1. Introduction
The human endothelium exerts a pivotal role in cardiovascular health and homeostasis. Indeed, it consists of a monolayer of endothelial cells (ECs), which line the internal surface of blood vessels and ensure vascular integrity, anti-platelet aggregation, and fibrinolytic properties [
1]. Furthermore, ECs regulate vascular hemostasis and alleviate the inflammatory process. Most of these functions are mediated by nitric oxide (NO), which is an endogenous vasodilator [
1]. Oxidative stress, inflammation, dyslipidemia, alterations in laminar blood flow, and shear stress are triggering factors in EC alterations leading to endothelial dysfunction [
2]. This condition is characterized by a functional impairment of ECs and represents a critical step in atherosclerosis pathogenesis. The loss of NO promotes low-density lipoprotein (LDL) oxidation and internalization by smooth muscle cells (SMCs) and macrophages in the well-known foam cells, which form the initial stage of atherosclerotic plaque [
3,
4,
5]. The inflammatory process exacerbation, vascular remodeling, and further complications, like plaque calcification, aggravate the atherosclerotic lesion, leading to high rupture risk. Considering the high incidence and mortality rate due to thromboembolic events, unveiling the main cellular and molecular mechanisms involved in atherosclerosis is crucial for novel and effective therapeutic strategies. Given the complexity of atherosclerosis, it is essential to develop advanced in vitro models that accurately replicate endothelial dysfunction to study disease mechanisms and explore novel therapeutic strategies.
Traditional in vitro studies rely on static 2D cell cultures, where ECs are grown as monolayers on plastic substrates [
6,
7]. However, these models present intrinsic limitations, as they fail to replicate the complex three-dimensional (3D) microenvironment of native tissues [
8,
9]. To address these limitations, various 3D models have been developed, including spheroids, microfluidic “vessel-on-a-chip” platforms [
10], and engineered tissues, which better mimic cell–cell and cell–extracellular matrix (ECM) interactions [
11,
12,
13]. Among these, hydrogel-based 3D models have emerged as promising platforms due to their ability to mimic the biochemical and mechanical properties of the ECM [
14]. Gelatin methacrylate (GelMa), a chemically modified gelatin, is widely used in tissue engineering applications due to its biocompatibility, tunable mechanical properties, and ability to support cell adhesion and proliferation [
15]. By adjusting the degree of methacrylation, key properties such as porosity, stiffness, and enzymatic degradability can be fine-tuned to meet specific target-tissue requirements [
16,
17]. Recent studies have leveraged these features to develop GelMa-based atherosclerosis models, such as two-layered GelMa hydrogel incorporating endothelial cells (ECs) and smooth muscle cells (SMCs), which has been used to investigate the role of zinc ions in atherosclerosis therapy [
18].
Building on the advantages of hydrogel-based 3D models, electrospinning technology provides an effective method to fabricate scaffolds that resemble the fibrous architecture of the ECM [
19,
20]. For instance, Zhao et al. developed a PCL/fibrin vascular scaffold using electrospinning, which exhibited good biomechanical properties, cell compatibility, and degradation characteristics. In vivo studies in abdominal aorta models showed that the PCL/fibrin grafts mimicked native artery function, with improved ECM deposition, endothelialization, and reduced calcification compared to PCL alone [
21]. In this study, we selected poly(L-lactide-co-ε-caprolactone) (PLCL) as the base polymer due to its biodegradability, mechanical resilience, and elastomeric properties, which are crucial for vascular tissue engineering applications [
22]. PLCL exhibits tunable mechanical properties that can be modulated by altering the copolymer ratio, which allows for the replication of the native vascular tissues [
23]. This mechanoelastic behavior supports endothelial cell proliferation under cyclic strain, which is relevant for mimicking the dynamic environment of blood vessels [
24]. Furthermore, PLCL has been successfully used in various soft tissue regeneration applications, such as blood vessels, tendons, skin, and cardiac tissues, where flexibility is essential to withstand high blood pressure [
25]. To enhance bioactivity, the electrospun PLCL mat was functionalized with type I collagen, a major ECM component that provides cell adhesion sites and biochemical cues essential for endothelial cell attachment and function [
26].
This study aimed to reproduce an in vitro biomimetic model of the human tunica intima to investigate the endothelial dysfunction under atherosclerotic conditions. To achieve this, a multilayered 3D composite scaffold was engineered by integrating electrospun PLCL nanofibers with a GelMa hydrogel core, creating a physiologically relevant microenvironment that replicates the structural, biomechanical, and biochemical features of native vascular tissue. To mimic endothelial dysfunction observed in early-stage atherosclerosis, human umbilical vein endothelial cells (HUVECs) were seeded onto the composite scaffold and exposed to oxidized low-density lipoproteins (ox-LDLs) and shear stress variations [
27]. Shear stress, a key biomechanical factor regulating endothelial homeostasis, plays a pivotal role in atherosclerosis progression. Low and oscillatory shear stress patterns have been associated with pro-inflammatory endothelial phenotypes, favoring lipid accumulation and plaque formation, while high laminar shear stress exerts protective effects on vascular function [
28,
29]. The multilayered electrospun PLCL-GelMa-PLCL composite scaffold represents an innovative approach for vascular tissue engineering, providing a biomimetic platform that closely resembles the human tunica intima. This model offers a promising tool for in vitro disease modeling, enabling the study of endothelial dysfunction under physiologically relevant conditions.
2. Materials and Methods
2.1. Materials
Gelatin from porcine skin Type A, carbonate–bicarbonate (CB) buffer, methacrylic anhydride (MAA) (94%), and dialysis cellulose membrane (12–14 kDa cutoff avg. flat width 25 mm) (94%) were supplied by Sigma Aldrich (St. Louis, MO, USA). Poly(L-lactide-co-ε-caprolactone) copolymer (PLCL, 70/30 LA/CL molar ratio) (PURASORB® PLC 7015, inherent viscosity midpoint of 1.5 dL/g) was purchased from Corbion (Amsterdam, The Netherlands). Dichloromethane (DCM) and dimethylformamide (DMF) were supplied by Sigma Aldrich and used without further purification. Type I collagen solution from calf skin (acetic acid 0.25%) was obtained from Sigma Aldrich (St. Louis, MO, USA).
For the cell culture, human umbilical vein endothelial cells (HUVECs), Endothelial Cell Growth Medium with SupplementMix (EGM), and Detachkits were purchased from Promocell (Heidelberg, Germany). Biolaminin 411 for simulating the basal membrane of the intima tunica was supplied by Voden Medical Instruments (Meda, MB, Italy). The human oxidized low-density lipoprotein (ox-LDL) employed for inducing endothelial damage was supplied by Kalen Biomedical (Montgomery Village, MD, USA). CD31 (Dako, Glostrup, Denmark), F-actin and Alexa-Fluor 488 antibodies, Prolong Gold Antifade reagent with DAPI, and PrestoBlue™ cell viability reagent were obtained from ThermoFisher Scientific (Waltham, MA, USA). The Total Nitric Oxide and Nitrate/Nitrite Parameter Assay kit was purchased from Bio-Techne (Minneapolis, MN, USA). Hexamethyldisilazane was purchased from Merck (Darmstadt, Germany).
2.2. Synthesis of Gelatin Methacrylate (GelMa) and Hydrogel Preparation
GelMa was synthesized through a methacrylation reaction between the hydroxyl and primary amino groups of gelatin chains and MAA, following an optimized procedure [
30,
31]. The reaction was conducted under carefully controlled conditions, including alkaline pH, temperature, reagent concentration, and stirring. Specifically, a 10% (
w/
v) solution of gelatin type A (20 g) was prepared in 200 mL of 0.25 M CB buffer. The gelatin was dissolved with vigorous stirring for 1 h in a 500 mL two-neck round-bottom flask immersed in an oil bath and maintained at 50 °C. Methacrylation was initiated by adding MAA at a ratio of 0.1 mL per gram of gelatin while maintaining a temperature of 50 °C and stirring at 500 rpm. The reaction was carried out for 1 h, during which periodic additions of 5 M NaOH were made to maintain the pH at 9, as methacrylic acid, a byproduct of the functionalization, was released. The reaction was quenched by adding a few drops of 37% (
w/
v) HCl to adjust the pH to 7.4. Residual byproducts were removed by purifying the solution through dialysis with a 12–14 kDa molecular weight cutoff membrane for 5 days. The purified GelMa was subsequently lyophilized, yielding a white porous solid, which was stored at 4 °C for further use.
The GelMa hydrogel was prepared using a sterile 5 mL disposable syringe fitted with a female/female Luer lock adapter, which facilitated the connection to a second syringe for homogenization, resulting in a 5% (w/v) solution. After dissolution, the photoinitiator Irgacure 2959 was incorporated into the GelMa solution at a concentration of 0.2% (w/v). Photocrosslinking was achieved by exposing the solution to UV light (365 nm) with an intensity of 12 mW/cm2 for 120 s.
The successful synthesis of GelMa was confirmed through Fourier transform infrared spectroscopy (FT-IR) analyses. FT-IR was carried out using a Spectrum Two instrument equipped with an attenuated total reflectance-Fourier transform infrared (ATR) accessory (Perkin-Elmer, diamond crystal, Milan, Italy) on both the gelatin and GelMa. All spectra were registered between 800 cm
−1 and 4000 cm
−1, with a resolution of 4 cm
−1, an accumulation of 16 scans, and a step size of 1 cm
−1. A methacrylation degree (MD) was calculated by
1H-NMR spectrometry, following the method reported in the literature [
32]. Analyses were conducted on a Bruker Advance III 400 spectrometer using 20 mg of GelMA dissolved in 0.70 mL of D
2O. The new signals observed at δ = 5.4 and 5.7 ppm, corresponding to the acrylic protons of the methacrylic groups in the methacrylic anhydride structure, validated the synthesis. The methacrylation degree was calculated based on the decrease in intensity of the arginine methyl group signal at δ = 2.85 ppm using the following Equation (1):
2.3. Fabrication of Polymeric Nanofibrous Mats
A custom-built electrospinning apparatus was employed, consisting of a high-voltage power supply (Spellman SL 50 P 10/CE/230), a syringe pump (KD Scientific 200 series), and a glass syringe connected to a stainless steel blunt-ended needle. The polymer solution was electrospun onto a rotating metallic drum collector (length = 120 mm, diameter = 50 mm) designed to produce scaffolds composed of uniaxially aligned nanofibers. Electrospinning was performed under controlled conditions at room temperature (RT) and a relative humidity of 50%. Polymer solutions were prepared by dissolving PLCL in a solvent mixture of DCM and N,N-DMF at a 65:35
v/
v ratio, with a polymer concentration of 20%
w/
v. The polymer solution was delivered at a constant flow rate (1.2 mL/h) through the needle, positioned 20 cm away from the collector. A voltage of 20 kV was applied to initiate fiber formation. Aligned nanofibers were produced by setting the collector to rotate at a linear speed of 6000 rpm. After 120 min of electrospinning, a mat with a thickness of 40–50 µm and dimensions of 15 × 8 cm was obtained. This procedure was optimized and described in detail in a previous study [
13], which was used as a basis for this work.
2.4. Rheology of GelMa Hydrogels
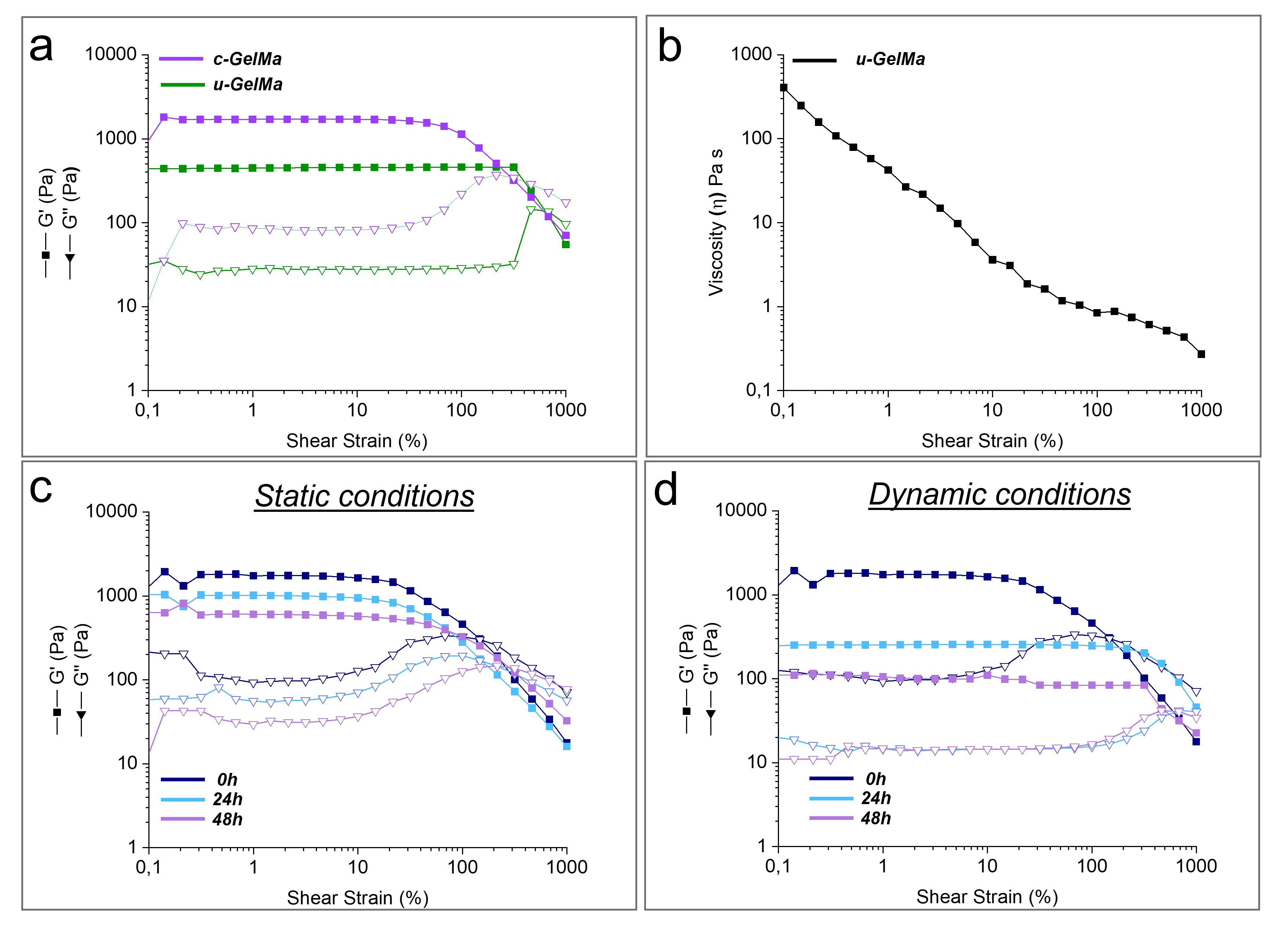
Rheological analyses on uncrosslinked (u-GelMa) and crosslinked (c-GelMa) GelMa hydrogels were conducted using an MCR 102 rheometer (Anton Paar) in a parallel-plate configuration with a PP-25 geometry (plate diameter: 25 mm, gap: 0.3 mm). Once the sample was in contact with the upper plate, the excess material was carefully removed with a spatula, and the surrounding trap was filled with distilled water to mitigate evaporation during the measurement. Amplitude sweep tests were conducted to investigate the viscoelastic properties of both uncrosslinked and crosslinked samples, with the crosslinked samples incubated in phosphate-buffered saline (PBS) at 37 °C to simulate physiological conditions. Rheological measurements were performed after specific time intervals, including a few minutes post-immersion and after 24, 48, and 72 h of incubation. During the tests, the hydrogel was subjected to a strain range from 0.01% to 1000%, maintaining a constant angular frequency at 1 rad/s to evaluate the storage modulus (G′) and the loss modulus (G′′) as a function of the applied strain (%). Flow curve tests were conducted to evaluate the processability and manufacturability of the u-GelMa hydrogel. The tests were performed in the rotational mode by applying a shear rate ranging from 0.1 s−1 to 1000 s−1 to examine the material’s viscosity as a function of both shear rates.
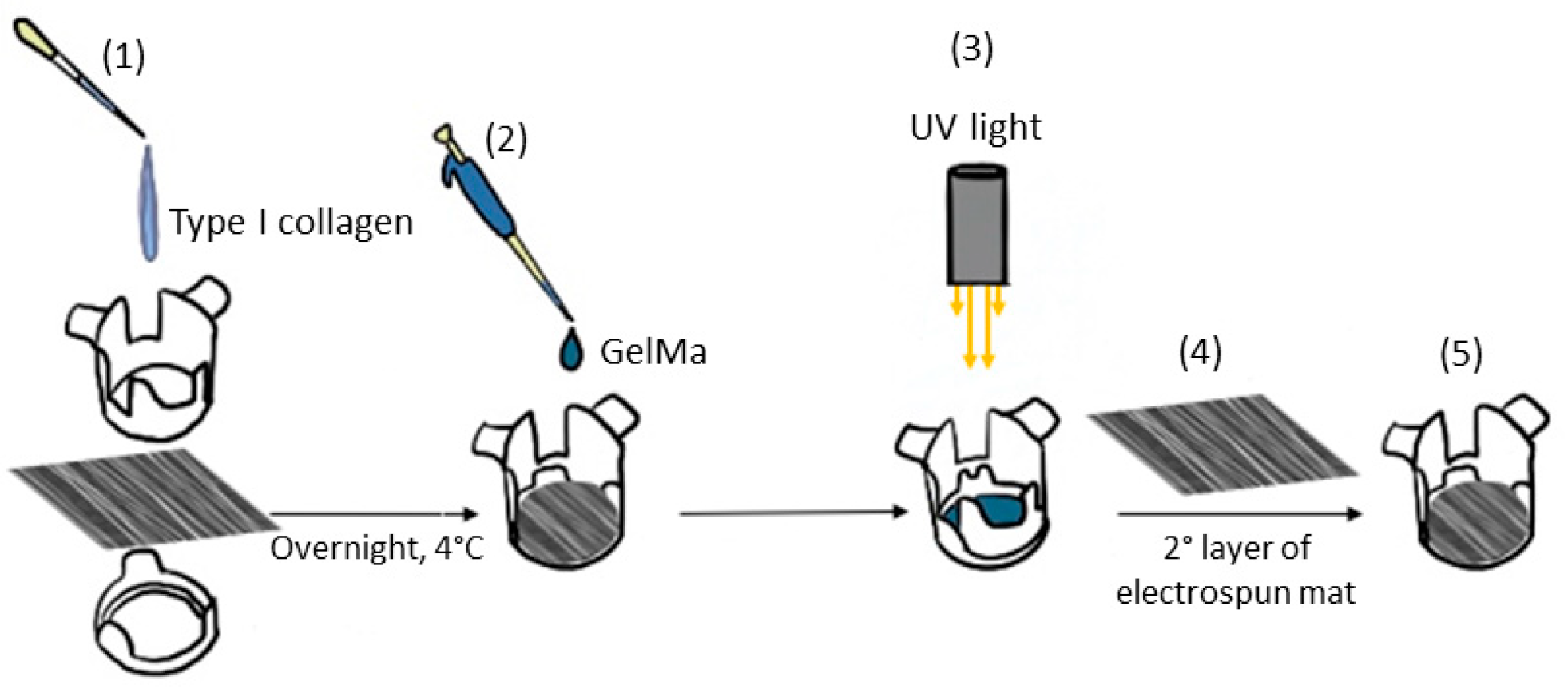
2.5. Composite Sandwich Assembling
Electrospun mats were prepared for cell seeding by cutting them into appropriately sized pieces and assembling them using CellCrown™ supports (Scaffdex, Tampere, Finland). The mats were sterilized with ethanol following a previously established protocol [
33] and subsequently coated with 0.01% type I collagen solution prepared by diluting collagen from calf skin 10-fold with sterile water. The collagen coating was applied overnight at 4 °C, after which the solution was removed, and the scaffolds were washed with PBS. After sterilization and functionalization, 500 µL of u-GelMa hydrogel was dispensed onto the mat surface and photocrosslinked within the fibers, following a previously described protocol. A second electrospun mat was then layered on top of the GelMa-coated mat, forming a composite scaffold with a sandwich-like structure (
Figure 1). Before the cell seeding, the upper mat of each sandwich-like structure was covered with biolaminin using a working concentration of 1 µg/cm
2 for 2 h in an incubator at 37 °C and 5% CO
2.
2.6. Dynamic Conditions with the LiveBox IVTech Bioreactor
The LiveBox bioreactor system (IVTech, Massarosa, Italy) was employed to facilitate dynamic, physiologically relevant conditions for the 3D culture of cells. The system features modular components, including a microfluidic system with hosting chambers and microtubing, allowing for the precise control of flow rates, shear stress, and media composition. This advanced device is specifically designed for interconnected dynamic cell cultures, incorporating a dual-chamber system (upper and lower chambers) with flow inlets and outlets and a holder for a porous PET membrane; then, the composite scaffolds were placed inside the hosting chambers. The bioreactor was connected to a programmable peristaltic pump, ensuring stable laminar flow at 50 µL/min and 500 µL/min flow rates, and maintained at 37 °C in a humidified environment with 5% CO2 for 24 h.
2.7. Cell Culture and Treatments
HUVECs were cultured in complete EGM and expanded until confluence, changing the medium every 2–3 days. For the static experiments, 2 × 10
5 cells were seeded on each sandwich-like scaffold and incubated at 37 °C and 5% CO
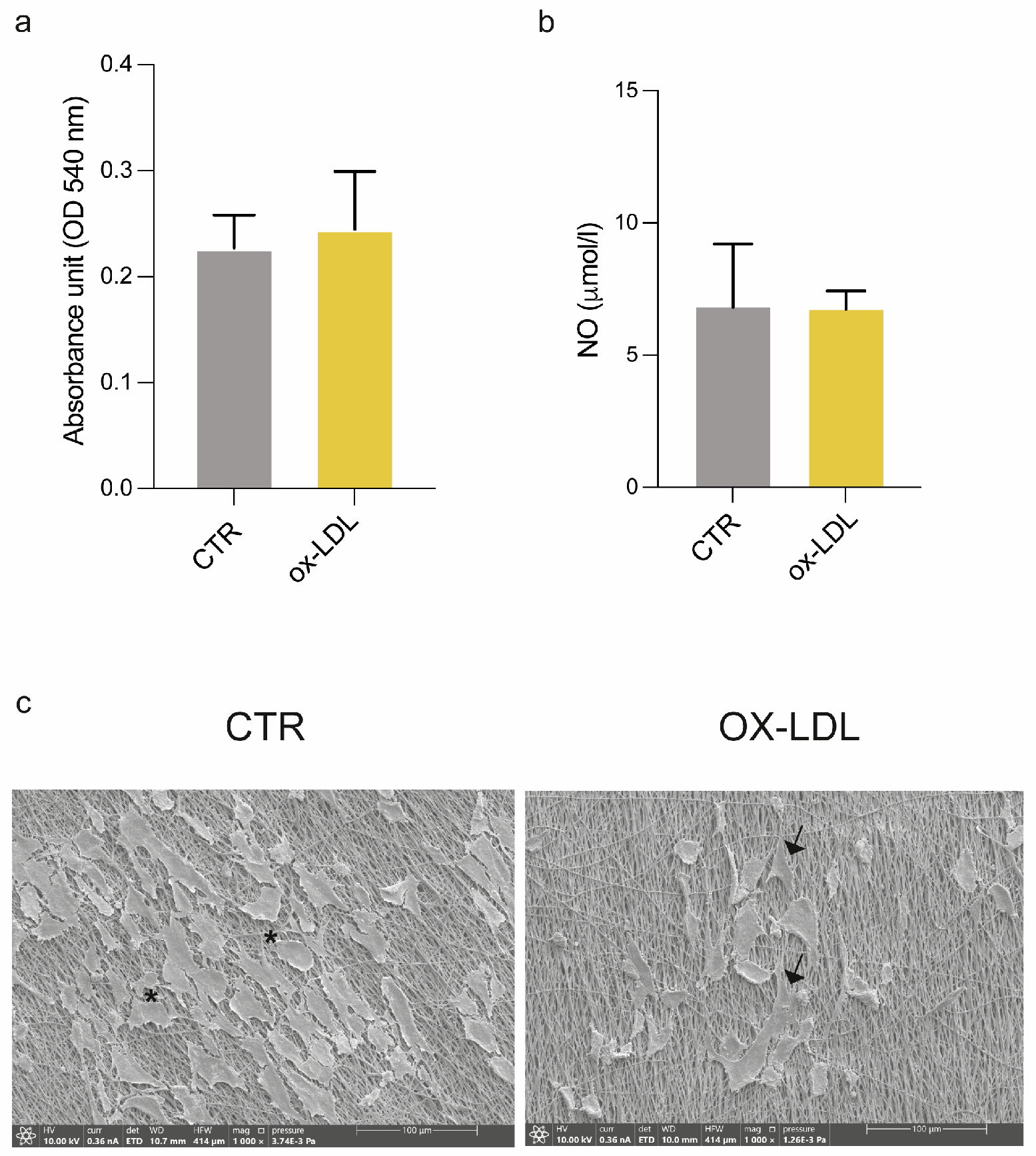
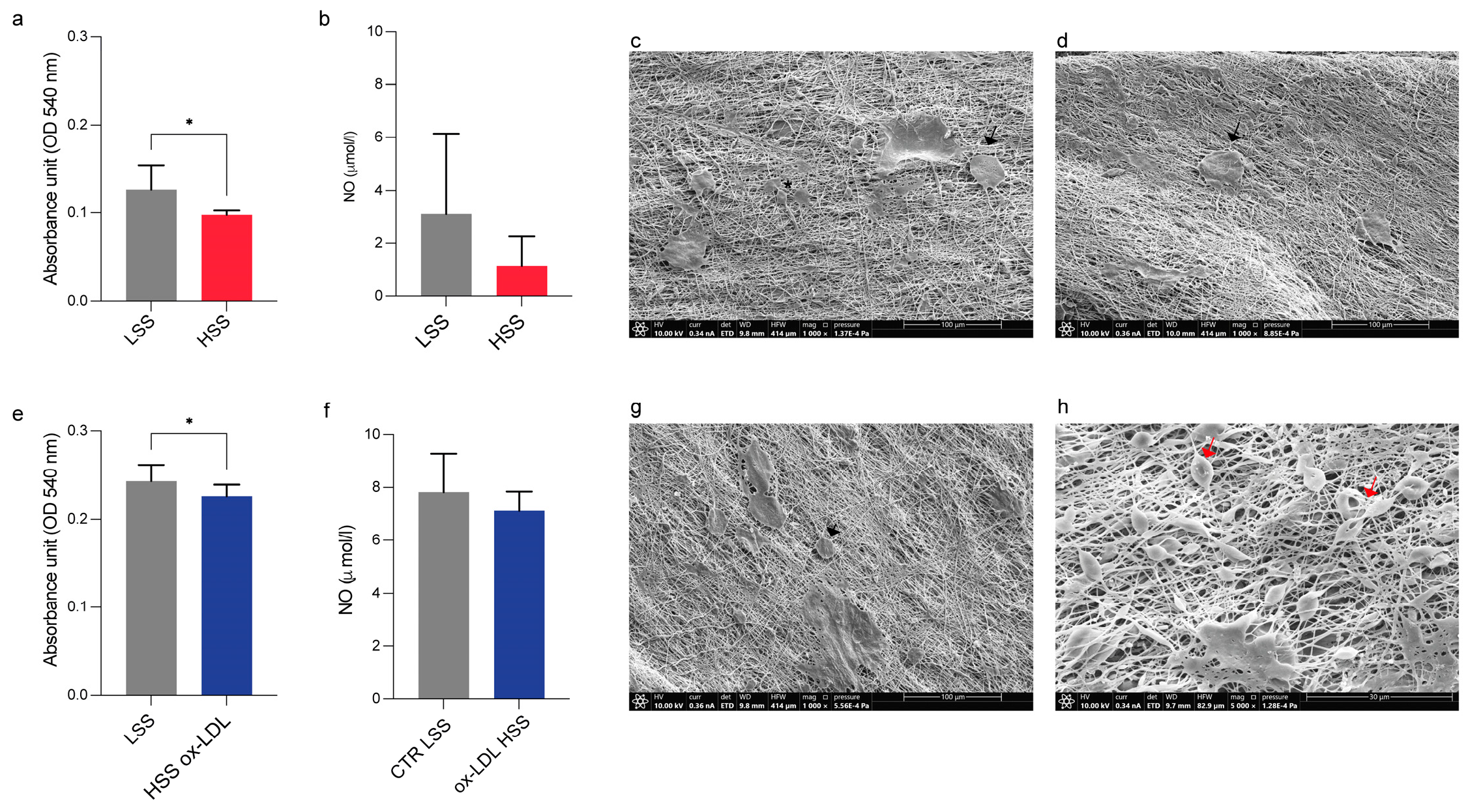
2 overnight to allow for cell adhesion. The following day, we induced the endothelial damage by treating HUVECs with 100 µg/mL of human ox-LDL added to complete EGM for 24 h (ox-LDL); the control cells were cultured in EGM without ox-LDL (CTR). For the dynamic experiments, 3.5 × 10
5 cells were seeded on scaffolds and left to adhere overnight in an incubator. After 24 h, scaffolds were placed in the bioreactor chambers according to the following experimental scheme: low shear stress (LSS) at a 50 µL/min flow rate and high shear stress (HSS) at a 500 µL/min flow rate; HSS with/without ox-LDL (
Figure 2). At the end of each experimental condition, the samples were processed for cellular and ultrastructural analysis, while the supernatants were collected and stored at −80 °C for an ELISA analysis.
2.8. Cell Viability
The HUVEC viability under culture on each sandwich-like scaffold in static and dynamic conditions was assessed using a commercial Presto Blue assay, in accordance with the manufacturer’s datasheet. Briefly, the medium of each scaffold was exchanged with a solution composed of fresh EGM with 10% of Presto Blue and incubated at 37 °C for 4 h. Then, 100 μL taken from each CTR and ox-LDL sandwich-like scaffold cultured in static and dynamic conditions was dispensed in a 96-well plate in triplicate, measuring the absorbance at 490 nm through a Multiskan SkyHigh (ThermoFisher Scientific) microplate reader.
2.9. Immunofluorescence
The endothelial and cytoskeleton molecule expression was evaluated using an immunofluorescence assay. Scaffolds cultured in static conditions were fixed with a solution of 2% paraformaldehyde in PBS with or without 1% Triton X-100 for 4 min at RT. Then, the samples were blocked with a solution of 1% bovine serum albumin (BSA) in PBS for 30 min at RT and subsequently labeled with CD31 (1:80, Dako, Glostrup, Denmark) primary antibody for 1 h, incubated at 37 °C, and Alexa Fluor 488 Phalloidin (1:500; ThermoFisher Scientific) fluorescent conjugated antibody for 30 min at RT. After several washings with PBS, scaffolds stained with CD31 were labeled with the Alexa Fluor 488 (1:250, ThermoFisher Scientific) secondary antibody for 1 h at 37 °C; all incubations were performed in a wet chamber in the dark, and all antibodies were diluted in 1% PBS/BSA. Then, the samples were rinsed and counterstained with Prolong Gold Antifade reagent with DAPI before the observation. The scaffolds were examined in a spectral confocal microscope (Nikon A1R mod. Ti2 Eclipse), acquiring digital images.
2.10. ELISA Assay to Determine the NO Levels
Nitric oxide (NO) levels were determined on culture media collected from HUVEC sandwich-like scaffolds in static and dynamic conditions, as summarized in
Figure 2. Cell culture supernatants were centrifuged at 1500 rpm for 10 min to remove particulates and debris.
The NO concentration was determined using a Total Nitric Oxide and Nitrate/Nitrite Assay, according to the manufacturer’s instructions. The optical density (OD) of each sample was determined using a Multiskan SkyHigh (ThermoFisher Scientific) microplate reader set at 540 nm with a wavelength correction at 690 nm. A standard curve was obtained from serial dilutions of NO standards, according to the manufacturer’s instructions.
2.11. Scanning Electron Microscopy
A scanning electron microscopy (SEM) analysis was carried out by using a desktop SEM (INCAx sight, Oxford Instrument, model: 7060; Abingdon, UK) set at a voltage of 20 kV on the samples fixed with a conducting bi-adhesive tape on an aluminum stub and coated with gold (by S150A Sputter Coater, Edwards, Burgess Hill, UK). The images were analyzed with the Image J software (version 1.53k, National Institutes of Health, USA), and the porosity was determined by measuring about 300 pores. The results are presented as the average pore sizes ± standard deviation.
SEM was also used to examine the HUVEC cells cultured on sandwich-like scaffolds in static and dynamic conditions with/without exposure to ox-LDL. After washing with PBS, the samples were fixed in 2.5% buffered glutaraldehyde overnight at 4 °C and subsequently post-fixed in 1% osmium tetroxide for 15 min. Then, the samples were washed in distilled water and dehydrated with ethanol at increasing concentrations (70%, 95%, and 100%) for 15 min for each passage. For the dry specimens, they were immersed in a 1:1 solution of absolute ethanol and hexamethyldisilazane (HMDS) and then in pure HMDS for 30 min each and finally air-dried; after glutaraldehyde fixation, all steps were performed at RT. Before the SEM analysis, the scaffolds were attached to aluminum stubs (Multilab type stub pin 1/2, Surrey, UK) using a conducting bi-adhesive tape for electron microscopy, coated with gold with the S150A Sputter Coater, and observed at 10 kV in an SEM microscope (Thermo Scientific Quattro S, Waltham, MA, USA) with secondary electrons.
2.12. Statistical Analysis
The GraphPad Prism 8 software was used for the statistical analysis and graph creations. Statistical differences between the control (CTR and LSS) and treated samples (ox-LDL, HSS, and HSS ox-LDL) were evaluated using an unpaired Student’s t-test. Each experiment was performed in duplicate or triplicate. The values are expressed as means ± standard deviation; a p-value < 0.05 was considered statistically significant.
4. Discussion
The PLCL 70/30 scaffolds exhibited physicochemical and mechanical properties suitable for applications aimed at mimicking the internal elastic membrane of tunica intima [
9]. The high degree of nanofiber alignment (68% within 0–18°), achieved during the electrospinning process, is particularly significant for recreating the anisotropic structure of the internal elastic membrane [
34], which plays a crucial role in tissue functionality. Furthermore, the stability of the fiber morphology after sterilization and a thermal treatment ensures the reliability of the scaffold under experimental and physiological conditions. The addition of type I collagen not only improved the biocompatibility but also significantly enhanced the hydrophilicity, as evidenced by the reduction in WCA, thus favoring cellular adhesion and proliferation [
13]. The coating with collagen type I was also critical for promoting stable integration with the GelMa hydrogel. The presence of collagen introduces reactive hydrophilic functional groups, which enhance physical interactions with GelMa, improving the adhesion and composite integrity. Beyond their structural advantages, PLCL nanofibers have also been shown to support co-culture strategies for vascular tissue engineering. For instance, Lee et al. [
35] demonstrated that electrospun PLCL scaffolds can be used to seed endothelial and smooth muscle cells, effectively recapitulating the layered configuration of native blood vessels. This provides a promising basis for future adaptations involving multiple vascular cell types, further increasing the physiological relevance of our engineered construct.
The FT-IR analysis confirmed that GelMa retains key structural features of pristine gelatin while introducing methacrylate groups. The appearance of the 1720 cm−1 peak in the GelMa spectrum, absent in the native gelatin, is particularly significant, as it signifies the introduction of ester bonds from methacrylate groups. Complementary to the FT-IR findings, the degree of methacrylation was quantified using 1H-NMR, revealing a methacrylation efficiency.
The morphological characteristics of u-GelMa and c-GelMa hydrogels were examined through SEM to evaluate how crosslinking influences their structural properties. U-GelMa displayed a relatively smooth surface morphology, indicative of a loosely structured internal network with minimal porosity. This compact morphology limits its ability to support cellular infiltration or efficient nutrient transport. On the other hand, c-GelMa exhibited a highly porous structure, characterized by a clear network of interconnected pores. This porosity is essential for promoting the effective diffusion of nutrients and oxygen, as well as supporting cell migration and infiltration within the hydrogel [
36]. The notable morphological shift observed post-crosslinking is attributed to the formation of covalent bonds between the acrylate groups in the GelMA, which not only stabilize the hydrogel network but also introduce controlled porosity to enhance its functional properties.
The rheological analyses of GelMA hydrogels demonstrated their ability to replicate key mechanical and viscoelastic properties of the ECM of the sub-endothelial layer in the tunica intima. The significant increase in the storage modulus after crosslinking highlights the enhanced stiffness and mechanical stability of the hydrogel. This increase reflects the formation of a robust crosslinked network, which is critical for maintaining structural integrity under physiological conditions (
Table S1). These values indicate that c-GelMa initially presents a stiffness slightly higher than that of the native vascular basement membrane (BM), whose reported elastic modulus ranges from 0.1 kPa to 1 kPa [
37]. However, rheological properties under culture conditions revealed a progressive decrease in the elastic modulus (G′) over time, particularly under dynamic flow (
Table S2), thereby approaching the mechanical range of the native BM. This time-dependent softening is particularly relevant, as it allows the hydrogel to transition toward a more compliant, biomimetic environment, which promotes proper cell functions.
Additionally, the observed shear-thinning behavior is another essential feature of GelMa hydrogels, as it facilitates their injectability and precise deposition. The decrease in viscosity from 405 Pa·s at 0.1 s−1 to 0.2 Pa·s at 1000 s−1 underscores the material’s ability to flow under applied shear stress, allowing it to form thin, uniform layers during deposition through a nozzle. The rheological stability of c-GelMa under both static and dynamic conditions highlights its potential for replicating physiological environments. The retention of gel-like behavior at 37 °C for up to 48 h under static conditions suggests that c-GelMa hydrogels possess sufficient mechanical integrity to support cellular and tissue interactions over short-term experimental time frames. The slight reduction in G′ and G″ values observed over time reflects a minor relaxation of the crosslinked network, which is expected as the hydrogel equilibrates with the surrounding environment. Under dynamic conditions, however, the hydrogel experienced a more pronounced decrease in mechanical properties, likely due to increased shear forces and mechanical stresses encountered during bioreactor operations. This reduction in G′ and G″ values underscores the impact of dynamic environments on the structural stability of hydrogels. Nevertheless, the hydrogel’s ability to preserve its gel-like characteristics, even under these more challenging conditions, demonstrates its capability to preserve the structural integrity of the scaffold.
To gain deep knowledge on the endothelial dysfunction and the early stage of atherosclerosis development, several 3D in vitro models have been developed [
38,
39] to overcome the limitations of 2D in vitro cellular systems, considered too simplified and unable to reproduce the cell−matrix interactions. In this contest, the sandwich-like configuration of our composite model was designed to mimic the multilayered organization of the human tunica intima, combining the mechanical strength and anisotropy of the PLCL nanofibrous mats with the viscoelastic properties and porosity of the c-GelMa hydrogel. According to our data, the 3D supports were suitable for cell culture and did not affect HUVEC viability and NO secretion, even in the presence of ox-LDL, a well-known trigger of endothelial damage and atherosclerosis initiation. The best experimental setting able to reproduce the endothelial dysfunction was the dynamic system induced by HUVECs grown under HSS in combination with ox-LDL. Shear stress is a well-known contributing factor to atherosclerosis, affecting endothelial cell gene expression and mechanical properties [
40]. Indeed, we found a detrimental effect of HSS on cell viability, both in standard growth medium and with ox-LDL addition. Unfortunately, NO data were not significant and only highlighted a decreasing trend following HSS and HSS culture with ox-LDL. According to the literature, ox-LDL and NO exert opposite effects on the human endothelium: ox-LDL promotes endothelial damage, atherosclerosis initiation, and all of the related downstream processes; conversely, NO performs a protective and anti-atherogenic role on the vasculature [
41].