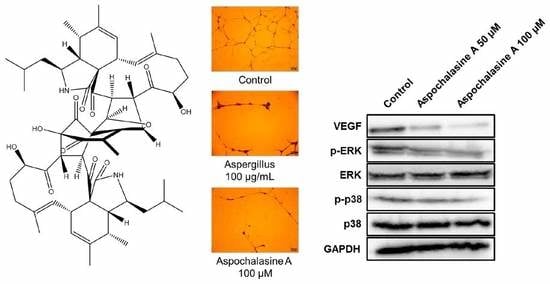
Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Proliferation Assay
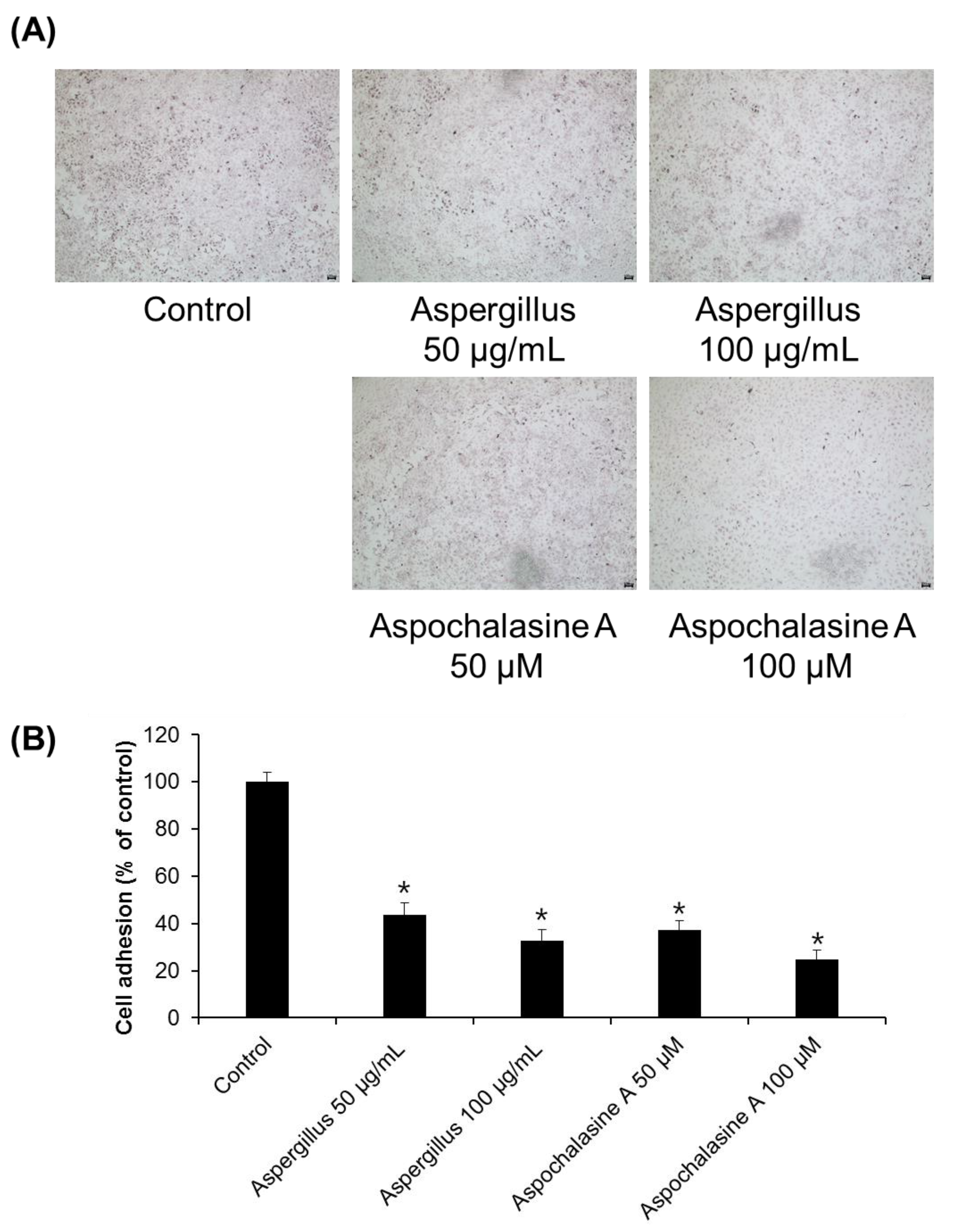
2.3. Cell Adhesion Assay
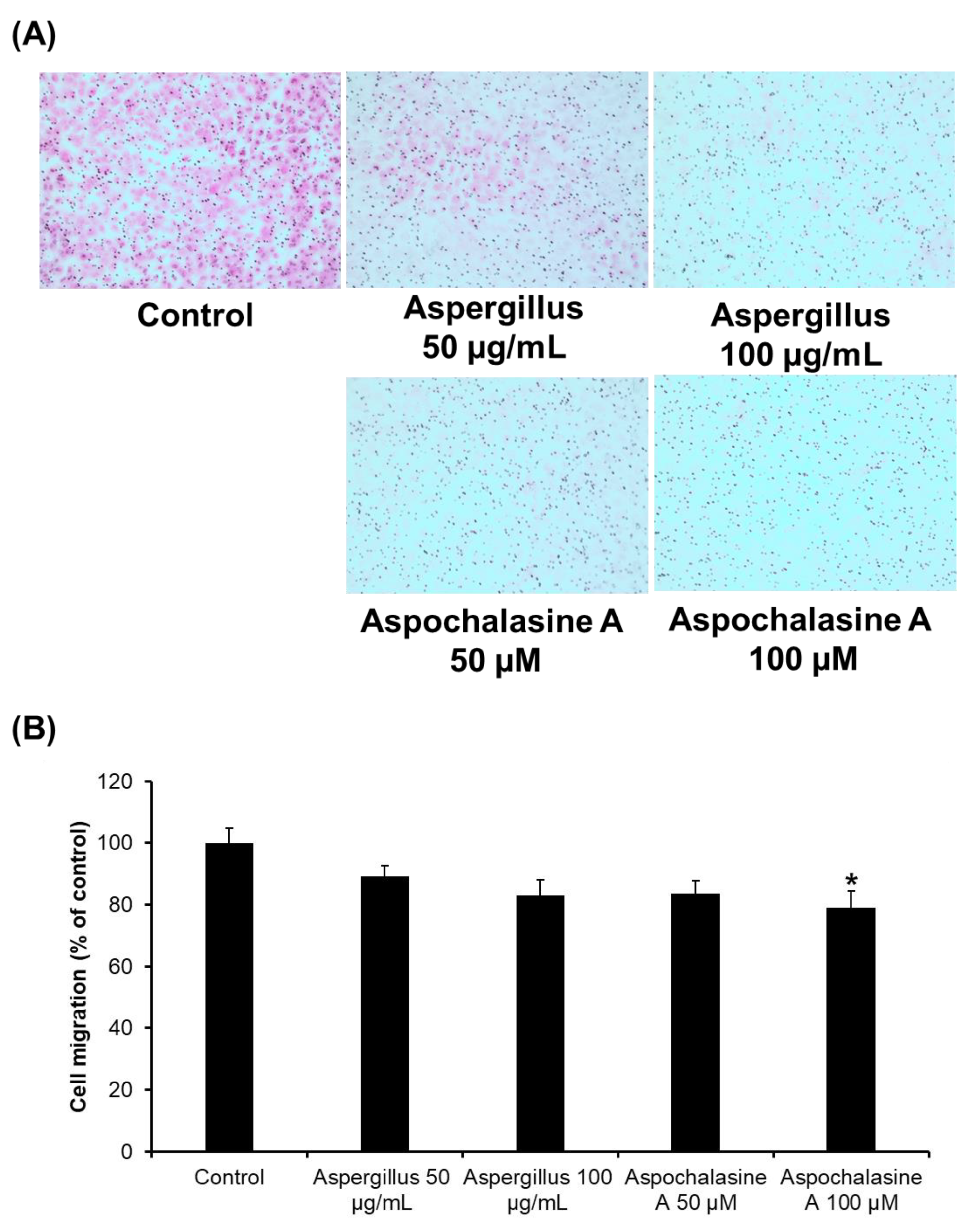
2.4. Cell Migration Assay
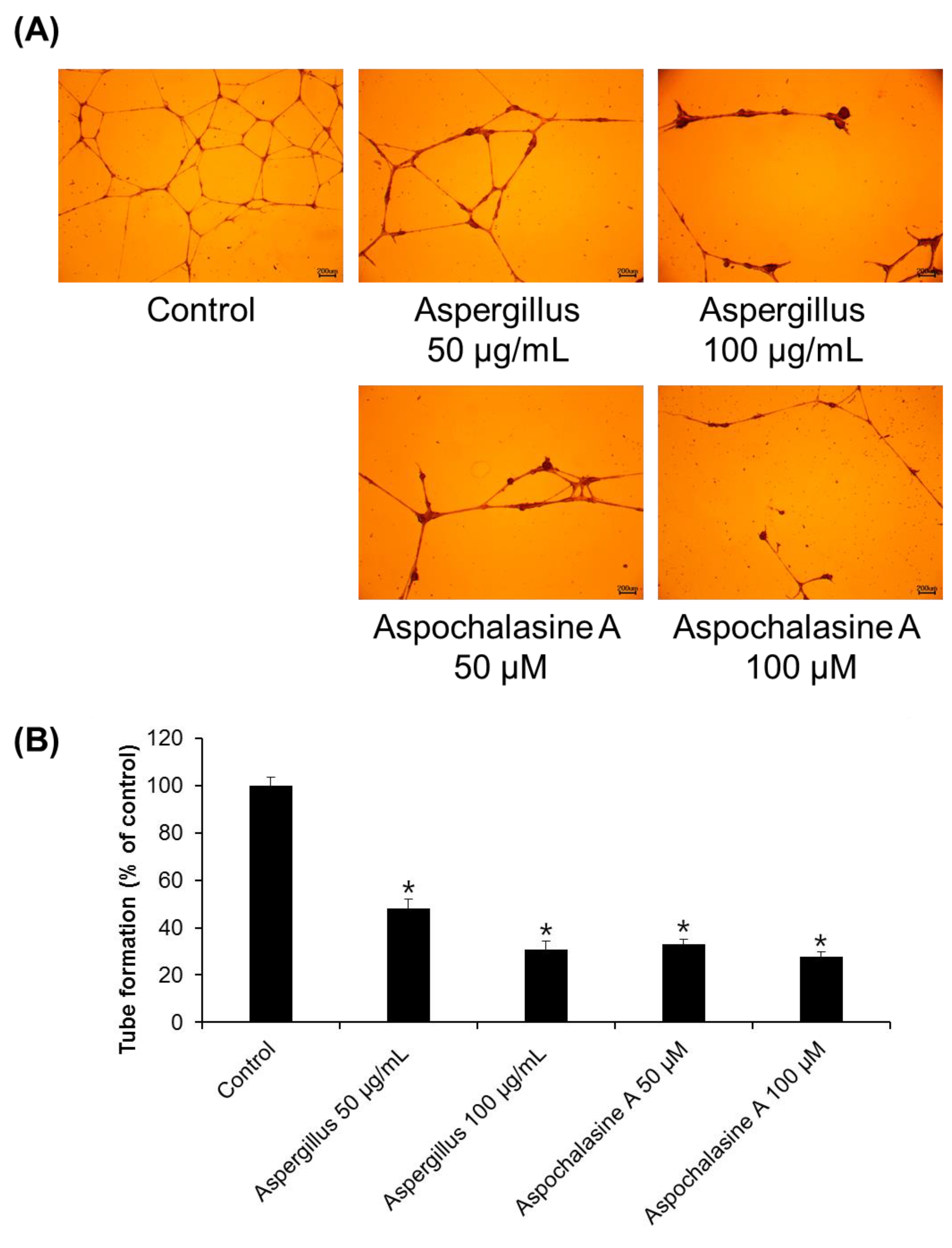
2.5. Tube Formation Assay
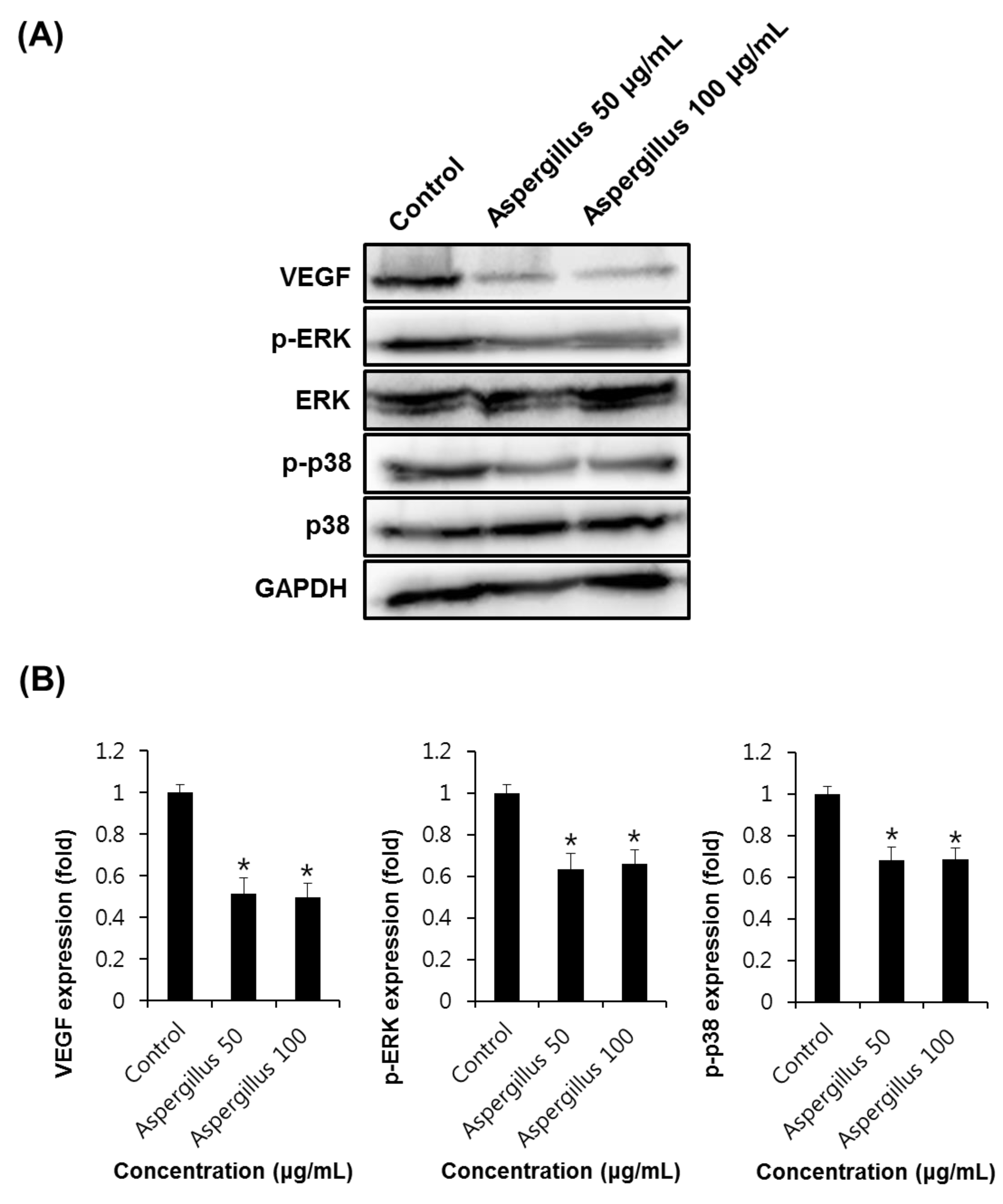
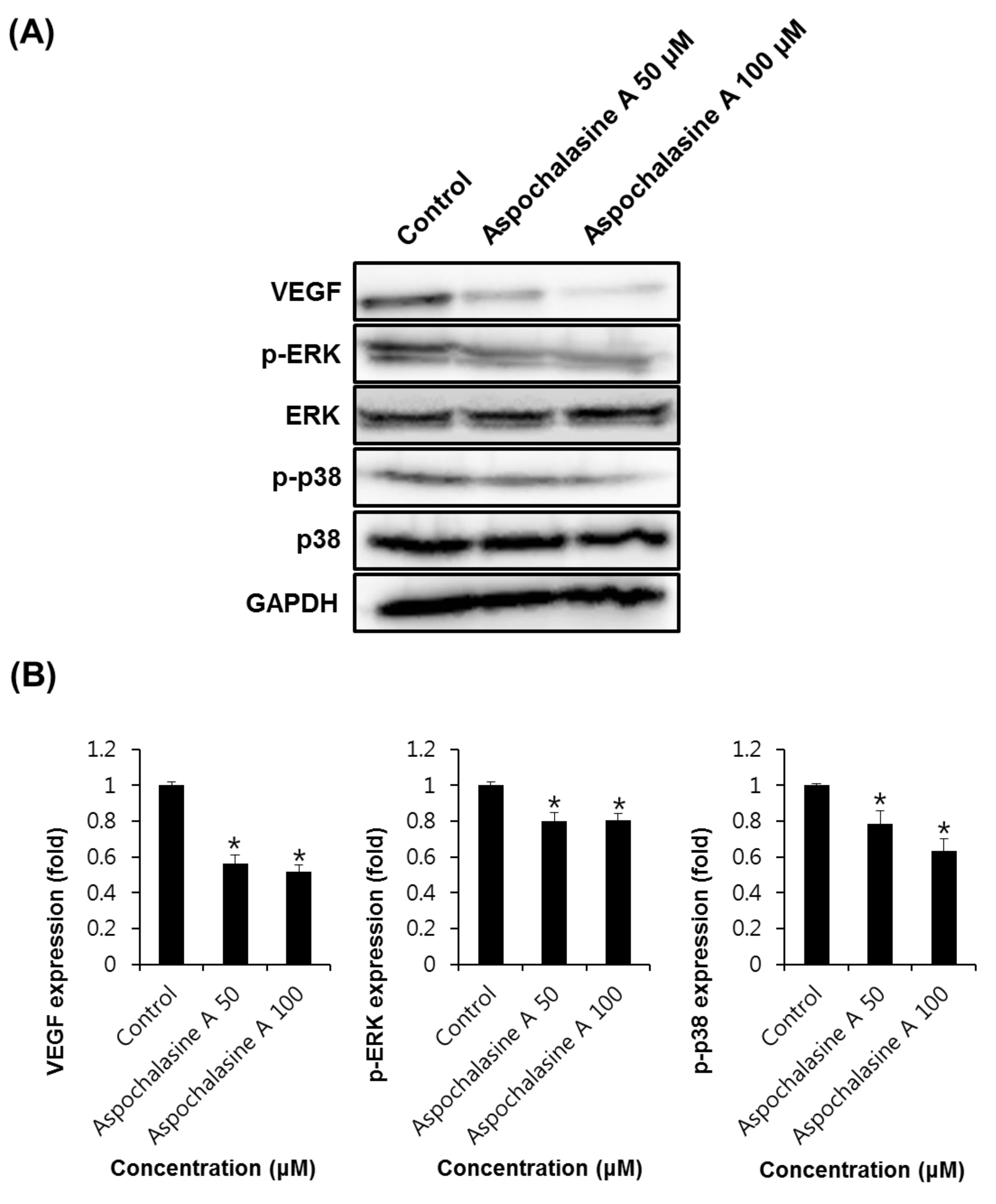
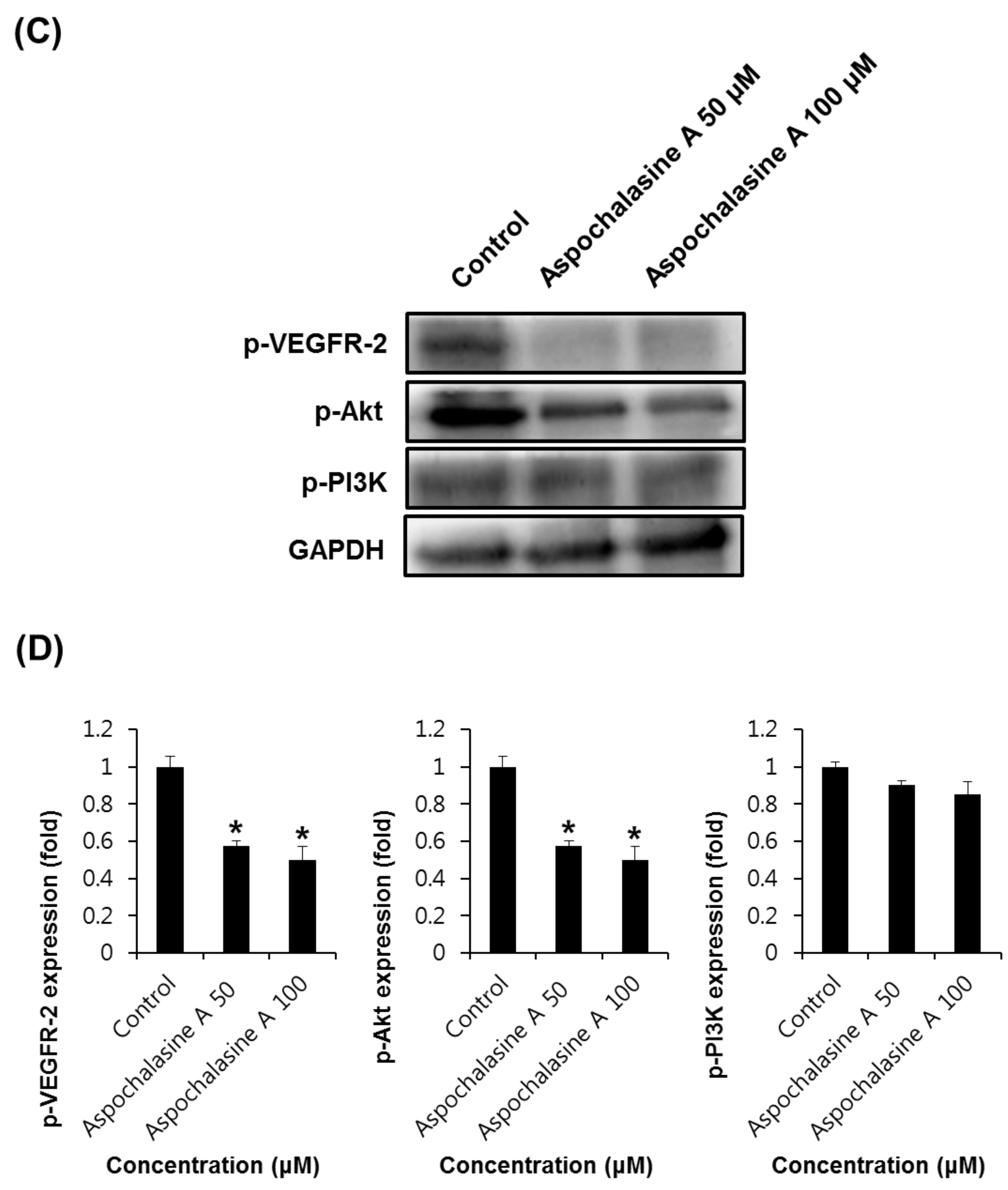
2.6. Western Blotting Analysis
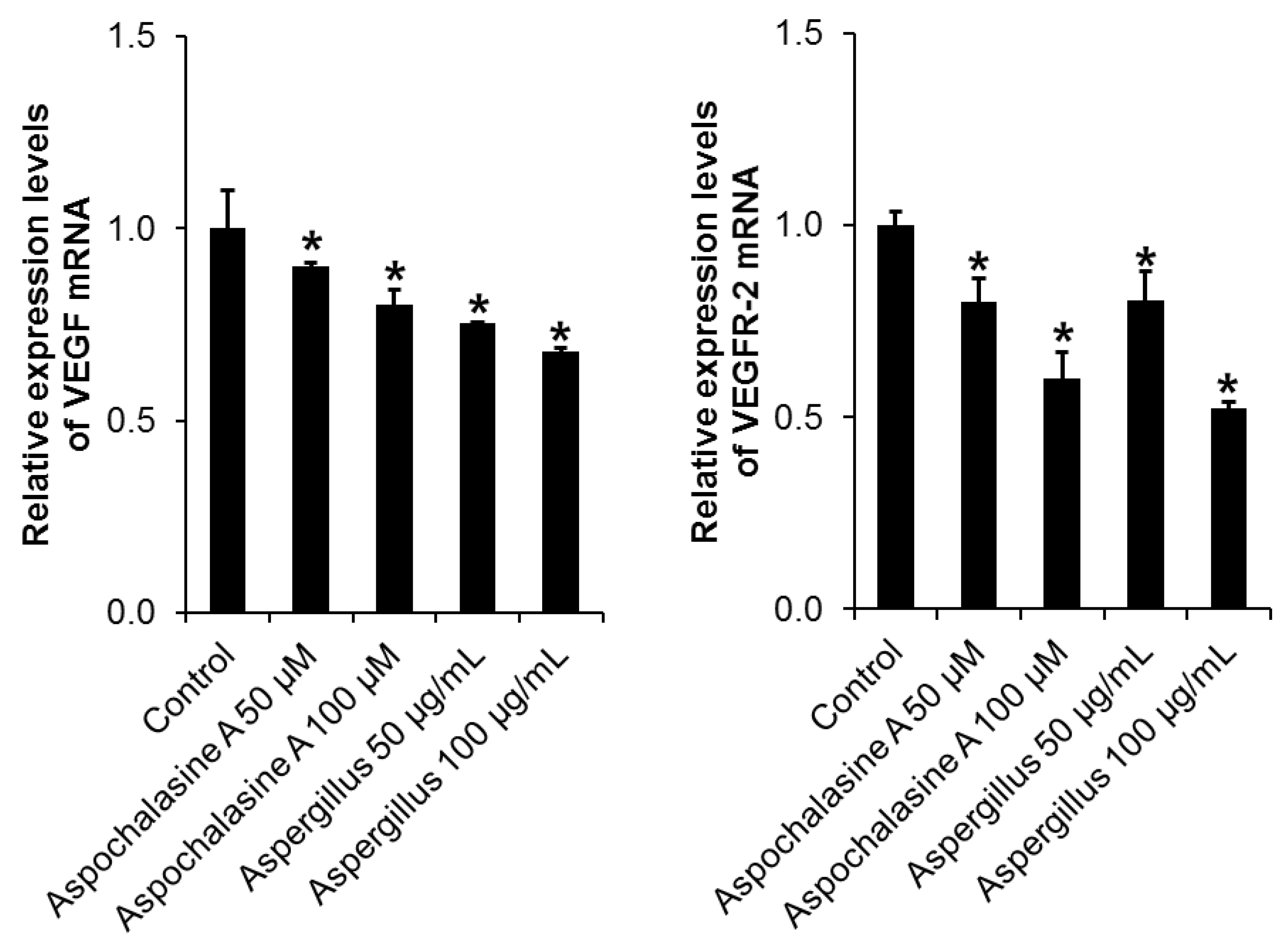
2.7. Semi-Quantitative Eeverse-Transcriptase PCR
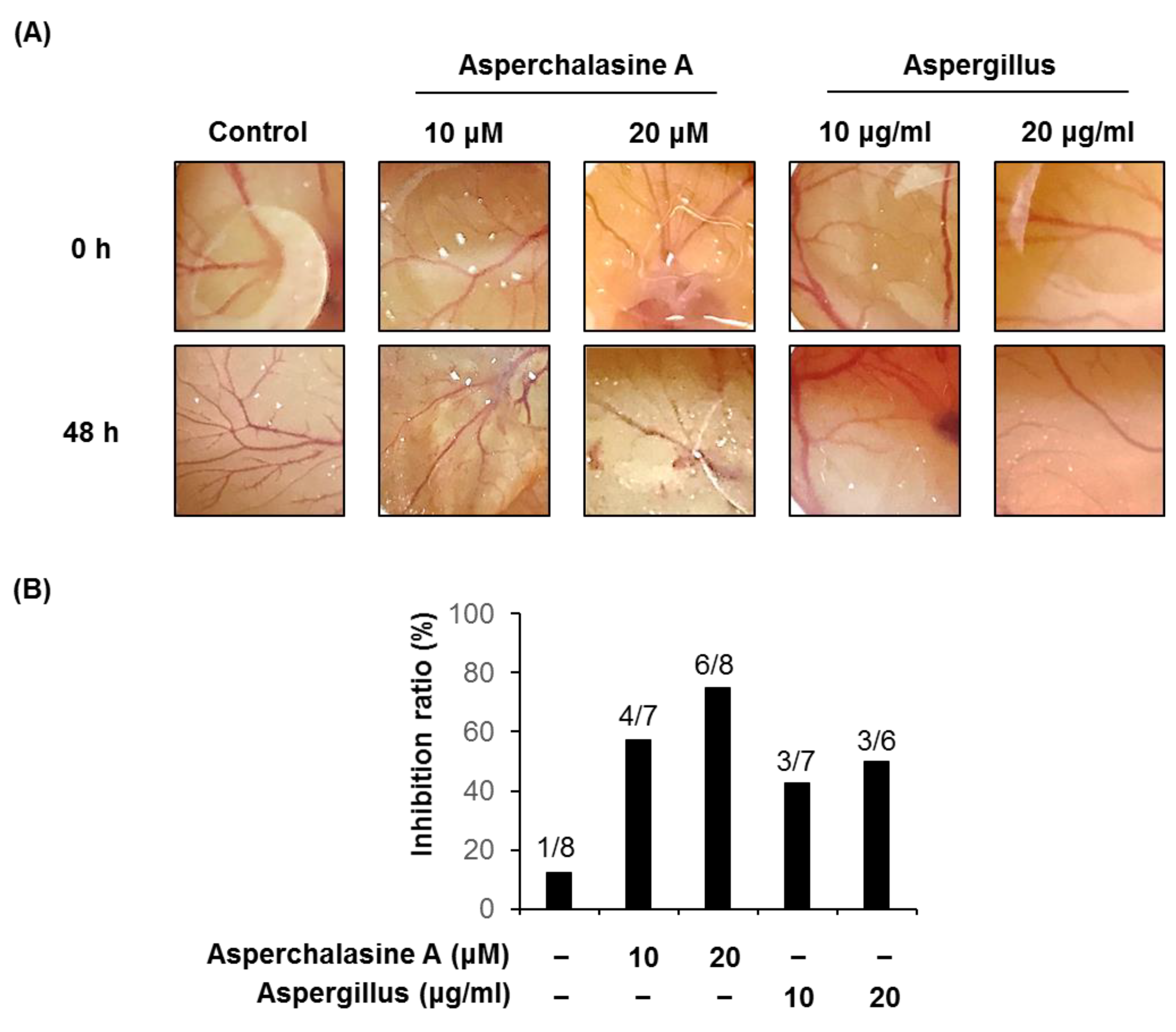
2.8. Chorioallantoic Membrane Assay
2.9. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Folkman, J.; Cotran, R. Relation of vascular proliferation to tumor growth. Int. Rev. Exp. Pathol. 1976, 16, 207–248. [Google Scholar] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Gohongi, T.; Kadambi, A.; Izumi, Y.; Ang, J.; Yun, C.O.; Buerk, D.G.; Huang, P.L.; Jain, R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA 2001, 98, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Park, J.Y.; Yoo, H.S.; Yoo, J.E.; Lee, H.L. Assessment of the anti-metastatic properties of sanguiin H-6 in HUVECs and MDA-MB-231 human breast cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 3291–3294. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Carroll, P.R.; Flax, J.; Blumenfeld, W.; Folkman, J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am. J. Pathol. 1993, 143, 401–409. [Google Scholar]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Berse, B.; Jackman, R.W.; Tognazzi, K.; Manseau, E.J.; Senger, D.R.; Dvorak, H.F. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993, 53, 4727–4735. [Google Scholar] [PubMed]
- Lee, T.K.; Park, J.Y.; Yu, J.S.; Jang, T.S.; Oh, S.T.; Pang, C.; Ko, Y.J.; Kang, K.S.; Kim, K.H. 7α,15-Dihydroxydehydroabietic acid from Pinus koraiensis inhibits the promotion of angiogenesis through downregulation of VEGF, p-Akt and p-ERK in HUVECs. Bioorg. Med. Chem. Lett. 2018, 28, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Ho, S.H.; Park, E.J.; Hahn, W.; Cho, H.; Jeong, J.G.; Lee, Y.W.; Kim, S. Angiostatin gene transfer as an effective treatment strategy in murine collagen-induced arthritis. Arthritis Rheum. 2002, 46, 793–801. [Google Scholar] [CrossRef]
- De Bandt, M.; Ben Mahdi, M.H.; Ollivier, V.; Grossin, M.; Dupuis, M.; Gaudry, M.; Bohlen, P.; Lipson, K.E.; Rice, A.; Wu, Y.; et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J. Immunol. 2003, 171, 4853–4859. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Knudsen, P.B.; Hanna, B.; Ohl, S.; Sellner, L.; Zenz, T.; Döhner, H.; Stilgenbauer, S.; Larsen, T.O.; Lichter, P.; Seiffert, M. Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton. Leukemia 2014, 28, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Yang, Y.; Sun, L.; Dou, H.; Tan, R.; Hou, Y. Chaetoglobosin F, a small molecule compound, possesses immunomodulatory properties on bone marrow-derived dendritic cells via TLR9 signaling pathway. Immunobiology 2013, 218, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Paradise, C.R.; Dudakovic, A.; Sen, B.; Nair, A.A.; Dietz, A.B.; Deyle, D.R.; Cool, S.M.; Rubin, J.; van Wijnen, A.J. Validation of Osteogenic Properties of Cytochalasin D by High-Resolution RNA-Sequencing in Mesenchymal Stem Cells Derived from Bone Marrow and Adipose Tissues. Stem Cells Dev. 2018, 27, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, W.; Zhang, P.; Ruan, W.; Zhu, X. Nematicidal activity of chaetoglobosin A poduced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Xue, Y.; Tong, Q.; Li, X.N.; Chen, X.; Wang, J.; Yao, G.; Luo, Z.; Zhang, Y. Asperchalasine A, a Cytochalasan Dimer with an Unprecedented Decacyclic Ring System, from Aspergillus flavipes. Angew. Chem. Int. Ed. Engl. 2015, 54, 13374–13378. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, Y.J. Inhibition of hypoxia-induced cyclooxygenase-2 by Korean Red Ginseng is dependent on peroxisome proliferator-activated receptor gamma. J. Ginseng Res. 2017, 41, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Martinelli, M.; Courty, J.; Cascone, I. Angiogenesis analyzer for ImageJ. In Proceedings of the 4th ImageJ User and Developer Conference, Luxembourg, 24–26 October 2012; pp. 198–201. [Google Scholar]
- Lee, H.; Kim, J.; Park, J.Y.; Kang, K.S.; Park, J.H.; Hwang, G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng Res. 2017, 41, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Han, C.; Fang, Y.; Gundeti, S.; Lee, I.-S.H.; Song, W.O.; Hwang, K.-C.; Kim, T.W.; Sung, G.-H.; Park, H. Inhibitory activity of Cordyceps bassiana extract on LPS-induced inflammation in RAW 264.7 cells by suppressing NF-κB activation. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
- Son, Y.; Kim, H.; Yang, B.; Kim, B.; Park, Y.C.; Park, C.; Kim, K. Inhibitory effects of methanol extract from Nardostachys chinensis on 27-hydroxycholesterol-induced differentiation of monocytic cells. Nat. Prod. Sci. 2017, 23, 239–246. [Google Scholar] [CrossRef]
- Guon, T.; Chung, H.S. Induction of apoptosis with Moringa oleifera fruits in HCT116 human colon cancer cells via intrinsic pathway. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
- Norton, K.-A.; Popel, A.S. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci. Rep. 2016, 6, 36992. [Google Scholar]
- Bischoff, J. Cell adhesion and angiogenesis. J. Clin. Investig. 1997, 99, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kwak, J.H.; Kang, K.S.; Jung, E.B.; Lee, D.S.; Lee, S.; Jung, Y.; Kim, K.H.; Hwang, G.S.; Lee, H.L.; et al. Wound healing effects of deoxyshikonin isolated from Jawoongo: In vitro and in vivo studies. J. Ethnopharmacol. 2017, 199, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Walker, T.G.; Wicky, S.; Hesketh, R. Angiogenesis and current antiangiogenic strategies for the treatment of cancer. J. Vasc. Interv. Radiol. 2010, 21, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2000, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.Y.; Lee, D.; Seok, S.; Kwon, Y.J.; Jang, T.S.; Kang, K.S.; Kim, K.H. Chemical constituents from the rare mushroom Calvatia nipponica inhibit the promotion of angiogenesis in HUVECs. Bioorg. Med. Chem. Lett. 2017, 27, 4122–4127. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Beane, T.J.; Quillien, A.; Male, I.; Zhu, L.J.; Lawson, N.D. Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development 2016, 143, 3796–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zhang, J.; Li, L.; Liu, C. The expression and clinical significance of PI3K, pAkt and VEGF in colon cancer. Oncol. Lett. 2012, 4, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kwon, K.A.; Lee, Y.E.; Kim, J.H.; Kim, S.H.; Kim, J.H. Korean Red Ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-κB and ERK1/2 pathways in colon cancer. J. Ginseng Res. 2018, 42, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Kang, S.; Zhao, L.; Vogt, P.K. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer. 2005, 12, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, M.S.; Hwang, G.S.; Yamabe, N.; Yoo, J.E.; Kang, K.S.; Kim, J.C.; Lee, J.G.; Ham, J.; Lee, H.L. Beneficial Effects of Deoxyshikonin on Delayed Wound Healing in Diabetic Mice. Int. J. Mol. Sci. 2018, 19, 11. [Google Scholar] [CrossRef]


| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| VEGF | 5′-TGCAGATTATGCGGATCAAACC-3′ | 5′-TGCATTCACATTTGTTGTGCTGTAG-3′ |
| VEGFR-2 | 5′-GGAAGCTCCTGAAGATCTGT-3′ | 5′-GAGGATATTTCGTGCCGC-3′ |
| GAPDH | 5′-CAAGATTGTCAGCAACGCAT-3′ | 5′-GTCTTCTGGGTGGCAGTGAT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Ji, Y.S.; Zhu, H.; Zhang, Y.; Park, D.H.; Kim, Y.-J.; Yoo, H.H.; Kang, K.S. Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling. Biomolecules 2019, 9, 358. https://doi.org/10.3390/biom9080358
Park JY, Ji YS, Zhu H, Zhang Y, Park DH, Kim Y-J, Yoo HH, Kang KS. Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling. Biomolecules. 2019; 9(8):358. https://doi.org/10.3390/biom9080358
Chicago/Turabian StylePark, Jun Yeon, Young Seok Ji, Hucheng Zhu, Yonghui Zhang, Do Hwi Park, Young-Joo Kim, Hye Hyun Yoo, and Ki Sung Kang. 2019. "Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling" Biomolecules 9, no. 8: 358. https://doi.org/10.3390/biom9080358
APA StylePark, J. Y., Ji, Y. S., Zhu, H., Zhang, Y., Park, D. H., Kim, Y.-J., Yoo, H. H., & Kang, K. S. (2019). Anti-Angiogenic Effect of Asperchalasine A Via Attenuation of VEGF Signaling. Biomolecules, 9(8), 358. https://doi.org/10.3390/biom9080358