The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion
Abstract
1. Introduction
2. LAIR1: Identification, Molecular Characteristics and Functional Properties
2.1. LAIR1 Discovery and Brief History
2.2. Main Molecular Features of LAIR1
2.3. Comparison of LAIR1 Expression and Other Leukocyte Inhibitory Receptors
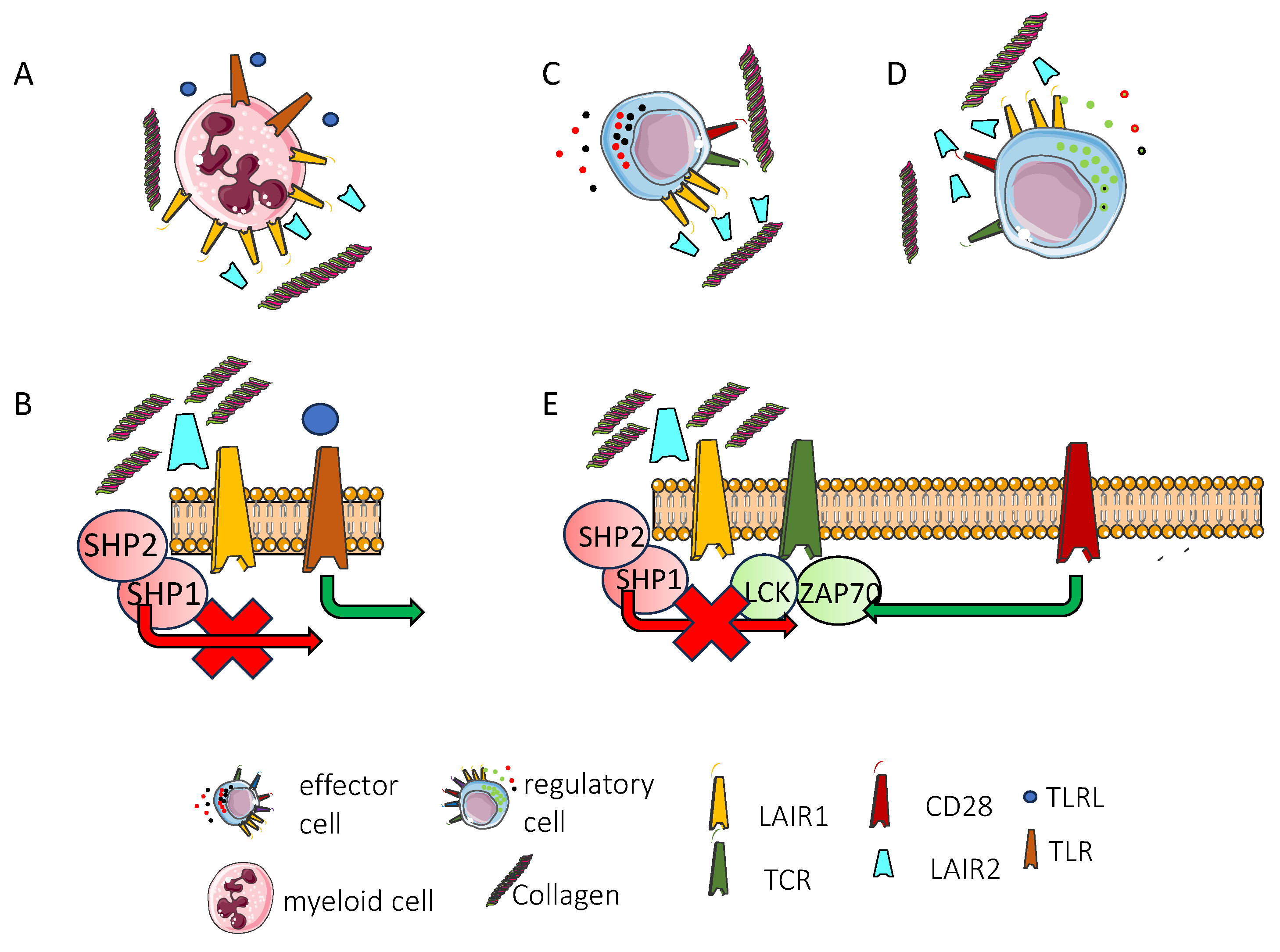
2.4. Lair1-Mediated Inhibitory Signal and Classical Immune Checkpoint Inhibitors Ctla4 and Pd1
3. LAIR1 in Leukocyte Subsets
3.1. LAIR1 Expression and Function in T Cells
3.2. LAIR1 and NK Cell Activities
3.3. LAIR1 and Antigen-Presenting Cells
3.4. LAIR1 Expression on B Cells in Healthy Individuals, Autoimmune Diseases, and Viral Infection
3.5. Lair1 in Myeloid and Innate Lymphoid Cells
4. LAIR2 and Atypical LAIR1 Expression
4.1. LAIR2: A Soluble Regulator of Immune Response with Therapeutic Potential, Highlighting the Functional Role of LAIR1
4.2. Expression of LAIR1 on Stromal Cells and Soluble Lair1 Presence in Some Diseases
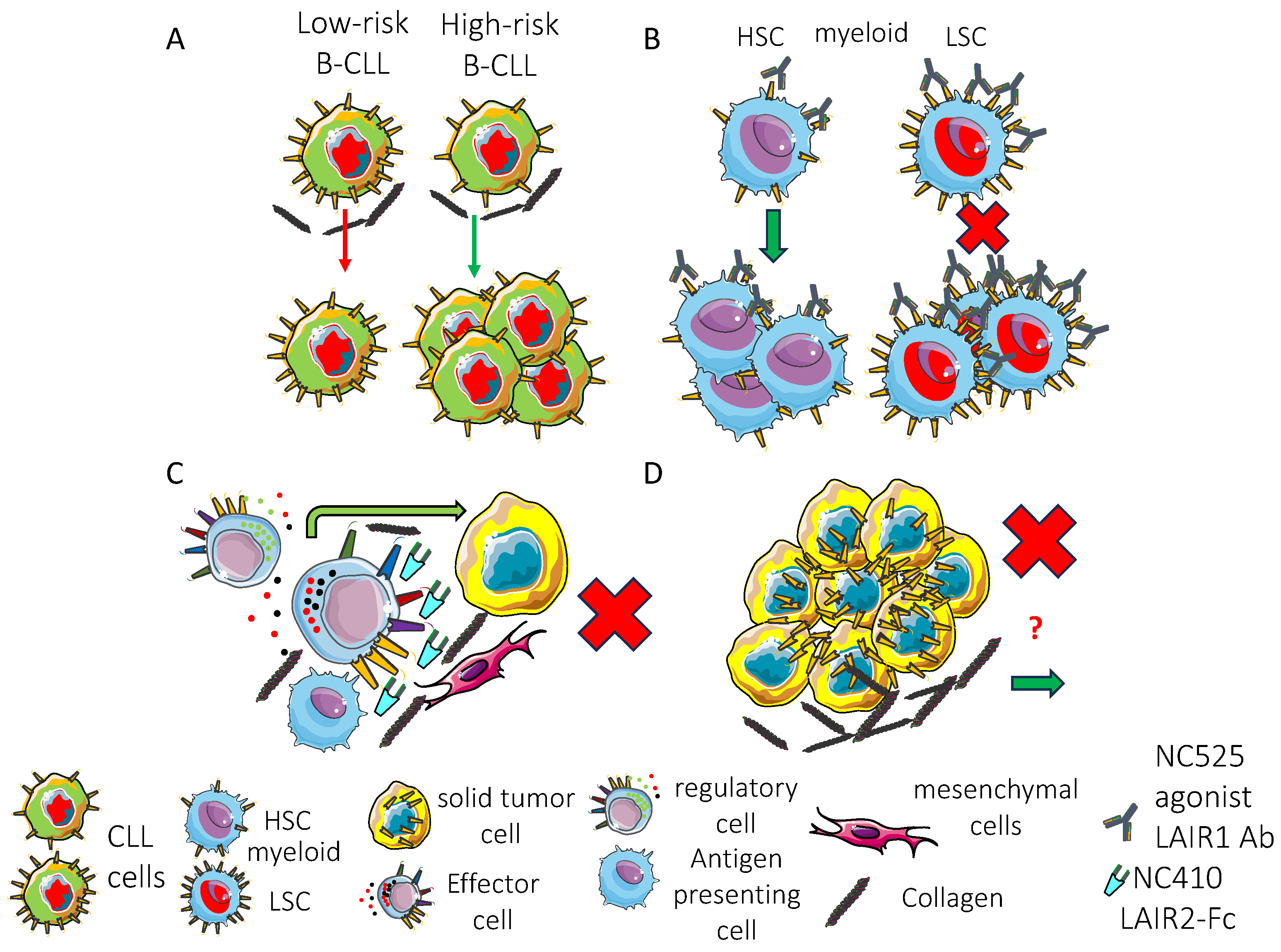
5. Expression of LAIR1 on Tumor Cells
5.1. LAIR1 and Hematological Malignancies
5.1.1. LAIR1 on Leukemic Myeloid Cells
5.1.2. LAIR1 on Leukemic/Lymphoma B Cells
5.2. LAIR1 Expression and Function on Solid Tumor Cells
6. LAIR1 Expression and Function in the Tumor Microenvironment
7. LAIR1: A Double-Edged Sword in Plasmodium falciparum Immune Evasion and Host Defense
8. LAIR1 Expression on Hematopoietic Cell Precursors and Role in the Regulation of Hematopoiesis and Cell Differentiation
9. Future Research to Identify the Knowledge Gaps of LAIR1 Function and Its Therapeutic Targeting
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTLA | cytotoxic T lymphocyte antigen |
| PD | programmed cell death |
| NSCLC | non-small cell lung cancer/carcinoma |
| KIR | Killer immunoglobulin-like receptor |
| CLIR | C lectin type inhibitory receptor |
| LAIR | leukocyte-associated immunoglobulin (Ig)-like receptor |
| CLL | chronic lymphocytic leukemia |
| mAb | monoclonal antibody |
| LCA | leukocyte common antigen |
| NK | natural killer |
| LFA1 | lymphocyte function-associated antigen |
| NKG2 | NK gene group 2 |
| NKB1/KIR3DL1 | killer cell immunoglobulin-like receptor 3DL1 |
| HLA-I | human leukocyte antigen I |
| MHC-I | major histocompatibility complex I |
| MHC-II | major histocompatibility complex II |
| CD | cluster of differentiation |
| IF | immunofluorescence |
| FC | flow cytometry |
| IHC | immunohistochemistry |
| FA | functional assay |
| IP | immunoprecipitation |
| WB | Western blot |
| ELISA | enzyme-linked immunosorbent assay |
| mo anti-h | mouse anti-human |
| LGL | large granular lymphocyte |
| AML | acute myeloid leukemia |
| ITIM | immunoreceptor tyrosine based inhibitory motif |
| ITAM | immunoreceptor tyrosine based activation motif |
| SHP | protein tyrosine phosphatase |
| SHIP | SH2 domain-containing inositol phosphatase |
| LILR | Leukocyte immunoglobulin-like receptor subfamily |
| VLA | very late antigen |
| IFN | interferon |
| CHB | chronic hepatitis B |
| Th | T helper |
| ILT | inhibitory lymphocyte transcript |
| LIR | leukocyte Ig-like receptor |
| Irp | inhibitory receptor protein |
| PE | phosphoethanolamine |
| PS | phosphatidylserine |
| IREM | immune receptor expressed on myeloid cells |
| Siglec | sialic acid-binding immunoglobulin-type lectin |
| RIFIN | Repetitive Interspersed Family of polypeptides |
| PDL | programmed death receptor ligand |
| HAVCR | hepatitis A virus cellular receptor |
| TIM | T-cell immunoglobulin and mucin-domain containing |
| HMGB | high motility group box |
| CEACAM | carcinoembryonic antigen related cell adhesion molecule |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| PVR | polio virus receptor |
| VISIG | V-set and immunoglobulin domain containing |
| LAG | lymphocyte activation gene |
| FGL | fibrinogen-like protein |
| TACTILE | T cell activation |
| increased late expression | |
| VISTA | V-domain Ig suppressor of T cell activation |
| PSGL | P selectin glycoprotein ligand |
| Sdc | syndecan |
| LRIG | leucine-rich repeats and immunoglobulin-like domains protein |
| MDSC | myeloid derived suppressor cells |
| pDC | plasmocytoid dendritic cell; |
| TCR | T cell antigen receptor |
| ZAP | zeta-chain-associated protein kinase |
| BCR | B cell antigen receptor |
| CSK | C-terminal Src kinase |
| SRC | Rous sarcoma virus kinase |
| IL2R | interleukin 2 receptor |
| MAPK | mitogen activated protein kinase |
| ERK | extracellular signal regulated kinase |
| LCK | lymphocyte specific protein tyrosine kinase |
| LYN | tyrosine protein kinase Lyn |
| COL | collagen |
| KLRB1 | killer cell lectin-like receptor subfamily B |
| member 1 | |
| NKRP1A | natural killer receptor protein 1A |
| NTSE | ecto-5′-nucleotidase |
| HBeAg | hepatitis B e-antigen |
| HBV | hepatitis B virus |
| FcγRIIIa | Fc gamma receptor IIIa |
| APC | antigen-presenting cells |
| DC | dendritic cells |
| GM-CSF | granulocyte-monocyte colony stimulating factor |
| TLR | Toll-like receptor |
| LPS | lipopolysaccharide |
| TNF | tumor necrosis factor |
| IGG | immunoglobulin G |
| SLE | systemic lupus erythematosus |
| MCTD | mixed connective tissue disease |
| SSc | systemic sclerosis |
| RA | rheumatoid arthritis |
| PWM | poke weed mitogen |
| MALP2 | macrophage activating lipopeptide 2 |
| IL | interleukin |
| ART | antiretroviral therapy |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| NFkB | nuclear factor k B |
| REL | v-rel avian reticuloendotheliosis viral oncogene homolog |
| STAT | signal transducer and activator of transcription |
| KO | knock-out |
| WT | wild type |
| ROS | reactive oxygen species |
| ECM | extracellular matrix component |
| CXCL | chemokine (c-x-c motif) ligand |
| CCL | chemokine (c-c motif) ligand |
| BMDM | bone-marrow-derived monocyte |
| LAIR-1−/− | LAIR1-negative mice |
| BDCA | blood dendritic cell antigen |
| CpG ODN-A | cytidine monophosphate guanosine oligodeoxynucleotides-A |
| PBMC | peripheral blood mononuclear cell |
| NKP | natural killer protein |
| COVID-19 | corona virus disease-19 |
| ISG-I | interferon stimulated genes-I |
| ILC | innate lymphoid cells |
| AHR | airway hyperreactivity |
| ARDS | airway respiratory distress syndrome |
| ICOS | inducible T cell co-stimulator |
| TNFR | tumor necrosis factor receptor |
| LAPTM5 | lysosomal protein transmembrane 5 |
| MAFB | V-maf musculoaponeurotic fibrosarcoma oncogene homolog B |
| SIRP | signal regulatory protein |
| AS | ankylosing spondylitis |
| FLS | fibroblast-like synoviocytes |
| LV | lentivirus |
| PHA | phytohemagglutinin A |
| PMA | phorbol myristate acetate |
| KD | Kawasaki disease |
| ICI | immune checkpoint inhibitor |
| AKT | a serine/threonine kinase |
| LSC | leukemia stem cells |
| BCLXL | B cell lymphoma extra large |
| PARP | poly (ADP-ribose) polymerases |
| HPC | hematopoietic precursor cells |
| BCL2 | B-cell lymphoma 2 |
| PECAM | platelet endothelial cell adhesion molecule |
| NSG | NOD scid gamma mouse (NOD. Cg-Prkdcscid Il2rgtm1Wjl/SzJ) |
| GRB | growth-factor-receptor-binding protein |
| CAMK | Ca2+/calmodulin-dependent protein kinase |
| cAMP | cyclic adenosine monophosphate |
| CREB | cAMP response element-binding protein |
| ALL | acute lymphoblastic leukemia |
| MRD | minimal residual disease |
| Ph+ | Philadelphia chromosome+ |
| Pecam1−/− | mice lacking PECAM1 |
| Cd300a−/− | mice lacking CD300 |
| Lair1fl/fl | homozygous floxed LAIR1 mice |
| HR | high risk |
| TTFT | time to first treatment |
| OS | overall survival |
| B-NHL | B-non-Hodgkin lymphoma |
| EOC | epithelial ovarian carcinoma |
| mTOR | molecular target of rapamycin |
| HER2 | human epidermal growth factor receptor 2 |
| Te-EVs | tissue exudative extracellular vesicles |
| RCC | renal cell carcinoma |
| CC | cervical carcinoma |
| LGG | low-grade glioma |
| GBM | glioblastoma multiforme |
| HCC | hepatocellular carcinoma |
| GSK | glycogen synthase kinase |
| MMP | metalloproteinase |
| TGF | transforming growth factor |
| SMA | smooth muscle actin |
| TME | tumor microenvironment |
| MK | megakaryocytes |
References
- Zhang, B.; Liu, J.; Mo, Y.; Zhang, K.; Huang, B.; Shang, D. CD8+ T cell exhaustion and its regulatory mechanisms in the tumor microenvironment: Key to the success of immunotherapy. Front. Immunol. 2024, 15, 1476904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poggi, A.; Zocchi, M.R. Natural killer cells and immune-checkpoint inhibitor therapy: Current knowledge and new challenges. Mol. Ther. Oncolytics 2021, 24, 26–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK cell exhaustion in the tumor microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baessler, A.; Vignali, D.A.A. T Cell Exhaustion. Annu. Rev. Immunol. 2024, 42, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nair, R.; Somasundaram, V.; Kuriakose, A.; Krishn, S.R.; Raben, D.; Salazar, R.; Nair, P. Deciphering T-cell exhaustion in the tumor microenvironment: Paving the way for innovative solid tumor therapies. Front. Immunol. 2025, 16, 1548234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalifeh, M.; Salman, H. Engineering resilient CAR T cells for immunosuppressive environment. Mol. Ther. 2025, 33, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, E.; Bléry, M.; Vély, F.; Vivier, E. Signaling pathways engaged by NK cell receptors: Double concerto for activating receptors, inhibitory receptors and NK cells. Semin. Immunol. 2000, 12, 139–147, Erratum in Semin. Immunol. 2000, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.H.; Zheng, J.Q.; Ding, J.Y.; Wu, Y.F.; Liu, L.; Yu, Z.L.; Chen, G. Exosome-Mediated Immunosuppression in Tumor Microenvironments. Cells 2022, 11, 1946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mincheva-Nilsson, L.; Baranov, V. Cancer exosomes and NKG2D receptor-ligand interactions: Impairing NKG2D-mediated cytotoxicity and anti-tumour immune surveillance. Semin. Cancer Biol. 2014, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Sarvnaz, H.; Arabpour, M.; Ramshe, S.M.; Asef-Kabiri, L.; Yousefi, H.; Akbari, M.E.; Eskandari, N. Cancer exosomes and natural killer cells dysfunction: Biological roles, clinical significance and implications for immunotherapy. Mol Cancer 2022, 21, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, X.; Hua, S.; Feng, T.; Ocansey, D.K. 2.; Yin, L. Hypoxia-Regulated Tumor-Derived Exosomes and Tumor Progression: A Focus on Immune Evasion. Int. J. Mol. Sci. 2022, 23, 11789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, L.N.; Alonso, M.A. Biogenesis and Function of T Cell-Derived Exosomes. Front. Cell Dev. Biol. 2016, 4, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sani, F.; Shojaei, S.; Tabatabaei, S.A.; Khorraminejad-Shirazi, M.; Latifi, M.; Sani, M.; Azarpira, N. CAR-T cell-derived exosomes: A new perspective for cancer therapy. Stem Cell Res. Ther. 2024, 15, 174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crescioli, S.; Kaplon, H.; Wang, L.; Visweswaraiah, J.; Kapoor, V.; Reichert, J.M. Antibodies to watch in 2025. MAbs 2025, 17, 2443538. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Ali, A.M.; Akhtar, M.T.; Fatima, T.; Shabbir, I.; Ul Haq, I. The journey of antibody-drug conjugates for revolutionizing cancer therapy: A review. Bioorg. Med. Chem. 2025, 117, 118010. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Chen, I.A. Antibody—Nanoparticle Conjugates in Therapy: Combining the Best of Two Worlds. Small 2025, 21, e2409635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laparra, A. Immuno-oncology in the daily practice. Curr. Opin. Oncol. 2025, 37, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Shang, S.; Chen, D. Recent advances in immunotherapy for small cell lung cancer. Curr. Opin. Oncol. 2025, 37, 17–26. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, B.; Janssen, J.C.; van Daele, P.L.A.; de Jonge, M.J.A.; Joosse, A.; Verheul, H.M.W.; Epker, J.L.; van der Veldt, A.A.M. From ICI to ICU: A systematic review of patients with solid tumors who are treated with immune checkpoint inhibitors (ICI) and admitted to the intensive care unit (ICU). Cancer Treat. Rev. 2025, 136, 102936. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edner, N.M.; Carlesso, G.; Rush, J.S.; Walker, L.S.K. Targeting co-stimulatory molecules in autoimmune disease. Nat. Rev. Drug Discov. 2020, 19, 860–883, Erratum in Nat. Rev. Drug Discov. 2021, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, J.; Ha, D.; Lee, J.; Kim, I.; Park, M.; Im, S.H.; Shin, K.; Kim, S. Network-based machine learning approach to predict immunotherapy response in cancer patients. Nat. Commun. 2022, 13, 3703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinicrope, F.A.; Turk, M.J. Immune checkpoint blockade: Timing is everything. J. Immunother. Cancer 2024, 12, e009722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moretta, A.; Bottino, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. What is a natural killer cell? Nat. Immunol. 2002, 3, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Muntasell, A.; Ochoa, M.C.; Cordeiro, L.; Berraondo, P.; López-Díaz de Cerio, A.; Cabo, M.; López-Botet, M.; Melero, I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr. Opin. Immunol. 2017, 45, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell receptors. Annu. Rev. Immunol. 1998, 16, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, O.A.; Fong, L.K.; Lanier, L.L. ITAM-based receptors in natural killer cells. Immunol. Rev. 2024, 323, 40–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Hatae, R.; Honjo, T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int. J. Clin. Oncol. 2020, 25, 790–800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okazaki, T.; Iwai, Y.; Honjo, T. New regulatory co-receptors: Inducible co-stimulator and PD-1. Curr. Opin. Immunol. 2002, 14, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.F.; Triglia, T.; Bartlett, P.F.; Mackay, I.R. Human natural killer cells, activated lymphocyte killer cells, and monocytes possess similar cytotoxic mechanisms. Proc. Natl. Acad. Sci. USA 1983, 80, 7606–7610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poggi, A.; Pella, N.; Morelli, L.; Spada, F.; Revello, V.; Sivori, S.; Augugliaro, R.; Moretta, L.; Moretta, A. p40, a novel surface molecule involved in the regulation of the non-major histocompatibility complex-restricted cytolytic activity in humans. Eur. J. Immunol. 1995, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Meyaard, L. The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 2008, 83, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Catellani, S.; Bruzzone, A.; Caligaris-Cappio, F.; Gobbi, M.; Zocchi, M.R. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia 2008, 22, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, M.R.; Pellegatta, F.; Pierri, I.; Gobbi, M.; Poggi, A. Leukocyte-associated Ig-like receptor-1 prevents granulocyte-monocyte colony stimulating factor-dependent proliferation and Akt1/PKB alpha activation in primary acute myeloid leukemia cells. Eur. J. Immunol. 2001, 31, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Crimini, E.; Boscolo Bielo, L.; Berton Giachetti, P.P.M.; Pellizzari, G.; Antonarelli, G.; Taurelli Salimbeni, B.; Repetto, M.; Belli, C.; Curigliano, G. Beyond PD(L)-1 Blockade in Microsatellite-Instable Cancers: Current Landscape of Immune Co-Inhibitory Receptor Targeting. Cancers 2024, 16, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Zeng, Z.; Chen, Z.; Yuan, N.; Ye, X.; Zhang, C.; Xia, N.; Luo, W. A new mechanism of antibody diversity: Formation of the natural antibodies containing LAIR1 and LILRB1 extracellular domains. Antib. Ther. 2024, 7, 157–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Werkmeister, J.A.; Burns, G.F.; Triglia, T. Anti-idiotype antibodies to the 9.1C3 blocking antibody used to probe the lethal hit stage of NK cell-mediated cytolysis. J. Immunol. 1984, 133, 1385–1390. [Google Scholar] [PubMed]
- Werkmeister, J.A.; Triglia, T.; Burns, G.F. The isolation of natural killer (NK)-resistant variants of the K562 cell line by mutagenesis and selection with antibodies which inhibit NK cell-mediated lysis. Cell Immunol. 1985, 92, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.F.; Werkmeister, J.A.; Triglia, T. A novel antigenic cell surface protein associated with T200 is involved in the post-activation stage of human NK cell-mediated lysis. J. Immunol. 1984, 133, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.A.; Cruijsen, C.W.; de Ruiter, T.; Nanlohy, N.; Willems, N.; Janssens-Korpela, P.L.; Meyaard, L. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur. J. Immunol. 2007, 37, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, L.; Shangguan, F.; Zhao, X.; Wang, W.; Gao, Z.; Zhou, H.; Qu, G.; Huang, Y.; An, J.; et al. LAIR-1 suppresses cell growth of ovarian cancer cell via the PI3K-AKT-mTOR pathway. Aging 2020, 12, 16142–16154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Zhang, Y.; Cheng, S.; Mu, Y.; Liu, Y.; Yi, X.; Jiang, D.; Ding, Y.; Zhuang, R. LAIR-1 overexpression inhibits epithelial-mesenchymal transition in osteosarcoma via GLUT1-related energy metabolism. World J. Surg. Oncol. 2020, 18, 136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, M.j.; Zhao, R.; Zhao, Z.J. Identification and characterization of leukocyte-associated Ig-like receptor-1 as a major anchor protein of tyrosine phosphatase SHP-1 in hematopoietic cells. J. Biol. Chem. 2000, 275, 17440–17446. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xu, Z.; Cui, J.; Jin, B. A non-stimulatory monoclonal antibody against the inhibitory immunoreceptor LAIR-1. Monoclon. Antib. Immunodiagn. Immunother. 2014, 33, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Jingushi, K.; Uemura, M.; Nakano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; Yamamoto, Y.; Hayashi, T.; Kinouchi, T.; Matsuzaki, K.; et al. Leukocyte-associated immunoglobulin-like receptor 1 promotes tumorigenesis in RCC. Oncol. Rep. 2019, 41, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dorando, H.K.; Mutic, E.C.; Li, J.Y.; Perrin, E.P.; Wurtz, M.K.; Quinn, C.C.; Payton, J.E. LPS and type I and II interferons have opposing effects on epigenetic regulation of LAIR1 expression in mouse and human macrophages. J. Leukoc. Biol. 2024, 115, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Huang, J.; Gibson, L.; Fradette, J.J.; Chen, H.H.; Koyano, K.; Cortez, C.; Li, B.; Ho, C.; Ashique, A.M.; et al. Antitumor Activity of a Novel LAIR1 Antagonist in Combination with Anti-PD1 to Treat Collagen-Rich Solid Tumors. Mol. Cancer Ther. 2024, 23, 1144–1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lovewell, R.R.; Hong, J.; Kundu, S.; Fielder, C.M.; Hu, Q.; Kim, K.W.; Ramsey, H.E.; Gorska, A.E.; Fuller, L.S.; Tian, L.; et al. LAIR-1 agonism as a therapy for acute myeloid leukemia. J. Clin. Investig. 2023, 133, e169519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.Y.; Zhang, Y.; Wang, D.L.; Song, C.J.; Han, W.N.; Yang, K.; Jin, B.Q. Generation of rat monoclonal antibodies against murine LAIR-1. Hybridoma 2007, 26, 316–321. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/gene/3903 (accessed on 3 June 2025).
- Poggi, A.; Tomasello, E.; Revello, V.; Nanni, L.; Costa, P.; Moretta, L. p40 molecule regulates NK cell activation mediated by NK receptors for HLA class I antigens and TCR-mediated triggering of T lymphocytes. Int. Immunol. 1997, 9, 1271–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poggi, A.; Tomasello, E.; Ferrero, E.; Zocchi, M.R.; Moretta, L. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur. J. Immunol. 1998, 28, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Maasho, K.; Masilamani, M.; Valas, R.; Basu, S.; Coligan, J.E.; Borrego, F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol. Immunol. 2005, 42, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Meyaard, L.; Hurenkamp, J.; Clevers, H.; Lanier, L.L.; Phillips, J.H. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J. Immunol. 1999, 162, 5800–5804. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=3903 (accessed on 3 June 2025).
- Ensembl. Available online: https://www.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000167613;r=19:54351384-54370558 (accessed on 3 June 2025).
- Van Laethem, F.; Donaty, L.; Tchernonog, E.; Lacheretz-Szablewski, V.; Russello, J.; Buthiau, D.; Almeras, M.; Moreaux, J.; Bret, C. LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms. Int. J. Mol. Sci. 2022, 23, 16136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravetch, J.V.; Lanier, L.L. Immune inhibitory receptors. Science 2000, 290, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Olcese, L.; Cambiaggi, A.; Semenzato, G.; Bottino, C.; Moretta, A.; Vivier, E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 1997, 158, 5083–5086. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lebbink, R.J.; de Ruiter, T.; Kaptijn, G.J.; Bihan, D.G.; Jansen, C.A.; Lenting, P.J.; Meyaard, L. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int. Immunol. 2007, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, R.J.; van den Berg, M.C.; de Ruiter, T.; Raynal, N.; van Roon, J.A.; Lenting, P.J.; Jin, B.; Meyaard, L. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J. Immunol. 2008, 180, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Rygiel, T.P.; Stolte, E.H.; de Ruiter, T.; van de Weijer, M.L.; Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 2011, 49, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Lebbink, R.J.; Raynal, N.; de Ruiter, T.; Bihan, D.G.; Farndale, R.W.; Meyaard, L. Identification of multiple potent binding sites for human leukocyte associated Ig-like receptor LAIR on collagens II and III. Matrix Biol. 2009, 28, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Brondijk, T.H.; de Ruiter, T.; Ballering, J.; Wienk, H.; Lebbink, R.J.; van Ingen, H.; Boelens, R.; Farndale, R.W.; Meyaard, L.; Huizinga, E.G. Crystal structure and collagen-binding site of immune inhibitory receptor LAIR-1: Unexpected implications for collagen binding by platelet receptor GPVI. Blood 2010, 115, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heino, J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000, 19, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Tomasello, E.; Costa, P. NKRP1A and p40 molecules are involved in regulation of activation and maturation of human NK cells. Res. Immunol. 1997, 148, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tsuji, T.; Jinushi, T.; Matsuzaki, J.; Sato, T.; Chamoto, K.; Togashi, Y.; Koda, T.; Nishimura, T. Differential regulation of VLA-2 expression on Th1 and Th2 cells: A novel marker for the classification of Th subsets. Int. Immunol. 2003, 15, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, N.; Xue, Y.; Zhang, M.; Li, Y.; Si, Y.; Bian, X.; Jia, Y.; Wang, Y. Expression of immunoglobulin-like transcript (ILT)2 and ILT3 in human gastric cancer and its clinical significance. Mol. Med. Rep. 2012, 5, 910–916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiroishi, M.; Tsumoto, K.; Amano, K.; Shirakihara, Y.; Colonna, M.; Braud, V.M.; Allan, D.S.; Makadzange, A.; Rowland-Jones, S.; Willcox, B.; et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. USA 2003, 100, 8856–8861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortez, V.S.; Colonna, M. Diversity and function of group 1 innate lymphoid cells. Immunol. Lett. 2016, 179, 19–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Döhring, C.; Colonna, M. Human natural killer cell inhibitory receptors bind to HLA class I molecules. Eur. J. Immunol. 1996, 26, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Navarro, F.; Bellón, T.; Llano, M.; García, P.; Samaridis, J.; Angman, L.; Cella, M.; López-Botet, M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 1997, 186, 1809–1818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aoukaty, A.; Lee, I.F.; Wu, J.; Tan, R. Chronic active Epstein-Barr virus infection associated with low expression of leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) on natural killer cells. J. Clin. Immunol. 2003, 23, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Zenarruzabeitia, O.; Vitallé, J.; Eguizabal, C.; Simhadri, V.R.; Borrego, F. The Biology and Disease Relevance of CD300a, an Inhibitory Receptor for Phosphatidylserine and Phosphatidylethanolamine. J. Immunol. 2015, 194, 5053–5060. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, X.; Su, D.; Zhang, Y.; Yu, L.; Jiang, F.; Zhou, X.; Feng, Y.; Ma, F. The Roles of Siglec7 and Siglec9 on Natural Killer Cells in Virus Infection and Tumour Progression. J. Immunol. Res. 2020, 2020, 6243819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simhadri, V.R.; Andersen, J.F.; Calvo, E.; Choi, S.C.; Coligan, J.E.; Borrego, F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 2012, 119, 2799–2809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez-Errico, D.; Sayós, J.; López-Botet, M. The IREM-1 (CD300f) inhibitory receptor associates with the p85alpha subunit of phosphoinositide 3-kinase. J. Immunol. 2007, 178, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Márquez, J.A.; Galfré, E.; Dupeux, F.; Flot, D.; Moran, O.; Dimasi, N. The crystal structure of the extracellular domain of the inhibitor receptor expressed on myeloid cells IREM-1. J. Mol. Biol. 2007, 367, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Walk, J.; Westerlaken, G.H.; van Uden, N.O.; Belderbos, M.E.; Meyaard, L.; Bont, L.J. Inhibitory receptor expression on neonatal immune cells. Clin. Exp. Immunol. 2012, 169, 164–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, N.; Zhang, K.; Gao, X.; Lv, M.; Luan, J.; Hu, Z.; Li, A.; Gou, X. Role and mechanism of LAIR-1 in the development of autoimmune diseases, tumors, and malaria: A review. Curr. Res. Transl. Med. 2020, 68, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Falco, M.; Moretta, L.; Moretta, A.; Bottino, C. KIR and KIR ligand polymorphism: A new area for clinical applications? Tissue Antigens. 2013, 82, 363–373. [Google Scholar] [CrossRef] [PubMed]
- López-Botet, M.; Muntasell, A.; Vilches, C. The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Semin. Immunol. 2014, 26, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Torkar, M.; Haude, A.; Milne, S.; Jones, T.; Sheer, D.; Beck, S.; Trowsdale, J. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc. Natl. Acad. Sci. USA 2000, 97, 4778–4783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colonna, M.; Nakajima, H.; Cella, M. Inhibitory and activating receptors involved in immune surveillance by human NK and myeloid cells. J. Leukoc. Biol. 1999, 66, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Borrego, F. The CD300 molecules: An emerging family of regulators of the immune system. Blood 2013, 121, 1951–1960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, L.; Choi, S.C.; Murakami, Y.; Allen, J.; Morse, H.C., 3rd; Qi, C.F.; Krzewski, K.; Coligan, J.E. p85α recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nat. Commun. 2014, 5, 3146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Houtum, E.J.H.; Büll, C.; Cornelissen, L.A.M.; Adema, G.J. Siglec Signaling in the Tumor Microenvironment. Front. Immunol. 2021, 12, 790317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chauvin, J.M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, A.P.; Tang, X.Y.; Xiong, Y.L.; Zheng, K.F.; Liu, Y.J.; Shi, X.G.; Lv, Y.; Jiang, T.; Ma, N.; Zhao, J.B. Immune Checkpoint LAG3 and Its Ligand FGL1 in Cancer. Front. Immunol. 2022, 12, 785091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, S.; Isayev, O.; Werner, J.; Bazhin, A.V. CD96 as a Potential Immune Regulator in Cancers. Int. J. Mol. Sci. 2023, 24, 1303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shekari, N.; Shanehbandi, D.; Kazemi, T.; Zarredar, H.; Baradaran, B.; Jalali, S.A. VISTA and its ligands: The next generation of promising therapeutic targets in immunotherapy. Cancer Cell Int. 2023, 23, 265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2022, 21, 509–528, Erratum in Nat. Rev. Drug Discov. 2022, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed] [PubMed Central]
- Marasco, M.; Berteotti, A.; Weyershaeuser, J.; Thorausch, N.; Sikorska, J.; Krausze, J.; Brandt, H.J.; Kirkpatrick, J.; Rios, P.; Schamel, W.W.; et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci. Adv. 2020, 6, eaay4458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verbrugge, A.; Rijkers, E.S.; de Ruiter, T.; Meyaard, L. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur. J. Immunol. 2006, 36, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, A.; de Ruiter, T.; Clevers, H.; Meyaard, L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int. Immunol. 2003, 15, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Parvini, S.; Majidpoor, J.; Mortezaee, K. The impact of PD-L1 as a biomarker of cancer responses to combo anti-PD-1/CTLA-4. Pathol. Res. Pract. 2023, 247, 154583. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, L.; Tang, S.; Zhao, J.; Zuo, L. Balancing Tumor Immunotherapy and Immune-Related Adverse Events: Unveiling the Key Regulators. Int. J. Mol. Sci. 2024, 25, 10919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93, Erratum in Mol. Cancer 2023, 22, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, X.; Zhang, Y.; Wang, S.; Wei, H.; Yu, J. Immune checkpoint inhibitor (ICI) combination therapy compared to monotherapy in advanced solid cancer: A systematic review. J. Cancer 2021, 12, 1318–1333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keerthivasan, S.; Şenbabaoğlu, Y.; Martinez-Martin, N.; Husain, B.; Verschueren, E.; Wong, A.; Yang, Y.A.; Sun, Y.; Pham, V.; Hinkle, T.; et al. Homeostatic functions of monocytes and interstitial lung macrophages are regulated via collagen domain-binding receptor LAIR1. Immunity 2021, 54, 1511–1526.e8. [Google Scholar] [CrossRef] [PubMed]
- Llinàs, L.; Lázaro, A.; de Salort, J.; Matesanz-Isabel, J.; Sintes, J.; Engel, P. Expression profiles of novel cell surface molecules on B-cell subsets and plasma cells as analyzed by flow cytometry. Immunol. Lett. 2011, 134, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Merlo, A.; Tenca, C.; Fais, F.; Battini, L.; Ciccone, E.; Grossi, C.E.; Saverino, D. Inhibitory receptors CD85j, LAIR-1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin. Vaccine Immunol. 2005, 12, 705–712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saverino, D.; Fabbi, M.; Merlo, A.; Ravera, G.; Grossi, C.E.; Ciccone, E. Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum. Immunol. 2002, 63, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Brand, D.D.; Rosloniec, E.F.; Yi, A.K.; Stuart, J.M.; Kang, A.H.; Myers, L.K. Leukocyte-associated immunoglobulin-like receptor 1 inhibits T-cell signaling by decreasing protein phosphorylation in the T-cell signaling pathway. J. Biol. Chem. 2020, 295, 2239–2247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, P.T.F.; Sandalova, E.; Jo, J.; Gill, U.; Ushiro-Lumb, I.; Tan, A.T.; Naik, S.; Foster, G.R.; Bertoletti, A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012, 143, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, T.; Marut, W.; Pascoal Ramos, M.I.; García, S.; Fleury, D.; Affandi, A.J.; Meijers, A.S.; Giovannone, B.; Tieland, R.G.; Elshof, E.; et al. Impaired LAIR-1-mediated immune control due to collagen degradation in fibrosis. J. Autoimmun. 2024, 146, 103219. [Google Scholar] [CrossRef] [PubMed]
- Agashe, V.V.; Jankowska-Gan, E.; Keller, M.; Sullivan, J.A.; Haynes, L.D.; Kernien, J.F.; Torrealba, J.R.; Roenneburg, D.; Dart, M.; Colonna, M.; et al. Leukocyte-Associated Ig-like Receptor 1 Inhibits Th1 Responses but Is Required for Natural and Induced Monocyte-Dependent Th17 Responses. J. Immunol. 2018, 201, 772–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez, M.W.; Paquet, A.C.; Yang, Y.H.; Erle, D.J. Differential gene expression by integrin beta 7+ and beta 7- memory T helper cells. BMC Immunol. 2004, 5, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Poggi, A.; Costa, P.; Zocchi, M.R.; Moretta, L. Phenotypic and functional analysis of CD4+ NKRP1A+ human T lymphocytes. Direct evidence that the NKRP1A molecule is involved in transendothelial migration. Eur. J. Immunol. 1997, 27, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Bouïs, D.; Liao, H.; Visovatti, S.H.; Pinsky, D.J. Ecto-5’ nucleotidase (CD73)-mediated adenosine generation and signaling in murine cardiac allograft vasculopathy. Circ. Res. 2008, 103, 1410–1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, Y.; Bi, Y.; Wei, H.; Li, J.; Huang, Z.; Liao, C.; Liao, W.; Huang, Y. Expression and clinical significance of inhibitory receptor Leukocyte-associated immunoglobulin-like receptor-1 on peripheral blood T cells of chronic hepatitis B patients: A cross-sectional study. Medicine 2021, 100, e26667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poggi, A.; Pardi, R.; Pella, N.; Morelli, L.; Sivori, S.; Vitale, M.; Revello, V.; Moretta, A.; Moretta, L. CD45-mediated regulation of LFA1 function in human natural killer cells. Anti-CD45 monoclonal antibodies inhibit the calcium mobilization induced via LFA1 molecules. Eur. J. Immunol. 1993, 23, 2454–2463. [Google Scholar] [CrossRef] [PubMed]
- Luri-Rey, C.; Teijeira, Á.; Wculek, S.K.; de Andrea, C.; Herrero, C.; Lopez-Janeiro, A.; Rodríguez-Ruiz, M.E.; Heras, I.; Aggelakopoulou, M.; Berraondo, P.; et al. Cross-priming in cancer immunology and immunotherapy. Nat. Rev. Cancer 2025, 25, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.Y.; Belabed, M.; Park, M.D.; Mattiuz, R.; Puleston, D.; Merad, M. Dendritic cell maturation in cancer. Nat. Rev. Cancer 2025, 25, 225–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carvalheiro, T.; Garcia, S.; Pascoal Ramos, M.I.; Giovannone, B.; Radstake, T.R.D.J.; Marut, W.; Meyaard, L. Leukocyte Associated Immunoglobulin Like Receptor 1 Regulation and Function on Monocytes and Dendritic Cells During Inflammation. Front. Immunol. 2020, 11, 1793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Son, M.; Santiago-Schwarz, F.; Al-Abed, Y.; Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl. Acad. Sci. USA 2012, 109, E3160–E3167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Vuurst de Vries, A.R.; Clevers, H.; Logtenberg, T.; Meyaard, L. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 1999, 29, 3160–3167. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.M.; Canevali, P.; Magnani, O.; Rossi, E.; Puppo, F.; Zocchi, M.R.; Poggi, A. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS ONE 2012, 7, e31903, Erratum in PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammad, R.; Hamdino, M.; El-Nasser, A.M.; Sobhy, A.; Eldesoky, N.A.; Mashaal, A.M.; Ali, H.F. Immunoregulatory complement receptor-1 and leukocyte-associated Ig-like receptor-1 expression on leukocytes in Psoriasis vulgaris. Innate Immun. 2020, 26, 683–692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rethi, B.; Sammicheli, S.; Amu, S.; Pensieroso, S.; Hejdeman, B.; Schepis, D.; Thang, P.H.; Chiodi, F. Concerted effect of lymphopenia, viraemia and T-cell activation on Fas expression of peripheral B cells in HIV-1-infected patients. AIDS 2013, 27, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bosenberg, M. Advances in Studying Cancer Immunology in Mice. Cold Spring Harb. Perspect. Med. 2025, 15, a041682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergsagel, P.L.; Chesi, M. Immunocompetent mouse models of multiple myeloma. Semin. Hematol. 2025, 62, 50–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sefik, E.; Xiao, T.; Chiorazzi, M.; Odell, I.; Zhang, F.; Agrawal, K.; Micevic, G.; Flavell, R.A. Engineering Mice to Study Human Immunity. Annu. Rev. Immunol. 2025, 43, 451–487. [Google Scholar] [CrossRef] [PubMed]
- Bubeck Wardenburg, J.; Williams, W.A.; Missiakas, D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. USA 2006, 103, 13831–13836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashimoto, M.; Tawaratsumida, K.; Kariya, H.; Kiyohara, A.; Suda, Y.; Krikae, F.; Kirikae, T.; Götz, F. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 2006, 177, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Carmona-Martínez, V.; Fernández-Fernández, M.D.; Antón, G.; Muñoz-Tornero, M.; Lencina, M.; Pagán, I.; de la Peña, J.; García-Peñarrubia, P. Expression of LAIR-1 (CD305) on Human Blood Monocytes as a Marker of Hepatic Cirrhosis Progression. J. Immunol. Res. 2019, 2019, 2974753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Sun, H.; Li, J.; Rong, Q.; Ji, X.; Li, B. The leukocyte-associated immunoglobulin (Ig)-like receptor-1 modulating cell apoptosis and inflammatory cytokines secretion in THP-1 cells after Helicobacter pylori infection. Microb. Pathog. 2017, 109, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, I.; Cantoni, C.; Carrega, P.; Oliveri, D.; Lui, G.; Conte, R.; Navarra, M.; Cavaliere, R.; Traggiai, E.; Gattorno, M.; et al. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNα production. PLoS ONE 2010, 5, e15080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, X.; Tian, L.; Esteso, G.; Choi, S.C.; Barrow, A.D.; Colonna, M.; Borrego, F.; Coligan, J.E. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J. Immunol. 2012, 188, 548–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ngo, C.; Garrec, C.; Tomasello, E.; Dalod, M. The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond. Cell. Mol. Immunol. 2024, 21, 1008–1035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Wijst, M.G.P.; Vazquez, S.E.; Hartoularos, G.C.; Bastard, P.; Grant, T.; Bueno, R.; Lee, D.S.; Greenland, J.R.; Sun, Y.; Perez, R.; et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 2021, 13, eabh2624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seery, V.; Raiden, S.C.; Algieri, S.C.; Grisolía, N.A.; Filippo, D.; De Carli, N.; Di Lalla, S.; Cairoli, H.; Chiolo, M.J.; Meregalli, C.N.; et al. 1 in behalf of the COVID-19 Naval Pediatric Workgroup and in behalf of the COVID-19, Fernández Pediatric Residency Workgroup. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 2021, 67, 103357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helou, D.G.; Quach, C.; Hurrell, B.P.; Li, X.; Li, M.; Akbari, A.; Shen, S.; Shafiei-Jahani, P.; Akbari, O. LAIR-1 limits macrophage activation in acute inflammatory lung injury. Mucosal Immunol. 2023, 16, 788–800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olivares-Martínez, E.; Hernández-Ramírez, D.F.; Núñez-Álvarez, C.A.; Meza-Sánchez, D.E.; Chapa, M.; Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; et al. Polymerized Type I Collagen Downregulates STAT-1 Phosphorylation Through Engagement with LAIR-1 in Circulating Monocytes, Avoiding Long COVID. Int. J. Mol. Sci. 2025, 26, 1018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Helou, D.G.; Shafiei-Jahani, P.; Hurrell, B.P.; Painter, J.D.; Quach, C.; Howard, E.; Akbari, O. LAIR-1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. J. Allergy Clin. Immunol. 2022, 149, 223–236.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maazi, H.; Akbari, O. Type two innate lymphoid cells: The Janus cells in health and disease. Immunol. Rev. 2017, 278, 192–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafiei-Jahani, P.; Helou, D.G.; Hurrell, B.P.; Howard, E.; Quach, C.; Painter, J.D.; Galle-Treger, L.; Li, M.; Loh, Y.E.; Akbari, O. CD200-CD200R immune checkpoint engagement regulates ILC2 effector function and ameliorates lung inflammation in asthma. Nat. Commun. 2021, 12, 2526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, C.; Kojo, S.; Yamashita, M.; Moro, K.; Lacaud, G.; Shiroguchi, K.; Taniuchi, I.; Ebihara, T. Runx/Cbfβ complexes protect group 2 innate lymphoid cells from exhausted-like hyporesponsiveness during allergic airway inflammation. Nat. Commun. 2019, 10, 447, Erratum in Nat. Commun. 2019, 10, 1075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hurrell, B.P.; Galle-Treger, L.; Jahani, P.S.; Howard, E.; Helou, D.G.; Banie, H.; Soroosh, P.; Akbari, O. TNFR2 Signaling Enhances ILC2 Survival, Function, and Induction of Airway Hyperreactivity. Cell Rep. 2019, 29, 4509–4524.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maazi, H.; Patel, N.; Sankaranarayanan, I.; Suzuki, Y.; Rigas, D.; Soroosh, P.; Freeman, G.J.; Sharpe, A.H.; Akbari, O. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 2015, 42, 538–551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helou, D.G.; Shafiei-Jahani, P.; Lo, R.; Howard, E.; Hurrell, B.P.; Galle-Treger, L.; Painter, J.D.; Lewis, G.; Soroosh, P.; Sharpe, A.H.; et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 2020, 11, 3998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, P.; Zhuang, W.; Zheng, Z.; Zhang, L.; Zhang, X.; Chen, Q. Dissecting T-cell heterogeneity in esophageal squamous cell carcinoma reveals the potential role of LAIR2 in antitumor immunity. Clin. Exp. Immunol. 2023, 214, 36–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos, M.I.P.; Tian, L.; de Ruiter, E.J.; Song, C.; Paucarmayta, A.; Singh, A.; Elshof, E.; Vijver, S.V.; Shaik, J.; Bosiacki, J.; et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. eLife 2021, 10, e62927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olde Nordkamp, M.J.; van Roon, J.A.; Douwes, M.; de Ruiter, T.; Urbanus, R.T.; Meyaard, L. Enhanced secretion of leukocyte-associated immunoglobulin-like receptor 2 (LAIR-2) and soluble LAIR-1 in rheumatoid arthritis: LAIR-2 is a more efficient antagonist of the LAIR-1-collagen inhibitory interaction than is soluble LAIR-1. Arthritis Rheum. 2011, 63, 3749–3757. [Google Scholar] [CrossRef]
- Ly, D.; Li, Q.; Navab, R.; Zeltz, C.; Fang, L.; Cabanero, M.; Zhu, C.Q.; Tsao, M.-S.; Zhang, L. Tumor associated regulatory T cell expression of LAIR2 is prognostic in lung adenocarcinoma. Cancers 2021, 14, 205. [Google Scholar] [CrossRef]
- Simone, R.; Pesce, G.; Antola, P.; Merlo, D.F.; Bagnasco, M.; Saverino, D. Serum LAIR-2 is increased in autoimmune thyroid diseases. PLoS ONE 2013, 8, e63282. [Google Scholar] [CrossRef]
- Camargo, C.M.; Augusto, D.G.; Petzl-Erler, M.L. Differential gene expression levels might explain association of LAIR2 polymorphisms with pemphigus. Hum. Genet. 2016, 135, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Farias, T.D.J.; Augusto, D.G.; de Almeida, R.C.; Malheiros, D.; Petzl-Erler, M.L. Screening the full leucocyte receptor complex genomic region revealed associations with pemphigus that might be explained by gene regulation. Immunology 2019, 156, 86–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, L.; Wang, S.; Li, J.; Li, J.; Li, B. Cancer immunotherapy based on blocking immune suppression mediated by an immune modulator LAIR-1. Oncoimmunology 2020, 9, 1740477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, S.; Dong, H.; Yi, X.; Zhang, J.; Liu, X.; Zhuang, R.; Ding, Y. LAIR-1 shedding from human fibroblast-like synoviocytes in rheumatoid arthritis following TNF-α stimulation. Clin. Exp. Immunol. 2018, 192, 193–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouyang, W.; Xue, J.; Liu, J.; Jia, W.; Li, Z.; Xie, X.; Liu, X.; Jian, J.; Li, Q.; Zhu, Y.; et al. Establishment of an ELISA system for determining soluble LAIR-1 levels in sera of patients with HFRS and kidney transplant. J. Immunol. Methods 2004, 292, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zeng, A.; Yang, P.; Zhang, J.; Liu, D.; Li, M.; Jing, F.; Yi, Q. Role of leukocyte-associated Ig-like receptor-1 in the pathogenesis of Kawasaki disease and coronary artery aneurysms. Immunol. Lett. 2025, 271, 106948. [Google Scholar] [CrossRef] [PubMed]
- Castellino, S.M.; Pei, Q.; Parsons, S.K.; Hodgson, D.; McCarten, K.; Horton, T.; Cho, S.; Wu, Y.; Punnett, A.; Dave, H.; et al. Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 1649–1660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garg, R.; Allen, K.J.H.; Dawicki, W.; Geoghegan, E.M.; Ludwig, D.L.; Dadachova, E. 225Ac-labeled CD33-targeting antibody reverses resistance to Bcl-2 inhibitor venetoclax in acute myeloid leukemia models. Cancer Med. 2021, 10, 1128–1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, X.; Lu, Z.; Cui, C.; Deng, M.; Fan, Y.; Dong, B.; Han, X.; Xie, F.; Tyner, J.W.; Coligan, J.E.; et al. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat. Cell Biol. 2015, 17, 665–677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Shojaee, S.; Buchner, M.; Geng, H.; Lee, J.W.; Klemm, L.; Titz, B.; Graeber, T.G.; Park, E.; Tan, Y.X.; et al. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature 2015, 521, 357–361, Erratum in Nature 2016, 534, 138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.; Bhatia, P.; Shandilya, J.K.; Rawat, A.; Varma, N.; Sachdeva, M.S.; Trehan, A.; Bansal, D.; Jain, R.; Totadri, S. Low Expression of Leucocyte Associated Immunoglobulin Like Receptor-1 (LAIR-1/CD305) in a Cohort of Pediatric Acute Lymphoblastic Leukemia Cases. Asian Pac. J. Cancer Prev. 2018, 19, 3131–3135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faure, G.C.; Amsellem, S.; Arnoulet, C.; Bardet, V.; Campos, L.; De Carvalho-Bittencourt, M.; de Labarthe, A.; Eischen, A.; Feuillard, J.; Fossat, C.; et al. Mutual benefits of B-ALL and HLDA/HCDM HLDA 9th Barcelona 2010. Immunol. Lett. 2011, 134, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Limnander, A.; Depeille, P.; Freedman, T.S.; Liou, J.; Leitges, M.; Kurosaki, T.; Roose, J.P.; Weiss, A. STIM1, PKC-δ and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat. Immunol. 2011, 12, 425–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammad, R.; Kandeel, E.Z.; Lambert, C.; Sack, U.; Kujumdshiev, S.; Kamhawy, A.; Abo-Elkheir, O.I.; Abd El Hakam, F.E.; Mashaal, A.; Ramadan, M.; et al. Leukemic B cells expression of CD200 and Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1, CD305) in Chronic Lymphocytic Leukemia patients in relation to Treg frequency. Pathol. Res. Pract. 2024, 263, 155669. [Google Scholar] [CrossRef] [PubMed]
- Rawstron, A.C.; Shingles, J.; de Tute, R.; Bennett, F.; Jack, A.S.; Hillmen, P. Chronic lymphocytic leukaemia (CLL) and CLL-type monoclonal B-cell lymphocytosis (MBL) show differential expression of molecules involved in lymphoid tissue homing. Cytom. Part B Clin. Cytom. 2010, 78 (Suppl. S1), S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Mirkowska, P.; Hofmann, A.; Sedek, L.; Slamova, L.; Mejstrikova, E.; Szczepanski, T.; Schmitz, M.; Cario, G.; Stanulla, M.; Schrappe, M.; et al. Leukemia surfaceome analysis reveals new disease-associated features. Blood 2013, 121, e149–e159. [Google Scholar] [CrossRef] [PubMed]
- Perbellini, O.; Falisi, E.; Giaretta, I.; Boscaro, E.; Novella, E.; Facco, M.; Fortuna, S.; Finotto, S.; Amati, E.; Maniscalco, F.; et al. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica 2014, 99, 881–887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coles, S.J.; Hills, R.K.; Wang, E.C.; Burnett, A.K.; Man, S.; Darley, R.L.; Tonks, A. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia 2012, 26, 2146–2148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pallasch, C.P.; Ulbrich, S.; Brinker, R.; Hallek, M.; Uger, R.A.; Wendtner, C.M. Disruption of T cell suppression in chronic lymphocytic leukemia by CD200 blockade. Leuk. Res. 2009, 33, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Devin, J.; Kassambara, A.; Bruyer, A.; Moreaux, J.; Bret, C. Phenotypic Characterization of Diffuse Large B-Cell Lymphoma Cells and Prognostic Impact. J. Clin. Med. 2019, 8, 1074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, A.; Patil, J.; Ghogale, S.G.; Deshpande, N.; Girase, K.; Shetye, N.; Rajpal, S.; Chatterjee, G.; Patkar, N.; Jain, D.; et al. Utility of leukocyte-associated immunoglobulin-like receptor-1 (CD305) in flow cytometric detection of minimal bone marrow involvement by B-cell non-Hodgkin lymphoma. Cytom. Part B Clin. Cytom. 2024, 106, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Fu, A.; Yang, S.; He, X.; Wang, Y.; Zhang, X.; Zhou, J.; Luan, X.; Yu, W.; Xue, J. Leukocyte-associated immunoglobulin-like receptor-1 expressed in epithelial ovarian cancer cells and involved in cell proliferation and invasion. Biochem. Biophys. Res. Commun. 2015, 458, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Alsaleem, M.A.; Toss, M.S.; Kariri, Y.A.; Althobiti, M.; Alsaeed, S.; Aljohani, A.I.; Narasimha, P.L.; Mongan, N.P.; Green, A.R.; et al. The ITIM-Containing Receptor: Leukocyte-Associated Immunoglobulin-Like Receptor-1 (LAIR-1) Modulates Immune Response and Confers Poor Prognosis in Invasive Breast Carcinoma. Cancers 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.L.; Zhang, M.J.; Wu, L.; Mao, L.; Chen, L.; Yu, G.T.; Deng, W.W.; Zhang, W.F.; Liu, B.; Sun, W.K.; et al. LAIR-1 overexpression and correlation with advanced pathological grade and immune suppressive status in oral squamous cell carcinoma. Head Neck 2019, 41, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, L.; Zhou, J.; Liu, L.; Fu, Q.; Fu, A.; Feng, X.; Xin, R.; Liu, H.; Gao, Y.; et al. Clinicopathologic significance of LAIR-1 expression in hepatocellular carcinoma. Curr. Probl. Cancer 2019, 43, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Carey, K.M.; Young, C.D.; Clark, A.J.; Dammer, E.B.; Singh, R.; Lillard, J.W., Jr. Subtype-specific analysis of gene co-expression networks and immune cell profiling reveals high grade serous ovarian cancer subtype linkage to variable immune microenvironment. J. Ovarian Res. 2024, 17, 240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poggi, A.; Pellegatta, F.; Leone, B.E.; Moretta, L.; Zocchi, M.R. Engagement of the leukocyte-associated Ig-like receptor-1 induces programmed cell death and prevents NF-kappaB nuclear translocation in human myeloid leukemias. Eur. J. Immunol. 2000, 30, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Miao, F.; Cao, Y.; Xue, J.; Cao, Q.; Zhang, X. Clinical significance of leukocyte-associated immunoglobulin-like receptor-1 expression in human cervical cancer. Exp. Ther. Med. 2016, 12, 3699–3705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, X.; Pan, S.; Wang, Z.; Chen, J.; Lu, L.; Cao, Q.; Song, S.; Zhang, H.; Liu, X.; Qu, X.; et al. LAIR1 drives glioma progression by nuclear focal adhesion kinase dependent expressions of cyclin D1 and immunosuppressive chemokines/cytokines. Cell Death Dis. 2023, 14, 684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, B.; Ke, X.; Qiu, J.; Ye, D.; Zhang, Z.; Zhang, X.; Luo, Y.; Yao, Y.; Wu, X.; Wang, X.; et al. LAIR1-mediated resistance of hepatocellular carcinoma cells to T cells through a GSK-3β/β-catenin/MYC/PD-L1 pathway. Cell. Signal. 2024, 115, 111039. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Varesano, S.; Zocchi, M.R. How to Hit Mesenchymal Stromal Cells and Make the Tumor Microenvironment Immunostimulant Rather Than Immunosuppressive. Front. Immunol. 2018, 9, 262, Erratum in Front. Immunol. 2018, 9, 1342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poggi, A.; Musso, A.; Dapino, I.; Zocchi, M.R. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunol. Lett. 2014, 159, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Chen, X.; Zhang, L.; Chen, M. Cancer associated fibroblasts in cancer development and therapy. J. Hematol. Oncol. 2025, 18, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vijver, S.V.; Singh, A.; Mommers-Elshof, E.T.A.M.; Meeldijk, J.; Copeland, R.; Boon, L.; Langermann, S.; Flies, D.; Meyaard, L.; Ramos, M.I.P. Collagen Fragments Produced in Cancer Mediate T Cell Suppression Through Leukocyte-Associated Immunoglobulin-Like Receptor 1. Front. Immunol. 2021, 12, 733561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horn, L.A.; Chariou, P.L.; Gameiro, S.R.; Qin, H.; Iida, M.; Fousek, K.; Meyer, T.J.; Cam, M.; Flies, D.; Langermann, S.; et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-β signaling enables PD-L1-mediated tumor eradication. J. Clin. Investig. 2022, 132, e155148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Li, Y.; Shen, M.; Xu, W.; Wu, S.; Yang, X.; Zhang, B.; Lin, N. Epigenetic Regulation of Stromal and Immune Cells and Therapeutic Targets in the Tumor Microenvironment. Biomolecules 2025, 15, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, M.M.; Zhao, Y.; Zhang, N.; Gong, F.Y.; Zhang, W.; Dong, C.S.; Dai, J.F.; Wang, J. Tumor Microenvironment: Obstacles and Opportunities for T Cell-Based Tumor Immunotherapies. Mol. Cancer Res. 2025, 23, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Guo, Q.; Guan, G.F.; Zhu, C.; Zou, C.Y.; Zhang, L.Y.; Cheng, W.; Wang, G.L.; Cheng, P.; Wu, A.H.; et al. Elevated lymphocyte specific protein 1 expression is involved in the regulation of leukocyte migration and immunosuppressive microenvironment in glioblastoma. Aging 2020, 12, 1656–1684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, J.H.; Lee, B.S.; Jang, J.Y.; Lee, Y.S.; Kim, H.J.; Roh, J.; Shin, Y.S.; Woo, H.G.; Kim, C.H. Single-cell transcriptome profiling of the stepwise progression of head and neck cancer. Nat. Commun. 2023, 14, 1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, L.; Kohno, M.; Murakami, J.; Zia, A.; Allen, J.; Yun, H.; Chan, M.; Baciu, C.; Liu, M.; Serre-Beinier, V.; et al. Defining and targeting tumor-associated macrophages in malignant mesothelioma. Proc. Natl. Acad. Sci. USA 2023, 120, e2210836120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.C. A perspective on LILRBs and LAIR1 as immune checkpoint targets for cancer treatment. Biochem. Biophys. Res. Commun. 2022, 633, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Bao, L.; Lu, X.; Hu, X.; Li, L.; Chen, J.; Jin, T.; Zhang, Y.; Tan, Z.; Huang, P.; et al. IL2RA+VSIG4+ tumor-associated macrophage is a key subpopulation of the immunosuppressive microenvironment in anaplastic thyroid cancer. Biochim. Biophys. Acta. Mol. Basis Dis. 2023, 1869, 166591. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Queirolo, P.; Boero, S.; Salvi, S.; Piccioli, P.; Boccardo, S.; Minghelli, S.; Morabito, A.; Fontana, V.; Pietra, G.; et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J. Transl. Med. 2013, 11, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Contardi, E.; Palmisano, G.L.; Tazzari, P.L.; Martelli, A.M.; Falà, F.; Fabbi, M.; Kato, T.; Lucarelli, E.; Donati, D.; Polito, L.; et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer 2005, 117, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zhang, H.; Preston, S.; Martin, K.; Zhou, B.; Vadalia, N.; Gamero, A.M.; Soboloff, J.; Tempera, I.; Zaidi, M.R. Interferon-γ Signaling in Melanocytes and Melanoma Cells Regulates Expression of CTLA-4. Cancer Res. 2018, 78, 436–450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomes, P.S.; Bhardwaj, J.; Rivera-Correa, J.; Freire-De-Lima, C.G.; Morrot, A. Immune Escape Strategies of Malaria Parasites. Front. Microbiol. 2016, 7, 1617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joannin, N.; Abhiman, S.; Sonnhammer, E.L.; Wahlgren, M. Sub-grouping and sub-functionalization of the RIFIN multi-copy protein family. BMC Genom. 2008, 9, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saito, F.; Hirayasu, K.; Satoh, T.; Wang, C.W.; Lusingu, J.; Arimori, T.; Shida, K.; Palacpac, N.M.Q.; Itagaki, S.; Iwanaga, S.; et al. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature 2017, 552, 101–105, Erratum in Nature 2018, 554, 554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pieper, K.; Tan, J.; Piccoli, L.; Foglierini, M.; Barbieri, S.; Chen, Y.; Silacci-Fregni, C.; Wolf, T.; Jarrossay, D.; Anderle, M.; et al. Public antibodies to malaria antigens generated by two LAIR1 insertion modalities. Nature 2017, 548, 597–601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, K.; Wang, Y.; Shen, C.H.; Chen, Y.; Zhang, B.; Liu, K.; Tsybovsky, Y.; Wang, S.; Farney, S.K.; Gorman, J.; et al. Structural basis of LAIR1 targeting by polymorphic Plasmodium RIFINs. Nat. Commun. 2021, 12, 4226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Li, X.; Chai, Y.; Song, H.; Qi, J.; Gao, G.F. Structural basis of malarial parasite RIFIN-mediated immune escape against LAIR1. Cell Rep. 2021, 36, 109600. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.W.; Siminovitch, K.A.; de Souza, L.; Tsui, F.W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 1993, 4, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Schweitzer, P.A.; Rajan, T.V.; Yi, T.; Ihle, J.N.; Matthews, R.J.; Thomas, M.L.; Beier, D.R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 1993, 73, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Ma, D.; Lin, D.; Sun, Y.; Liu, X.; Li, Q.; Jia, W.; Cao, Y.; Zhu, Y.; Jin, B. 9.1C3 is identical to LAIR-1, which is expressed on hematopoietic progenitors. Biochem. Biophys. Res. Commun. 2003, 310, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, X.; Zhao, H.; Fu, Q.; Cao, Y.; Wang, Y.; Feng, X.; Fu, A. Leukocyte-associated immunoglobulin-like receptor-1 is expressed on human megakaryocytes and negatively regulates the maturation of primary megakaryocytic progenitors and cell line. Biochem. Biophys. Res. Commun. 2011, 405, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.; Mahadevan, D.; Mohammad, S.; Kazmi, A.; Manda, S.; Sohal, D.; Hutson, T.E.; Fu, S.; Martz, A.; Kordahi, S.; et al. A phase 1b study of NC410 in combination with pembrolizumab in immune checkpoint inhibitor (ICI) naïve, and refractory microsatellite stable (MSS)/microsatellite instability-low (MSI-L) colorectal cancer (CRC) and ovarian cancer. J. Clin. Oncol. 2024, 42 (Suppl. S16), 2538. [Google Scholar] [CrossRef]



| Antibody Clone Name | Antibody Feature | Immunization with | Assays Applied | Preclinical/ Clinical Application | Main Reference |
|---|---|---|---|---|---|
| 9.1C3 | IgG2a mo anti-h LAIR1 | LGL | FC, IHC, FA | ND/ND | [41] |
| NKTA255 | IgG1 mo anti-h LAIR1 | NK cells and CD2- thymocytes | FC, WB, FA | ND/ND | [42] |
| NKTA72 | IgG1 mo anti-h LAIR1 | NK cells and CD2- thymocytes | FC, WB, FA | ND/ND | [42] |
| 1B1 | IgG1 mo anti-h LAIR1 | NK cell clones | FC, WB, FA | ND/ND | [42] |
| 1F1 | IgG1 mo anti-h LAIR1 | NK cell clones | FC, WB, FA | ND/ND | [42] |
| DX26 | IgG1 mo anti-h LAIR1 | NK cells | FC, WB, FA | ND/ND | [43] |
| 8A8 | IgG1k mo anti-h LAIR1 | Not determined | FA | ND/ND | [51] |
| lc12 (ab14826) | IgG mo anti-h LAIR1 | Abcam, not defined | IF | Yes/ND | [52] |
| (F-5) sc-398141 | IgG2a mo anti-h LAIR1 | Amino acids 182–287 mapping at the C-terminus of LAIR1 of human origin | WB, IP, IF, ELISA | ND/ND | [53] |
| Antisera 145 Antisera 148 | Polyclonal rabbit anti-h LAIR1 | 145 * 148 ** | IP * WB ** | ND/ND | [54] |
| 3G4 | Mo anti-h IgG1 | LAIR2-GST fusion protein | WB/FA ^ | ND/ND | [55] |
| HPA011155 | Polyclonal rabbit anti-h LAIR1 | Not defined | FC, WB, IHC | ND/ND | [56] |
| 342219 FAB2664JF525 | IgG2b mo anti-h LAIR1 | rh LAIR1 isoform 1 Gln22-His163 | WB, FC, CyTOF-ready | ND/ND | [57] |
| Monoclonal 113 | IgG Armenian hamster anti-h | Abcam Not determined | WB, IP, FC, FA | ND/ND | [58] |
| NGM438 | IgG1 humanized anti-h LAIR1 | Not determined | High-affinity therapeutic mAb to antagonize LAIR1 in solid cancers | ND/ND | [59] |
| NGM438 | Modified to react with mouse LAIR1 | Not determined | Therapeutic antibody in mouse models | Yes/ND | [59] |
| NC525 | IgG1k Humanized anti-h LAIR1 | Not determined | Reactivity with LAIR1+ AML cells | Yes/Yes | [60] |
| FMU-mLAIR-1.1, FMU-mLAIR-1.2, FMU-mLAIR-1.3 | IgM IgG1 IgM Rat anti-mouse LAIR1 | Murine LAIR1-Fc | Reactivity with murine LAIR1 cells IHC, WB, FC | Possible use in murine models | [61] |
| Receptors Designation Cluster Differentiation | Ligand | Cell Expression | Ligand Expression | Reference |
|---|---|---|---|---|
| LAIR1/CD305 LAIR2/CD306 | Collagen, C1q, adiponectin, surfactant protein D, RIFIN | T, B, NK, myeloid cells, tumor cells | Every tissue | [96] |
| Inhibitory KIR/CD158 | MHC-I allele | NK, T cells | Almost all nucleated cells | [97] |
| Activating KIR/CD158 | MHC-I allele | NK, T cells | Almost all nucleated cells | [97] |
| Inhibitory CLIR CD94/NKG2B CD94/NKG2A | MHC-I allele | NK, T cells | Almost all nucleated cells | [71] |
| Activating CLIR CD94/NKG2C | MHC-I allele | NK, T cells | Almost all nucleated cells | [98] |
| ILT/LILRB/LIR/CD85 | Several HLA-I antigens, HLA-G | Myeloid cells, B cells | Almost all nucleated cells | [99,100] |
| Irp60/CD300a | PE, PS | Myeloid cells, B cells | activated, infected, transformed, or apoptotic cells | [101] |
| IREM1/CD300f | Not determined in humans, norovirus receptor in mice | Myeloid cells and mast cells | [102] | |
| Siglec1-13, 15-17 inhibiting | Glycans with sialic acid | Myeloid cells, B cells, osteoclasts | Different cell types | [103] |
| Siglec14 activating | Glycans with sialic acid | Primary and secondary lymphoid organs, subsets of innate cells | Different cell types | [103] |
| CTLA4/CD152 | CD80/CD86 | Activated T cells several subsets (Treg), tumor cells of different histotypes | Antigen-presenting cells | [26] |
| PD1/CD274 | PDL1/PDL2 | T, NK cells | Antigen-presenting cells, several tumor cells | [104] |
| TIM3/CD366/HAVCR2 | Hepatitis virus A cellular receptor 2, Galectin 9, phosphatidyl serine, HMGB1, CEACAM1 | T, NK cells | Different cell types, antigen-presenting cells, epithelial cells | [105] |
| TIGIT/ VSIG9 | CD155/PVR, CD112/PVRL2 | T, NK cells | High expression on tumor cells, low expression in normal cells | [106] |
| LAG3/CD223 | MHC-II, Galectin 3, FGL1 | T cells, NK cells, pDC B cells | Antigen-presenting cells, activated lymphocytes | [107] |
| CD96/TACTILE | CD155/PVR | NK cells, T cells | High expression on tumor cells, low expression in normal cells | [108] |
| VISTA | VSIG-3, PSGL-1, VISIG-8, Galectin 9, Sdc-2, LRIG-1 | T subsets, Treg, MDSC | Several types of cells, immune cells | [109] |
| Solid Tumor Type | LAIR1 Expression | LAIR1-Mediated Function After Silencing | LAIR1-Mediated Function After Overexpression | LAIR1 Association with Biological Features of Solid Tumor | Reference DOI |
|---|---|---|---|---|---|
| Ovarian carcinoma | Tumor tissues Some cell lines | Increase in proliferation, colony formation, matrix invasion | Inhibition of proliferation, migration, induction apoptosis | Correlates with grade | [52,193] |
| Breast carcinoma | Tumor tissues, some cell lines | Reduces proliferation and invasion | ND | Correlates with shorter patient survival, and grade | [194] |
| Renal cell carcinoma | Tumor tissues | Reduces proliferation | Increase in proliferation | Correlates with shorter survival | [56] |
| Cervical carcinoma | Tumor tissues | ND | Reduction in proliferation and anti-apoptosis capacity | Correlates with the grade | [199] |
| Osteosarcoma | Tumor tissues and cell lines and healthy osteoblasts | ND | Inhibition of migration and EMT | Correlates with the stage | [53] |
| Glioblastoma | Low-grade glioma, some cell lines | ND | In murine models, large tumor with LAIR1 overexpression | [200] | |
| Hepatocellular carcinoma | Tumor tissues, association with low differentiation | Increasing PDL1 expression | Worse OS | [196,201] | |
| Oral squamous cell carcinoma | Tumor tissue, mainly associated with leukocyte infiltration * | ND | ND | Association with grade and immunosuppressive cells (MDSC, M2) | [195] |
| Gene/Agent | Isoform/Molecule | RefSeq (mRNA/protein) | Functional Role | Therapeutic Relevance | Clinical/Preclinical Tools | PMID/Trial |
|---|---|---|---|---|---|---|
| LAIR1 | Isoform A (canonical) | NM_002287.4/NP_002278.2 | Full-length inhibitory receptor with ITIM motifs | Immune checkpoint in tumors, infections, inflammation | Antagonist mAbs – preclinical | PMID: 38648067 PMID: 36211388 |
| MSPHPTALLGLVLCLAQTIHTQEEDLPRPSISAEPGTVIPLGSHVTFVCRGPVGVQTFRLERDSRSTYNDTEDVSQASPSESEARFRIDSVREGNAGLYRCIYYKPPKWSEQSDYLELLVKESSGGPDSPDTEPGSSAGPTQRPSDNSHNEHAPASQGLKAEHLYI LIGVSVVFLFCLLLLVLFCLHRQNQIKQGPPRSKDEEQKPQQRPDLAVDVLERTADKATVNGLPEKDRETDTSALAAGSSQEVTYAQLDHWALTQRTARAVSPQSTKPMAESITYAAVARH | ||||||
| LAIR1 | Isoform B | NM_001289025.3/NP_001275954.2 | C-terminal variant | Unknown | None known | — |
| MSPHPTALLGLVLCLAQTIHTQEEDLPRPSISAEPGTVIPLGSHVTFVCRGPVGVQTFRLERDSRSTYNDTEDVSQASPSESEARFRIDSVREGNAGLYRCIYYKPPKWSEQSDYLELLVKXXXXXXXXXXXXXXXXXGPTQRPSDNSHNEHAPASQGLKAEHLYI LIGVSVVFLFCLLLLVLFCLHRQNQIKQGPPRSKDEEQKPQQRPDLAVDVLERTADKATVNGLPEKDRETDTSALAAGSSQEVTYAQLDHWALTQRTARAVSPQSTKPMAESITYAAVARH | ||||||
| LAIR1 | Isoform C | NM_001289026.3/NP_001275955.2 | Truncated cytoplasmic tail | Not established | None known | — |
| MSPHPTALLGLVLCLAQTIHTQEXDLPRPSISAEPGTVIPLGSHVTFVCRGPVGVQTFRLERDSRSTYNDTEDVSQASPSESEARFRIDSVREGNAGLYRCIYYKPPKWSEQSDYLELLVKXXXXXXXXXXXXXXXXXGPTQRPSDNSHNEHAPASQGLKAEHLYI LIGVSVVFLFCLLLLVLFCLHRQNQIKQGPPRSKDEEQKPQQRPDLAVDVLERTADKATVNGLPEKDRETDTSALAAGSSQEVTYAQLDHWALTQRTARAVSPQSTKPMAESITYAAVARH | ||||||
| LAIR1 | Isoform D | NM_001289027.3/NP_001275956.2 | Lacks ITIMs; potential decoy | No validated role | None known | — |
| MSPHPTALLGLVLCLAQTIHTQEEDLPRPSISAEPGTVIPLGSHVTFVCRGPVGVQTFRLERDSRSTYNDTEDVSQASPSESEARFRIDSVREGNAGLYRCIYYKPPKWSEQSDYLELLVKESSGGPDSPDTEPGSSAGPTQRPSDNSHNEHAPASQGLKAEHLYI LIGVSVVFLFCLLLLVLFCLHRQNQIKQGPPRSKDEEQKPQQR | ||||||
| LAIR2 | Soluble isoform | NM_001014835.2/NP_001014835.1 | Secreted decoy receptor that binds collagen and C1q | Potential immune modulator and biomarker | NC410 (LAIR-2–Fc)–Phase I clinical trial | PMID: 34121658 PMID:38236251 NCT04408599 NCT05572684 NCT06941857 |
| MSPHLTALLGLVLCLAQTIHTQEGALPRPSISAEPGTVISPGSHVTFMCRGPVGVQTFRLEREDRAKYKDSYNVFRLGPSESEARFHIDSVSEGNAGLYRCLYYKPPGWSEHSDFLELLVKESSGGPDSPDTEPGSSAGTVPGTEASGFDAP | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggi, A.; Matis, S.; Uras, C.R.M.; Raffaghello, L.; Benelli, R.; Zocchi, M.R. The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion. Biomolecules 2025, 15, 866. https://doi.org/10.3390/biom15060866
Poggi A, Matis S, Uras CRM, Raffaghello L, Benelli R, Zocchi MR. The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion. Biomolecules. 2025; 15(6):866. https://doi.org/10.3390/biom15060866
Chicago/Turabian StylePoggi, Alessandro, Serena Matis, Chiara Rosa Maria Uras, Lizzia Raffaghello, Roberto Benelli, and Maria Raffaella Zocchi. 2025. "The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion" Biomolecules 15, no. 6: 866. https://doi.org/10.3390/biom15060866
APA StylePoggi, A., Matis, S., Uras, C. R. M., Raffaghello, L., Benelli, R., & Zocchi, M. R. (2025). The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion. Biomolecules, 15(6), 866. https://doi.org/10.3390/biom15060866








