueling Hou, Jianguo Cao, Xiling Dai, Junjie Yu, and Guozheng Huang. 2019. "Synthesis of Novel Analogs of Thieno[2,3-d] Pyrimidin-4(3H)-ones as Selective Inhibitors of Cancer Cell Growth" Biomolecules 9, no. 10: 631. https://doi.org/10.3390/biom9100631
APA StyleZhang, S., Liu, F., Hou, X., Cao, J., Dai, X., Yu, J., & Huang, G. (2019). Synthesis of Novel Analogs of Thieno[2,3-d] Pyrimidin-4(3H)-ones as Selective Inhibitors of Cancer Cell Growth. Biomolecules, 9(10), 631. https://doi.org/10.3390/biom9100631