A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling by Homology: Computational Procedures
2.2. Molecular Docking Procedure
2.3. Expression of Bovine Recombinant IFN-τ in the Pichia Pastoris System
2.4. Purification of Bovine Recombinant IFN-τ by Ion Exchange
2.5. Inhibition of the Cytopathic Effect of Mengo Virus in MDBK Cells
2.6. Antiviral Markers Activation Analysis in MDBK Cells
2.7. Antiviral Activity of brIFN-τ
2.8. Encapsulating brIFN-τ Using Chitosan as a Low Molecular Weight Polymer
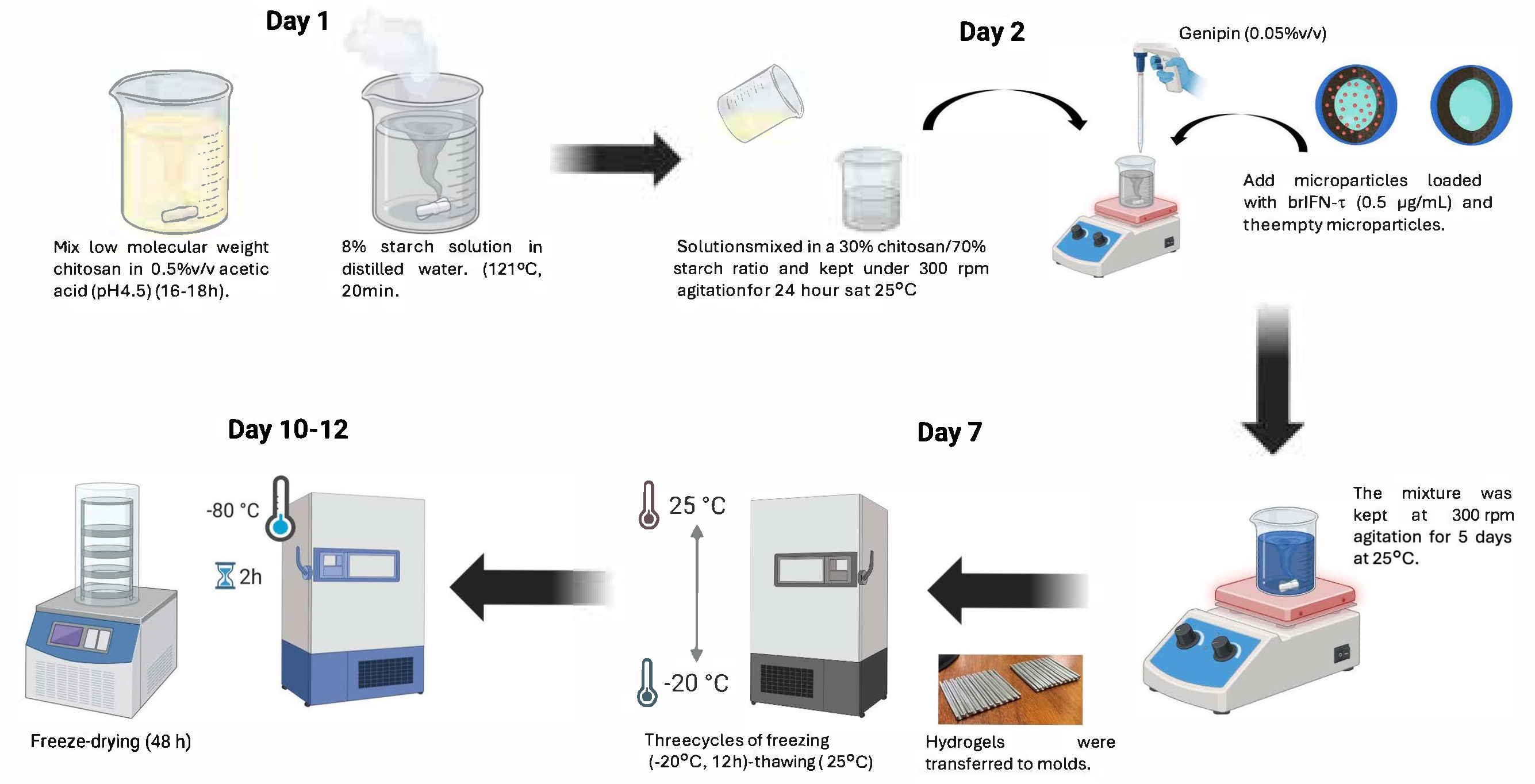
2.8.1. Microencapsulation of rbIFN-τ in a Chitosan Matrix
2.8.2. Preparation of the Samples to Be Microencapsulated
2.9. Microencapsulated brIFN-τ Release Assay Generated at Three Temperatures
2.10. Release Assay of brIFN-τ from Chitosan Microparticle from 120 °C
2.11. Incorporation of Microencapsulated brIFN-τ into a Semi-Solid Matrix (Starch/Chitosan, Hydrogel)
2.12. The Release Assay of Microencapsulated brIFN-τ Associated with Hydrogel
2.13. Safety Assessment of Intrauterine Device in Ovine Model
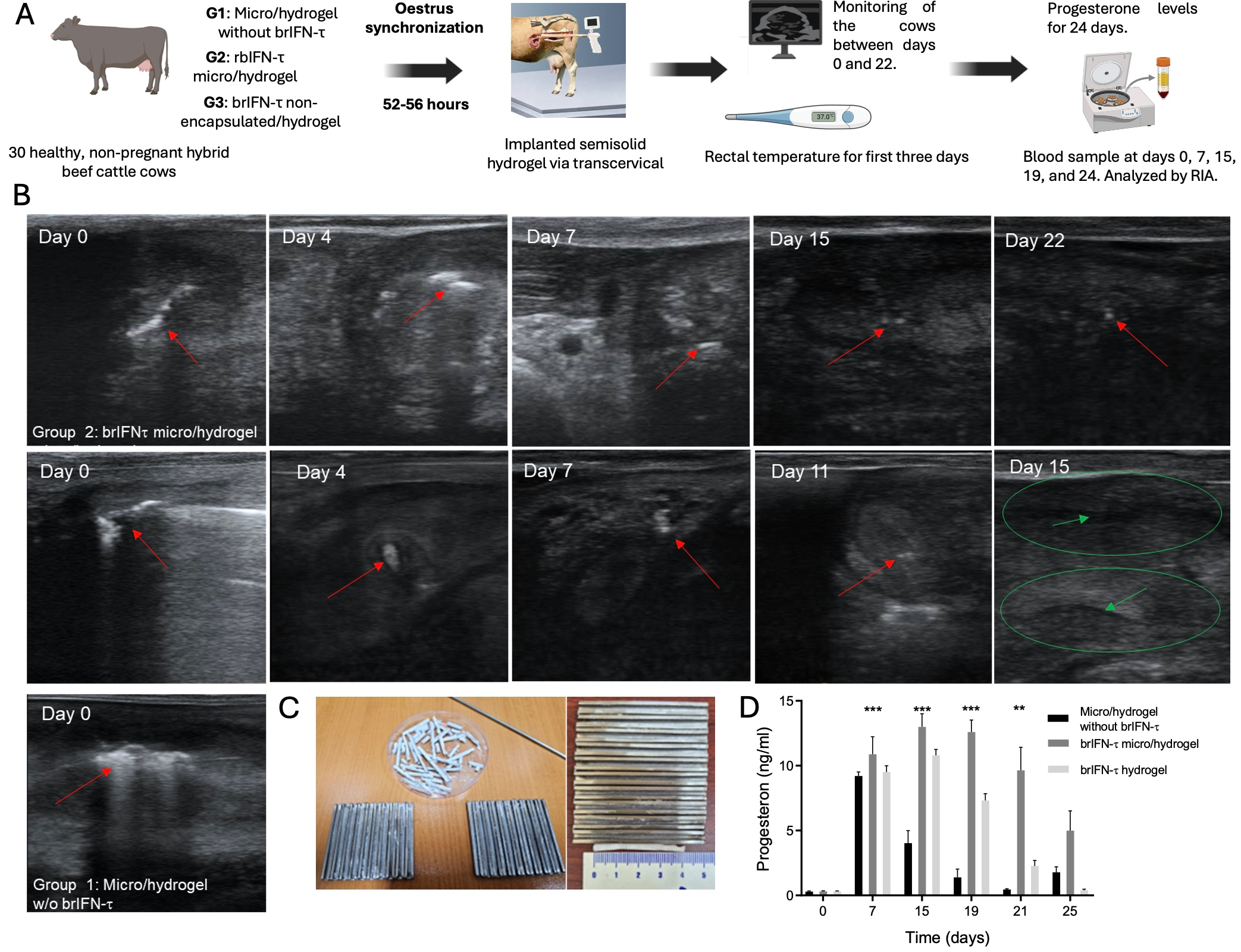
2.14. Anti-Luteolytic Activity in Cows
2.15. Statistical Analysis
3. Results
3.1. Computational Modeling of the 3D Structure of the Interferon Tau
3.1.1. Homology Modeling
3.1.2. Docking Assays
3.2. Expression, Characterization, and Purification of brIFN-τ
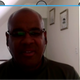
3.2.1. brIFN-τ Expression
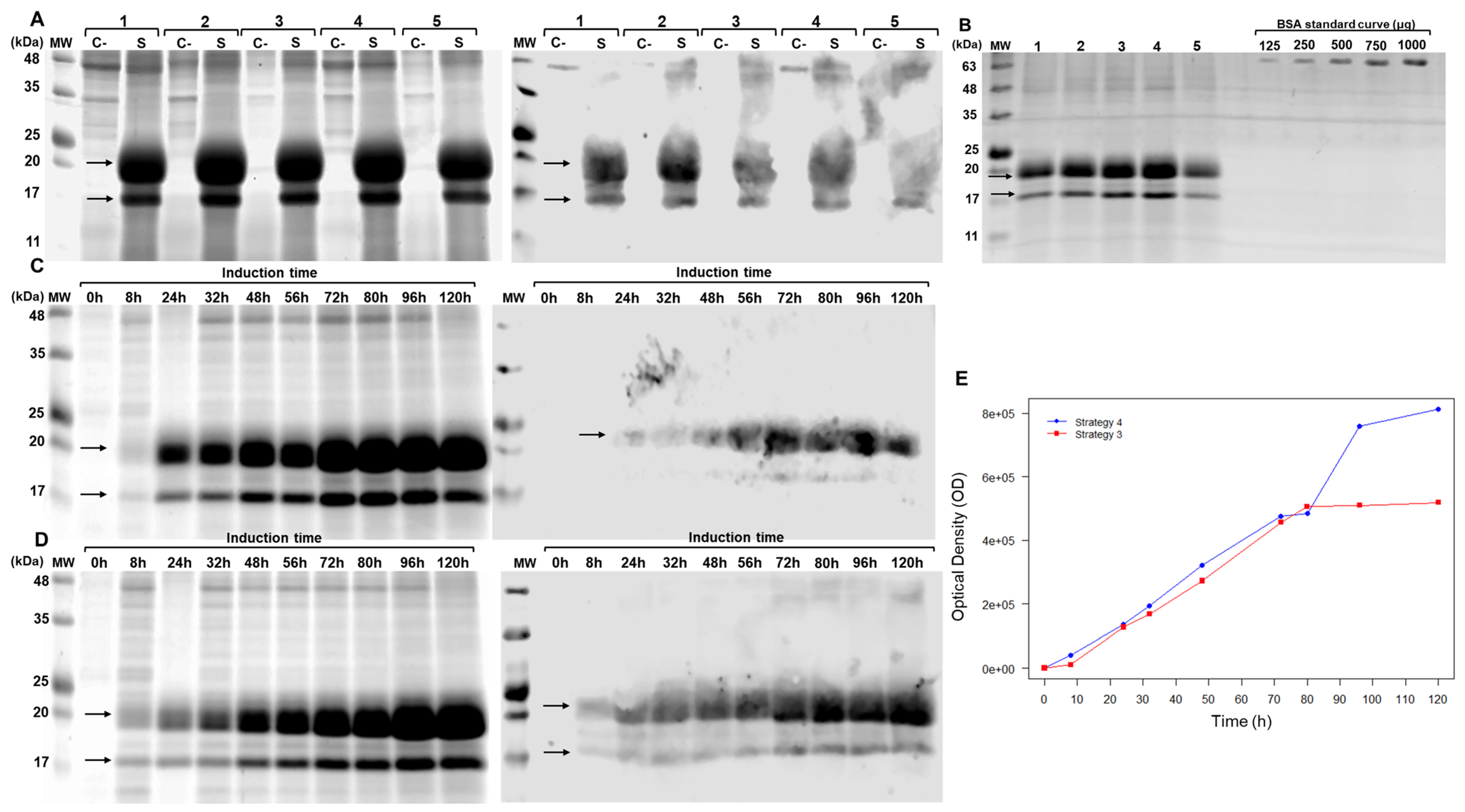
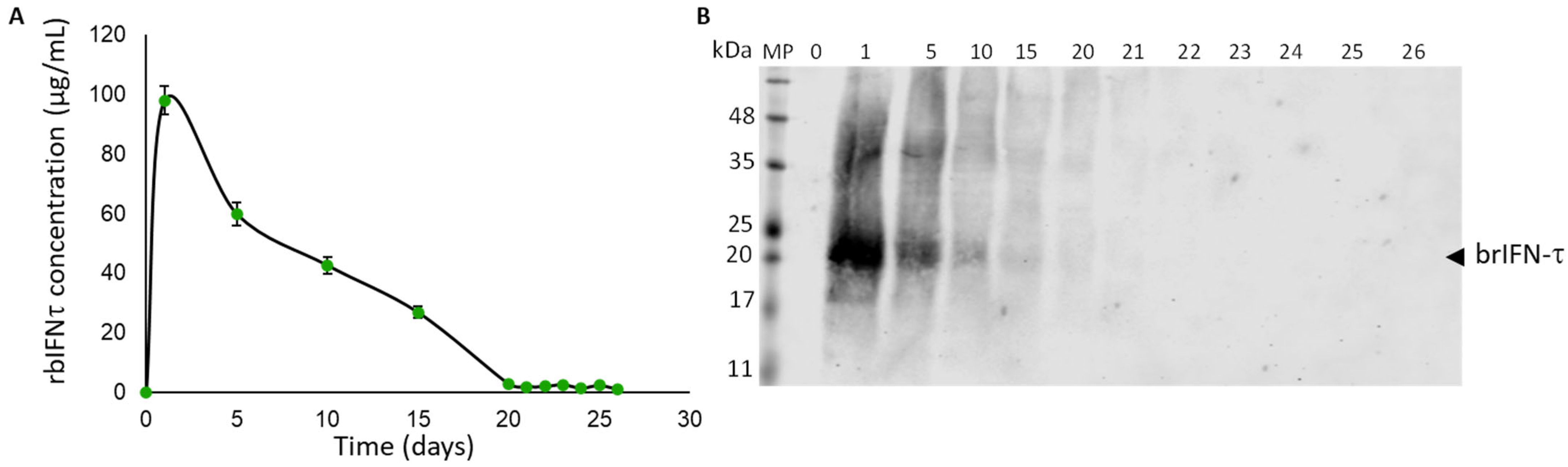
3.2.2. Purification of brIFN-τ
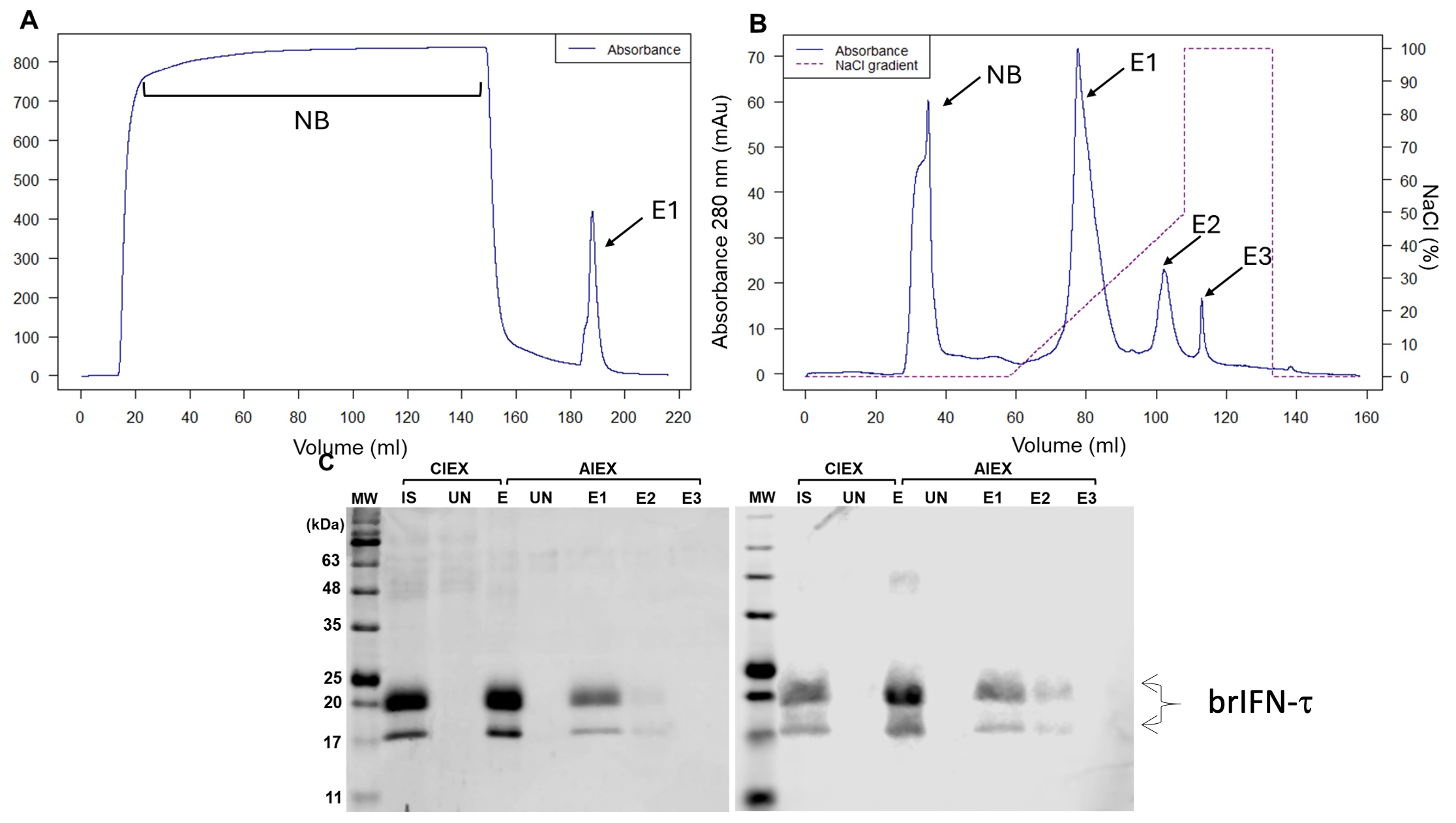
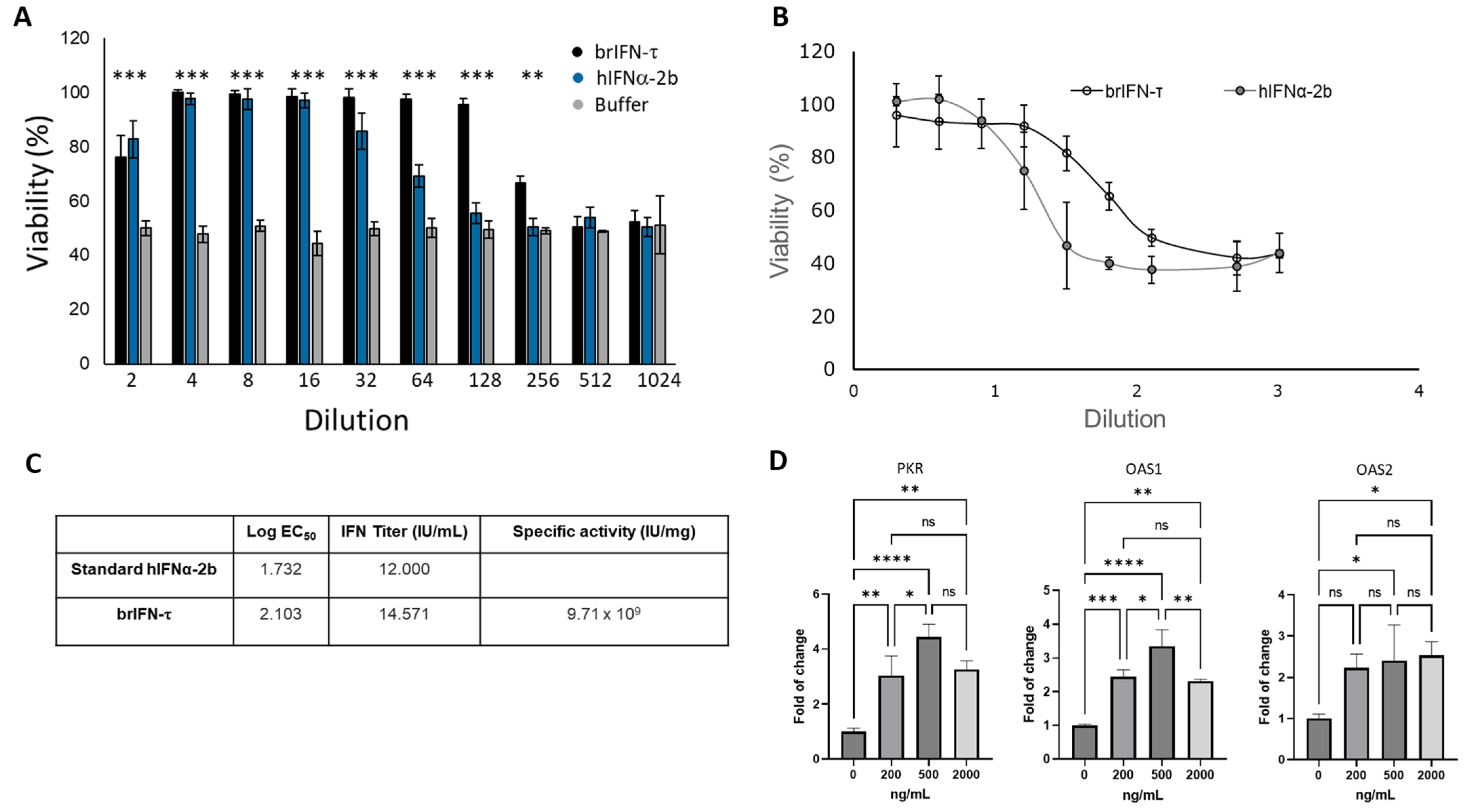
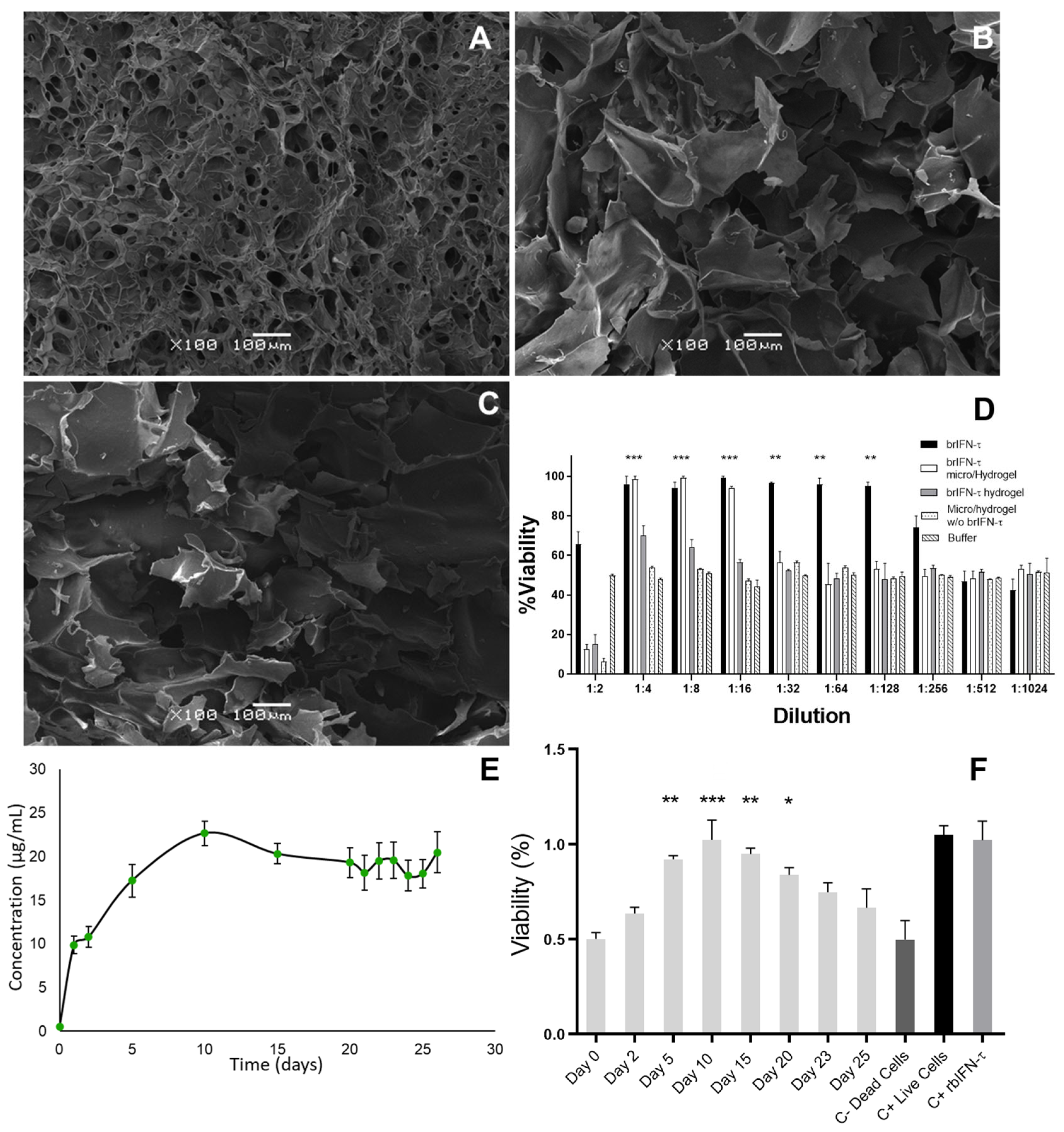
3.2.3. Cell Viability, Antiviral Markers, and Antiviral Activity of brIFN-τ
3.3. Microencapsulation of brIFN-τ and Packaging in Semi-Solid Matrix
3.3.1. Microparticles Obtained by Spray Drying Procedure
3.3.2. Antiviral Activity of Encapsulated brIFN-τ In Vitro
3.3.3. brIFN-τ Release In Vitro, Simulating Bovine Uterine Environment Conditions
3.4. Semi-Solid Starch/Chitosan Matrix with Incorporated Microencapsulated brIFN-τ
3.4.1. Stable Hydrogel Matrix Synthesis
3.4.2. Hydrogel Characterization
3.4.3. brIFN-τ Release from Hydrogel In Vitro
3.5. Drug Safety and Anti-Luteolytic Activity
3.5.1. Device Safety in the Ovine Model
3.5.2. Anti-Luteolytic Activity Validation in Cows
4. Discussion
4.1. Homology Modeling of Bovine Recombinant IFN-τ
4.2. Docking Experiment of IFN-τ with the α/β-Receptor 1 and 2
4.3. Expression, Purification, and Biological Activity Evaluation of brIFN-τ
4.4. Microencapsulation of brIFN-τ
4.5. Semi-Solid Matrix brIFN-τ Incorporation
4.6. Safety and Anti-Luteolytic Effects In Vivo
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IFN-τ | Interferon tau |
| brIFN-τ | Bovine recombinant interferon tau |
| FBS | Fetal bovine serum |
| hIFNα-2b | Human interferon alpha-2b |
| MDBK | Madin–Darby bovine kidney |
| NMR | Nuclear magnetic resonance |
| OAS1 | 2′-5′-oligoadenylate synthetase 1 |
| OAS2 | 2′-5′-oligoadenylate synthetase 2 |
| PGF2α | Prostaglandin F2α |
| PKR | Protein kinase R |
| qPCR | Quantitative polymerase chain reaction |
| YP | Yeast extract–peptone |
| YPG | Yeast extract–peptone–glycerol |
References
- Ealy, A.D. Pregnancy Losses in Livestock: An Overview of the Physiology and Endocrinology Symposium for the 2020 Asas-Csas-Wsasas Virtual Meeting. J. Anim. Sci. 2020, 98, skaa277. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, A.; Bisinotto, R.S.; Peñagaricano, F. Genes and Pathways Associated with Pregnancy Loss in Dairy Cattle. Sci. Rep. 2021, 11, 13329. [Google Scholar] [CrossRef]
- Williams, G.W.; Anderson, D.P. Choices Magazine 1 The Latin American Livestock Industry: Growth and Challenges. 2020. Available online: http://www.choicesmagazine.org/choices-magazine/submitted-articles/the-latin-american-livestock-industry-growth-and-challenges (accessed on 1 January 2020).
- Gädicke, P.; Monti, G. Factors Related to the Level of Occurrence of Bovine Abortion in Chilean Dairy Herds. Prev. Vet. Med. 2013, 110, 183–189. [Google Scholar] [CrossRef]
- Gädicke L’Huissier, P.; Chihuailaf, R.; Letelier, R.; Allende, R.; Ruiz, A.; Junod, T.; Gädicke L’Huissier, P.; Chihuailaf, R.; Letelier, R.; Allende, R.; et al. Monitoring Different Causal Patterns of Bovine Abortion Syndrome. Vet. México OA 2022, 9, e652. [Google Scholar] [CrossRef]
- Lonergan, P.; Sánchez, J.M. Symposium Review: Progesterone Effects on Early Embryo Development in Cattle. J. Dairy. Sci. 2020, 103, 8698–8707. [Google Scholar] [CrossRef] [PubMed]
- Pohler, K.G.; Franco, G.A.; Reese, S.T.; Smith, M.F. Physiology and Pregnancy of Beef Cattle. Anim. Agric. Sustain. Chall. Innov. 2020, 37–55. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.J.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.P.; Sartori, R. Pivotal Periods for Pregnancy Loss during the First Trimester of Gestation in Lactating Dairy Cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef]
- Reese, S.T.; Franco, G.A.; Poole, R.K.; Hood, R.; Fernadez Montero, L.; Oliveira Filho, R.V.; Cooke, R.F.; Pohler, K.G. Pregnancy Loss in Beef Cattle: A Meta-Analysis. Anim. Reprod. Sci. 2020, 212, 106251. [Google Scholar] [CrossRef]
- Bazer, F.W.; Thatcher, W.W. Chronicling the Discovery of Interferon Tau. Reproduction 2017, 154, F11–F20. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Monteiro, P.L.J.; Domingues, R.R.; Andrade, J.P.N.; Mezera, M.A. Review: Maintenance of the Ruminant Corpus Luteum during Pregnancy: Interferon-Tau and Beyond. Animal 2023, 17, 100827. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Czerniawska-Piątkowska, E.; Wrzecińska, M. The Importance of Interferon-Tau in the Diagnosis of Pregnancy. Biomed. Res. Int. 2021, 2021, 9915814. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.P.; Thatcher, W.W.; Chebel, R.C.; Cerri, R.L.A.; Galvão, K.N. The Effect of Embryonic Death Rates in Cattle on the Efficacy of Estrus Synchronization Programs. Anim. Reprod. Sci. 2004, 82–83, 513–535. [Google Scholar] [CrossRef]
- Forde, N.; Lonergan, P. Interferon-Tau and Fertility in Ruminants. Reproduction 2017, 154, F33–F43. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus Elongation in Ruminants: Roles of Progesterone, Prostaglandin, Interferon Tau and Cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Saugandhika, S.; Sharma, V.; Malik, H.; Saini, S.; Bag, S.; Kumar, S.; Singh, N.K.; Mohanty, A.K.; Malakar, D. Expression and Purification of Buffalo Interferon-Tau and Efficacy of Recombinant Buffalo Interferon-Tau for in Vitro Embryo Development. Cytokine 2015, 75, 186–196. [Google Scholar] [CrossRef]
- Kerbler, T.L.; Buhr, M.M.; Jordan, L.T.; Leslie, K.E.; Walton, J.S. Relationship between Maternal Plasma Progesterone Concentration and Interferon-Tau Synthesis by the Conceptus in Cattle. Theriogenology 1997, 47, 703–714. [Google Scholar] [CrossRef]
- Green, J.C.; Okamura, C.S.; Poock, S.E.; Lucy, M.C. Measurement of Interferon-Tau (IFN-τ) Stimulated Gene Expression in Blood Leukocytes for Pregnancy Diagnosis within 18-20d after Insemination in Dairy Cattle. Anim. Reprod. Sci. 2010, 121, 24–33. [Google Scholar] [CrossRef]
- Lemley, C.O.; Camacho, L.E.; Vonnahme, K.A. Maternal Recognition and Physiology of Pregnancy. Bov. Reprod. 2021, 324–338. [Google Scholar] [CrossRef]
- Monaco, C.F.; Davis, J.S. Mechanisms of Angioregression of the Corpus Luteum. Front. Physiol. 2023, 14, 1254943. [Google Scholar] [CrossRef]
- Nagaya, H.; Kanaya, T.; Kaki, H.; Tobita, Y.; Takahashi, M.; Takahashi, H.; Yokomizo, Y.; Inumaru, S. Establishment of a Large-Scale Purification Procedure for Purified Recombinant Bovine Interferon-τ Produced by a Silkworm-Baculovirus Gene Expression System. J. Vet. Med. Sci. 2004, 66, 1395–1401. [Google Scholar] [CrossRef][Green Version]
- Takahashi, H.; Tsunazaki, M.; Hamano, T.; Takahashi, M.; Okuda, K.; Inumaru, S.; Okano, A.; Geshi, M.; Hirako, M. Biological Activity of Recombinant Bovine Interferon τ Produced by a Silkworm- Baculovirus Gene Expression System. J. Vet. Med. Sci. 2014, 76, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia Pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.I.; Villacis-Aguirre, C.A.; Santiago Vispo, N.; Santiago Padilla, L.; Santana, S.P.; Parra, N.C.; Toledo Alonso, J.R. Forms and Methods for Interferon’s Encapsulation. Pharmaceutics 2021, 13, 1533. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N.; Velasco, H.; Mecerreyes, D.; Antonio, R.; et al. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zuo, X.; Zhou, Z.; Gu, Y.; Zheng, H.; Wang, X.; Wang, G.; Xu, C.; Wang, F. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 4333. [Google Scholar] [CrossRef]
- Procopio, A.; Lagreca, E.; Jamaledin, R.; La Manna, S.; Corrado, B.; Di Natale, C.; Onesto, V. Recent Fabrication Methods to Produce Polymer-Based Drug Delivery Matrices (Experimental and In Silico Approaches). Pharmaceutics 2022, 14, 872. [Google Scholar] [CrossRef]
- Koppisetti, H.; Abdella, S.; Nakmode, D.D.; Abid, F.; Afinjuomo, F.; Kim, S.; Song, Y.; Garg, S. Unveiling the Future: Opportunities in Long-Acting Injectable Drug Development for Veterinary Care. Pharmaceutics 2025, 17, 626. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An Automated Protein Homology-Modeling Server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Kumar, N.; Saluja, K.; Mehta, V.; Anand, M.; Sharma, M.; Chaurasia, R.; Gupta, A.K.; Kumar, J.; Chourasia, M. Bioinformatics Tools to Study Homology Modeling. Comput. Biol. Drug Discov. Repurposing 2024, 75–108. [Google Scholar] [CrossRef]
- Pandiyan, S.; Ruan, T.; Zhong, Z.; Yao, M.; Wang, L. Modeling 3D Structures of PIK3CA and PIK3R1 Genes Based on Homology Modeling, Molecular Docking, Molecular Dynamics and MM-GBSA Study against Breast Cancer: Insights from an in-Silico Approach. J. Mol. Struct. 2025, 1331, 141580. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Remmert, M.; Biegert, A.; Hauser, A.; Söding, J. HHblits: Lightning-Fast Iterative Protein Sequence Searching by HMM-HMM Alignment. Nat. Methods 2012, 9, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Benkert, P.; Tosatto, S.C.E.; Schomburg, D. QMEAN: A Comprehensive Scoring Function for Model Quality Assessment. Proteins Struct. Funct. Genet. 2008, 71, 261–277. [Google Scholar] [CrossRef]
- Oduselu, G.O.; Ajani, O.O.; Ajamma, Y.U.; Brors, B.; Adebiyi, E. Homology Modelling and Molecular Docking Studies of Selected Substituted Benzo[d]Imidazol-1-Yl)Methyl)Benzimidamide Scaffolds on Plasmodium Falciparum Adenylosuccinate Lyase Receptor. Bioinform. Biol. Insights 2019, 13, 1177932219865533. [Google Scholar] [CrossRef]
- Gasteiger, E.; Jung, E.; Bairoch, A. SWISS-PROT: Connecting Biomolecular Knowledge Via a Protein Database. Curr. Issues Mol. Biol. 2001, 3, 47–55. [Google Scholar] [CrossRef]
- Delano, W.L. PyMOL: An Open-Source Molecular Graphics Tool. 2002, Volume 40, pp. 82–92. Available online: http://subdude-site.com/WebPages_Local/RefInfo/Computer/Linux/LinuxGuidesOfOthers/linuxProgrammingGuides/pdfs/Molecular/Mol_PyMOL_desc_2000_9pgs.pdf (accessed on 1 January 2020).
- Yuan, S.; Chan, H.C.S.; Hu, Z. Using PyMOL as a Platform for Computational Drug Design. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Yadav, U.; Pandey, J. Molecular Docking Studies of Rifampicin—Rpo B Complex: Repurposing Drug Design Implications for against Plasmodium Falciparum Malaria through a Computational Approach. Drug Res. 2023, 73, 164–169. [Google Scholar] [CrossRef]
- Oyedele, A.Q.K.; Ogunlana, A.T.; Boyenle, I.D.; Adeyemi, A.O.; Rita, T.O.; Adelusi, T.I.; Abdul-Hammed, M.; Elegbeleye, O.E.; Odunitan, T.T. Docking Covalent Targets for Drug Discovery: Stimulating the Computer-Aided Drug Design Community of Possible Pitfalls and Erroneous Practices. Mol. Divers. 2022, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; de Azevedo, W., Jr. Molecular Docking Algorithms. Curr. Drug Targets 2008, 9, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and Scoring in Virtual Screening for Drug Discovery: Methods and Applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U.; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Adesina, A.; Ahmad, S.; Bowler-Barnett, E.H.; Bye-A-Jee, H.; Carpentier, D.; et al. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting Modern Challenges in Visualization and Analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.Y. The HDOCK Server for Integrated Protein–Protein Docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.Y. HDOCK: A Web Server for Protein-Protein and Protein-DNA/RNA Docking Based on a Hybrid Strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Lamazares Arcia, E.; Fleitas-Salazar, N.; Gancino Guevara, M.; Mansilla, R.; Gómez-Gaete, C.; Altamirano, C.; Fernandez, K.; Ruiz, A.; Toledo Alonso, J.R. Polymeric Nanoencapsulation of Alpha Interferon Increases Drug Bioavailability and Induces a Sustained Antiviral Response in Vivo. Mater. Sci. Eng. C 2020, 116, 111260. [Google Scholar] [CrossRef] [PubMed]
- Interferon α-2b European Pharmacopoeia (EP) Reference Standard|99210-65-8. Available online: https://www.sigmaaldrich.com/CL/es/product/sial/i0320301 (accessed on 16 May 2025).
- Di Veroli, G.Y.; Fornari, C.; Goldlust, I.; Mills, G.; Koh, S.B.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. An Automated Fitting Procedure and Software for Dose-Response Curves with Multiphasic Features. Sci. Rep. 2015, 5, 14701. [Google Scholar] [CrossRef]
- Team Medical & Scientific Website, Buchi Buchi Mini Spray Dryer B-290. Available online: https://www.tms-lab.com/product/buchi-mini-spray-dryer-b-290/ (accessed on 29 May 2025).
- Morton, D.B.; Griffiths, P.H. Guidelines on the Recognition of Pain, Distress and Discomfort in Experimental Animals and an Hypothesis for Assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Espinoza-Villavicencio, J.L.; Palacios-Espinosa, A.; Ortega-Pérez, R.; Guillén-Trujillo, A.; Manríquez-Hirales, E.; Espinoza-Villavicencio, J.L.; Palacios-Espinosa, A.; Ortega-Pérez, R.; Guillén-Trujillo, A.; Manríquez-Hirales, E. Inseminación Artificial a Tiempo Fijo y Reinseminación de Vacas Para Carne Tratadas Con y Sin Gonadotropina Coriónica Equina. Nova Sci. 2021, 13, 2021. [Google Scholar] [CrossRef]
- Fleitas-Salazar, N.; Lamazares, E.; Pedroso-Santana, S.; Kappes, T.; Pérez-Alonso, A.; Hidalgo, Á.; Altamirano, C.; Sánchez, O.; Fernández, K.; Toledo, J.R. Long-Term Release of Bioactive Interferon-Alpha from PLGA-Chitosan Microparticles: In Vitro and in Vivo Studies. Biomater. Adv. 2022, 143, 213167. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Aznawi, A.; Basyuni, M.; Hanafiah, D.; Mubaraq, A.; Leopardas, V. Bioinformatic of Binding Protein by PHYRE2 and SWISS-MODEL Software from Sonneratia Alba and Sonneratia Caseolaris. In Proceedings of the IOP Conference Series Earth and Environmental Science: 6th International Conference on Natural Resources and Technology, Medan, Indonesia, 27 August 2024. [Google Scholar] [CrossRef]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A Web Server for Automatic Structure Matching and RMSD Calculations among Identical and Similar Compounds in Protein-Ligand Docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef]
- Lin, C.C.J.; Chang, J.Y. Pathway of Oxidative Folding of Bovine α-Interferon: Predominance of Native Disulfide-Bonded Folding Intermediates. Biochemistry 2007, 46, 3925–3932. [Google Scholar] [CrossRef]
- Zheng, X.; Bo, X.; Jin, K.; He, X.; Jia, Y.; Zhou, Z.; Xu, C.; Nan, Y.; Wu, C. Porcine ISG15 Fused IFN-Λ3 as a Novel Antiviral Agent for Treating Porcine Reproductive and Respiratory Syndrome Virus Infection in Vivo. Int. J. Biol. Macromol. 2025, 287, 138242. [Google Scholar] [CrossRef]
- Wang, T.; Liu, B.; Huang, J.; Zhao, Q.; Shen, H.; Bi, T.; Liu, Z.; Dai, Y.; Sun, Q. IFN-γ-Mediated Inhibition of JAK/STAT Signaling via Nano-Scutellarin Treatment Is an Efficient Strategy for Ameliorating Liver Fibrosis. J. Transl. Med. 2025, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Contes, K.; Bility, M.T.; Tang, Q. Chasing Virus Replication and Infection: PAMP-PRR Interaction Drives Type I Interferon Production, Which in Turn Activates ISG Expression and ISGylation. Viruses 2025, 17, 528. [Google Scholar] [CrossRef]
- Dharmarathna, C.S.; Kumar, S.; Gunawardena, Y.I.N.S.; Dassanayake, R.S.; Hettiarachchi, C. AOX1 Promoter-Driven Expression of Yeast-Enhanced Green Fluorescent Protein in Pichia Pastoris. Microbe 2023, 1, 100012. [Google Scholar] [CrossRef]
- García-Suárez, J. Usos de La Levadura Pichia Pastoris En La Producción de Proteínas Recombinantes. Vaccimonitor 2021, 30, 153–163. [Google Scholar]
- Allampalli, S.S.P.; Sekhar, S.; Sivaprakasam, S. Enhanced Production of Human Interferon A2b in Glycoengineered Pichia Pastoris by Robust Control of Methanol Feeding and Implications of Various Control Strategies. Biochem. Eng. J. 2024, 201, 109152. [Google Scholar] [CrossRef]
- Naseem, M.U.; Tajti, G.; Gaspar, A.; Szanto, T.G.; Borrego, J.; Panyi, G. Optimization of Pichia Pastoris Expression System for High-Level Production of Margatoxin. Front. Pharmacol. 2021, 12, 733610. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, F.; Peng, Y.; Chi, Z. Optimization for High-Level Expression of the Pichia Guilliermondii Recombinant Inulinase in Pichia Pastoris and Characterization of the Recombinant Inulinase. Process Biochem. 2009, 44, 1335–1339. [Google Scholar] [CrossRef]
- Dietzsch, C.; Spadiut, O.; Herwig, C. A Fast Approach to Determine a Fed Batch Feeding Profile for Recombinant Pichia Pastoris Strains. Microb. Cell Fact. 2011, 10, 85. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Li, Z.; Wang, F. Combined Strategies for Improving the Production of Recombinant Rhizopus Oryzae Lipase in Pichia Pastoris. Bioresources 2013, 8, 2867–2880. [Google Scholar] [CrossRef]
- He, H.; Zhai, C.; Mei, M.; Rao, Y.; Liu, Y.; Wang, F.; Ma, L.; Jiang, Z.; Zhang, G.; Yi, L. Functional Expression of Porcine Interferon-α Using a Combinational Strategy in Pichia Pastoris GS115. Enzyme Microb. Technol. 2019, 122, 55–63. [Google Scholar] [CrossRef]
- Wang, S.; Dai, H.; Tang, Q.; Yu, Y.; Xie, Y.; Wang, T.; Huang, Y. Engineering Protein Translocation Pathway to Improve Recombinant Proteins in Pichia Pastoris. Curr. Res. Biotechnol. 2024, 7, 100182. [Google Scholar] [CrossRef]
- Geier, E.A.; Simon, E.H. Growth of Wild-Type and Interferon-Sensitive Mengovirus in Tk- L Cells. J. Interferon Res. 1985, 5, 297–304. [Google Scholar] [CrossRef]
- Shaw, A.E.; Hughes, J.; Gu, Q.; Behdenna, A.; Singer, J.B.; Dennis, T.; Orton, R.J.; Varela, M.; Gifford, R.J.; Wilson, S.J.; et al. Fundamental Properties of the Mammalian Innate Immune System Revealed by Multispecies Comparison of Type I Interferon Responses. PLoS Biol. 2017, 15, e2004086. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, S.; Tsochantaridis, I.; Tokamani, M.; Kokkinopliti, K.D.; Tsomakidis, P.; Giannakakis, A.; Galanis, A.; Pappa, A.; Sandaltzopoulos, R. Expression and Purification of Human Interferon Alpha 2a (IFNα2a) in the Methylotrophic Yeast Pichia Pastoris. Protein Expr. Purif. 2023, 211, 106339. [Google Scholar] [CrossRef]
- Ingram, Z.; Patkar, A.; Oh, D.; Authors Zoe Ingram, A.; Zhang, K.K.; Chung, C.; Lin-Cereghino, J.; Lin-Cereghino, G.P. Overcoming Obstacles in Protein Expression in the Yeast Pichia Pastoris: Interviews of Leaders in the Pichia Field. Pac. J. Health 2021, 4, 2. [Google Scholar] [CrossRef]
- Li, J.; Roberts, R.M. Structure-Function Relationships in the Interferon-Tau (IFN-Tau). Changes in Receptor Binding and in Antiviral and Antiproliferative Activities Resulting from Site-Directed Mutagenesis Performed near the Carboxyl Terminus. J. Biol. Chem. 1994, 269, 24826–24833. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.; Bogunovic, D. A Non-Canonical Function of OAS1 to Combat Viral and Bacterial Infections. Immunity 2024, 57, 1721–1723. [Google Scholar] [CrossRef]
- Harioudh, M.K.; Perez, J.; Chong, Z.; Nair, S.; So, L.; McCormick, K.D.; Ghosh, A.; Shao, L.; Srivastava, R.; Soveg, F.; et al. Oligoadenylate Synthetase 1 Displays Dual Antiviral Mechanisms in Driving Translational Shutdown and Protecting Interferon Production. Immunity 2024, 57, 446–461.e7. [Google Scholar] [CrossRef]
- Maskouni, E.J.; Abbasi, S.; Mousavi, E.; Najafimemar, Z.; Arabzadeh, A.M.; Farrokhnia, M.; Ebrahimi, S. Expression Level of Interferon-Stimulated Genes PKR, OAS1, MX1, and ISG15 in Peripheral Blood Mononuclear Cells of COVID-19 Patients: A Retrospective Study. J. Acute Dis. 2024, 13, 111–115. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Scherbik, S.V.; Brinton, M.A. Activation of Oas1a Gene Expression by Type I IFN Requires Both STAT1 and STAT2 While Only STAT2 Is Required for Oas1b Activation. Virology 2012, 425, 71–81. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A Review on the Encapsulation of Bioactive Components Using Spray-Drying and Freeze-Drying Techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Tangarife, D.P.C.; Arias, L.P.P.; Zapata, A.M.O. Aspectos Tecnológicos de La Microencapsulación de Compuestos Bioactivos En Alimentos Mediante Secado Por Aspersión. Cienc. Y Tecnol. Agropecu. 2021, 22, 1–21. [Google Scholar] [CrossRef]
- de la Cruz Pech-Canul, A.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R.L. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings 2020, 10, 197. [Google Scholar] [CrossRef]
- Hernández-Coronado, C.; Rosales-Torres, A.; Vázquez-Lopez, S.; Guzmán-Sánchez, A. Sincronización del estro y ovulación en hembras bovinas de razas cárnicas. Bases endocrinas y protocolos usados. Abanico Vet. 2023, 13, 1–37. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Fan, Z.; Cheng, P.; Zhang, P.; Zhang, G.; Han, J. Rheological Insight of Polysaccharide/Protein Based Hydrogels in Recent Food and Biomedical Fields: A Review. Int. J. Biol. Macromol. 2022, 222, 1642–1664. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Ucar, O. Update on Factors Affecting the Success of Intrauterine Insemination in Ruminants. In Proceedings of the SMSLF 2021: 5th International Conference on Sustainable Management of Smart Livestock’s Farming 2021 (organised by University of Alexandria, EGYPT, Virtual-Invited Presentation), Alexandria, Egypt, 3–5 November 2021. [Google Scholar]
- Lanctôt, S.; Fustier, P.; Taherian, A.R.; Bisakowski, B.; Zhao, X.; Lacasse, P. Effect of Intramammary Infusion of Chitosan Hydrogels at Drying-off on Bovine Mammary Gland Involution. J. Dairy. Sci. 2017, 100, 2269–2281. [Google Scholar] [CrossRef]




| Strategy | 0 h | 24 h | 48 h |
|---|---|---|---|
| 1 | 0.3% | 0.4% | 0.5% |
| 2 | 0.5% | 0.75% | 1% |
| 3 | 0.5% | 1% | 1.5% |
| 4 | 0.5% | 1% | 1% |
| 5 | 1% | 1% | 1% |
| Strategy | 0 h | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|---|
| 3 | 0.5% | 1% | 1.5% | 1.5% | 1.5% |
| 4 | 0.5% | 1% | 1% | 1% | 1% |
| Gene Name-Gene ID (NCBI Gene) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Tm | Product Size (bp) |
|---|---|---|---|---|
| Bos taurus 2′-5′-oligoadenylate synthetase 1 (OAS1) mRNA-NM_001040606.1 | AAATAGCTGGGAGCGGCTTG | CTGTGTTCTTGGGGCGACAC | 60 | 114 |
| Bos taurus 2′-5′-oligoadenylate synthetase 2 (OAS2), mRNA-NM_001024557.1 | GCCTTCAATGCTCTGGGCTT | CAGGCCTGGCTTTCACCATA | 58 | 90 |
| Bos taurus eukaryotic translation initiation factor 2 alpha kinase 2 (PKR), mRNA-NM_178109.3 | TGGAGACACGGAAGAGCTGT | ATGTCACGGACGATTTCCGC | 56 | 122 |
| Bos taurus beta-actin (ACTB) mRNA-AY141970.1 | GCCCATCTATGAGGGGTACG | ATGTCACGGACGATTTCCGC | 60 | 147 |
| Inlet Temperature (°C) | Inlet Temperature (°C) | Aspiration (%) | Feed Flow Rate (mL/min) | Air Flow Rate (L/h) |
|---|---|---|---|---|
| 100 | 63 | 95 | 6.0 | 536 |
| 120 | 76 | 95 | 6.0 | 536 |
| 140 | 88 | 95 | 6.0 | 536 |
| Template | Seq Identity (%) | Found by | Method | Seq Similarity | Coverage | Description |
|---|---|---|---|---|---|---|
| A0A7R8C394.1.A | 100.00 | AFDB | AlphaFold v2 | 0.61 | 0.98 | Interferon 1BE10 |
| 1b5l.1.A | 68.82 | BLAST | X-ray | 0.50 | 0.87 | Interferon Tau |
| 1b5l.1.A | 68.64 | HHblits | X-ray | 0.50 | 0.87 | Interferon tau |
| 3oq3.1.A | 53.61 | HHblits | X-ray | 0.45 | 0.85 | Interferon alpha-5 |
| 3se4.1.B | 66.08 | HHblits | X-ray | 0.50 | 0.88 | Interferon omega-1 |
| 3se4.1.B | 66.07 | BLAST | X-ray | 0.50 | 0.86 | Interferon omega-1 |
| 3oq3.1.A | 54.94 | BLAST | X-ray | 0.45 | 0.83 | Interferon alpha-5 |
| 2kz1.1.A | 56.10 | HHblits | NMR | 0.46 | 0.84 | Interferon alpha-2 |
| 7e0e.1.A | 50.30 | HHblits | X-ray | 0.43 | 0.86 | Interferon alpha-2 |
| 7e0e.1.A | 51.85 | BLAST | X-ray | 0.44 | 0.83 | Interferon alpha-2 |
| 2lag.1.B | 56.10 | HHblits | NMR | 0.46 | 0.84 | Interferon alpha-2 |
| 4z5r.1.A | 56.10 | HHblits | X-ray | 0.46 | 0.84 | Interferon alpha-2 |
| 1itf.1.A | 56.10 | HHblits | NMR | 0.46 | 0.84 | Interferon Alpha-2a |
| 2hym.1.B | 56.10 | HHblits | NMR | 0.46 | 0.84 | Interferon alpha-2 |
| 3ux9.1.A | 57.67 | BLAST | X-ray | 0.46 | 0.84 | Interferon alpha-1/13 |
| 1au1.1.A | 34.15 | HHblits | X-ray | 0.36 | 0.84 | Interferon-Beta |
| 3ux9.1.A | 56.63 | HHblits | X-ray | 0.45 | 0.85 | Interferon alpha-1/13 |
| 3se3.1.B | 54.55 | HHblits | X-ray | 0.45 | 0.85 | Interferon alpha 2b |
| 3s9d.1.A | 54.55 | HHblits | X-ray | 0.45 | 0.85 | Interferon alpha-2 |
| 1au1.1.B | 34.15 | HHblits | X-ray | 0.36 | 0.84 | Interferon-Beta |
| 2lms.1.A | 55.76 | HHblits | NMR | 0.45 | 0.85 | Interferon alpha-2 |
| 6jhd.1.A | 56.36 | HHblits | NMR | 0.45 | 0.85 | Interferon alpha-8 |
| 6jhd.1.A | 57.06 | BLAST | NMR | 0.45 | 0.84 | Interferon alpha-8 |
| 1au1.1.A | 37.50 | BLAST | X-ray | 0.39 | 0.70 | Interferon-beta |
| 1au1.1.B | 37.50 | BLAST | X-ray | 0.39 | 0.70 | Interferon-beta |
| Poses | IFN-τ-α/β-Receptor-1 | IFN-τ-α/β-Receptor-2 | ||
|---|---|---|---|---|
| Docking Score | Confidence Score | Docking Score | Confidence Score | |
| Pose-1 | −259.84 | 0.9000 | −271.82 | 0.9196 |
| Pose-2 | −249.51 | 0.8798 | −270.79 | 0.9180 |
| Pose-3 | −240.63 | 0.8597 | −266.38 | 0.9111 |
| Pose-4 | −238.00 | 0.8532 | −256.82 | 0.8944 |
| Pose-5 | −236.13 | 0.8485 | −251.72 | 0.8844 |
| Sample | Mass (g) | Yield (%) |
|---|---|---|
| 100 °C | 0.96 | 56.1 |
| 120 °C | 1.14 | 66.1 |
| 140 °C | 1.16 | 67.8 |
| Sample | Mass (g) | Yield (%) |
|---|---|---|
| 100 °C | 0.96 | 56.1 |
| 120 °C | 1.14 | 66.1 |
| 140 °C | 1.16 | 67.8 |
| Hematocrit | Total Leukocytes | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Day 0 | Day 3 | Day 7 | Day 14 | Day 0 | Day 3 | Day 7 | Day 14 |
| Control Group | 10.16 ± 0.46 a | 10.48 ± 0.83 a | 10.47 ± 0.95 a | 10.66 ± 0.95 a | 6876 ± 1443 a | 8560 ± 2211 a | 10,148 ± 4071 a | 8926 ± 2749 a |
| Treated Group | 9.37 ± 1.08 a | 10.02 ± 1.35 a | 10.23 ± 1.36 a | 9.84 ± 1.18 a | 6374 ± 2834 a | 7440 ± 2721 a | 8306 ± 2513 a | 7082 ± 2906 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamazares, E.; Vásquez, A.; Gancino, K.; Sandoval, F.; Yáñez-Torres, J.; Gutierrez-Reinoso, M.A.; García-Herreros, M.; Gädicke, P.; Cabezas, I.; Hugues, F.; et al. A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle. Biomolecules 2025, 15, 1009. https://doi.org/10.3390/biom15071009
Lamazares E, Vásquez A, Gancino K, Sandoval F, Yáñez-Torres J, Gutierrez-Reinoso MA, García-Herreros M, Gädicke P, Cabezas I, Hugues F, et al. A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle. Biomolecules. 2025; 15(7):1009. https://doi.org/10.3390/biom15071009
Chicago/Turabian StyleLamazares, Emilio, Aleikar Vásquez, Kelly Gancino, Felipe Sandoval, Javiera Yáñez-Torres, Miguel A. Gutierrez-Reinoso, Manuel García-Herreros, Paula Gädicke, Ignacio Cabezas, Florence Hugues, and et al. 2025. "A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle" Biomolecules 15, no. 7: 1009. https://doi.org/10.3390/biom15071009
APA StyleLamazares, E., Vásquez, A., Gancino, K., Sandoval, F., Yáñez-Torres, J., Gutierrez-Reinoso, M. A., García-Herreros, M., Gädicke, P., Cabezas, I., Hugues, F., Ramos, T. I., Camacho, F., Mena-Ulecia, K., & Toledo, J. R. (2025). A Novel Microencapsulated Bovine Recombinant Interferon Tau Formulation for Luteolysis Modulation in Cattle. Biomolecules, 15(7), 1009. https://doi.org/10.3390/biom15071009