Biomolecule-Based Coacervation: Mechanisms, Applications, and Future Perspectives in Biomedical and Biotechnological Fields
Abstract
1. Introduction
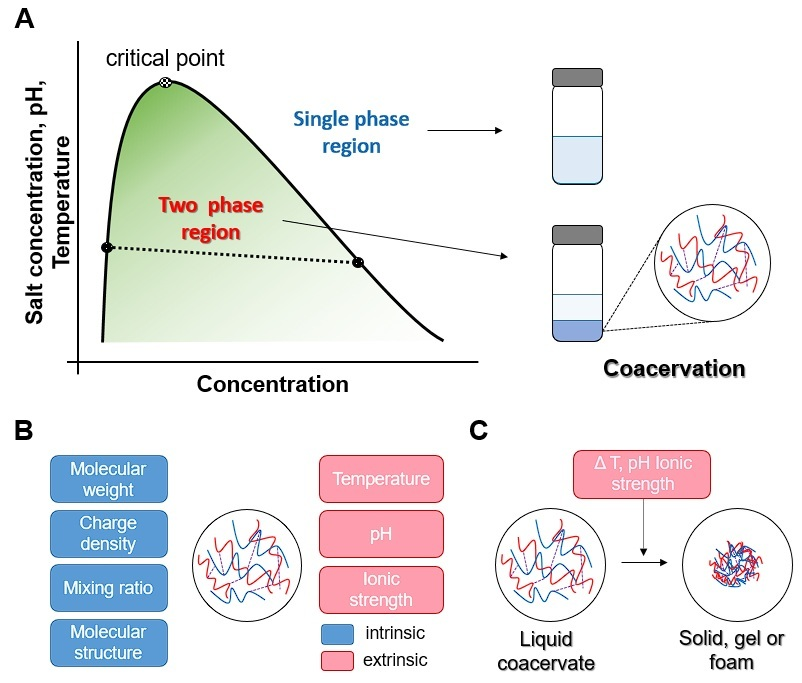
2. Mechanism of Coacervation
3. The Type of Coacervation
3.1. Simple Coacervation
3.2. Complex Coacervation—Binary and Ternary
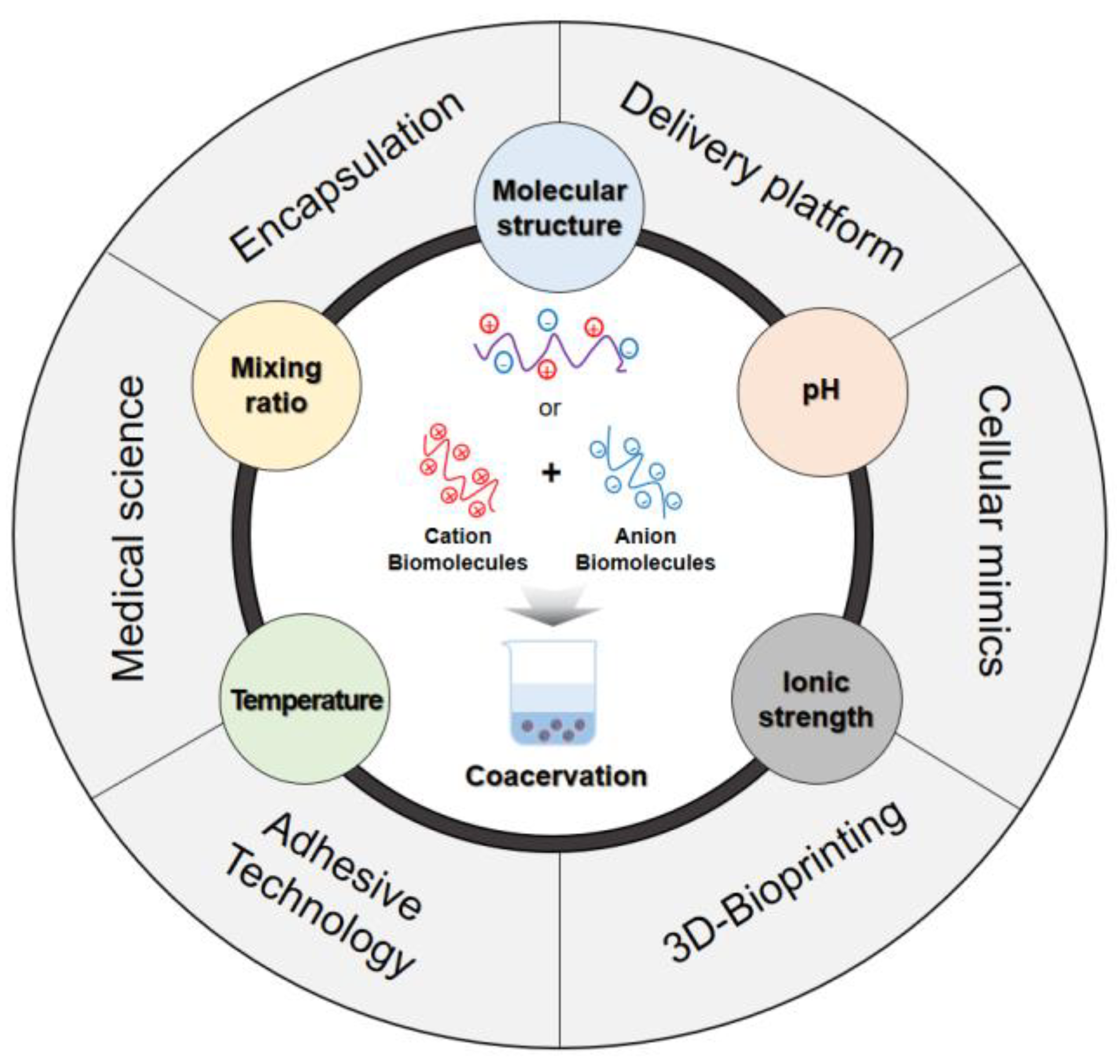
4. Factors Influencing the Coacervation Process
4.1. Molecular Structure
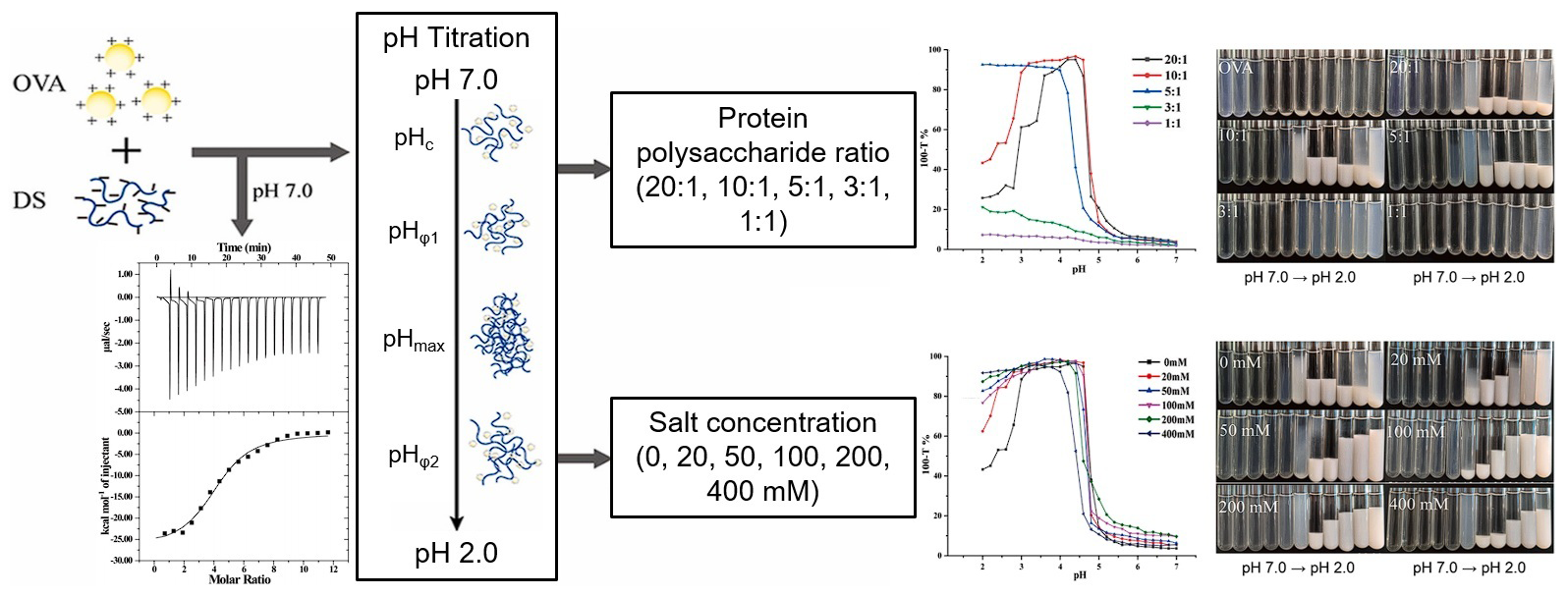
4.2. pH
4.3. Temperature
4.4. Mixing Ratio
4.5. Ionic Strength
5. Biomolecule-Based Coacervation
5.1. Proteins
5.2. Nucleacids

5.3. Peptides
5.4. Polysaccharides
6. Application of Biomolecule-Based Coacervation
| Bioapplication | Biomolecule Component | Cargo | Study Objective | Ref. |
|---|---|---|---|---|
| Encapsulation | SA/CS | Walnut oil | Improved higher loading capacity and oxidation stability | [117] |
| Encapsulation | GA/CS or GA/trehalose/CS | Lacticaseibacillus rhamnosus | Promoted the stability of probiotic bacteria | [118] |
| Encapsulation | Gelatin/chia mucilage | Oregano essential oil | Developed encapsulation system for spray drying and improved encapsulation efficiency | [119] |
| Encapsulation | Zein/CS | Resveratrol | Improved encapsulation efficiency and dispersion stability via atmospheric cold plasma | [120] |
| Encapsulation | Plum seed protein isolate/polysaccharides | Essential oils | Enhanced stability, storage, emulsification, and encapsulation | [121] |
| Encapsulation | Zein–gallic acid/CS | Gallic acid | Induced structural modifications of encapsulation and enhanced thermal stability | [122] |
| Encapsulation | SPI/CS | Deer oil | Enhanced the stability of encapsulation against oxidative stress and encapsulation efficiency. | [123] |
| Encapsulation | β-conglycinin/lysozyme | Curcumin | Improved encapsulation efficiency, loading capacity, and stability against light and heat treatment | [124] |
| Encapsulation | GA/Krill protein isolate | Antarctic Krill oil | Developed stable and biocompatible encapsulation for oil | [65] |
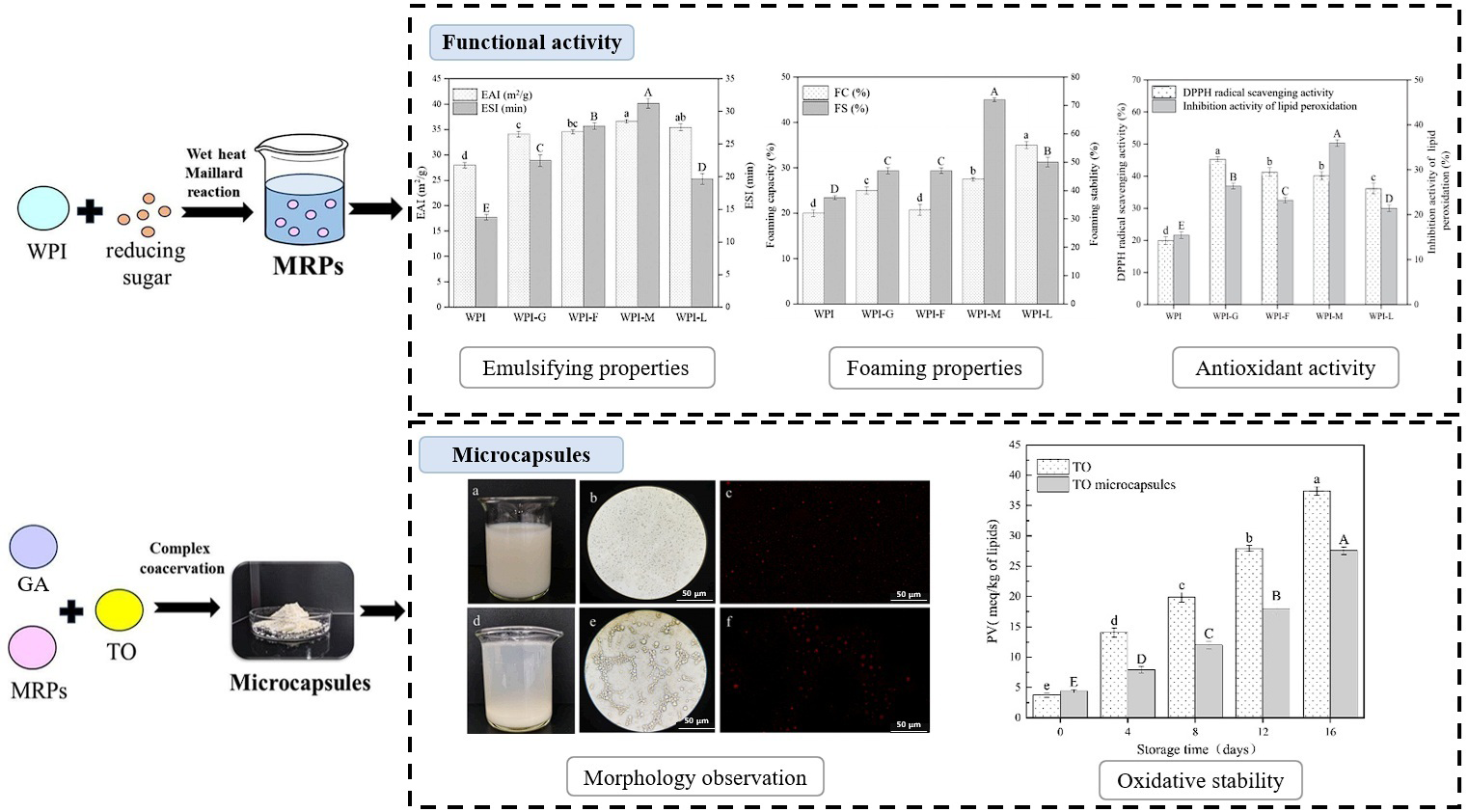
| Encapsulation | WPI/GA | Tuna oil | Enhanced oxidative stability and made encapsulation more thermosensitively suitable | [125] |
| Encapsulation | WPI/flaxseed gum/monodiglyceride fatty acids | Resveratrol | Improved stability, encapsulation efficiency, and sustained antioxidant ability | [126] |
| Delivery platform | Cholesterol-modified DNA/histone | Virus particle, mRNA, cytokines, peptides | Enhanced the stability of the delivery vehicle biological agents | [127] |
| Delivery platform | Fungal CS/GA | α-tocopherol | Promoted stable and easy-to-prepare encapsulation materials for harsh conditions | [128] |
| Delivery platform | LMWG or HMWG/SA | miRNA-497 | Developed a biocompatible delivery system to enhance cellular uptake and stability | [129] |
| Delivery platform | Heparin GAG/tyrosine- and arginine-based peptide | Tannic acid | Developed a stable and permeable delivery system that released drugs in response to biological triggers | [130] |
| Delivery platform | Dextran graft copolymer | DNA | Enhanced release capabilities and transfection | [131] |
| Delivery platform | Ellagic acid/casein | Ellagic acid | Enhanced oral absorption and improved solubility | [132] |
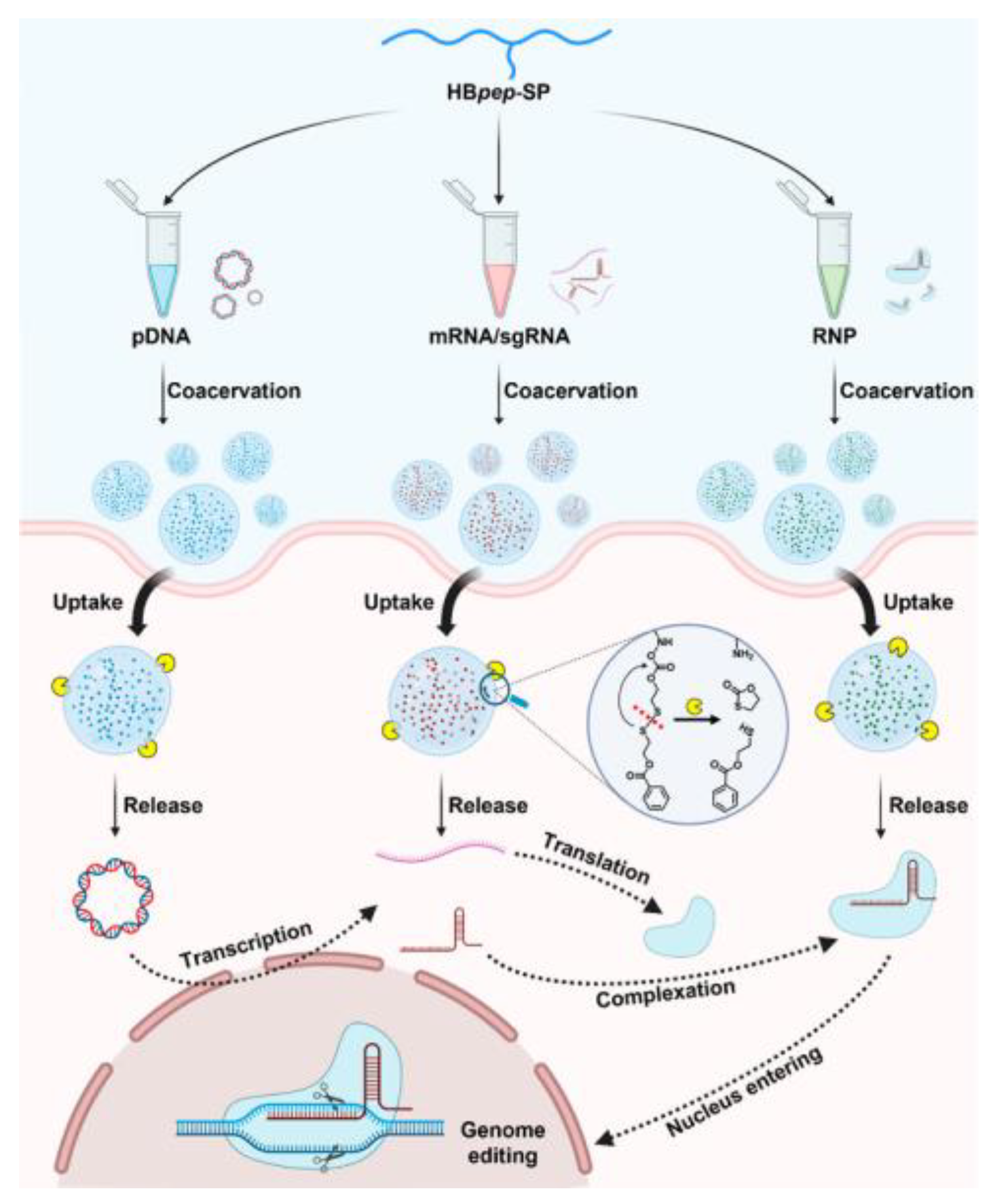
| Delivery platform | Peptide | pDNA, mRNA/sgRNA, RNP | Developed a redox-triggered delivery vehicle for CRISRP-Cas9 genome editing | [133] |
| Delivery platform | Peptide | siRNA, pDNA, mRNA | Developed a pH-responsive delivery nanocarrier for cancer therapy | [134] |
| Delivery platform | Single-stranded DNA/PLL | Emamectin benzoates | Improved loading capacities and stability against photodegradation | [135] |
| Delivery platform | PEAD/heparin | BMP-2 | Developed a protein delivery platform with enhanced colloidal stability | [136] |
| Tissue engineering | CMC/gelatin | - | Developed 3D extrusion printing hydrogel with higher printing fidelity and without any discontinuities during the printing process | [137] |
| Tissue engineering | Gleatin/GA/CMC | β-carotene | Improved thermal, pH, and ionic strength stability and evaluated its potential applications in surimi | [138] |
| Tissue engineering | HA/CS | Rat BMSCs | Developed chondro-inductivity scaffold for encapsulating BMSCs | [139] |
| Tissue engineering | Gelatin/QHECE | Glucose oxidase | Developed glucose-responsive microneedle loaded with glucose oxidase and enhanced drug release | [140] |
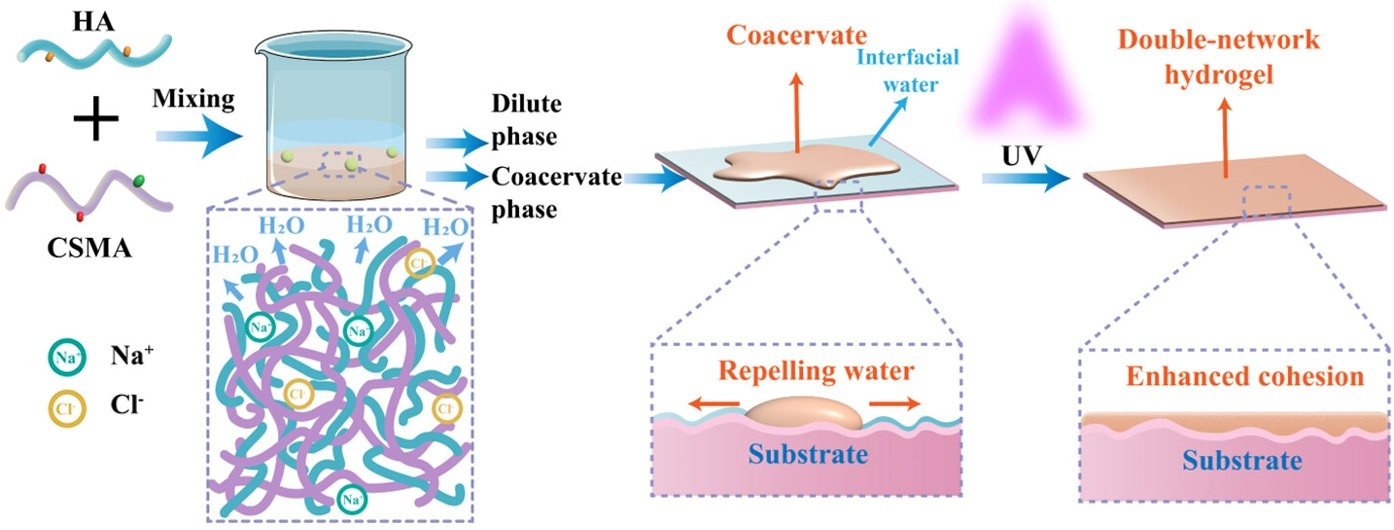
| Tissue engineering | LMWC/HA or HMWC/HA | - | Developed biocompatible hydrogel with shape adaptability and enhanced wet adhesion | [141] |
| Tissue engineering | SPI/chelator-soluble pectin | β-conglycinin, glycinin | Developed food inks for 3D printing with enhanced particle distribution and mechanical properties | [142] |
| Tissue engineering | Theabrownin/whey protein isolate | - | Developed coacervate for modulating energy metabolism and mitochondrial apoptosis to strengthen muscle cells | [64] |
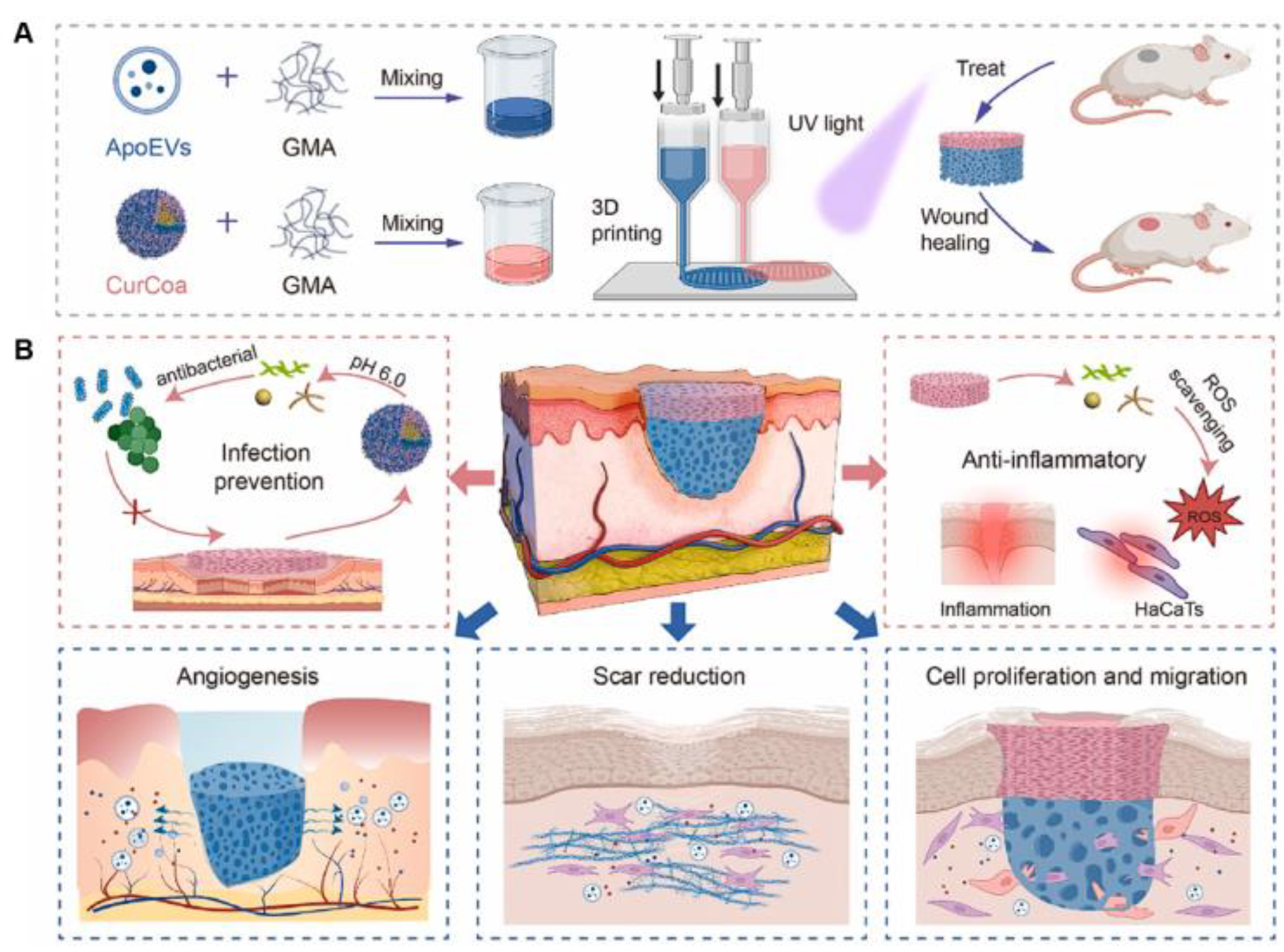
| Tissue engineering | ApoEVs/GelMA or curcumin/CMCS/GelMA | Apoptotic extracellular vesicles, curcumin | Developed multifunctional 3D-printed scaffold for enhancing skin regeneration and promoting antibacterial activity and ROS scavenging activity | [143] |
| Tissue engineering | PEAD/heparin | Cargo IGF-1 | Enhanced bioactivity of cargo IGF-1 and sustained release to embed in cartilage regeneration hydrogel | [144] |
| Tissue engineering | Egg yolk/CMC | β-carotene | Enhanced stability of interfacial layer and structural strength | [145] |
| Adhesive technology | Methacrylated LMWC/HA | - | Developed coacervate with enhanced wet tissue adhesion and tunable properties | [146] |
| Adhesive technology | CS/HA | - | Enhanced underwater adhesion strength against salt switch conditions and promoted antibacterial properties | [147] |
| Adhesive technology | Peptide/polyoxometalate | - | Developed injectable, self-solidifying underwater adhesion and enhanced its properties | [148] |
| Adhesive technology | Tyramine-conjugated alginate/RGD peptide | Calcium phosphate | Developed a photo-mineralized hydrogel and enhanced adhesiveness and bioactivity of bones | [149] |
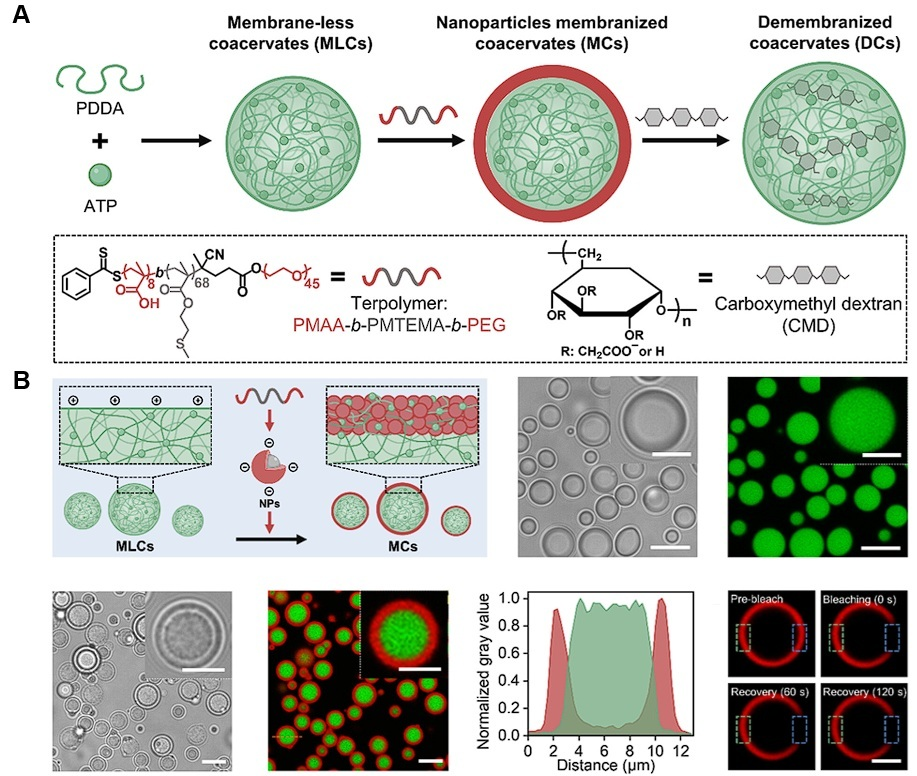
| Cellular mimicking | PDDA/ATP | Dextran | Developed a demembranization system that reconfigures in response to biological signals with enhanced permeability | [150] |
| Cellular mimicking | RNA/peptide | RNA | Developed fuel-dependent RNA-containing coacervation that mimics membraneless organelles | [83] |
| Cellular mimicking | Dextran/polyaspartic acid | DNA, enzymes | Developed a biomimetic platform capable of biomacromolecule segregation, reaction control, and morphological reconfiguration | [151] |
| Cellular mimicking | PEG/dextran | DNA | Developed compartmentalized artificial cell structures to mimic and investigate spatiotemporal control mechanisms | [152] |
6.1. Encapsulation
6.2. Delivery Platform
6.3. Tissue Engineering
6.4. Adhesive Technology
6.5. Cellular Mimicking
7. Fundamental Challenges of Coacervation
8. Future Perspective for Coacervation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ApoEV | Apoptotic extracellular vesicle |
| ATP | Adenosine 5′-triphosphate |
| BMP-2 | Bone morphogenetic protein 2 |
| BMSC | Bone marrow mesenchymal stem cell |
| CLSM | Confocal laser scanning microscope |
| CMC | Carboxymethyl cellulose |
| CMCS | Carboxymethyl chitosan |
| CMD | Carboxymethyl dextran |
| CS | Chitosan |
| DC | Demembranized coacervate |
| ECM | Extracellular matrix |
| ELP | Elastin-like polypeptide |
| FG | Fish gelatin |
| GA | Arabic gum, gum arabic |
| GAG | Glycosaminoglycan |
| HA | Hyaluronic acid |
| HMWC | High-molecular-weight chitosan |
| HMWG | High-molecular-weight cationized gelatin |
| HRV | Rhinovirus |
| IGF-1 | Insulin-like growth factor-1 |
| LLPS | Liquid–liquid phase separation |
| LMWC | Low-molecular-weight chitosan |
| LMWG | Low-molecular-weight cationized gelatin |
| MAP | Mussel adhesive protein |
| MC | Nanoparticle membranized coacervate |
| MLC | Membraneless coacervate |
| OVA | Ovalbumin |
| PDDA | Poly(diallyldimethylammonium chloride) |
| PLL | Poly-L-lysine |
| PP | Pea protein |
| PPV | Procine parvovirus |
| QHECE | Hydroxyethylcellulose ethoxylate |
| RNP | Ribonucleoprotein |
| ROS | Reactive oxygen species |
| SA | Sodium alginate |
| SPI | Soy protein isolate |
| SPP | Soluble pea protein |
| SSPS | Soluble soybean polysaccharide |
| WPI | Whey protein isolate |
References
- Zhou, L.; Shi, H.; Li, Z.; He, C. Recent Advances in Complex Coacervation Design from Macromolecular Assemblies and Emerging Applications. Macromol. Rapid Commun. 2020, 41, 2000149. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, H.; Bungenberg de Jong, H. Chemistry–Coacervation (Partial Miscibility in Colloid Systems); Koninklijke Nederlandse Akademie van Wetenschappen: Amsterdam, The Netherlands, 1929; pp. 849–856. [Google Scholar]
- Nakashima, K.K.; Vibhute, M.A.; Spruijt, E. Biomolecular Chemistry in Liquid Phase Separated Compartments. Front. Mol. Biosci. 2019, 6, 21. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Khalifa, H.O.; Ki, M.-R.; Pack, S.P. Nanoengineered Silica-Based Biomaterials for Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 6125. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ki, M.-R.; Nguyen, T.K.M.; Min, K.H.; Pack, S.P. In vivo self-assembly of an intact functional cage protein: Intracellular generation of chimeric ferritins without disassembly-involved damage. J. Ind. Eng. Chem. 2025, 141, 67–71. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Son, R.G.; Ki, M.-R.; Pack, S.P. Biosilica-coated carbonic anhydrase displayed on Escherichia coli: A novel design approach for efficient and stable biocatalyst for CO2 sequestration. Int. J. Biol. Macromol. 2024, 277, 134058. [Google Scholar] [CrossRef] [PubMed]
- Kayitmazer, A.B.; Seeman, D.; Minsky, B.B.; Dubin, P.L.; Xu, Y. Protein–polyelectrolyte interactions. Soft Matter 2013, 9, 2553–2583. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Yewdall, N.A.; André, A.A.M.; Lu, T.; Spruijt, E. Coacervates as models of membraneless organelles. Curr. Opin. Colloid Interface Sci. 2021, 52, 101416. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, T.; Tavares, G.M.; Bouhallab, S. Heteroprotein complex coacervation: A generic process. Adv. Colloid Interface Sci. 2017, 239, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide-protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Moschakis, T.; Biliaderis, C.G. Biopolymer-based coacervates: Structures, functionality and applications in food products. Curr. Opin. Colloid Interface Sci. 2017, 28, 96–109. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Kato, M.; Xiang, S.; Wu, L.; Theodoropoulos, P.; Mirzaei, H.; Han, T.; Xie, S.; Corden, J.L.; McKnight, S.L. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 2013, 155, 1049–1060. [Google Scholar] [CrossRef]
- Lin, Y.; Protter, D.S.; Rosen, M.K.; Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 2015, 60, 208–219. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Ban, E.; Kim, A. Coacervates: Recent developments as nanostructure delivery platforms for therapeutic biomolecules. Int. J. Pharm. 2022, 624, 122058. [Google Scholar] [CrossRef]
- Blocher, W.C.; Perry, S.L. Complex coacervate-based materials for biomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2017, 9, e1442. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, J.T.; Voorn, M.J. Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Comp. Physiol. 1957, 49, 7–26. [Google Scholar] [CrossRef]
- Gooch, J.W. Flory-Huggins Theory. In Encyclopedic Dictionary of Polymers; Springer Science & Business Media: New York, NY, USA, 2011; p. 315. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Black, J.R. Debye-Hückel Equation. In Encyclopedia of Geochemistry; Encyclopedia of Earth Sciences Series; Springer: Cham, Switzerland, 2016; pp. 1–3. [Google Scholar]
- Michaeli, I.; Overbeek, J.T.G.; Voorn, M. Phase separation of polyelectrolyte solutions. J. Polym. Sci. 1957, 23, 443–450. [Google Scholar] [CrossRef]
- Veis, A. A review of the early development of the thermodynamics of the complex coacervation phase separation. Adv. Colloid Interface Sci. 2011, 167, 2–11. [Google Scholar] [CrossRef]
- Veis, A.; Aranyi, C. Phase separation in polyelectrolyte systems. I. Complex coacervates of gelatin. J. Phys. Chem. 1960, 64, 1203–1210. [Google Scholar] [CrossRef]
- Tainaka, K.I. Effect of counterions on complex coacervation. Biopolym. Orig. Res. Biomol. 1980, 19, 1289–1298. [Google Scholar] [CrossRef]
- Sing, C.E. Development of the modern theory of polymeric complex coacervation. Adv. Colloid Interface Sci. 2017, 239, 2–16. [Google Scholar] [CrossRef]
- Astoricchio, E.; Alfano, C.; Rajendran, L.; Temussi, P.A.; Pastore, A. The Wide World of Coacervates: From the Sea to Neurodegeneration. Trends Biochem. Sci. 2020, 45, 706–717. [Google Scholar] [CrossRef]
- Furlani, F.; Donati, I.; Marsich, E.; Sacco, P. Characterization of chitosan/hyaluronan complex coacervates assembled by varying polymers weight ratio and chitosan physical-chemical composition. Colloids Interfaces 2020, 4, 12. [Google Scholar] [CrossRef]
- Furlani, F.; Parisse, P.; Sacco, P. On the formation and stability of chitosan/hyaluronan-based complex coacervates. Molecules 2020, 25, 1071. [Google Scholar] [CrossRef]
- Kim, S.; Huang, J.; Lee, Y.; Dutta, S.; Yoo, H.Y.; Jung, Y.M.; Jho, Y.; Zeng, H.; Hwang, D.S. Complexation and coacervation of like-charged polyelectrolytes inspired by mussels. Proc. Natl. Acad. Sci. USA 2016, 113, E847–E853. [Google Scholar] [CrossRef]
- Najafi, S.; Lin, Y.; Longhini, A.P.; Zhang, X.; Delaney, K.T.; Kosik, K.S.; Fredrickson, G.H.; Shea, J.-E.; Han, S. Liquid–liquid phase separation of Tau by self and complex coacervation. Protein Sci. 2021, 30, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Nguyen, T.K.M.; Park, T.-I.; Park, H.-M.; Pack, S.P. Biomimetic Silica Particles with Self-Loading BMP-2 Knuckle Epitope Peptide and Its Delivery for Bone Regeneration. Pharmaceutics 2023, 15, 1061. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Gabryelczyk, B.; Manimekalai, M.S.S.; Grüber, G.; Salentinig, S.; Miserez, A. Self-coacervation of modular squid beak proteins—A comparative study. Soft Matter 2017, 13, 7740–7752. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Du, X.; Mo, L.; Wang, H. Self-coacervation of carboxymethyl chitosan as a pH-responsive encapsulation and delivery strategy. Int. J. Biol. Macromol. 2021, 192, 1169–1177. [Google Scholar] [CrossRef]
- Kaushik, P.; Rawat, K.; Bohidar, H.B. Heat-induced coacervation of elastin and its possible thermoreversibility. Colloid Polym. Sci. 2019, 297, 947–956. [Google Scholar] [CrossRef]
- Gulão, E.d.S.; de Souza, C.J.F.; Andrade, C.T.; Garcia-Rojas, E.E. Complex coacervates obtained from peptide leucine and gum arabic: Formation and characterization. Food Chem. 2016, 194, 680–686. [Google Scholar] [CrossRef]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein Encapsulation via Polypeptide Complex Coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Sing, C.E.; Perry, S.L. Recent progress in the science of complex coacervation. Soft Matter 2020, 16, 2885–2914. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Cao, Y.; Xue, B. Liquid-liquid phase separation: Fundamental physical principles, biological implications, and applications in supramolecular materials engineering. Supramol. Mater. 2023, 2, 100049. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, J.; Li, C.; Ren, J.; Wang, Z.; Zhang, R.; Liu, X. Heteroprotein complex coacervation of ovalbumin and lysozyme: Phase behavior, microstructure and processing properties. Food Hydrocoll. 2023, 144, 109013. [Google Scholar] [CrossRef]
- Li, L.; Srivastava, S.; Andreev, M.; Marciel, A.B.; de Pablo, J.J.; Tirrell, M.V. Phase Behavior and Salt Partitioning in Polyelectrolyte Complex Coacervates. Macromolecules 2018, 51, 2988–2995. [Google Scholar] [CrossRef]
- Kuroyanagi, S.; Shimada, N.; Fujii, S.; Furuta, T.; Harada, A.; Sakurai, K.; Maruyama, A. Highly Ordered Polypeptide with UCST Phase Separation Behavior. J. Am. Chem. Soc. 2019, 141, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xia, X.; Mao, J.; Wang, C.; Dawadi, M.B.; Modarelli, D.A.; Zacharia, N.S. Composition and property tunable ternary coacervate: Branched polyethylenimine and a binary mixture of a strong and weak polyelectrolyte. Mol. Syst. Des. Eng. 2019, 4, 110–121. [Google Scholar] [CrossRef]
- Perry, S.L.; Leon, L.; Hoffmann, K.Q.; Kade, M.J.; Priftis, D.; Black, K.A.; Wong, D.; Klein, R.A.; Pierce, C.F., 3rd; Margossian, K.O.; et al. Chirality-selected phase behaviour in ionic polypeptide complexes. Nat. Commun. 2015, 6, 6052. [Google Scholar] [CrossRef]
- Pramanik, U.; Das, A.; Brown, E.; Struckman, H.L.; Wang, H.; Stealey, S.; Sprunger, M.L.; Wasim, A.; Fascetti, J.; Mondal, J.; et al. Histidine-rich enantiomeric peptide coacervates enhance antigen sequestration and presentation to T cells. Chem. Sci. 2025, 16, 7523–7536. [Google Scholar] [CrossRef]
- Vieregg, J.R.; Lueckheide, M.; Marciel, A.B.; Leon, L.; Bologna, A.J.; Rivera, J.R.; Tirrell, M.V. Oligonucleotide-Peptide Complexes: Phase Control by Hybridization. J. Am. Chem. Soc. 2018, 140, 1632–1638. [Google Scholar] [CrossRef]
- Nichols, M.K.; Kumar, R.K.; Bassindale, P.G.; Tian, L.; Barnes, A.C.; Drinkwater, B.W.; Patil, A.J.; Mann, S. Fabrication of Micropatterned Dipeptide Hydrogels by Acoustic Trapping of Stimulus-Responsive Coacervate Droplets. Small 2018, 14, 1800739. [Google Scholar] [CrossRef]
- Huang, G.-Q.; Han, X.-N.; Xiao, J.-X. Glutaraldehyde-crosslinked O-carboxymethyl chitosan–gum Arabic coacervates: Characteristics versus complexation acidity. J. Dispers. Sci. Technol. 2017, 38, 1607–1612. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Koksal, A.F.; Kilic Iyilik, E. Complex coacervation of hyaluronic acid and chitosan: Effects of pH, ionic strength, charge density, chain length and the charge ratio. Soft Matter 2015, 11, 8605–8612. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Venema, P.; van der Linden, E.; de Vries, R. The generality of pH-induced liquid-liquid phase separation in plant proteins extends to commercial legume flours. Food Hydrocoll. 2025, 162, 110927. [Google Scholar] [CrossRef]
- Jamshidian, H.; Rafe, A. Complex coacervate of wheat germ protein/high methoxy pectin in encapsulation of d-limonene. Chem. Biol. Technol. Agric. 2024, 11, 60. [Google Scholar] [CrossRef]
- Varanko, A.K.; Su, J.C.; Chilkoti, A. Elastin-like polypeptides for biomedical applications. Annu. Rev. Biomed. Eng. 2020, 22, 343–369. [Google Scholar] [CrossRef]
- Poudyal, R.R.; Pir Cakmak, F.; Keating, C.D.; Bevilacqua, P.C. Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry. Biochemistry 2018, 57, 2509–2519. [Google Scholar] [CrossRef]
- Aumiller, W.M., Jr.; Keating, C.D. Experimental models for dynamic compartmentalization of biomolecules in liquid organelles: Reversible formation and partitioning in aqueous biphasic systems. Adv. Colloid Interface Sci. 2017, 239, 75–87. [Google Scholar] [CrossRef]
- Adhikari, S.; Prabhu, V.M.; Muthukumar, M. Lower critical solution temperature behavior in polyelectrolyte complex coacervates. Macromolecules 2019, 52, 6998–7004. [Google Scholar] [CrossRef]
- Priftis, D.; Laugel, N.; Tirrell, M. Thermodynamic Characterization of Polypeptide Complex Coacervation. Langmuir 2012, 28, 15947–15957. [Google Scholar] [CrossRef]
- Fu, X.; Xing, C.; Sun, J. Tunable LCST/UCST-Type Polypeptoids and Their Structure–Property Relationship. Biomacromolecules 2020, 21, 4980–4988. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, X.; Hu, Z.; Wang, W.; Schroer, M.A.; Ren, J.; Svergun, D.; Chen, A.; Yang, P.; Zeng, A.-P. A globular protein exhibits rare phase behavior and forms chemically regulated orthogonal condensates in cells. Nat. Commun. 2025, 16, 2449. [Google Scholar] [CrossRef]
- Kaushik, P.; Rawat, K.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. Mixing ratio dependent complex coacervation versus bicontinuous gelation of pectin with in situ formed zein nanoparticles. Soft Matter 2018, 14, 6463–6475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, H.; Ma, N.; Zhong, L.; Ma, G.; Pei, F.; Chen, H.; Hu, Q. Effect of ionic strength and mixing ratio on complex coacervation of soy protein isolate/Flammulina velutipes polysaccharide. Food Sci. Hum. Wellness 2023, 12, 183–191. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.; Wen, C.; Wei, K.; Peng, L.; Wei, X. Theabrownin/whey protein isolate complex coacervate strengthens C2C12 cell proliferation via modulation of energy metabolism and mitochondrial apoptosis. Int. J. Biol. Macromol. 2024, 283, 137686. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, K.; Chen, K.; Li, Y.; Jiang, L. The complex coacervation of gum Arabic and krill protein isolate and their application for Antarctic krill oil encapsulation. Carbohydr. Polym. 2025, 348, 122831. [Google Scholar] [CrossRef]
- Li, L.; Lai, B.; Yan, J.-N.; Yambazi, M.H.; Wang, C.; Wu, H.-T. Characterization of complex coacervation between chia seed gum and whey protein isolate: Effect of pH, protein/polysaccharide mass ratio and ionic strength. Food Hydrocoll. 2024, 148, 109445. [Google Scholar] [CrossRef]
- Mirlohi, K.; Blocher McTigue, W.C. Coacervation for biomedical applications: Innovations involving nucleic acids. Soft Matter 2025, 21, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Sabadini, J.B.; Oliveira, C.L.P.; Loh, W. Assessing the Structure and Equilibrium Conditions of Complex Coacervate Core Micelles by Varying Their Shell Composition and Medium Ionic Strength. Langmuir 2024, 40, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Soussi Hachfi, R.; Hamon, P.; Rousseau, F.; Famelart, M.-H.; Bouhallab, S. Ionic Strength Dependence of the Complex Coacervation between Lactoferrin and β-Lactoglobulin. Foods 2023, 12, 1040. [Google Scholar] [CrossRef]
- Xiao, K.; Yang, Y.; Xu, X.; Szymanowski, J.E.S.; Zhou, Y.; Sigmon, G.E.; Burns, P.C.; Liu, T. Coacervate Formation in Dilute Aqueous Solutions of Inorganic Molecular Clusters with Simple Divalent Countercations. Inorg. Chem. 2024, 63, 15331–15339. [Google Scholar] [CrossRef]
- Holkar, A.; Gao, S.; Villaseñor, K.; Lake, M.; Srivastava, S. Quantitative turbidimetric characterization of stabilized complex coacervate dispersions. Soft Matter 2024, 20, 5060–5070. [Google Scholar] [CrossRef]
- Sathyavageeswaran, A.; Bonesso Sabadini, J.; Perry, S.L. Self-Assembling Polypeptides in Complex Coacervation. Acc. Chem. Res. 2024, 57, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Onuchic, P.L.; Milin, A.N.; Alshareedah, I.; Deniz, A.A.; Banerjee, P.R. Divalent cations can control a switch-like behavior in heterotypic and homotypic RNA coacervates. Sci. Rep. 2019, 9, 12161. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein–polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Boeynaems, S.; Holehouse, A.S.; Weinhardt, V.; Kovacs, D.; Van Lindt, J.; Larabell, C.; Van Den Bosch, L.; Das, R.; Tompa, P.S.; Pappu, R.V.; et al. Spontaneous driving forces give rise to protein− RNA condensates with coexisting phases and complex material properties. Proc. Natl. Acad. Sci. USA 2019, 116, 7889–7898. [Google Scholar] [CrossRef]
- Rousi, Z.; Malhiac, C.; Fatouros, D.G.; Paraskevopoulou, A. Complex coacervates formation between gelatin and gum Arabic with different arabinogalactan protein fraction content and their characterization. Food Hydrocoll. 2019, 96, 577–588. [Google Scholar] [CrossRef]
- Simon, J.R.; Eghtesadi, S.A.; Dzuricky, M.; You, L.; Chilkoti, A. Engineered ribonucleoprotein granules inhibit translation in protocells. Mol. Cell 2019, 75, 66–75.e65. [Google Scholar] [CrossRef]
- Gracia, P.; Polanco, D.; Tarancón-Díez, J.; Serra, I.; Bracci, M.; Oroz, J.; Laurents, D.V.; García, I.; Cremades, N. Molecular mechanism for the synchronized electrostatic coacervation and co-aggregation of alpha-synuclein and tau. Nat. Commun. 2022, 13, 4586. [Google Scholar] [CrossRef]
- Allahyartorkaman, M.; Chan, T.-H.; Chen, E.H.L.; Ng, S.-T.; Chen, Y.-A.; Wen, J.-K.; Ho, M.-R.; Yen, H.-Y.; Kuan, Y.-S.; Kuo, M.-H.; et al. Phosphorylation-Induced Self-Coacervation versus RNA-Assisted Complex Coacervation of Tau Proteins. J. Am. Chem. Soc. 2025, 147, 10172–10187. [Google Scholar] [CrossRef]
- Liu, J.; Chai, J.; Zhang, T.; Yuan, Y.; Saini, R.K.; Xu, M.; Li, S.; Shang, X. Phase behavior, thermodynamic and rheological properties of ovalbumin/dextran sulfate: Effect of biopolymer ratio and salt concentration. Food Hydrocoll. 2021, 118, 106777. [Google Scholar] [CrossRef]
- Archut, A.; Klost, M.; Drusch, S.; Kastner, H. Complex coacervation of pea protein and pectin: Contribution of different protein fractions to turbidity. Food Hydrocoll. 2023, 134, 108032. [Google Scholar] [CrossRef]
- Park, K.S.; Park, T.-I.; Lee, J.E.; Hwang, S.-Y.; Choi, A.; Pack, S.P. Aptamers and Nanobodies as New Bioprobes for SARS-CoV-2 Diagnostic and Therapeutic System Applications. Biosensors 2024, 14, 146. [Google Scholar] [CrossRef]
- Donau, C.; Späth, F.; Sosson, M.; Kriebisch, B.A.K.; Schnitter, F.; Tena-Solsona, M.; Kang, H.-S.; Salibi, E.; Sattler, M.; Mutschler, H.; et al. Active coacervate droplets as a model for membraneless organelles and protocells. Nat. Commun. 2020, 11, 5167. [Google Scholar] [CrossRef] [PubMed]
- Douliez, J.P.; Martin, N.; Gaillard, C.; Beneyton, T.; Baret, J.C.; Mann, S.; Beven, L. Catanionic coacervate droplets as a surfactant-based membrane-free protocell model. Angew. Chem. Int. Ed. 2017, 56, 13689–13693. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Sabatino, V.; Ward, T.R.; Walther, A. Functional and morphological adaptation in DNA protocells via signal processing prompted by artificial metalloenzymes. Nat. Nanotechnol. 2020, 15, 914–921. [Google Scholar] [CrossRef]
- Liu, W.; Lupfer, C.; Samanta, A.; Sarkar, A.; Walther, A. Switchable hydrophobic pockets in DNA protocells enhance chemical conversion. J. Am. Chem. Soc. 2023, 145, 7090–7094. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakamoto, T.; Nomura, S.M.; Murata, S.; Yanagisawa, M.; Takinoue, M. Liquid DNA Coacervates form Porous Capsular Hydrogels via Viscoelastic Phase Separation on Microdroplet Interface. Adv. Mater. Interfaces 2024, 11, 2300898. [Google Scholar] [CrossRef]
- van Haren, M.H.I.; Helmers, N.S.; Verploegen, L.; Beckers, V.A.C.; Spruijt, E. Shape transformations in peptide–DNA coacervates driven by enzyme-catalyzed deacetylation. Soft Matter 2024, 20, 9493–9502. [Google Scholar] [CrossRef]
- Liu, Q.; Wan, K.; Shang, Y.; Wang, Z.G.; Zhang, Y.; Dai, L.; Wang, C.; Wang, H.; Shi, X.; Liu, D.; et al. Cofactor-free oxidase-mimetic nanomaterials from self-assembled histidine-rich peptides. Nat. Mater. 2021, 20, 395–402. [Google Scholar] [CrossRef]
- Ma, L.; Fang, X.; Wang, C. Peptide-based coacervates in therapeutic applications. Front. Bioeng. Biotechnol. 2023, 10, 1100365. [Google Scholar] [CrossRef]
- Sheehan, F.; Sementa, D.; Jain, A.; Kumar, M.; Tayarani-Najjaran, M.; Kroiss, D.; Ulijn, R.V. Peptide-Based Supramolecular Systems Chemistry. Chem. Rev. 2021, 121, 13869–13914. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, L.; Zou, Y.; Yang, Y.; Wang, C. Steric Dependence of Chirality Effect in Surface-Mediated Peptide Assemblies Identified with Scanning Tunneling Microscopy. Nano Lett. 2019, 19, 5403–5409. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, K.; Yu, W.; Wang, Y.; Hua, J.; Liu, B.; Gong, Z.; Wang, J.; Xu, A.; You, Z.; et al. Sustained release of GDF5 from a designed coacervate attenuates disc degeneration in a rat model. Acta Biomater. 2019, 86, 300–311. [Google Scholar] [CrossRef]
- Sun, Y.; Lau, S.Y.; Lim, Z.W.; Chang, S.C.; Ghadessy, F.; Partridge, A.; Miserez, A. Phase-separating peptides for direct cytosolic delivery and redox-activated release of macromolecular therapeutics. Nat. Chem. 2022, 14, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Kim, S.H.; Kim, K.H.; Pack, S.P. Bio-inspired formation of silica particles using the silica-forming peptides found by silica-binding motif sequence, RRSSGGRR. Process Biochem. 2021, 111, 262–269. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cheng, Y.; Goessling, L.; Obermeyer, A.C. Formation and Gelation of Elastin-like Polypeptide Complex Coacervates. bioRxiv 2025, bioRxiv:2025.2001.2015.633264. [Google Scholar] [CrossRef]
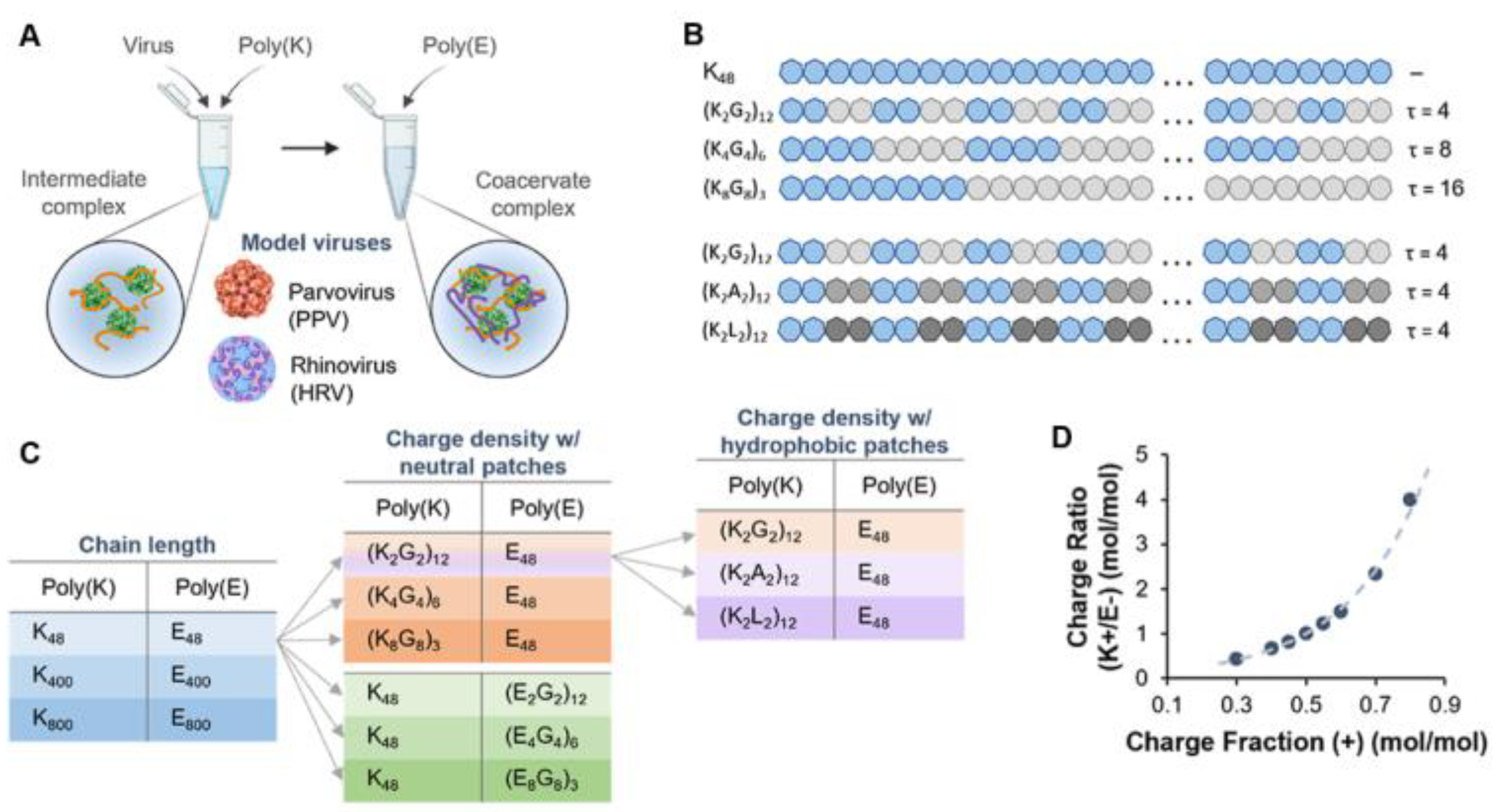
- Joshi, P.U.; Decker, C.; Zeng, X.; Sathyavageeswaran, A.; Perry, S.L.; Heldt, C.L. Design Rules for the Sequestration of Viruses into Polypeptide Complex Coacervates. Biomacromolecules 2024, 25, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Feng, Y.; Wan, H.; Li, Z.; Sun, K.; Ye, S. Construction and characterization of probiotic intestinal-targeted delivery system based on complex coacervation and double-emulsion structure. Food Hydrocoll. 2025, 160, 110814. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, J.; Song, K.; Wan, Z.; Guo, J.; Yang, X. Plant protein-based complex coacervation via protein deamidation. Food Hydrocoll. 2025, 159, 110660. [Google Scholar] [CrossRef]
- Aktaş, H.; Custodio-Mendoza, J.; Szpicer, A.; Pokorski, P.; Samborska, K.; Kurek, M.A. Polysaccharide-potato protein coacervates for enhanced anthocyanin bioavailability and stability. Int. J. Biol. Macromol. 2024, 282, 136829. [Google Scholar] [CrossRef]
- Li, G.-Y.; Chen, Q.-H.; Su, C.-R.; Wang, H.; He, S.; Liu, J.; Nag, A.; Yuan, Y. Soy protein-polysaccharide complex coacervate under physical treatment: Effects of pH, ionic strength and polysaccharide type. Innov. Food Sci. Emerg. Technol. 2021, 68, 102612. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Z.-Y.; Sun, Y.-E.; Zeng, Q.-Z.; Yang, X.-Q. Complex coacervation of soy protein with chitosan: Constructing antioxidant microcapsule for algal oil delivery. LWT 2017, 75, 171–179. [Google Scholar] [CrossRef]
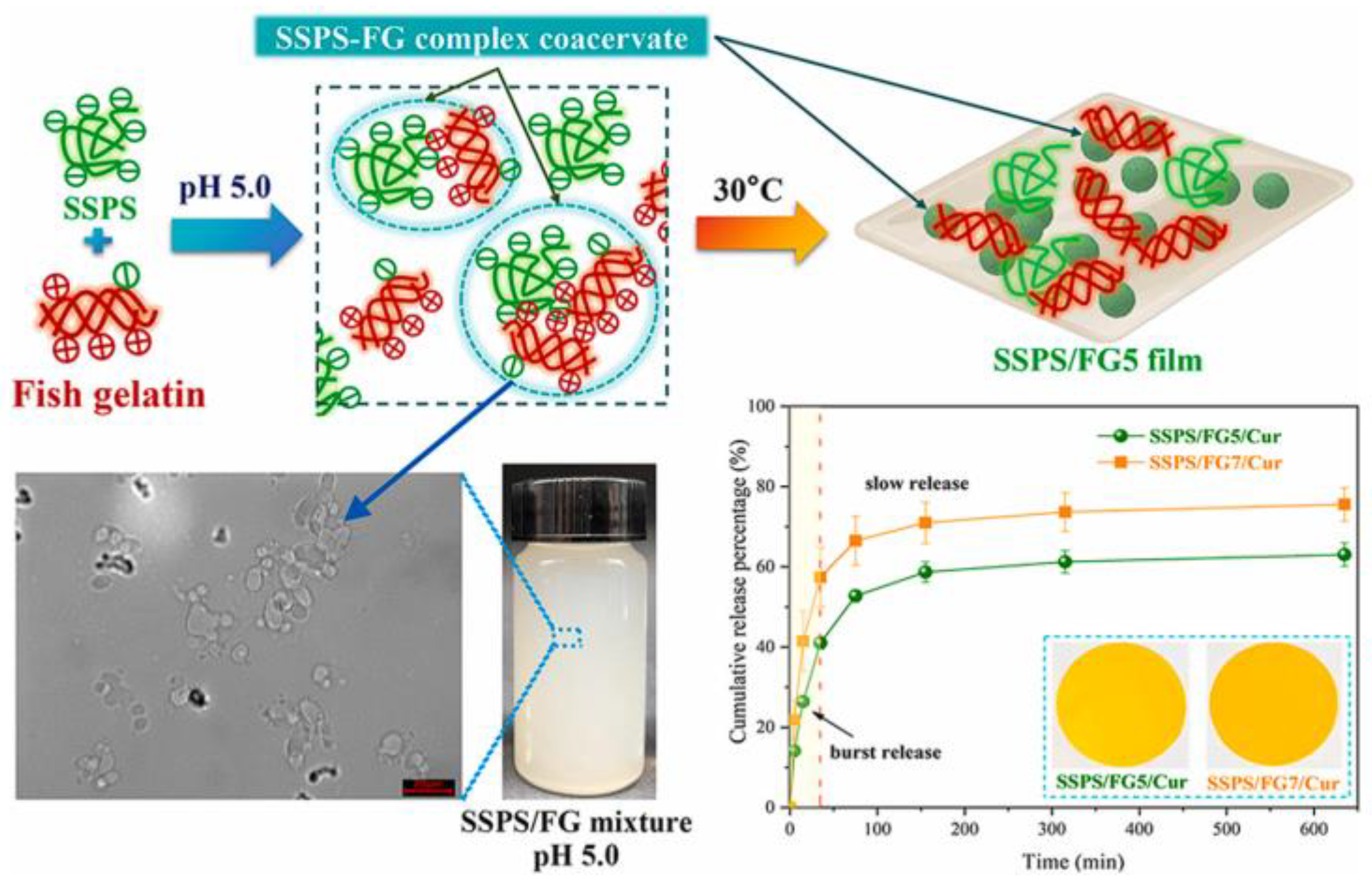
- Wang, Y.; Liu, J.; Ding, Y.; Zheng, X.; Jiang, Y.; Tang, K. Fabrication and characterization of fish gelatin/soluble soybean polysaccharide edible blend films through complex coacervation. Food Hydrocoll. 2024, 155, 110226. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Zheng, J.; Van der Meeren, P.; Sun, W. New insights into protein–polysaccharide complex coacervation: Dynamics, molecular parameters, and applications. Aggregate 2024, 5, e449. [Google Scholar] [CrossRef]
- Fraccia, T.P.; Martin, N. Non-enzymatic oligonucleotide ligation in coacervate protocells sustains compartment-content coupling. Nat. Commun. 2023, 14, 2606. [Google Scholar] [CrossRef]
- Johnson, N.R.; Wang, Y. Coacervate delivery systems for proteins and small molecule drugs. Expert Opin. Drug Deliv. 2014, 11, 1829–1832. [Google Scholar] [CrossRef]
- Roy, P.S. Complex Coacervate-Based Materials for Biomedicine: Recent Advancements and Future Prospects. Ind. Eng. Chem. Res. 2024, 63, 5414–5487. [Google Scholar] [CrossRef]
- Pathak, J.; Priyadarshini, E.; Rawat, K.; Bohidar, H.B. Complex coacervation in charge complementary biopolymers: Electrostatic versus surface patch binding. Adv. Colloid Interface Sci. 2017, 250, 40–53. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Amiri, S.; Mousavi Khaneghah, A. The application of the coacervation technique for microencapsulation bioactive ingredients: A critical review. J. Agric. Food Res. 2024, 18, 101431. [Google Scholar] [CrossRef]
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Mar. Drugs 2024, 22, 496. [Google Scholar] [CrossRef]
- Ki, M.-R.; Youn, S.; Kim, D.H.; Pack, S.P. Natural Compounds for Preventing Age-Related Diseases and Cancers. Int. J. Mol. Sci. 2024, 25, 7530. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Yuyang, H.; Uriho, A.; Harindintwali, J.D.; Liu, Q.; Li, Y. Spray-and freeze-drying of microcapsules prepared by complex coacervation method: A review. Food Hydrocoll. 2023, 140, 108650. [Google Scholar] [CrossRef]
- Kwant, A.N.; Es Sayed, J.S.; Kamperman, M.; Burgess, J.K.; Slebos, D.-J.; Pouwels, S.D. Sticky Science: Using Complex Coacervate Adhesives for Biomedical Applications. Adv. Healthc. Mater. 2025, 14, 2402340. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Wang, C.S.; Shao, H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 2011, 167, 85–93. [Google Scholar] [CrossRef]
- Cook, A.B.; Novosedlik, S.; van Hest, J.C.M. Complex Coacervate Materials as Artificial Cells. Acc. Mater. Res. 2023, 4, 287–298. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, C.; Ma, X.; Yan, W.; Wang, F. Preparation and characterization of walnut oil microcapsules by complex coacervation with sodium alginate and chitosan. LWT 2025, 222, 117630. [Google Scholar] [CrossRef]
- Barajas-Álvarez, P.; Haro-González, J.N.; González-Ávila, M.; Espinosa-Andrews, H. Gum Arabic/Chitosan Coacervates for Encapsulation and Protection of Lacticaseibacillus rhamnosus in Storage and Gastrointestinal Environments. Probiotics Antimicrob. Proteins 2024, 16, 2073–2084. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Chen, Y.; Gao, Y.; Zhang, Z.; Li, S.; Chen, Y. Complex coacervation of zein-chitosan via atmospheric cold plasma treatment: Improvement of encapsulation efficiency and dispersion stability. Food Hydrocoll. 2020, 107, 105943. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, X.; Li, C.; Adhikari, B. Modification of plum seed protein isolate via enzymatic hydrolysis, polyphenol conjugation and polysaccharide complexation to enhance emulsification and encapsulation of essential oils. Int. J. Biol. Macromol. 2025, 306, 141812. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wu, X.; Piao, S.; Zhang, Q.; Zhou, D. Covalent modulation of zein surface potential by gallic acid to enhance the formation of electrostatic-driven ternary antioxidant complex coacervates with chitosan. Food Chem. 2025, 475, 143233. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zong, Y.; Chen, W.; Zhao, Y.; Geng, J.; He, Z.; Du, R. Microencapsulation of Deer Oil in Soy Protein Isolate–Chitosan Complex Coacervate—Preparation, Characterization, and Simulated Digestion. Foods 2025, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Gao, Q.; Ge, G.; Sun, W.; Van der Meeren, P.; Zhao, M. Encapsulation behavior of curcumin in heteroprotein complex coacervates and precipitates fabricated from β-conglycinin and lysozyme. Food Hydrocoll. 2022, 133, 107964. [Google Scholar] [CrossRef]
- Wang, K.-L.; Yu, B.-K.; Zhao, H.-F.; Liu, Y.-X.; Wu, C.-Y.; Zhang, Y.-H.; Mu, Z.-S. Preparation and characterization of microcapsules for tuna oil by maillard reaction products of whey protein isolate and Arabic gum via complex coacervation. Food Chem. 2025, 475, 143269. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Q.; Xu, M.; An, S.; Chu, L.; Yan, T. Preparation and characterization of resveratrol-loaded microcapsules with whey protein and flaxseed gum by membrane emulsification and complex coacervation methods. Int. J. Biol. Macromol. 2025, 306, 141783. [Google Scholar] [CrossRef]
- Wen, P.; Huang, H.; Zhang, R.; Zheng, H.; Liang, T.; Zhuang, C.; Wu, Q.; Wang, J.; Liu, F.; Zhang, K.; et al. Coacervate vesicles assembled by liquid–liquid phase separation improve delivery of biopharmaceuticals. Nat. Chem. 2025, 17, 279–288. [Google Scholar] [CrossRef]
- Delaporte, A.; Paraskevopoulou, A.; Grisel, M.; Gore, E. Animal-free coacervates: The combination of fungal chitosan-gum Arabic for the encapsulation of lipophilic compounds. Int. J. Biol. Macromol. 2025, 299, 140003. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Park, M.; Kim, Y.; Park, J.; Kim, A. Enhanced delivery of anti-inflammatory miRNA-497 to dermal fibroblasts using cationized gelatin-sodium alginate coacervates. J. Drug Deliv. Sci. Technol. 2024, 97, 105767. [Google Scholar] [CrossRef]
- Yim, W.; Jin, Z.; Chang, Y.-C.; Brambila, C.; Creyer, M.N.; Ling, C.; He, T.; Li, Y.; Retout, M.; Penny, W.F.; et al. Polyphenol-stabilized coacervates for enzyme-triggered drug delivery. Nat. Commun. 2024, 15, 7295. [Google Scholar] [CrossRef]
- Chenglong, W.; Shuhan, X.; Jiayi, Y.; Wencai, G.; Guoxiong, X.; Hongjing, D. Dextran-based coacervate nanodroplets as potential gene carriers for efficient cancer therapy. Carbohydr. Polym. 2020, 231, 115687. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Dai, W.; Zhang, Q. Enhanced oral absorption and anti-inflammatory activity of ellagic acid via a novel type of case in nanosheets constructed by simple coacervation. Int. J. Pharm. 2021, 594, 120131. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, X.; Chen, L.; Chew, W.L.; Ping, Y.; Miserez, A. Redox-Responsive Phase-Separating Peptide as a Universal Delivery Vehicle for CRISPR/Cas9 Genome Editing Machinery. ACS Nano 2023, 17, 16597–16606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Han, M.; Jiao, X.; Zhou, J.; Wang, X.; Su, R.; Wang, Y.; Qi, W. An ultra pH-responsive peptide nanocarrier for cancer gene therapy. J. Mater. Chem. B 2023, 11, 8974–8984. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, L.; Han, H.; Zhang, Y.; Zhu, W.; Xu, Y.; Xu, Z.; Yang, Y.; Qian, X. Highly Stable, Excellent Foliar Adhesion and Anti-Photodegradation Nucleic Acid-Peptide Coacervates for Broad Agrochemicals Delivery. Small 2025, 21, 2500044. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Gajendiran, M.; Kim, K. Development of Polymer Coacersome Structure with Enhanced Colloidal Stability for Therapeutic Protein Delivery. Macromol. Biosci. 2019, 19, e1900207. [Google Scholar] [CrossRef]
- Gharanjig, H.; Najaf Zadeh, H.; Stevens, C.; Abhayawardhana, P.; Huber, T.; Nazmi, A.R. Development of Extrudable Hydrogels Based on Carboxymethyl Cellulose–Gelatin Complex Coacervates. Gels 2025, 11, 51. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Song, L.; Jiao, J.; Prakash, S.; Dong, X. Encapsulation of β-carotene in gelatin-gum Arabic-sodium carboxymethylcellulose complex coacervates: Enhancing surimi gel properties and exploring 3D printing potential. Int. J. Biol. Macromol. 2024, 278, 134129. [Google Scholar] [CrossRef]
- Karabıyık Acar, Ö.; Bedir, S.; Kayitmazer, A.B.; Kose, G.T. Chondro-inductive hyaluronic acid/chitosan coacervate-based scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2021, 188, 300–312. [Google Scholar] [CrossRef]
- George Joy, J.; Son, H.K.; Lee, S.-J.; Kim, J.-C. Glucose-responsive soluble microneedle prepared with “Gelatin—Hydroxyethylcellulose ethoxylate complex coacervate” for the transdermal drug delivery. J. Ind. Eng. Chem. 2024, 134, 432–447. [Google Scholar] [CrossRef]
- Wu, M.; Deng, D.; Peng, D.; Tan, C.; Lv, J.; Zhang, W.; Liu, Y.; Tian, H.; Zhao, Y. Development of low-molecular-weight polysaccharide-based wound dressings for full-thickness cutaneous wound healing via coacervate formation. Carbohydr. Polym. 2025, 348, 122851. [Google Scholar] [CrossRef]
- Xie, J.; Bi, J.; Liu, X.; Blecker, C.; Jacquet, N.; Lyu, J. Development of soy protein isolate-chelator soluble pectin composite gels as extrusion-based 3D food printing inks: Effects of mingling strategy. Food Hydrocoll. 2025, 162, 110904. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, J.; Jiang, M.; Tan, W.; Zeng, Y.; Liu, X.; Wang, P.; Jiang, H.; Zhou, J.; Liu, X.; et al. 3D-printed multifunctional bilayer scaffold with sustained release of apoptotic extracellular vesicles and antibacterial coacervates for enhanced wound healing. Biomaterials 2025, 318, 123196. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Kim, S.; Jung, Y.C.; Wang, Y.; Kang, B.-J.; Kim, K. Dual delivery of stem cells and insulin-like growth factor-1 in coacervate-embedded composite hydrogels for enhanced cartilage regeneration in osteochondral defects. J. Control. Release 2020, 327, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, Y.; Ma, Y.; Zhang, H.; Xia, N.; Li, H.; Wang, Z.; Rayan, A.M.; Ghamry, M.; Mohamed, T.A. High internal phase Pickering emulsions stabilized by egg yolk-carboxymethyl cellulose as an age-friendly dysphagia food: Tracking the dynamic transition from co-solubility to coacervates. Carbohydr. Polym. 2024, 342, 122430. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Peng, D.; Lv, J.; Zhang, W.; Tian, H.; Wang, T.; Wu, M.; Zhao, Y. Double-Network Hydrogel Based on Methacrylated Chitosan/Hyaluronic Acid Coacervate for Enhanced Wet-Tissue Adhesion. Biomacromolecules 2025, 26, 2317–2330. [Google Scholar] [CrossRef]
- Galland, P.; Iqbal, M.H.; Favier, D.; Legros, M.; Schaaf, P.; Boulmedais, F.; Vahdati, M. Tuning the underwater adhesiveness of antibacterial polysaccharides complex coacervates. J. Colloid Interface Sci. 2024, 661, 196–206. [Google Scholar] [CrossRef]
- Ji, F.; Li, Y.; Zhao, H.; Wang, X.; Li, W. Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion. Molecules 2024, 29, 681. [Google Scholar] [CrossRef]
- Yun, J.; Woo, H.T.; Lee, S.; Cha, H.J. Visible light-induced simultaneous bioactive amorphous calcium phosphate mineralization and in situ crosslinking of coacervate-based injectable underwater adhesive hydrogels for enhanced bone regeneration. Biomaterials 2025, 315, 122948. [Google Scholar] [CrossRef]
- Zhou, Y.; Maitz, M.F.; Zhang, K.; Voit, B.; Appelhans, D. Dynamic and Diverse Coacervate Architectures by Controlled Demembranization. J. Am. Chem. Soc. 2025, 147, 12239–12250. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Liu, J.; Huang, Y.; Zhu, M.; Chen, C.; Ji, W.; Huang, X. Alternating Binary Droplets-Based Protocell Networks Driven by Heterogeneous Liquid–Liquid Phase Separation. Angew. Chem. Int. Ed. 2025, 64, e202422175. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-N.; Huck, W.T.S. Microfluidic Formation of Monodisperse Coacervate Organelles in Liposomes. Angew. Chem. 2017, 129, 9868–9872. [Google Scholar] [CrossRef]
- Williams, D.S.; Koga, S.; Hak, C.R.C.; Majrekar, A.; Patil, A.J.; Perriman, A.W.; Mann, S. Polymer/nucleotide droplets as bio-inspired functional micro-compartments. Soft Matter 2012, 8, 6004–6014. [Google Scholar] [CrossRef]
- Drobot, B.; Iglesias-Artola, J.M.; Le Vay, K.; Mayr, V.; Kar, M.; Kreysing, M.; Mutschler, H.; Tang, T.Y.D. Compartmentalised RNA catalysis in membrane-free coacervate protocells. Nat. Commun. 2018, 9, 3643. [Google Scholar] [CrossRef]
- Zhao, M.; Zacharia, N.S. Protein encapsulation via polyelectrolyte complex coacervation: Protection against protein denaturation. J. Chem. Phys. 2018, 149, 163326. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Williams, D.S.; Perriman, A.W.; Mann, S. Peptide–nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011, 3, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, E.; Sprakel, J.; Cohen Stuart, M.A.; van der Gucht, J. Interfacial tension between a complex coacervate phase and its coexisting aqueous phase. Soft Matter 2010, 6, 172–178. [Google Scholar] [CrossRef]
- Altenburg, W.J.; Yewdall, N.A.; Vervoort, D.F.M.; van Stevendaal, M.H.M.E.; Mason, A.F.; van Hest, J.C.M. Programmed spatial organization of biomacromolecules into discrete, coacervate-based protocells. Nat. Commun. 2020, 11, 6282. [Google Scholar] [CrossRef]
- Pippa, N.; Karayianni, M.; Pispas, S.; Demetzos, C. Complexation of cationic-neutral block polyelectrolyte with insulin and in vitro release studies. Int. J. Pharm. 2015, 491, 136–143. [Google Scholar] [CrossRef]
- Schuster, B.S.; Reed, E.H.; Parthasarathy, R.; Jahnke, C.N.; Caldwell, R.M.; Bermudez, J.G.; Ramage, H.; Good, M.C.; Hammer, D.A. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 2018, 9, 2985. [Google Scholar] [CrossRef]
- Yewdall, N.A.; Buddingh, B.C.; Altenburg, W.J.; Timmermans, S.B.P.E.; Vervoort, D.F.M.; Abdelmohsen, L.K.E.A.; Mason, A.F.; van Hest, J.C.M. Physicochemical Characterization of Polymer-Stabilized Coacervate Protocells. ChemBioChem 2019, 20, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- Margossian, K.O.; Brown, M.U.; Emrick, T.; Muthukumar, M. Coacervation in polyzwitterion-polyelectrolyte systems and their potential applications for gastrointestinal drug delivery platforms. Nat. Commun. 2022, 13, 2250. [Google Scholar] [CrossRef] [PubMed]
- Park, U.; Lee, M.S.; Jeon, J.; Lee, S.; Hwang, M.P.; Wang, Y.; Yang, H.S.; Kim, K. Coacervate-mediated exogenous growth factor delivery for scarless skin regeneration. Acta Biomater. 2019, 90, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Koide, A.; Osada, K.; Yamasaki, Y.; Kataoka, K. Encapsulation of Myoglobin in PEGylated Polyion Complex Vesicles Made from a Pair of Oppositely Charged Block Ionomers: A Physiologically Available Oxygen Carrier. Angew. Chem. Int. Ed. 2007, 46, 6085–6088. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, K.; Li, S. Nucleic Acids Based Polyelectrolyte Complexes: Their Complexation Mechanism, Morphology, and Stability. Chem. Mater. 2021, 33, 7923–7943. [Google Scholar] [CrossRef]
- Zhao, M.; Eghtesadi, S.A.; Dawadi, M.B.; Wang, C.; Huang, S.; Seymore, A.E.; Vogt, B.D.; Modarelli, D.A.; Liu, T.; Zacharia, N.S. Partitioning of Small Molecules in Hydrogen-Bonding Complex Coacervates of Poly(acrylic acid) and Poly(ethylene glycol) or Pluronic Block Copolymer. Macromolecules 2017, 50, 3818–3830. [Google Scholar] [CrossRef]
- Zhao, M.; Zacharia, N.S. Sequestration of Methylene Blue into Polyelectrolyte Complex Coacervates. Macromol. Rapid Commun. 2016, 37, 1249–1255. [Google Scholar] [CrossRef]
- Obermeyer, A.C.; Mills, C.E.; Dong, X.-H.; Flores, R.J.; Olsen, B.D. Complex coacervation of supercharged proteins with polyelectrolytes. Soft Matter 2016, 12, 3570–3581. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/Chitosan Nanoparticles are Effective for Oral Insulin Delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Control. Release 2000, 69, 149–158. [Google Scholar] [CrossRef]
- Malmo, J.; Sørgård, H.; Vårum, K.M.; Strand, S.P. siRNA delivery with chitosan nanoparticles: Molecular properties favoring efficient gene silencing. J. Control. Release 2012, 158, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.P.; Lelu, S.; Reitan, N.K.; de Lange Davies, C.; Artursson, P.; Vårum, K.M. Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking. Biomaterials 2010, 31, 975–987. [Google Scholar] [CrossRef]
- Li, M.-F.; Chen, L.; Xu, M.-Z.; Zhang, J.-L.; Wang, Q.; Zeng, Q.-Z.; Wei, X.-C.; Yuan, Y. The formation of zein-chitosan complex coacervated particles: Relationship to encapsulation and controlled release properties. Int. J. Biol. Macromol. 2018, 116, 1232–1239. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Voo, Z.X.; Chin, W.; Ono, R.J.; Yang, C.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Coacervate Hydrogel for Delivery of Anticancer Drug-Loaded Nanoparticles in vivo. ACS Appl. Mater. Interfaces 2018, 10, 13274–13282. [Google Scholar] [CrossRef]
- Huei, G.O.S.; Muniyandy, S.; Sathasivam, T.; Veeramachineni, A.K.; Janarthanan, P. Iron cross-linked carboxymethyl cellulose–gelatin complex coacervate beads for sustained drug delivery. Chem. Pap. 2016, 70, 243–252. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Kim, K.H.; Seo, J.H.; Pack, S.P. Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration. Biomimetics 2024, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Min, K.H.; Pack, S.P. Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite. Biomimetics 2024, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Kumar Sharma, P.; Malviya, R. Pharmaceutical and tissue engineering applications of polyelectrolyte complexes. Curr. Smart Mater. 2018, 3, 21–31. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Hwang, D.S.; Zeng, H.; Lu, Q.; Israelachvili, J.; Waite, J.H. Adhesion mechanism in a DOPA-deficient foot protein from green mussels. Soft Matter 2012, 8, 5640–5648. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Tan, Y.; Martinez Rodriguez, N.R.; Yu, J.; Israelachvili, J.N.; Waite, J.H. A mussel-derived one component adhesive coacervate. Acta Biomater. 2014, 10, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Dompé, M.; Vahdati, M.; van Ligten, F.; Cedano-Serrano, F.J.; Hourdet, D.; Creton, C.; Zanetti, M.; Bracco, P.; van der Gucht, J.; Kodger, T.; et al. Enhancement of the Adhesive Properties by Optimizing the Water Content in PNIPAM-Functionalized Complex Coacervates. ACS Appl. Polym. Mater. 2020, 2, 1722–1730. [Google Scholar] [CrossRef]
- Winslow, B.D.; Shao, H.; Stewart, R.J.; Tresco, P.A. Biocompatibility of adhesive complex coacervates modeled after the sandcastle glue of Phragmatopoma californica for craniofacial reconstruction. Biomaterials 2010, 31, 9373–9381. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jin, S.; Park, Y.; Jung, Y.M.; Cha, H.J. Coacervation of Interfacial Adhesive Proteins for Initial Mussel Adhesion to a Wet Surface. Small 2018, 14, e1803377. [Google Scholar] [CrossRef]
- Lim, S.; Choi, Y.S.; Kang, D.G.; Song, Y.H.; Cha, H.J. The adhesive properties of coacervated recombinant hybrid mussel adhesive proteins. Biomaterials 2010, 31, 3715–3722. [Google Scholar] [CrossRef]
- Kim, B.J.; Oh, D.X.; Kim, S.; Seo, J.H.; Hwang, D.S.; Masic, A.; Han, D.K.; Cha, H.J. Mussel-Mimetic Protein-Based Adhesive Hydrogel. Biomacromolecules 2014, 15, 1579–1585. [Google Scholar] [CrossRef]
- Zhong, C.; Gurry, T.; Cheng, A.A.; Downey, J.; Deng, Z.; Stultz, C.M.; Lu, T.K. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat. Nanotechnol. 2014, 9, 858–866. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Cohen Stuart, M.A. Electrostatic Free Energy of Weakly Charged Macromolecules in Solution and Intermacromolecular Complexes Consisting of Oppositely Charged Polymers. Langmuir 2004, 20, 2785–2791. [Google Scholar] [CrossRef]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef]
- Oparin, A.I. The Origin of Life; Dover Pubns: Garden City, NY, USA, 1953. [Google Scholar]
- Spector, D.L. Nuclear domains. J. Cell Sci. 2001, 114, 2891–2893. [Google Scholar] [CrossRef] [PubMed]
- Nott, T.J.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.D.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol. Cell 2015, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Weber, S.C.; Vaidya, N.; Haataja, M.; Brangwynne, C.P. RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. USA 2015, 112, E5237–E5245. [Google Scholar] [CrossRef]
- Hubstenberger, A.; Noble, S.L.; Cameron, C.; Evans, T.C. Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 2013, 27, 161–173. [Google Scholar] [CrossRef]
- Yewdall, N.A.; Mason, A.F.; van Hest, J.C.M. The hallmarks of living systems: Towards creating artificial cells. Interface Focus 2018, 8, 20180023. [Google Scholar] [CrossRef]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K.; Cleaves, H.J. Membraneless polyester microdroplets as primordial compartments at the origins of life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830–15835. [Google Scholar] [CrossRef]
- Longo, L.M.; Despotović, D.; Weil-Ktorza, O.; Walker, M.J.; Jabłońska, J.; Fridmann-Sirkis, Y.; Varani, G.; Metanis, N.; Tawfik, D.S. Primordial emergence of a nucleic acid-binding protein via phase separation and statistical ornithine-to-arginine conversion. Proc. Natl. Acad. Sci. USA 2020, 117, 15731–15739. [Google Scholar] [CrossRef] [PubMed]
- Kembaren, R.; Kleijn, J.M.; Borst, J.W.; Kamperman, M.; Hofman, A.H. Enhanced stability of complex coacervate core micelles following different core-crosslinking strategies. Soft Matter 2022, 18, 3052–3062. [Google Scholar] [CrossRef]
- Zhao, M.; Cho, S.-H.; Wu, X.; Mao, J.; Vogt, B.D.; Zacharia, N.S. Covalently crosslinked coacervates: Immobilization and stabilization of proteins with enhanced enzymatic activity. Soft Matter 2024, 20, 7623–7633. [Google Scholar] [CrossRef]
- Chen, S.; Zou, G.; Guo, Q.; Qian, X.; Li, H.; Gao, H.; Yu, J. Extreme pH Tolerance in Peptide Coacervates Mediated by Multivalent Hydrogen Bonds for Enzyme-Triggered Oral Drug Delivery. J. Am. Chem. Soc. 2025, 147, 9704–9715. [Google Scholar] [CrossRef]
- Li, C.-L.; Qin, F.; Li, R.-r.; Zhuan, J.; Zhu, H.-Y.; Wang, Y.; Wang, K. Preparation and In Vivo Expression of CS-PEI/pCGRP Complex for Promoting Fracture Healing. Int. J. Polym. Sci. 2019, 2019, 9432194. [Google Scholar] [CrossRef]
- Santos, J.L.; Ren, Y.; Vandermark, J.; Archang, M.M.; Williford, J.-M.; Liu, H.-W.; Lee, J.; Wang, T.-H.; Mao, H.-Q. Continuous Production of Discrete Plasmid DNA-Polycation Nanoparticles Using Flash Nanocomplexation. Small 2016, 12, 6214–6222. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, X.; Xu, X.; Leung, K.K.C.; Rai, A.; Deng, Y.; Yang, B.; Lai, H.; Peng, X.; Shi, P.; et al. Nanoparticle-assembled bioadhesive coacervate coating with prolonged gastrointestinal retention for inflammatory bowel disease therapy. Nat. Commun. 2021, 12, 7162. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duan, L.; Liu, G.; Sun, J.; Shahbazi, M.-A.; Kundu, S.C.; Reis, R.L.; Xiao, B.; Yang, X. Bioinspired Polyacrylic Acid-Based Dressing: Wet Adhesive, Self-Healing, and Multi-Biofunctional Coacervate Hydrogel Accelerates Wound Healing. Adv. Sci. 2023, 10, 2207352. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Vernejoul, F.; Cambois, G.; Lulka, H.; Hanoun, N.; Dufresne, M.; Meulle, A.; Vignolle-Vidoni, A.; Ligat, L.; et al. First-in-man phase 1 clinical trial of gene therapy for advanced pancreatic cancer: Safety, biodistribution, and preliminary clinical findings. Mol. Ther. 2015, 23, 779–789. [Google Scholar] [CrossRef]
- Marvin, L.; Paiva, W.; Gill, N.; Morales, M.A.; Halpern, J.M.; Vesenka, J.; Balog, E.R.M. Flow imaging microscopy as a novel tool for high-throughput evaluation of elastin-like polymer coacervates. PLoS ONE 2019, 14, e0216406. [Google Scholar] [CrossRef]
- Pei, Y.; Zheng, Y.; Li, Z.; Liu, J.; Zheng, X.; Tang, K.; Kaplan, D.L. Ethanol-induced coacervation in aqueous gelatin solution for constructing nanospheres and networks: Morphology, dynamics and thermal sensitivity. J. Colloid Interface Sci. 2021, 582, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, T.; Yang, D.; Peng, X.; Zhang, H.; Zeng, H. Recent advances in coacervation and underlying noncovalent molecular interaction mechanisms. Prog. Polym. Sci. 2024, 153, 101827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.H.; Ki, M.-R.; Chung, D.Y.; Pack, S.P. Biomolecule-Based Coacervation: Mechanisms, Applications, and Future Perspectives in Biomedical and Biotechnological Fields. Biomolecules 2025, 15, 861. https://doi.org/10.3390/biom15060861
Kim DH, Ki M-R, Chung DY, Pack SP. Biomolecule-Based Coacervation: Mechanisms, Applications, and Future Perspectives in Biomedical and Biotechnological Fields. Biomolecules. 2025; 15(6):861. https://doi.org/10.3390/biom15060861
Chicago/Turabian StyleKim, Dong Hyun, Mi-Ran Ki, Da Yeon Chung, and Seung Pil Pack. 2025. "Biomolecule-Based Coacervation: Mechanisms, Applications, and Future Perspectives in Biomedical and Biotechnological Fields" Biomolecules 15, no. 6: 861. https://doi.org/10.3390/biom15060861
APA StyleKim, D. H., Ki, M.-R., Chung, D. Y., & Pack, S. P. (2025). Biomolecule-Based Coacervation: Mechanisms, Applications, and Future Perspectives in Biomedical and Biotechnological Fields. Biomolecules, 15(6), 861. https://doi.org/10.3390/biom15060861






