A Scoping Review of Sarcoglycan Expression in Non-Muscle Organs: Beyond Muscles
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- •
- Which SG subunits have been detected in non-muscle organs?
- •
- What experimental or observational methods have been employed to assess SG expression?
- •
- Are there reported associations between SG expression levels and specific pathological or physiological states in non-muscle organs?
- •
- Are there molecular features, such as glycosylation, membrane lipid associations, or scaffold protein interactions, described for SGs in these organs?
- •
- What are the main research gaps identified in the literature?
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Clinical and ex vivo human studies | Studies focusing exclusively on muscle |
| Animal studies | Studies on the expression of SGs in non-muscle organs identified in the muscular coat |
| Cell-based models | Studies involving sarcoglycanopathies and myoclonic dystonia |
| Studies that investigate the expression of one or more SG subunits (α, β, γ, δ, ε, and ζ) in non-muscle organs | Case reports, case series, reviews, meta-analyses, letters, editorials, commentaries, communications, supplements, and proceedings papers |
| Research articles as experimental studies, observational studies, and in vitro/in vivo analyses | Papers without full-text availability |
| Papers published in English | Papers published in languages other than English |
2.3. Information Sources
| Database | Search Strategy |
|---|---|
| PubMed/MEDLINE “https://pubmed.ncbi.nlm.nih.gov/ (accessed on 19 February 2025)” | (Sarcoglycan complex OR Sarcoglycans) AND (Non-Muscle Tissue OR Epithelial tissue OR Gland OR Prostate OR Breast OR Liver OR Pancreas OR Spleen OR Nervous system OR Kidney OR Lung OR Connective tissue OR Adipose organ) AND (Immunohistochemistry OR RT-PCR OR Immunofluorescence OR mRNA) |
| Scopus “https://www.scopus.com/home.uri (accessed on 19 February 2025)” | (“Sarcoglycans” OR sarcoglycan*) AND (“Non-Muscle Tissues”) AND (expression OR “immunohistochemistry” OR “immunofluorescence” OR “RT-PCR” OR mRNA) |
| Web of Science “https://www.webofscience.com/wos/ (accessed on 19 February 2025)” | (Sarcoglycan complex OR Sarcoglycans) AND (Non-Muscle Tissue OR Epithelial tissue OR Gland OR Prostate OR Breast OR Liver OR Pancreas OR Spleen OR Nervous system OR Kidney OR Lung OR Connective tissue OR Adipose organ) AND (Immunohistochemistry OR RT-PCR OR Immunofluorescence OR mRNA) |
| ScienceDirect “https://www.sciencedirect.com/ (accessed on 19 February 2025)” | (Sarcoglycan complex OR Sarcoglycans) AND “Non-muscle tissue” AND (Immunohistochemistry OR “RT-PCR” OR Immunofluorescence OR mRNA) |
| Google Scholar “https://scholar.google.com/ (accessed on 19 February 2025)” | (Sarcoglycan complex OR Sarcoglycans) AND “Non-muscle tissue” AND (Immunohistochemistry OR “RT-PCR” OR Immunofluorescence OR mRNA) |
2.4. Screening and Selection Process
2.5. Data Extraction
2.6. Data Synthesis
3. Results
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Overview of SG Expression Across Non-Muscle Organs
3.4. Central Nervous System (CNS)
3.4.1. Animal Studies
3.4.2. Human Studies
| Study (Authors, Year) | Study Design | Tissue/Organ/Cell Types Examined | Types of SG Proteins Studied | Species/Models |
|---|---|---|---|---|
| Xiao and LeDoux (2003) [38] | Molecular study (Northern analysis, RT-PCR and ISH) | Neural (cerebellar cortex, striatum, cerebral cortex, thalamus, hippocampus) | ε | Rat [16] |
| Vermiglio et al. (2011) [24] | Immunofluorescence study | Cerebral and cerebellar cortex (neurons and glial cells) | α, β, γ, δ, ε | |
| Cutroneo et al. (2015) [37] | Immunohistochemical study | hippocampus, cerebral and cerebellar cortex | α, β, γ, δ, ε, ζ | |
| Rizzo et al. (2018) [31] | Immunofluorescence study | Cerebral cortex (neurons and glial cells) Cerebellar cortex (neurons and glial cells) | α, β, γ, δ, ε, ζ | |
| Chan et al. (2005) [16] | Immunohistochemistry and molecular (FISH) study | Olfactory bulb (mitral cell layer) Cerebellum (Purkinje cell) Hippocampal formation and neocortex Monoaminergic cell groups and brainstem nuclei | ε | Mouse |
| Shiga et al. (2006) [30] | Immunofluorescence and molecular (RT-PCR and immunoprecipitation) study | Brain | γ, ζ | |
| Fort et al. (2005) [34] | Immunohistochemistry and molecular (RT-PCR) study | Retina (Müller and ganglion cells) | α, β, γ, δ, ε | |
| Boulay et al. (2015) [26] | Immunofluorescence and molecular (RT-PCR, Western blot) study | Cerebrovascular system (brain vessels, cortex, and hippocampus) | α, β, γ, δ, ε, ζ | |
| Anastasi et al. (2012) [28] | Immunohistochemical and molecular study (RT-PCR, Western Blot) | Cerebral cortex (neurons and astrocytes) | α, β, γ, δ, ε, ζ | Human |
3.5. Peripheral Nervous System (PNS)
3.5.1. Animal Studies
3.5.2. Human Studies
| Study (Authors, Year) | Study Design | Tissue/Organ/Cell Types Examined | Types of SG Proteins Studied | Species/Models |
|---|---|---|---|---|
| Cai et al. (2007) [25] | Immunofluorescence and molecular study (RT-PCR and Western blot) | Peripheral nervous system (sciatic nerves and Schwann cell cultures) | α, β, γ, δ, ε, ζ | Rat Hamster |
| Imamura (2000) [33] | Immunofluorescence and molecular (immunoprecipitation and Western blot) study | Peripheral nerves (sciatic, femoral and tibial) | α, β, γ, δ, ε | Rabbit |
3.6. Glands
3.6.1. Animal Studies
3.6.2. Human Studies
3.7. Oral Mucosa
3.7.1. Animal Studies
3.7.2. Human Studies
3.8. Adipose
3.8.1. Animal Studies
3.8.2. Human Studies
3.9. Other Organs
3.9.1. Animal Studies
3.9.2. Human Studies
4. Discussion
4.1. Expression and Functional Roles of SGs in Non-Muscle Organs
4.1.1. CNS
4.1.2. PNS
4.1.3. Glands and Oral Mucosa
4.1.4. Adipose
4.1.5. Other Organs
4.2. Focus of the Post-Translational Modifications (PTMs) of SGs: Glycosylation
5. Limitations and Gaps in the Literature
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durbeej, M.; Campbell, K.P. Biochemical Characterization of the Epithelial Dystroglycan Complex. J. Biol. Chem. 1999, 274, 26609–26616. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Saito, F.; Yamada, H.; Hase, A.; Sunada, Y.; Shimizu, T. Sarcoglycan Complex: A Muscular Supporter of Dystroglycan-Dystrophin Interplay? Cell. Mol. Biol. 1999, 45, 751–762. [Google Scholar] [PubMed]
- Noguchi, S.; Wakabayashi, E.; Imamura, M.; Yoshida, M.; Ozawa, E. Formation of Sarcoglycan Complex with Differentiation in Cultured Myocytes. Eur. J. Biochem. 2000, 267, 640–648. [Google Scholar] [CrossRef]
- Younus, M.; Ahmad, F.; Malik, E.; Bilal, M.; Kausar, M.; Abbas, S.; Shaheen, S.; Kakar, M.U.; Alfadhel, M.; Umair, M. SGCD Homozygous Nonsense Mutation (p.Arg97∗) Causing Limb-Girdle Muscular Dystrophy Type 2F (LGMD2F) in a Consanguineous Family, a Case Report. Front. Genet. 2019, 9, 727. [Google Scholar] [CrossRef]
- Xiao, H.F.; Gong, S.Z.; Zhao, Y.; LeDoux, M.S. Developmental Expression of Rat TorsinA Transcript and Protein. Dev. Brain Res. 2004, 152, 47–60. [Google Scholar] [CrossRef]
- Wolburg, H.; Wolburg-Buchholz, K.; Fallier-Becker, P.; Noell, S.; Mack, A.F. Structure and Functions of Aquaporin-4-Based Orthogonal Arrays of Particles. In International Review of Cell and Molecular Biology; Kwang, W.J., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 287, pp. 1–41. [Google Scholar]
- Cirak, S.; Arechavala-Gomeza, V.; Guglieri, M.; Feng, L.; Torelli, S.; Anthony, K.; Abbs, S.; Garralda, M.E.; Bourke, J.; Wells, D.J.; et al. Exon Skipping and Dystrophin Restoration in Patients with Duchenne Muscular Dystrophy after Systemic Phosphorodiamidate Morpholino Oligomer Treatment: An Open-Label, Phase 2, Dose-Escalation Study. Lancet 2011, 378, 595–605. [Google Scholar] [CrossRef]
- Chawla, G.; Lin, C.-H.; Han, A.; Shiue, L.; Ares, M.J.; Black, D.L. Sam68 Regulates a Set of Alternatively Spliced Exons during Neurogenesis. Mol. Cell. Biol. 2009, 29, 201–213. [Google Scholar] [CrossRef]
- Henry, M.D.; Campbell, K.P. Dystroglycan: An Extracellular Matrix Receptor Linked to the Cytoskeleton. Curr. Opin. Cell Biol. 1996, 8, 625–631. [Google Scholar] [CrossRef]
- Straub, V.; Campbell, K.P. Muscular Dystrophies and the Dystrophin-Glycoprotein Complex. Curr. Opin. Neurol. 1997, 10, 168–175. [Google Scholar] [CrossRef]
- Ceccarini, M.; Grasso, M.; Veroni, C.; Gambara, G.; Artegiani, B.; Macchia, G.; Ramoni, C.; Torreri, P.; Mallozzi, C.; Petrucci, T.C.; et al. Association of Dystrobrevin and Regulatory Subunit of Protein Kinase A: A New Role for Dystrobrevin as a Scaffold for Signaling Proteins. J. Mol. Biol. 2007, 371, 1174–1187. [Google Scholar] [CrossRef]
- Straub, V.; Ettinger, A.J.; Durbeej, M.; Venzke, D.P.; Cutshall, S.; Sanes, J.R.; Campbell, K.P. Epsilon-Sarcoglycan Replaces Alpha-Sarcoglycan in Smooth Muscle to Form a Unique Dystrophin-Glycoprotein Complex. J. Biol. Chem. 1999, 274, 27989–27996. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.T.; McNally, E.M. Sarcoglycans in Vascular Smooth and Striated Muscle. Trends Cardiovasc. Med. 2003, 13, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.T.; Zarnegar, S.; McNally, E.M. Zeta-Sarcoglycan, a Novel Component of the Sarcoglycan Complex, Is Reduced in Muscular Dystrophy. Hum. Mol. Genet. 2002, 11, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Chen, Z.; Schottenfeld, J.; Stahl, R.C.; Kunkel, L.M.; Chan, Y.-M. Specific Assembly Pathway of Sarcoglycans Is Dependent on Beta- and Delta-Sarcoglycan. Muscle Nerve 2004, 29, 409–419. [Google Scholar] [CrossRef]
- Chan, P.; Gonzalez-Maeso, J.; Ruf, F.; Bishop, D.F.; Hof, P.R.; Sealfon, S.C. Epsilon-Sarcoglycan Immunoreactivity and MRNA Expression in Mouse Brain. J. Comp. Neurol. 2005, 482, 50–73. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Sharma, M.C.; Gulati, S.; Kabra, M.; Pandey, R.M.; Sarkar, C. Skin Biopsy: A New Tool to Diagnose Sarcoglycanopathy. J. Child Neurol. 2014, 29, NP5–NP8. [Google Scholar] [CrossRef]
- Arena, S.; Favaloro, A.; Cutroneo, G.; Consolo, A.; Arena, F.; Anastasi, G.; Di Benedetto, V. Sarcoglycan Subcomplex Expression in Refluxing Ureteral Endings. J. Urol. 2008, 179, 1980–1986. [Google Scholar] [CrossRef]
- Arena, S.; Cutroneo, G.; Favaloro, A.; Sinatra, M.T.; Trimarchi, F.; Scarvaglieri, S.; Mallamace, A.; Arena, F.; Anastasi, G.; Di Benedetto, V. Abnormal Distribution of Sarcoglycan Subcomplex in Colonic Smooth Muscle Cells of Aganglionic Bowel. Int. J. Mol. Med. 2010, 25, 353–359. [Google Scholar] [CrossRef]
- Anastasi, G.; Cutroneo, G.; Sidoti, A.; Rinaldi, C.; Bruschetta, D.; Rizzo, G.; D’Angelo, R.; Tarone, G.; Amato, A.; Favaloro, A. Sarcoglycan Subcomplex Expression in Normal Human Smooth Muscle. J. Histochem. Cytochem. 2007, 55, 831–843. [Google Scholar] [CrossRef]
- Muni-Lofra, R.; Juanola-Mayos, E.; Schiava, M.; Moat, D.; Elseed, M.A.; Michel-Sodhi, J.; Harris, E.; McCallum, M.; Moore, U.; Richardson, M.T.; et al. Longitudinal Analysis of Respiratory Function of Different Types of Limb Girdle Muscular Dystrophies Reveals Independent Trajectories. Neurol. Genet. 2023, 9, e200084. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, Q.; Huang, X.; Zhou, D.; Xu, H.; Zhao, F.; Mi, X.; Wang, R.; Jia, F.; et al. Mutation in Ε-Sarcoglycan Induces a Myoclonus-Dystonia Syndrome-Like Movement Disorder in Mice. Neurosci. Bull. 2020, 37, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Vermiglio, G.; Runci, M.; Scibilia, A.; Biasini, F.; Cutroneo, G. Preliminary Study on Sarcoglycan Sub-Complex in Rat Cerebral and Cerebellar Cortex. Ital. J. Anat. Embryol. 2012, 117, 54–64. [Google Scholar]
- Cai, H.; Erdman, R.A.; Zweier, L.; Chen, J.; Shaw, J.H.; Baylor, K.A.; Stecker, M.M.; Carey, D.J.; Chan, Y.M. The Sarcoglycan Complex in Schwann Cells and Its Role in Myelin Stability. Exp. Neurol. 2007, 205, 257–269. [Google Scholar] [CrossRef]
- Boulay, A.; Saubaméa, B.; Cisternino, S.; Mignon, V.; Mazeraud, A.; Jourdren, L.; Blugeon, C.; Cohen-Salmon, M. The Sarcoglycan Complex Is Expressed in the Cerebrovascular System and Is Specifically Regulated by Astroglial Cx30 Channels. Front. Cell. Neurosci. 2015, 9, 9. [Google Scholar] [CrossRef]
- Arco, A.; Favaloro, A.; Gioffrè, M.; Santoro, G. Sarcoglycans in the Normal and Pathological Breast Tissue of Humans: An Immunohistochemical and Molecular Study. Cells Tissues Organs 2012, 195, 550–562. [Google Scholar] [CrossRef]
- Anastasi, G.; Tomasello, F.; Di Mauro, D.; Cutroneo, G.; Favaloro, A.; Conti, A.; Ruggeri, A.; Rinaldi, C.; Trimarchi, F. Expression of Sarcoglycans in the Human Cerebral Cortex: An Immunohistochemical and Molecular Study. Cells Tissues Organs 2012, 196, 470–480. [Google Scholar] [CrossRef]
- Favaloro, A.; Rizzo, G.; Santoro, G.; Pergolizzi, S.; Furci, A.; Centofanti, A.; Cutroneo, G. Sarcoglycans and Integrins in Human Thyrocytes: An Immunofluorescence Study. Ital. J. Anat. Embryol. 2022, 126, 125–138. [Google Scholar] [CrossRef]
- Shiga, K.; Yoshioka, H.; Matsumiya, T.; Kimura, I.; Takeda, S.; Imamura, M. Zeta-Sarcoglycan Is a Functional Homologue of Gamma-Sarcoglycan in the Formation of the Sarcoglycan Complex. Exp. Cell Res. 2006, 312, 2083–2092. [Google Scholar] [CrossRef]
- Rizzo, G.; Di Mauro, D.; Cutroneo, G.; Schembri-Wismayer, P.; Brunetto, D.; Spoto, C.; Vermiglio, G.; Centofanti, A.; Favaloro, A. An Immunofluorescence Study About Staining Pattern Variability of Sarcoglycans in Rat’s Cerebral and Cerebellar Cortex. Eur. J. Exp. Biol. 2018, 8, 7. [Google Scholar] [CrossRef]
- Nastro Siniscalchi, E.; Cutroneo, G.; Catalfamo, L.; Santoro, G.; Allegra, A.; Oteri, G.; Cicciù, D.; Alonci, A.; Penna, G.; Musolino, C.; et al. Immunohistochemical Evaluation of Sarcoglycans and Integrins in Gingival Epithelium of Multiple Myeloma Patients with Bisphosphonate-Induced Osteonecrosis of the Jaw. Oncol. Rep. 2010, 24, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Araishi, K.; Noguchi, S.; Ozawa, E. A Sarcoglycan-Dystroglycan Complex Anchors Dp116 and Utrophin in the Peripheral Nervous System. Hum. Mol. Genet. 2000, 9, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.; Estrada, F.-J.; Bordais, A.; Mornet, D.; Sahel, J.-A.; Picaud, S.; Vargas, H.R.; Coral-Vázquez, R.M.; Rendon, A. The Sarcoglycan-Sarcospan Complex Localization in Mouse Retina Is Independent from Dystrophins. Neurosci. Res. 2005, 53, 25–33. [Google Scholar] [CrossRef] [PubMed]
- De Ponte, F.S.; Favaloro, A.; Siniscalchi, E.N.; Centofanti, A.; Runci, M.; Cutroneo, G.; Catalfamo, L. Sarcoglycans and Integrins in Bisphosphonate Treatment: Immunohistochemical and Scanning Electron Microscopy Study. Oncol. Rep. 2013, 30, 2639–2646. [Google Scholar] [CrossRef]
- Cutroneo, G.; Bramanti, P.; Favaloro, A.; Anastasi, G.; Trimarchi, F.; Di Mauro, D.; Rinaldi, C.; Speciale, F.; Inferrera, A.; Santoro, G.; et al. Sarcoglycan Complex in Human Normal and Pathological Prostatic Tissue: An Immunohistochemical and RT-PCR Study. Anat. Rec. 2014, 297, 327–336. [Google Scholar] [CrossRef]
- Cutroneo, G.; Bramanti, P.; Anastasi, G.; Bruschetta, D.; Favaloro, A.; Vermiglio, G.; Trimarchi, F.; Di Mauro, D.; Rizzo, G. Sarcoglycans and Gaba(a) Receptors in Rat Central Nervous System: An Immunohistochemical Study. Ital. J. Anat. Embryol. 2015, 120, 105–116. [Google Scholar]
- Xiao, J.; LeDoux, M.S. Cloning, Developmental Regulation and Neural Localization of Rat Epsilon-Sarcoglycan. Brain Res. Mol. Brain Res. 2003, 119, 132–143. [Google Scholar] [CrossRef]
- Romo-Yáñez, J.; Montañez, C.; Salazar-Olivo, L.A. Dystrophins and DAPs Are Expressed in Adipose Tissue and Are Regulated by Adipogenesis and Extracellular Matrix. Biochem. Biophys. Res. Commun. 2011, 404, 717–722. [Google Scholar] [CrossRef]
- Groh, S.; Zong, H.; Goddeeris, M.M.; Lebakken, C.S.; Venzke, D.; Pessin, J.E.; Campbell, K.P. Sarcoglycan Complex: Implications for Metabolic Defects in Muscular Dystrophies. J. Biol. Chem. 2009, 284, 19178–19182. [Google Scholar] [CrossRef]
- Cazurro-Gutiérrez, A.; Marcé-Grau, A.; Correa-Vela, M.; Salazar, A.; Vanegas, M.I.; Macaya, A.; Bayés, À.; Pérez-Dueñas, B. ε-Sarcoglycan: Unraveling the Myoclonus-Dystonia Gene. Mol. Neurobiol. 2021, 58, 3938–3952. [Google Scholar] [CrossRef]

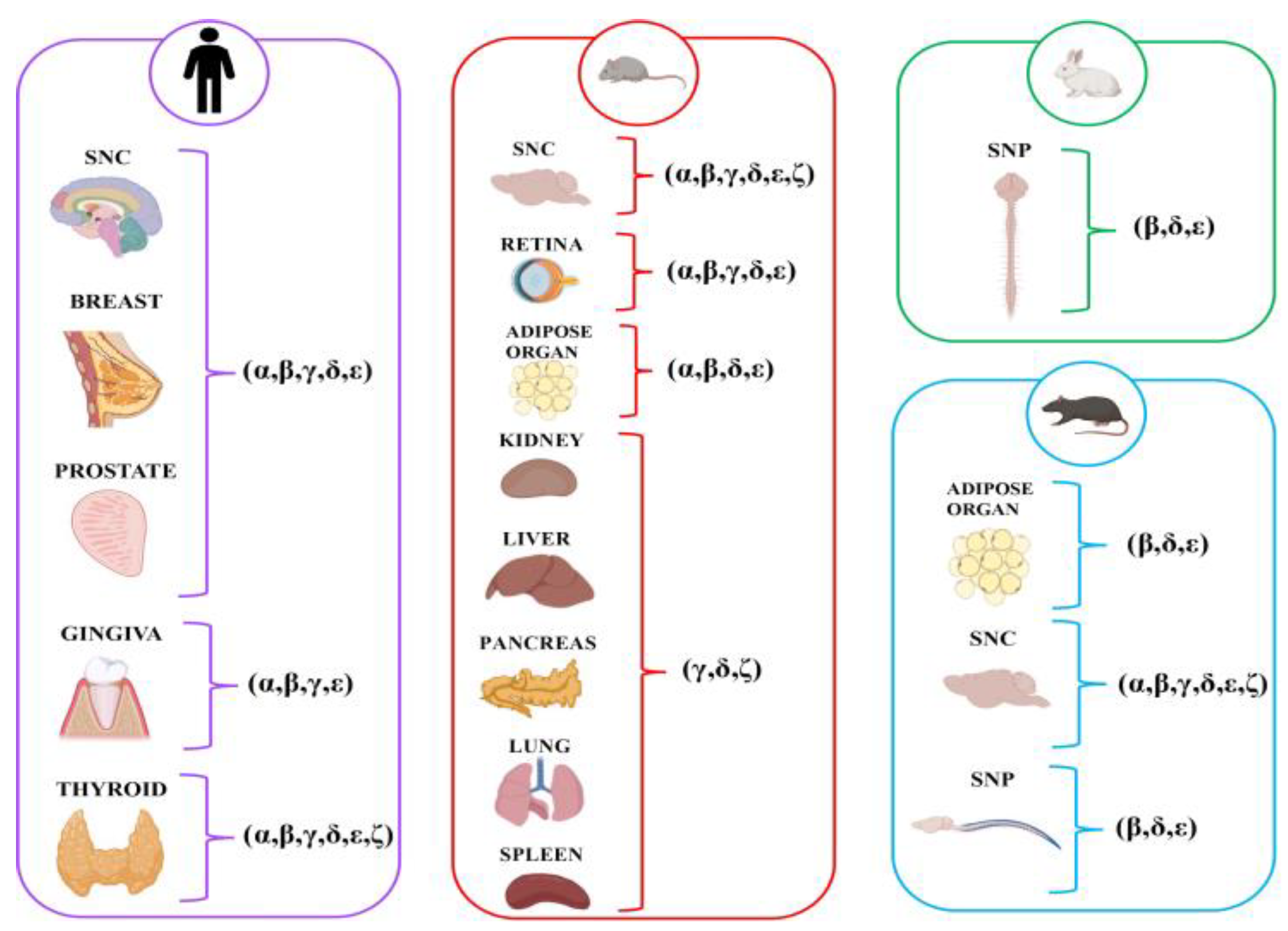

| Study (Authors, Year) | Study Design | Tissue/Organ/Cell Types Examined | Types of SG Proteins Studied | Species/Models |
|---|---|---|---|---|
| Shiga et al. (2006) [30] | Immunofluorescence and molecular (RT-PCR and immunoprecipitation) study | Pancreas | δ, ζ | Mouse |
| Arco et al. (2012) [27] | Immunohistochemical and molecular study (RT-PCR) | Glandular breast (epithelial and myoepithelial cells) | α, β, γ, δ, ε | Human |
| Cutroneo et al. (2014) [36] | Immunohistochemical and molecular (RT-PCR) study | Prostate gland (epithelial and myoepithelial cells) | α, β, γ, δ, ε | |
| Favaloro et al. (2022) [29] | Immunofluorescence study | Thyroid | α, β, γ, δ, ε, ζ |
| Study (Authors, Year) | Study Design | Tissue/Organ/Cell Types Examined | Types of SG Proteins Studied | Species/Models |
|---|---|---|---|---|
| Nastro Siniscalchi et al. (2010) [32] | Immunohistochemical study | Gingival epithelium | β, γ, ε | Human |
| De Ponte et al. (2013) [35] | Immunohistochemical study | Gingival epithelium | α, γ, ε |
| Study (Authors, Year) | Study Design | Tissue/Organ/Cell Types Examined | Types of SG Proteins Studied | Species/Models |
|---|---|---|---|---|
| Groh et al. (2009) [40] | Molecular study (RT-PCR and Western blot) | White adipocytes | α, β, δ | Mouse |
| Romo-Yáñez et al. (2011) [39] | Molecular study (RT-PCR and Western blot) | Adipose tissue (differentiation adipocyte) | β, δ, ε | Rat |
| Study (Authors, Year) | Study Design | Tissue/Organ/Cell Types Examined | Types of SG Proteins Studied | Species/Models |
|---|---|---|---|---|
| Xiao and LeDoux (2003) [38] | Molecular study (Northern analysis, RT-PCR and ISH) | Liver Kidney Lung Spleen Testis | ε | Rat |
| Shiga et al. (2006) [30] | Immunofluorescence and molecular (RT-PCR and immunoprecipitation) study | Kidney Liver Lung Spleen | δ, γ, ζ | Mouse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicita, F.; Freni, J.; Centofanti, A.; Favaloro, A.; Labellarte, D.; Cutroneo, G.; Anastasi, M.R.; Vermiglio, G. A Scoping Review of Sarcoglycan Expression in Non-Muscle Organs: Beyond Muscles. Biomolecules 2025, 15, 1020. https://doi.org/10.3390/biom15071020
Nicita F, Freni J, Centofanti A, Favaloro A, Labellarte D, Cutroneo G, Anastasi MR, Vermiglio G. A Scoping Review of Sarcoglycan Expression in Non-Muscle Organs: Beyond Muscles. Biomolecules. 2025; 15(7):1020. https://doi.org/10.3390/biom15071020
Chicago/Turabian StyleNicita, Fabiana, Josè Freni, Antonio Centofanti, Angelo Favaloro, Davide Labellarte, Giuseppina Cutroneo, Michele Runci Anastasi, and Giovanna Vermiglio. 2025. "A Scoping Review of Sarcoglycan Expression in Non-Muscle Organs: Beyond Muscles" Biomolecules 15, no. 7: 1020. https://doi.org/10.3390/biom15071020
APA StyleNicita, F., Freni, J., Centofanti, A., Favaloro, A., Labellarte, D., Cutroneo, G., Anastasi, M. R., & Vermiglio, G. (2025). A Scoping Review of Sarcoglycan Expression in Non-Muscle Organs: Beyond Muscles. Biomolecules, 15(7), 1020. https://doi.org/10.3390/biom15071020










