Drug Transporters and Metabolizing Enzymes in Antimicrobial Drug Pharmacokinetics: Mechanisms, Drug–Drug Interactions, and Clinical Implications
Abstract
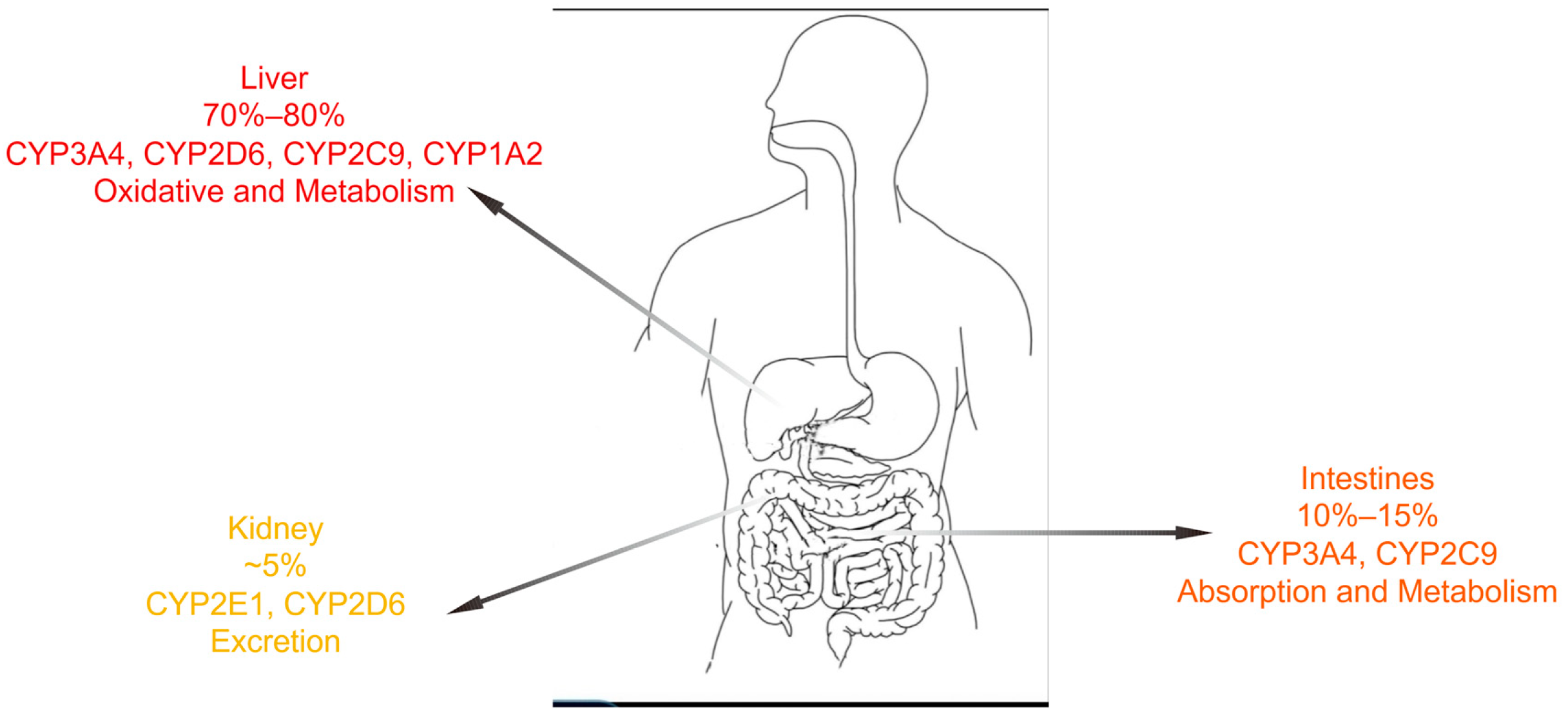
1. Introduction
2. Uptake Transporters with Antibacterial Agents
2.1. Organic Anion Transporter (OAT)
2.2. Organic Anion Transporting Polypeptide (OATP)
2.3. Organic Cation Transporter (OCT)
2.4. Oligopeptide Transporter (PEPT)
| Uptake Transporters | Substrates | Inhibitors |
|---|---|---|
| OAT1 | Cefazolin [33], cefotiam [33,34], cephalexin [33,34], meropenem [37], nemonoxacin [38] | Tetracycline [33], oxytetracycline [33], minocycline [33], doxycycline [33], meropenem–vaborbactam [37] |
| OAT2 | Cefotaxime [36], erythromycin [36], tetracycline [36] | Tetracycline [33], oxytetracycline [33], minocycline [33] |
| OAT3 | Cefdinir [35], cefotiam [35], meropenem [37], nemonoxacin [38], penicillin [40] | Meropenem–vaborbactam [37] |
| OAT4 | Tetracycline [33] | |
| OATP1A1 | Nafcillin [54] | |
| OATP1A2 | Ciprofloxacin [47], levofloxacin [47] | |
| OATP1A4 | Nafcillin [54,55], cefradine [54], cefazolin [54], cefmetazole [54], cefoperazone [54], cefsulodin [54] | |
| OATP1A5 | Ciprofloxacin [48] | Azithromycin [51], clarithromycin [51], clindamycin [51] |
| OATP1B1 | Erythromycin [51], clarithromycin [51] | Rifampicin [49], macrolides (except for azithromycin) [52] |
| OATP1B3 | Clarithromycin [51], erythromycin [51] | Rifampicin [50], bilirubin [52], macrolides (except for azithromycin) [52] |
| OCT1 | Trimethoprim [64], ciprofloxacin [65], fleroxacin [65], gatifloxacin [65], levofloxacin [64,65], moxifloxacin [65], norfloxacin [65], ofloxacin [65], pefloxacin [65], prulifloxacin [65], sparfloxacin [65] | Ciprofloxacin [65], fleroxacin [65], gatifloxacin [65], levofloxacin [65], moxifloxacin [3,65], norfloxacin [65], ofloxacin [65], pefloxacin [65], prulifloxacin [65], sparfloxacin [65] |
| OCT2 | Nemonoxacin [38], trimethoprim [64], gentamicin [13,64] | |
| OCT3 | Moxifloxacin [3] | |
| PEPT1 | Cefuroxime axetil [81], amoxicillin [82], cefixime [88], cephalexin [83,90], amoxicillin [82,91] | Cefixime [88] |
| PEPT2 | Cephalexin [83,85], cefotaxime [83,85], cefaclor [83,84,85,89], cephaloridine [83,85], cefixime [88] | Cefixime [88] |
3. Efflux Transporters with Antibacterial Agents
3.1. P-Glycoprotein (P-Gp)
3.2. Breast Cancer Resistance Protein (BCRP)
3.3. Multidrug Resistance-Associated Protein (MRP)
3.4. Mammal Multidrug and Toxin Extrusion Protein (MATE)
| Efflux Transporters | Substrates | Inhibitors |
|---|---|---|
| P-gp | Tobramycin [98], minocycline [98], oxytetracycline [99], ivermectin [99], gemifloxacin [107] | Clarithromycin [94,96], telithromycin [96], roxithromycin [96], azithromycin [96], erythromycin [96], minocycline [98], oxytetracycline [99], gatifloxacin [102], gemifloxacin [107] |
| BCRP | Ciprofloxacin [100,101,103], ofloxacin [101,103], norfloxacin [101], grepafloxacin [103], prulifloxacin [103], cefoperazone [104], cefamandole [104], ceftriaxone [104], cefotiam [104] | |
| MRP2 | Cefoperazone [102], cefpiramide [102], ceftriaxone [102], cefotetan [102], cefotiam [102], erythromycin [102], gemifloxacin [107], danofloxacin mesylate [108] | Gatifloxacin [102], gemifloxacin [107] |
| MATE1 | Norfloxacin [109], ciprofloxacin [109], kanamycin [109], erythromycin [109] | Moxifloxacin [3] |
| MATE2-K | Norfloxacin [109], ciprofloxacin [109], kanamycin [109], erythromycin [109] | Moxifloxacin [3] |
4. Cytochrome P450 Enzymes with Antibacterial Agents
| CYP450 Enzymes | Substrates | Inhibitors |
|---|---|---|
| CYP1A2 | Ciprofloxacin [115] | |
| CYP2C9 | Fluconazole [34,114,116,126] | |
| CYP2C19 | Fluconazole [34,114,116,126], | |
| CYP3A4 | Erythromycin [114], rifampicin [125], fluconazole [126] | Erythromycin [114], ciprofloxacin [114], enoxacin [114], fluconazole [34,114,116,126], itraconazole [114], voriconazole [114], clarithromycin [119,124], erythromycin [119], isoniazid [119], ritonavir [119], delavirdine [119] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, T.; Zeng, S.; Zheng, Q.; Zhu, F. The Important Role of Transporter Structures in Drug Disposition, Efficacy, and Toxicity. Drug Metab. Dispos. 2023, 51, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. S10), S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Te Brake, L.H.; van den Heuvel, J.J.; Buaben, A.O.; van Crevel, R.; Bilos, A.; Russel, F.G.; Aarnoutse, R.E.; Koenderink, J.B. Moxifloxacin Is a Potent In Vitro Inhibitor of OCT- and MATE-Mediated Transport of Metformin and Ethambutol. Antimicrob. Agents Chemother. 2016, 60, 7105–7114. [Google Scholar] [CrossRef] [PubMed]
- Momper, J.D.; Nigam, S.K. Developmental regulation of kidney and liver solute carrier and ATP-binding cassette drug transporters and drug metabolizing enzymes: The role of remote organ communication. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Lee, J.; Beers, J.L.; Geffert, R.M.; Jackson, K.D. A Review of CYP-Mediated Drug Interactions: Mechanisms and In Vitro Drug-Drug Interaction Assessment. Biomolecules 2024, 14, 99. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Metabolism and Excretion Prediction: Recent Advances, Challenges, and Future Perspectives. Pharmaceutics 2023, 15, 1260. [Google Scholar] [CrossRef]
- Liu, X. Transporter-Mediated Drug-Drug Interactions and Their Significance. Adv. Exp. Med. Biol. 2019, 1141, 241–291. [Google Scholar] [CrossRef]
- Gyimesi, G.; Hediger, M.A. Transporter-Mediated Drug Delivery. Molecules 2023, 28, 1151. [Google Scholar] [CrossRef]
- Zhang, Y.; Hagenbuch, B. Protein-protein interactions of drug uptake transporters that are important for liver and kidney. Biochem. Pharmacol. 2019, 168, 384–391. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, X.; Liu, H.; Yuan, Y.; Xiao, Y.; Nan, J.; Zhang, W.; Song, W.; Wang, J.; Wei, F.; et al. Cryo-EM structures of human organic anion transporting polypeptide OATP1B1. Cell Res. 2023, 33, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Fan, Y.; Yu, Z.; You, G. Regulation of organic anion transporters: Role in physiology, pathophysiology, and drug elimination. Pharmacol. Ther. 2021, 217, 107647. [Google Scholar] [CrossRef]
- Samodelov, S.L.; Kullak-Ublick, G.A.; Gai, Z.; Visentin, M. Organic Cation Transporters in Human Physiology, Pharmacology, and Toxicology. Int. J. Mol. Sci. 2020, 21, 7890. [Google Scholar] [CrossRef]
- Zhou, S.; Shu, Y. Transcriptional Regulation of Solute Carrier (SLC) Drug Transporters. Drug Metab. Dispos. 2022, 50, 1238–1250. [Google Scholar] [CrossRef]
- Mora Lagares, L.; Novič, M. Recent Advances on P-Glycoprotein (ABCB1) Transporter Modelling with In Silico Methods. Int. J. Mol. Sci. 2022, 23, 14804. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; de Wolf, C.; van de Wetering, K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007, 453, 661–673. [Google Scholar] [CrossRef]
- Nies, A.T.; Damme, K.; Kruck, S.; Schaeffeler, E.; Schwab, M. Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch. Toxicol. 2016, 90, 1555–1584. [Google Scholar] [CrossRef] [PubMed]
- Mehendale-Munj, S.; Sawant, S. Breast Cancer Resistance Protein: A Potential Therapeutic Target for Cancer. Curr. Drug Targets 2021, 22, 420–428. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Khardori, N.; Stevaux, C.; Ripley, K. Antibiotics: From the Beginning to the Future: Part 2. Indian. J. Pediatr. 2020, 87, 43–47. [Google Scholar] [CrossRef]
- Méndez, R.; Latorre, A.; González-Jiménez, P. Ceftobiprole medocaril. Rev. Esp. Quimioter. 2022, 35 (Suppl. S1), 25–27. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, M.; Mensa, J. Ceftazidime-avibactam. Rev. Esp. Quimioter. 2021, 34 (Suppl. S1), 38–40. [Google Scholar] [CrossRef]
- Novelli, A.; Del Giacomo, P.; Rossolini, G.M.; Tumbarello, M. Meropenem/vaborbactam: A next generation β-lactam β-lactamase inhibitor combination. Expert. Rev. Anti Infect. Ther. 2020, 18, 643–655. [Google Scholar] [CrossRef]
- Keam, S.J. Sulbactam/Durlobactam: First Approval. Drugs 2023, 83, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, Y.; Qu, X.; Bian, X.; Hu, J.; Xu, X.; Xiao, L.; Liu, Y.; Zhang, J. In vitro pharmacodynamics of nemonoxacin and other antimicrobial agents against Mycoplasma pneumoniae. Microbiol. Spectr. 2023, 11, e0243123. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.M.A.; Sominsky, L.A.; Chittò, M.; Schwarz, E.M.; Moriarty, T.F. Antibacterial Mechanisms and Clinical Impact of Sitafloxacin. Pharmaceuticals 2024, 17, 1537. [Google Scholar] [CrossRef]
- Huang, P.Y.; Hsu, C.K.; Tang, H.J.; Lai, C.C. Eravacycline: A comprehensive review of in vitro activity, clinical efficacy, and real-world applications. Expert. Rev. Anti Infect. Ther. 2024, 22, 387–398. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Ianevski, A.; Ahmad, S.; Anunnitipat, K.; Oksenych, V.; Zusinaite, E.; Tenson, T.; Bjørås, M.; Kainov, D.E. Seven classes of antiviral agents. Cell Mol. Life Sci. 2022, 79, 605. [Google Scholar] [CrossRef]
- Marques, L.; Vale, N. Prediction of CYP-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Modeling: A Case Study of Salbutamol and Fluvoxamine. Pharmaceutics 2023, 15, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zeng, S.; Shu, Y. Drug-Drug Interactions at Organic Cation Transporter 1. Front. Pharmacol. 2021, 12, 628705. [Google Scholar] [CrossRef] [PubMed]
- Babu, E.; Takeda, M.; Narikawa, S.; Kobayashi, Y.; Yamamoto, T.; Cha, S.H.; Sekine, T.; Sakthisekaran, D.; Endou, H. Human organic anion transporters mediate the transport of tetracycline. Jpn. J. Pharmacol. 2002, 88, 69–76. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef]
- Baietto, L.; Corcione, S.; Pacini, G.; Perri, G.D.; D’Avolio, A.; De Rosa, F.G. A 30-years review on pharmacokinetics of antibiotics: Is the right time for pharmacogenetics? Curr. Drug Metab. 2014, 15, 581–598. [Google Scholar] [CrossRef]
- Shen, H.; Lai, Y.; Rodrigues, A.D. Organic Anion Transporter 2: An Enigmatic Human Solute Carrier. Drug Metab. Dispos. 2017, 45, 228–236. [Google Scholar] [CrossRef]
- Shoulders, B.R.; Casapao, A.M.; Venugopalan, V. An Update on Existing and Emerging Data for Meropenem-Vaborbactam. Clin. Ther. 2020, 42, 692–702. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dai, X.J.; Yang, Y.; Chen, X.Y.; Wang, T.; Tang, Y.B.; Tsai, C.Y.; Chang, L.W.; Chang, Y.T.; Zhong, D.F. Effects of probenecid and cimetidine on the pharmacokinetics of nemonoxacin in healthy Chinese volunteers. Drug Des. Devel Ther. 2016, 10, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef]
- Burckhardt, G.; Burckhardt, B.C. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb. Exp. Pharmacol. 2011, 201, 29–104. [Google Scholar] [CrossRef]
- Barza, M.; Weinstein, L. Pharmacokinetics of the penicillins in man. Clin. Pharmacokinet. 1976, 1, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Meng, Q.; Huo, X.; Sun, H.; Peng, J.; Ma, X.; Sun, P.; Liu, K. MDR1 and OAT1/OAT3 mediate the drug-drug interaction between puerarin and methotrexate. Pharm. Res. 2014, 31, 1120–1132. [Google Scholar] [CrossRef]
- Gessner, A.; König, J.; Fromm, M.F. Clinical Aspects of Transporter-Mediated Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1386–1394. [Google Scholar] [CrossRef]
- Ciută, A.D.; Nosol, K.; Kowal, J.; Mukherjee, S.; Ramírez, A.S.; Stieger, B.; Kossiakoff, A.A.; Locher, K.P. Structure of human drug transporters OATP1B1 and OATP1B3. Nat. Commun. 2023, 14, 5774. [Google Scholar] [CrossRef]
- Niemi, M.; Pasanen, M.K.; Neuvonen, P.J. Organic anion transporting polypeptide 1B1: A genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol. Rev. 2011, 63, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, J.; Czubacka, A.; Oleszek, W. Dietary phytochemicals and human health. Adv. Exp. Med. Biol. 2010, 698, 74–98. [Google Scholar] [CrossRef]
- Maeda, T.; Takahashi, K.; Ohtsu, N.; Oguma, T.; Ohnishi, T.; Atsumi, R.; Tamai, I. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol. Pharm. 2007, 4, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Shirasaka, Y.; Li, Y.; Shibue, Y.; Kuraoka, E.; Spahn-Langguth, H.; Kato, Y.; Langguth, P.; Tamai, I. Concentration-dependent effect of naringin on intestinal absorption of beta(1)-adrenoceptor antagonist talinolol mediated by p-glycoprotein and organic anion transporting polypeptide (Oatp). Pharm. Res. 2009, 26, 560–567. [Google Scholar] [CrossRef]
- Litjens, C.H.C.; van den Heuvel, J.; Russel, F.G.M.; Aarnoutse, R.E.; Te Brake, L.H.M.; Koenderink, J.B. Rifampicin Transport by OATP1B1 Variants. Antimicrob. Agents Chemother. 2020, 64, 1–6. [Google Scholar] [CrossRef]
- Pahwa, S.; Alam, K.; Crowe, A.; Farasyn, T.; Neuhoff, S.; Hatley, O.; Ding, K.; Yue, W. Pretreatment With Rifampicin and Tyrosine Kinase Inhibitor Dasatinib Potentiates the Inhibitory Effects Toward OATP1B1- and OATP1B3-Mediated Transport. J. Pharm. Sci. 2017, 106, 2123–2135. [Google Scholar] [CrossRef]
- Garver, E.; Hugger, E.D.; Shearn, S.P.; Rao, A.; Dawson, P.A.; Davis, C.B.; Han, C. Involvement of intestinal uptake transporters in the absorption of azithromycin and clarithromycin in the rat. Drug Metab. Dispos. 2008, 36, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Seithel, A.; Eberl, S.; Singer, K.; Auge, D.; Heinkele, G.; Wolf, N.B.; Dörje, F.; Fromm, M.F.; König, J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab. Dispos. 2007, 35, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- Nakakariya, M.; Shimada, T.; Irokawa, M.; Koibuchi, H.; Iwanaga, T.; Yabuuchi, H.; Maeda, T.; Tamai, I. Predominant contribution of rat organic anion transporting polypeptide-2 (Oatp2) to hepatic uptake of beta-lactam antibiotics. Pharm. Res. 2008, 25, 578–585. [Google Scholar] [CrossRef]
- Nakakariya, M.; Shimada, T.; Irokawa, M.; Maeda, T.; Tamai, I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics. Drug Metab. Pharmacokinet. 2008, 23, 347–355. [Google Scholar] [CrossRef]
- Alam, K.; Crowe, A.; Wang, X.; Zhang, P.; Ding, K.; Li, L.; Yue, W. Regulation of Organic Anion Transporting Polypeptides (OATP) 1B1- and OATP1B3-Mediated Transport: An Updated Review in the Context of OATP-Mediated Drug-Drug Interactions. Int. J. Mol. Sci. 2018, 19, 855. [Google Scholar] [CrossRef]
- Shitara, Y.; Hirano, M.; Sato, H.; Sugiyama, Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: Analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J. Pharmacol. Exp. Ther. 2004, 311, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, B.; Drouot, S.; Barrail-Tran, A.; Taburet, A.M. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin. Pharmacokinet. 2013, 52, 815–831. [Google Scholar] [CrossRef]
- Olsson, A.G.; McTaggart, F.; Raza, A. Rosuvastatin: A highly effective new HMG-CoA reductase inhibitor. Cardiovasc. Drug Rev. 2002, 20, 303–328. [Google Scholar] [CrossRef]
- Schneck, D.W.; Birmingham, B.K.; Zalikowski, J.A.; Mitchell, P.D.; Wang, Y.; Martin, P.D.; Lasseter, K.C.; Brown, C.D.; Windass, A.S.; Raza, A. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin. Pharmacol. Ther. 2004, 75, 455–463. [Google Scholar] [CrossRef]
- Wenzel, C.; Drozdzik, M.; Oswald, S. Organic Cation Transporter 1 an Intestinal Uptake Transporter: Fact or Fiction? Front. Pharmacol. 2021, 12, 648388. [Google Scholar] [CrossRef] [PubMed]
- Angenoorth, T.J.F.; Maier, J.; Stankovic, S.; Bhat, S.; Sucic, S.; Freissmuth, M.; Sitte, H.H.; Yang, J.W. Rescue of Misfolded Organic Cation Transporter 3 Variants. Cells 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.H. Molecular and cellular physiology of organic cation transporter 2. Am. J. Physiol. Renal Physiol. 2019, 317, F1669–F1679. [Google Scholar] [CrossRef] [PubMed]
- Redeker, K.M.; Jensen, O.; Gebauer, L.; Meyer-Tönnies, M.J.; Brockmöller, J. Atypical Substrates of the Organic Cation Transporter 1. Biomolecules 2022, 12, 1664. [Google Scholar] [CrossRef]
- Mulgaonkar, A.; Venitz, J.; Gründemann, D.; Sweet, D.H. Human organic cation transporters 1 (SLC22A1), 2 (SLC22A2), and 3 (SLC22A3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob. Agents Chemother. 2013, 57, 2705–2711. [Google Scholar] [CrossRef]
- Jung, N.; Lehmann, C.; Rubbert, A.; Knispel, M.; Hartmann, P.; van Lunzen, J.; Stellbrink, H.J.; Faetkenheuer, G.; Taubert, D. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab. Dispos. 2008, 36, 1616–1623. [Google Scholar] [CrossRef]
- Nies, A.T.; Koepsell, H.; Damme, K.; Schwab, M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb. Exp. Pharmacol. 2011, 201, 105–167. [Google Scholar] [CrossRef]
- Tahara, H.; Kusuhara, H.; Endou, H.; Koepsell, H.; Imaoka, T.; Fuse, E.; Sugiyama, Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 2005, 315, 337–345. [Google Scholar] [CrossRef]
- Umehara, K.I.; Iwatsubo, T.; Noguchi, K.; Usui, T.; Kamimura, H. Effect of cationic drugs on the transporting activity of human and rat OCT/Oct 1-3 in vitro and implications for drug-drug interactions. Xenobiotica 2008, 38, 1203–1218. [Google Scholar] [CrossRef]
- Bachmakov, I.; Glaeser, H.; Endress, B.; Mörl, F.; König, J.; Fromm, M.F. Interaction of beta-blockers with the renal uptake transporter OCT2. Diabetes Obes. Metab. 2009, 11, 1080–1083. [Google Scholar] [CrossRef]
- Zolk, O.; Solbach, T.F.; König, J.; Fromm, M.F. Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab. Dispos. 2009, 37, 1312–1318. [Google Scholar] [CrossRef]
- Bachmakov, I.; Glaeser, H.; Fromm, M.F.; König, J. Interaction of oral antidiabetic drugs with hepatic uptake transporters: Focus on organic anion transporting polypeptides and organic cation transporter 1. Diabetes 2008, 57, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Ayrton, A.; Morgan, P. Role of transport proteins in drug discovery and development: A pharmaceutical perspective. Xenobiotica 2008, 38, 676–708. [Google Scholar] [CrossRef] [PubMed]
- Kindla, J.; Fromm, M.F.; König, J. In vitro evidence for the role of OATP and OCT uptake transporters in drug-drug interactions. Expert. Opin. Drug Metab. Toxicol. 2009, 5, 489–500. [Google Scholar] [CrossRef]
- Moore, K.H.; Yuen, G.J.; Raasch, R.H.; Eron, J.J.; Martin, D.; Mydlow, P.K.; Hussey, E.K. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin. Pharmacol. Ther. 1996, 59, 550–558. [Google Scholar] [CrossRef]
- Tanihara, Y.; Masuda, S.; Katsura, T.; Inui, K. Protective effect of concomitant administration of imatinib on cisplatin-induced nephrotoxicity focusing on renal organic cation transporter OCT2. Biochem. Pharmacol. 2009, 78, 1263–1271. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstädt, H.; Lanvers-Kaminsky, C.; am Zehnhoff-Dinnesen, A.; Schinkel, A.H.; et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef]
- Türk, D.; Müller, F.; Fromm, M.F.; Selzer, D.; Dallmann, R.; Lehr, T. Renal Transporter-Mediated Drug-Biomarker Interactions of the Endogenous Substrates Creatinine and N(1)-Methylnicotinamide: A PBPK Modeling Approach. Clin. Pharmacol. Ther. 2022, 112, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, M. Transport of drugs by proton-coupled peptide transporters: Pearls and pitfalls. Expert. Opin. Drug Metab. Toxicol. 2009, 5, 887–905. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Daniel, H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 2008, 38, 1022–1042. [Google Scholar] [CrossRef]
- Kramer, W. Transporters, Trojan horses and therapeutics: Suitability of bile acid and peptide transporters for drug delivery. Biol. Chem. 2011, 392, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, M. Drug transport via the intestinal peptide transporter PepT1. Curr. Opin. Pharmacol. 2013, 13, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Liu, Q.; Meng, Q.; Cang, J.; Sun, H.; Gao, Y.; Kaku, T.; Liu, K. Pharmacokinetic interaction between JBP485 and cephalexin in rats. Drug Metab. Dispos. 2010, 38, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, M.E.; Prasad, P.D.; Mackenzie, B.; Ganapathy, V.; Leibach, F.H. Interaction of anionic cephalosporins with the intestinal and renal peptide transporters PEPT 1 and PEPT 2. Biochim. Biophys. Acta 1997, 1324, 296–308. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Wu, J.; Wang, C.; Peng, J.; Ma, X.; Liu, K. PEPT1 involved in the uptake and transepithelial transport of cefditoren in vivo and in vitro. Eur. J. Pharmacol. 2009, 612, 9–14. [Google Scholar] [CrossRef]
- Song, F.; Hu, Y.; Jiang, H.; Smith, D.E. Species Differences in Human and Rodent PEPT2-Mediated Transport of Glycylsarcosine and Cefadroxil in Pichia Pastoris Transformants. Drug Metab. Dispos. 2017, 45, 130–136. [Google Scholar] [CrossRef]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Liu, Q.; Meng, Q.; Huo, X.; Sun, P.; Yang, X.; Sun, H.; Zhen, Y.; Peng, J.; et al. PEPT1- and OAT1/3-mediated drug-drug interactions between bestatin and cefixime in vivo and in vitro in rats, and in vitro in human. Eur. J. Pharm. Sci. 2014, 63, 77–86. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, I.B.; Noh, C.K.; Quach, H.P.; Yoon, I.S.; Chow, E.C.Y.; Kim, M.; Jin, H.E.; Cho, K.H.; Chung, S.J.; et al. Effects of 1α,25-dihydroxyvitamin D3, the natural vitamin D receptor ligand, on the pharmacokinetics of cefdinir and cefadroxil, organic anion transporter substrates, in rat. J. Pharm. Sci. 2014, 103, 3793–3805. [Google Scholar] [CrossRef]
- Ingersoll, S.A.; Ayyadurai, S.; Charania, M.A.; Laroui, H.; Yan, Y.; Merlin, D. The role and pathophysiological relevance of membrane transporter PepT1 in intestinal inflammation and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G484–G492. [Google Scholar] [CrossRef]
- Foley, D.W.; Pathak, R.B.; Phillips, T.R.; Wilson, G.L.; Bailey, P.D.; Pieri, M.; Senan, A.; Meredith, D. Thiodipeptides targeting the intestinal oligopeptide transporter as a general approach to improving oral drug delivery. Eur. J. Med. Chem. 2018, 156, 180–189. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Müller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, H.; Yano, I.; Ito, T.; Hashida, T.; Futami, T.; Nohara, R.; Sasayama, S.; Inui, K. Effect of clarithromycin on renal excretion of digoxin: Interaction with P-glycoprotein. Clin. Pharmacol. Ther. 1998, 64, 123–128. [Google Scholar] [CrossRef]
- Rengelshausen, J.; Göggelmann, C.; Burhenne, J.; Riedel, K.D.; Ludwig, J.; Weiss, J.; Mikus, G.; Walter-Sack, I.; Haefeli, W.E. Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin-clarithromycin interaction. Br. J. Clin. Pharmacol. 2003, 56, 32–38. [Google Scholar] [CrossRef]
- Eberl, S.; Renner, B.; Neubert, A.; Reisig, M.; Bachmakov, I.; König, J.; Dörje, F.; Mürdter, T.E.; Ackermann, A.; Dormann, H.; et al. Role of p-glycoprotein inhibition for drug interactions: Evidence from in vitro and pharmacoepidemiological studies. Clin. Pharmacokinet. 2007, 46, 1039–1049. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Jagannath, C.; Hunter, R.L.; Dasgupta, A. Bioavailability of tobramycin after oral delivery in FVB mice using CRL-1605 copolymer, an inhibitor of P-glycoprotein. Life Sci. 2000, 67, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Milane, A.; Fernandez, C.; Vautier, S.; Bensimon, G.; Meininger, V.; Farinotti, R. Minocycline and riluzole brain disposition: Interactions with p-glycoprotein at the blood-brain barrier. J. Neurochem. 2007, 103, 164–173. [Google Scholar] [CrossRef]
- Schrickx, J.; Fink-Gremmels, J. P-glycoprotein-mediated transport of oxytetracycline in the Caco-2 cell model. J. Vet. Pharmacol. Ther. 2007, 30, 25–31. [Google Scholar] [CrossRef]
- Haslam, I.S.; Wright, J.A.; O’Reilly, D.A.; Sherlock, D.J.; Coleman, T.; Simmons, N.L. Intestinal ciprofloxacin efflux: The role of breast cancer resistance protein (ABCG2). Drug Metab. Dispos. 2011, 39, 2321–2328. [Google Scholar] [CrossRef]
- Merino, G.; Alvarez, A.I.; Pulido, M.M.; Molina, A.J.; Schinkel, A.H.; Prieto, J.G. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab. Dispos. 2006, 34, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Vadlapatla, R.K.; Vadlapudi, A.D.; Pal, D.; Mitra, A.K. Interaction of gatifloxacin with efflux transporters: A possible mechanism for drug resistance. Int. J. Pharm. 2010, 395, 114–121. [Google Scholar] [CrossRef]
- Ando, T.; Kusuhara, H.; Merino, G.; Alvarez, A.I.; Schinkel, A.H.; Sugiyama, Y. Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab. Dispos. 2007, 35, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.J.; Hua, W.X.; Jian, Z.; Wei, P.H.; Ni, L.Y.; Hua, L.Y.; Wen, C.D.; Ying, Z.; Li, C. The Role of Drug Transporters in the Pharmacokinetics of Antibiotics. Curr. Drug Metab. 2016, 17, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, Y.; Jang, Y.; Cho, J.Y.; Yoon, S.; Chung, J.Y. Comprehensive Evaluation of OATP- and BCRP-Mediated Drug-Drug Interactions of Methotrexate Using Physiologically-Based Pharmacokinetic Modeling. Clin. Pharmacol. Ther. 2024, 116, 1013–1022. [Google Scholar] [CrossRef]
- Keppler, D. Multidrug resistance proteins (MRPs, ABCCs): Importance for pathophysiology and drug therapy. Handb. Exp. Pharmacol. 2011, 201, 299–323. [Google Scholar] [CrossRef]
- Vadlapatla, R.K.; Vadlapudi, A.D.; Kwatra, D.; Pal, D.; Mitra, A.K. Differential effect of P-gp and MRP2 on cellular translocation of gemifloxacin. Int. J. Pharm. 2011, 420, 26–33. [Google Scholar] [CrossRef]
- Schrickx, J.A.; Fink-Gremmels, J. Danofloxacin-mesylate is a substrate for ATP-dependent efflux transporters. Br. J. Pharmacol. 2007, 150, 463–469. [Google Scholar] [CrossRef]
- Claxton, D.P.; Jagessar, K.L.; McHaourab, H.S. Principles of Alternating Access in Multidrug and Toxin Extrusion (MATE) Transporters. J. Mol. Biol. 2021, 433, 166959. [Google Scholar] [CrossRef]
- Shen, H.; Yao, M.; Sinz, M.; Marathe, P.; Rodrigues, A.D.; Zhu, M. Renal Excretion of Dabigatran: The Potential Role of Multidrug and Toxin Extrusion (MATE) Proteins. Mol. Pharm. 2019, 16, 4065–4076. [Google Scholar] [CrossRef]
- Saad, A.A.A.; Zhang, F.; Mohammed, E.A.H.; Wu, X. Clinical Aspects of Drug-Drug Interaction and Drug Nephrotoxicity at Renal Organic Cation Transporters 2 (OCT2) and Multidrug and Toxin Exclusion 1, and 2-K (MATE1/MATE2-K). Biol. Pharm. Bull. 2022, 45, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, X.; Badawy, S.; Ihsan, A.; Liu, Z.; Xie, C.; Wang, X. Metabolism and Mechanism of Human Cytochrome P450 Enzyme 1A2. Curr. Drug Metab. 2021, 22, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Shakya, S.; Shrestha, S.; Aryal, D.; Timalsina, K.P.; Dhakal, D.; Khatri, Y.; Parajuli, N. Biocatalytic role of cytochrome P450s to produce antibiotics: A review. Biotechnol. Bioeng. 2023, 120, 3465–3492. [Google Scholar] [CrossRef]
- Molinaro, C.; Kawasaki, Y.; Wanyoike, G.; Nishioka, T.; Yamamoto, T.; Snedecor, B.; Robinson, S.J.; Gosselin, F. Engineered Cytochrome P450-Catalyzed Oxidative Biaryl Coupling Reaction Provides a Scalable Entry into Arylomycin Antibiotics. J. Am. Chem. Soc. 2022, 144, 14838–14845. [Google Scholar] [CrossRef]
- Zhou, S.F.; Yang, L.P.; Zhou, Z.W.; Liu, Y.H.; Chan, E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009, 11, 481–494. [Google Scholar] [CrossRef]
- Zhou, S.F.; Zhou, Z.W.; Yang, L.P.; Cai, J.P. Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr. Med. Chem. 2009, 16, 3480–3675. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.F. The Metabolism of Clopidogrel: CYP2C19 Is a Minor Pathway. J. Clin. Pharmacol. 2016, 56, 1474–1483. [Google Scholar] [CrossRef]
- Rotzinger, S.; Fang, J.; Coutts, R.T.; Baker, G.B. Human CYP2D6 and metabolism of m-chlorophenylpiperazine. Biol. Psychiatry 1998, 44, 1185–1191. [Google Scholar] [CrossRef]
- Wei, W.; Li, Z.; Li, H.J.; An, Y.; Qu, H.; Yao, C.; Zhang, J.; Li, J.; Zhang, G.; Ma, X.; et al. The inhibitory effect of 225 frequently-used traditional Chinese medicines for CYP3A4 metabolic enzyme by isoform-specific probe. Fitoterapia 2021, 152, 104858. [Google Scholar] [CrossRef]
- Lou, Y.; Song, F.; Cheng, M.; Hu, Y.; Chai, Y.; Hu, Q.; Wang, Q.; Zhou, H.; Bao, M.; Gu, J.; et al. Effects of the CYP3A inhibitors, voriconazole, itraconazole, and fluconazole on the pharmacokinetics of osimertinib in rats. PeerJ 2023, 11, e15844. [Google Scholar] [CrossRef]
- Jänne, P.A.; Yang, J.C.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Deng, Z.; Lai, C.; Lu, H.; Huang, M.; Wen, Y.; Shi, L. Inhibitory effect of ketoconazole, quinidine and 1-aminobenzotriazole on pharmacokinetics of l-tetrahydropalmatine and its metabolite in rats. Xenobiotica 2021, 51, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Gilani, B.; Cassagnol, M. Biochemistry, Cytochrome P450. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wen, J.; ChenYang; Zhao, M.; Hu, W.; Xiao, Y.W. Effects of clarithromycin on the pharmacokinetics of tacrolimus and expression of CYP3A4 and P-glycoprotein in rats. Fundam. Clin. Pharmacol. 2023, 37, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. CYP induction-mediated drug interactions: In vitro assessment and clinical implications. Pharm. Res. 2006, 23, 1089–1116. [Google Scholar] [CrossRef]
- Gardin, A.; Ufer, M.; Legangneux, E.; Rossato, G.; Jin, Y.; Su, Z.; Pal, P.; Li, W.; Shakeri-Nejad, K. Effect of Fluconazole Coadministration and CYP2C9 Genetic Polymorphism on Siponimod Pharmacokinetics in Healthy Subjects. Clin. Pharmacokinet. 2019, 58, 349–361. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Wang, R.; Li, T.; Zuo, Y.; Yang, S.; Dong, D.; Zhu, Y. Drug Transporters and Metabolizing Enzymes in Antimicrobial Drug Pharmacokinetics: Mechanisms, Drug–Drug Interactions, and Clinical Implications. Biomolecules 2025, 15, 864. https://doi.org/10.3390/biom15060864
Lin K, Wang R, Li T, Zuo Y, Yang S, Dong D, Zhu Y. Drug Transporters and Metabolizing Enzymes in Antimicrobial Drug Pharmacokinetics: Mechanisms, Drug–Drug Interactions, and Clinical Implications. Biomolecules. 2025; 15(6):864. https://doi.org/10.3390/biom15060864
Chicago/Turabian StyleLin, Kaili, Ruoqing Wang, Tong Li, Yawen Zuo, Shilei Yang, Deshi Dong, and Yanna Zhu. 2025. "Drug Transporters and Metabolizing Enzymes in Antimicrobial Drug Pharmacokinetics: Mechanisms, Drug–Drug Interactions, and Clinical Implications" Biomolecules 15, no. 6: 864. https://doi.org/10.3390/biom15060864
APA StyleLin, K., Wang, R., Li, T., Zuo, Y., Yang, S., Dong, D., & Zhu, Y. (2025). Drug Transporters and Metabolizing Enzymes in Antimicrobial Drug Pharmacokinetics: Mechanisms, Drug–Drug Interactions, and Clinical Implications. Biomolecules, 15(6), 864. https://doi.org/10.3390/biom15060864







