Context Matters: Divergent Roles of Exercise-Induced and Tumor-Derived Lactate in Cancer
Abstract
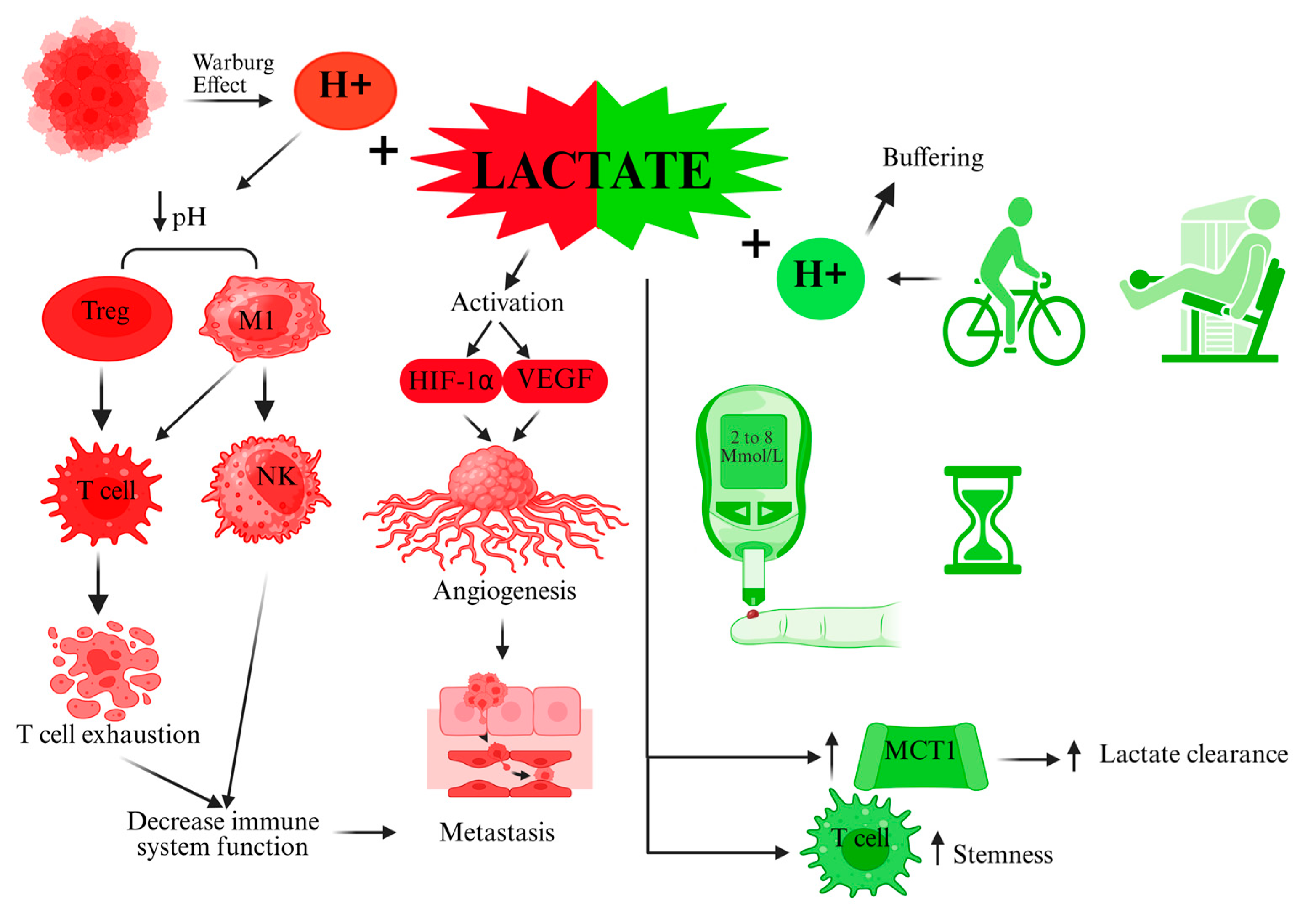
1. Introduction
2. Mechanistic and Metabolic Perspectives of Lactate in Cancer vs. Exercise
3. Immunological Effects of Lactate: Suppression in the TME vs. Activation with Exercise
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gladden, L.B. Lactate as a key metabolic intermediate in cancer. Ann. Transl. Med. 2019, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, F.; Afonso, J.; Costa, M.; Granja, S. Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy. Front. Oncol. 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 492088. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef]
- Dubińska, M.; Paduch-Jakubczyk, W.; Bilska, W.; Ciułek, U.; Dobosz, A.; Zduńczyk, W. Physical Activity and Its Effects on Cancer Prevention, Survival Rates, and Recovery. Qual. Sport 2024, 22, 54321. [Google Scholar] [CrossRef]
- Brown, J.C.; Winters-Stone, K.; Lee, A.; Schmitz, K.H. Cancer, physical activity, and exercise. Compr. Physiol. 2012, 2, 2775–2809. [Google Scholar] [CrossRef]
- McTiernan, A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer 2008, 8, 205–211. [Google Scholar] [CrossRef]
- Hofmann, P. Cancer and Exercise: Warburg Hypothesis, Tumour Metabolism and High-Intensity Anaerobic Exercise. Sports 2018, 6, 10. [Google Scholar] [CrossRef]
- Khoramipour, K.; Soltany, A.; Khosravi, P.; Rezaei, M.H.; Madadizadeh, E.; García-Chico, C.; Maroto-Izquierdo, S.; Khoramipour, K. High intensity interval training as a therapy: Mitophagy restoration in breast cancer. Arch. Biochem. Biophys. 2024, 762, 110213. [Google Scholar] [CrossRef]
- Coma, N.; Moral, S.; Ballesteros, E.; Eraso, A.; Ventura, M.; Pujol, E.; Brugada, R. Current Evidence on the Benefit of Exercise in Cancer Patients: Effects on Cardiovascular Mortality, Cardiotoxicity, and Quality of Life. Rev. Cardiovasc. Med. 2023, 24, 160. [Google Scholar] [CrossRef]
- Sun, K.; Shen, Y.; Xiao, X.; Xu, H.; Zhang, Q.; Li, M. Crosstalk between lactate and tumor-associated immune cells: Clinical relevance and insight. Front. Oncol. 2024, 14, 1506849. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Pereira-Nunes, A.; Baltazar, F.; Granja, S. Lactate as a Regulator of Cancer Inflammation and Immunity. Immunometabolism 2019, 1, e190015. [Google Scholar] [CrossRef]
- Mortazavi Farsani, S.S.; Verma, V. Lactate mediated metabolic crosstalk between cancer and immune cells and its therapeutic implications. Front. Oncol. 2023, 13, 1175532. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Hekmatikar, A.H.; Moqhadasi, A. Lactate can promote metastasis in cancer, what about physical exercise? J. Exerc. Organ Cross Talk 2024, 4, 67–73. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- De Berardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Wang, M.; Yao, L.; Li, X.; Dong, H.; Li, M.; Sun, T.; Liu, X.; Liu, Y.; et al. Role of Proton-Coupled Monocarboxylate Transporters in Cancer: From Metabolic Crosstalk to Therapeutic Potential. Front. Cell Dev. Biol. 2020, 8, 651. [Google Scholar] [CrossRef]
- Boidot, R.; Veǵran, F.; Meulle, A.; Le Breton, A.; Dessy, C.; Sonveaux, P.; Lizard-Nacol, S.; Feron, O. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012, 72, 939–948. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family—Role and regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef]
- Draoui, N.; Feron, O. Lactate shuttles at a glance: From physiological paradigms to anti-cancer treatments. DMM Dis. Model. Mech. 2011, 4, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058.e4. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Liu, X.; Wang, H.; Zhu, X.; Huang, W.; Liu, L.; Qiu, H.; Yang, Y. Lactate aggravates inflammation through the upregulation of ICAM-1 via GPR81-HSPA12B-mediated signaling in acute lung injury. Chin. Med. J. 2024, 137, 1885–1887. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Q.; Zheng, J.; Yang, Y.; Zhang, X.; Ma, A.; Qin, Y.; Qin, Z.; Zheng, X. The function and mechanism of lactate and lactylation in tumor metabolism and microenvironment. Genes Dis. 2023, 10, 2029–2037. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Mendes, C.; Serpa, J. Revisiting lactate dynamics in cancer—A metabolic expertise or an alternative attempt to survive? J. Mol. Med. 2020, 98, 1397–1414. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 10145. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Luo, Z.; Su, M.; Yu, C. Recent Progress in the Therapeutic Modulation of Lactate for Cancer Treatment. Adv. Ther. 2023, 6, 2200254. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and lactylation in cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef]
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- Stallknecht, B.; Vissing, J.; Galbo, H. Lactate production and clearance in exercise. Effects of training. A mini-review. Scand. J. Med. Sci. Sport. 1998, 8, 127–131. [Google Scholar] [CrossRef]
- Anaerobiosis, Lactate, and Gas Exchange During Exercise: The Issues—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/3536588/ (accessed on 20 May 2025).
- Hubbard, J.L. The effect of exercise on lactate metabolism. J. Physiol. 1973, 231, 1–18. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Zhao, E.; Li, B.; Li, S.; Dong, X.; Yuan, L.; Yang, H. Prognostic Value of Lactate Dehydrogenase in Second-Line Immunotherapy for Advanced Esophageal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610245. [Google Scholar] [CrossRef]
- Bozzetti, F. Potential Benefits from Physical Exercise in Advanced Cancer Patients Undergoing Systemic Therapy? A Narrative Review of the Randomized Clinical Trials. Curr. Oncol. 2024, 31, 7631–7646. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, N.; Niels, T.; Tewes, M.; Götte, M. A Systematic Review of the Safety, Feasibility and Benefits of Exercise for Patients with Advanced Cancer. Cancers 2021, 13, 4478. [Google Scholar] [CrossRef]
- Gresham, G.; Raines, C.; Asher, A.; Freedland, S.J.; Shirazipour, C.H.; Sleight, A.G. Can high-intensity interval training impact tumor suppression and inflammatory response in prostate cancer survivors? Prostate Cancer Prostatic Dis. 2023, 26, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Koivula, T.; Lempiäinen, S.; Rinne, P.; Rannikko, J.H.; Hollmén, M.; Sundberg, C.J.; Rundqvist, H.; Minn, H.; Heinonen, I. The effect of acute exercise on circulating immune cells in newly diagnosed breast cancer patients. Sci. Rep. 2023, 13, 6561. [Google Scholar] [CrossRef]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Jedlička, M.; Feglarová, T.; Janstová, L.; Hortová-Kohoutková, M.; Frič, J. Lactate from the tumor microenvironment—A key obstacle in NK cell-based immunotherapies. Front. Immunol. 2022, 13, 932055. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, Y.; Zhao, X.; Yu, J. From metabolic byproduct to immune modulator: The role of lactate in tumor immune escape. Front. Immunol. 2024, 15, 1492050. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F.; Kzhyshkowska, J.; Supuran, C.T. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.L.J.; Vignali, P.D.A.; Mullett, S.J.; Overacre-Delgoffe, A.E.; Peralta, R.M.; Grebinoski, S.; Menk, A.V.; Rittenhouse, N.L.; DePeaux, K.; Whetstone, R.D.; et al. Metabolic support of tumor-infiltrating regulatory T cells by lactic acid. Nature 2021, 591, 645. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Yang, J.L.; Lai, R.; Zhou, Z.J.; Tang, D.; Hu, L.; Zhao, L.J. Impact of lactate on immune cell function in the tumor microenvironment: Mechanisms and therapeutic perspectives. Front. Immunol. 2025, 16, 1563303. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Vaupel, P. Lactate-avid regulatory T cells: Metabolic plasticity controls immunosuppression in tumour microenvironment. Signal Transduct. Target. Ther. 2021, 6, 171. [Google Scholar] [CrossRef]
- Kumagai, S.; Koyama, S.; Itahashi, K.; Tanegashima, T.; Lin, Y.; Togashi, Y.; Kamada, T.; Irie, T.; Okumura, G.; Kono, H.; et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022, 40, 201–218.e9. [Google Scholar] [CrossRef]
- Kim, J.; Li, J.; Wei, J.; Lim, S.A. Regulatory T Cell Metabolism: A Promising Therapeutic Target for Cancer Treatment? Immune Netw. 2025, 25, e13. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Yu, X.; Huang, T.; Chen, J.; Wang, J.; Wilhelm, J.; Li, S.; Song, J.; Li, W.; et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 2022, 13, 4981. [Google Scholar] [CrossRef]
- Lactate Appears to Rejuvenate Cancer-Fighting CD8+ T Cells. Available online: https://www.genengnews.com/news/lactate-appears-to-rejuvenate-cancer-fighting-cd8-t-cells/ (accessed on 21 May 2025).
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’Acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate regulates metabolic and proinflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef]
- Adammek, F.; Wences, T.; Walzik, D.; Trebing, S.; Belen, S.; Renpening, D.; Zimmer, P.; Joisten, N. Kinetics of immune cell mobilization during acute aerobic exercise in healthy adults. Int. J. Sports Med. 2023, 45, 908–916. [Google Scholar] [CrossRef]
- Koivula, T.; Lempiäinen, S.; Neuvonen, J.; Norha, J.; Hollmén, M.; Sundberg, C.J.; Rundqvist, H.; Minn, H.; Rinne, P.; Heinonen, I. The effect of exercise and disease status on mobilization of anti-tumorigenic and pro-tumorigenic immune cells in women with breast cancer. Front. Immunol. 2024, 15, 1394420. [Google Scholar] [CrossRef]
- Stampley, J.E.; Cho, E.; Wang, H.; Theall, B.; Johannsen, N.M.; Spielmann, G.; Irving, B.A. Impact of maximal exercise on immune cell mobilization and bioenergetics. Physiol. Rep. 2023, 11, e15753. [Google Scholar] [CrossRef]
- Gouez, M.; Rébillard, A.; Thomas, A.; Beaumel, S.; Matera, E.L.; Gouraud, E.; Orfila, L.; Martin, B.; Pérol, O.; Chaveroux, C.; et al. Combined effects of exercise and immuno-chemotherapy treatments on tumor growth in MC38 colorectal cancer-bearing mice. Front. Immunol. 2024, 15, 1368550. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Exercise Reduces Tumor Initiation and Progression in Mouse Models. Cancer Discov. 2016, 6, 339. [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Bay, M.L.; Heywood, S.; Wedell-Neergaard, A.S.; Schauer, T.; Lehrskov, L.L.; Christensen, R.H.; Legård, G.E.; Jensen, P.Ø.; Krogh-Madsen, R.; Ellingsgaard, H. Human immune cell mobilization during exercise: Effect of IL-6 receptor blockade. Exp. Physiol. 2020, 105, 2086–2098. [Google Scholar] [CrossRef]
- Zhou, Y.; Lou, J.; Tian, Y.; Ding, J.; Wang, X.; Tang, B. How lactate affects immune strategies in lymphoma. Front. Mol. Biosci. 2024, 11, 1480884. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate Is a Metabolic Mediator That Shapes Immune Cell Fate and Function. Front. Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef]
- Hao, Z.N.; Tan, X.P.; Zhang, Q.; Li, J.; Xia, R.; Ma, Z. Lactate and Lactylation: Dual Regulators of T-Cell-Mediated Tumor Immunity and Immunotherapy. Biomolecules 2024, 14, 1646. [Google Scholar] [CrossRef]
- Harmon, C.; O’Farrelly, C.; Robinson, M.W. The Immune Consequences of Lactate in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1259, 113–124. [Google Scholar] [CrossRef]
- Mandadzhiev, N. The contemporary role of lactate in exercise physiology and exercise prescription—A review of the literature. Folia Med. 2025, 67, e144693. [Google Scholar] [CrossRef]
- Reese, K.L.; Pantel, K.; Smit, D.J. Multibiomarker panels in liquid biopsy for early detection of pancreatic cancer—A comprehensive review. J. Exp. Clin. Cancer Res. 2024, 43, 250. [Google Scholar] [CrossRef] [PubMed]
- Home Page | Journal of Experimental & Clinical Cancer Research. Available online: https://jeccr.biomedcentral.com/?gad_source=1&gad_campaignid=22642321321&gbraid=0AAAAApIOJzrNiOMyKOkcSQGy4wbKHojd2&gclid=CjwKCAjwmenCBhA4EiwAtVjzmqCWdSjrzNuqQDzvuRZqmkEooS4htjUBA4-ucBR0OvxlVTlSN1VE_BoC89MQAvD_BwE (accessed on 24 June 2025).
- Walker, R.C.; Pezeshki, P.; Barman, S.; Ngan, S.; Whyte, G.; Lagergren, J.; Gossage, J.; Kelly, M.; Baker, C.; Knight, W.; et al. Exercise During Chemotherapy for Cancer: A Systematic Review. J. Surg. Oncol. 2024, 130, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N.; Kaplan, M.A.; Küçüköner, M.; Urakçı, Z.; Laçin, Ş.; Ceylan, E.H.; Işıkdoğan, A. The effect of exercise on disease-free survival and overall survival in patients with breast cancer. Ir. J. Med. Sci. 2022, 191, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S.; Hamaue, Y.; Fukushima, T.; Tanaka, T.; Fu, J.B.; Nakano, J. Effect of Exercise on Mortality and Recurrence in Patients with Cancer: A Systematic Review and Meta-Analysis. Integr. Cancer Ther. 2020, 19, 250. [Google Scholar] [CrossRef]
- Nieman, D.C. Immune response to heavy exertion. J. Appl. Physiol. 1997, 82, 1385–1394. [Google Scholar] [CrossRef]
- de Rezende, L.F.M.; de Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical activity and cancer: An umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef]
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; De Gonzalez, A.B.; Hartge, P.; et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Wang, Q.; Yu, X.; Ouyang, L. Lactate-induced protein lactylation in cancer: Functions, biomarkers and immunotherapy strategies. Front. Immunol. 2024, 15, 1513047. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A Metabolic Key Player in Cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Luo, M.; Wei, H.; Qiu, M.; Su, C.; Ning, R.; Zhou, S. Prognostic value of the lactate dehydrogenase to albumin ratio in advanced non-small cell lung cancer patients treated with the first-line PD-1 checkpoint inhibitors combined with chemotherapy. Front. Immunol. 2025, 16, 1473962. [Google Scholar] [CrossRef] [PubMed]
- Priest, J.B.; Oei, T.O.; Moorehead, W.R. Exercise-induced changes in common laboratory tests. Am. J. Clin. Pathol. 1982, 77, 285–289. [Google Scholar] [CrossRef]
- García-Suárez, P.C.; Rentería, I.; Moncada-Jiménez, J.; Fry, A.C.; Jiménez-Maldonado, A. Acute Systemic Response of BDNF, Lactate and Cortisol to Strenuous Exercise Modalities in Healthy Untrained Women. Dose-Response 2020, 18, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Al Akko, M.; Maher, M.; Airia, P. The Impact of Acute Exercise on Blood Work Parameters: A Case Report of a Healthy Male University Student. Cureus 2024, 16, e70018. [Google Scholar] [CrossRef]
- Azevedo, M.J.; Viamonte, S.; Castro, A. Exercise prescription in oncology patients: General principles. Rehabilitacion 2013, 47, 170–178. [Google Scholar] [CrossRef]
- Yang, L.; Morielli, A.R.; Heer, E.; Kirkham, A.A.; Cheung, W.Y.; Usmani, N.; Friedenreich, C.M.; Courneya, K.S. Effects of Exercise on Cancer Treatment Efficacy: A Systematic Review of Preclinical and Clinical Studies. Cancer Res. 2021, 81, 4889. [Google Scholar] [CrossRef]
- Gouez, M.; Pérol, O.; Pérol, M.; Caux, C.; Ménétrier-Caux, C.; Villard, M.; Walzer, T.; Delrieu, L.; Saintigny, P.; Marijnen, P.; et al. Effect of acute aerobic exercise before immunotherapy and chemotherapy infusion in patients with metastatic non-small-cell lung cancer: Protocol for the ERICA feasibility trial. BMJ Open 2022, 12, e056819. [Google Scholar] [CrossRef]
- Li, Q.; Guo, C.; Cao, B.; Zhou, F.; Wang, J.; Ren, H.; Li, Y.; Wang, M.; Liu, Y.; Zhang, H.; et al. Safety and efficacy evaluation of personalized exercise prescription during chemotherapy for lung cancer patients. Thorac. Cancer 2024, 15, 906–918. [Google Scholar] [CrossRef]
- White, K.R.; Lu, J.; Ibrahim, Z.; Furth, P.A. Enabling exercise prescription for survivors of cancer. Sci. Rep. 2021, 11, 9557. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Cui, Q.; Guo, B.; Ding, W.; Liu, J.; Quan, L.; Li, X.; Xie, P.; Jin, L.; et al. Activation of GPR81 by lactate drives tumour-induced cachexia. Nat. Metab. 2024, 6, 708–723. [Google Scholar] [CrossRef]
- Rodríguez-Cañamero, S.; Cobo-Cuenca, A.I.; Carmona-Torres, J.M.; Pozuelo-Carrascosa, D.P.; Santacruz-Salas, E.; Rabanales-Sotos, J.A.; Cuesta-Mateos, T.; Laredo-Aguilera, J.A. Impact of physical exercise in advanced-stage cancer patients: Systematic review and meta-analysis. Cancer Med. 2022, 11, 3714. [Google Scholar] [CrossRef]
| Context | Estimated Blood Lactate Levels (mmol/L) | Cancer Type | Reference | Note |
|---|---|---|---|---|
| Rest (sedentary cancer patient) | 1.5 | Advanced solid tumors (general) | [41] | May be elevated due to tumor metabolism or cachexia |
| Low-intensity walking (<40% HRmax) | 2 | Breast, colorectal, and prostate | [42] | Mild exertion; lactate slightly increases with fatigue |
| Moderate aerobic (55–70% HRmax) | 3 | Breast, lung, and colorectal | [43] | Safe and well-tolerated; modest transient lactate rise |
| Supervised HIIT (85–90% HRmax) | 5 | Prostate, lung, and lymphoma (survivors) | [44] | Requires high fitness or supervision; buffered rise |
| Maximal effort test (e.g., CPET) | 6 | Breast and lung (during CPET or trials) | [45] | Rarely performed unless in a trial; peak still lower than healthy due to lower capacity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi Hekmatikar, A.h.; Zolfaghari, G.; Basereh, A.; Awang Daud, D.M.; Khoramipour, K. Context Matters: Divergent Roles of Exercise-Induced and Tumor-Derived Lactate in Cancer. Biomolecules 2025, 15, 1010. https://doi.org/10.3390/biom15071010
Ahmadi Hekmatikar Ah, Zolfaghari G, Basereh A, Awang Daud DM, Khoramipour K. Context Matters: Divergent Roles of Exercise-Induced and Tumor-Derived Lactate in Cancer. Biomolecules. 2025; 15(7):1010. https://doi.org/10.3390/biom15071010
Chicago/Turabian StyleAhmadi Hekmatikar, Amir hossein, Ghazal Zolfaghari, Aref Basereh, D. Maryama Awang Daud, and Kayvan Khoramipour. 2025. "Context Matters: Divergent Roles of Exercise-Induced and Tumor-Derived Lactate in Cancer" Biomolecules 15, no. 7: 1010. https://doi.org/10.3390/biom15071010
APA StyleAhmadi Hekmatikar, A. h., Zolfaghari, G., Basereh, A., Awang Daud, D. M., & Khoramipour, K. (2025). Context Matters: Divergent Roles of Exercise-Induced and Tumor-Derived Lactate in Cancer. Biomolecules, 15(7), 1010. https://doi.org/10.3390/biom15071010






