Physiological Mechanisms and Core Genes in Response to Saline-Alkali Stress in Foxtail Millet (Setaria italica L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Salt-Alkali Treatment
2.2. Measurement of Growth Indicators and Physiological Indicators
2.3. Observation of the Ultrastructure of Seedling Leaf Cells Under Saline-Alkali Stress
2.4. RNA Extraction, Transcriptome Sequencing, and Data Analysis
2.5. Weighted Gene Co-Expression Network Analysis
2.6. Quantitative PCR Analysis
2.7. Data Statistical Analysis
3. Results
3.1. Effects of Saline-Alkali Stress on Foxtail Millet Growth and Development
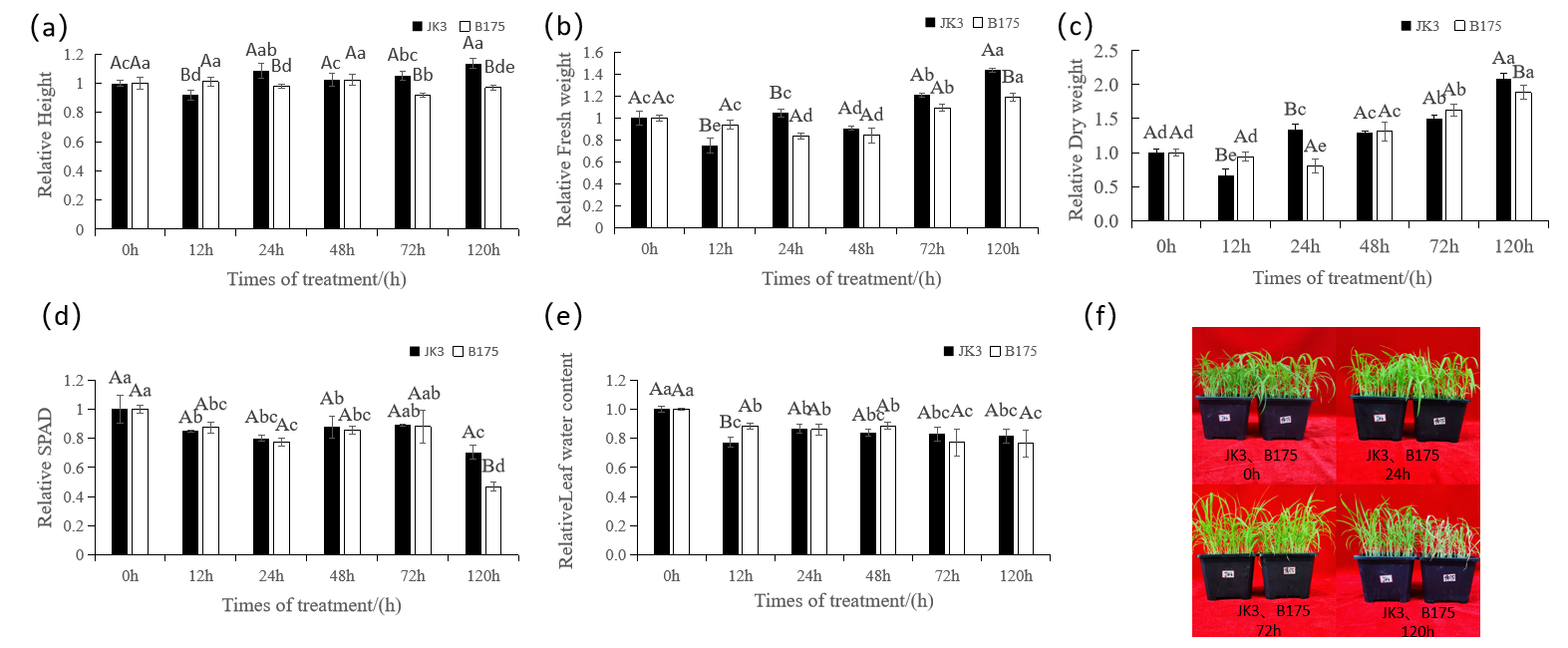
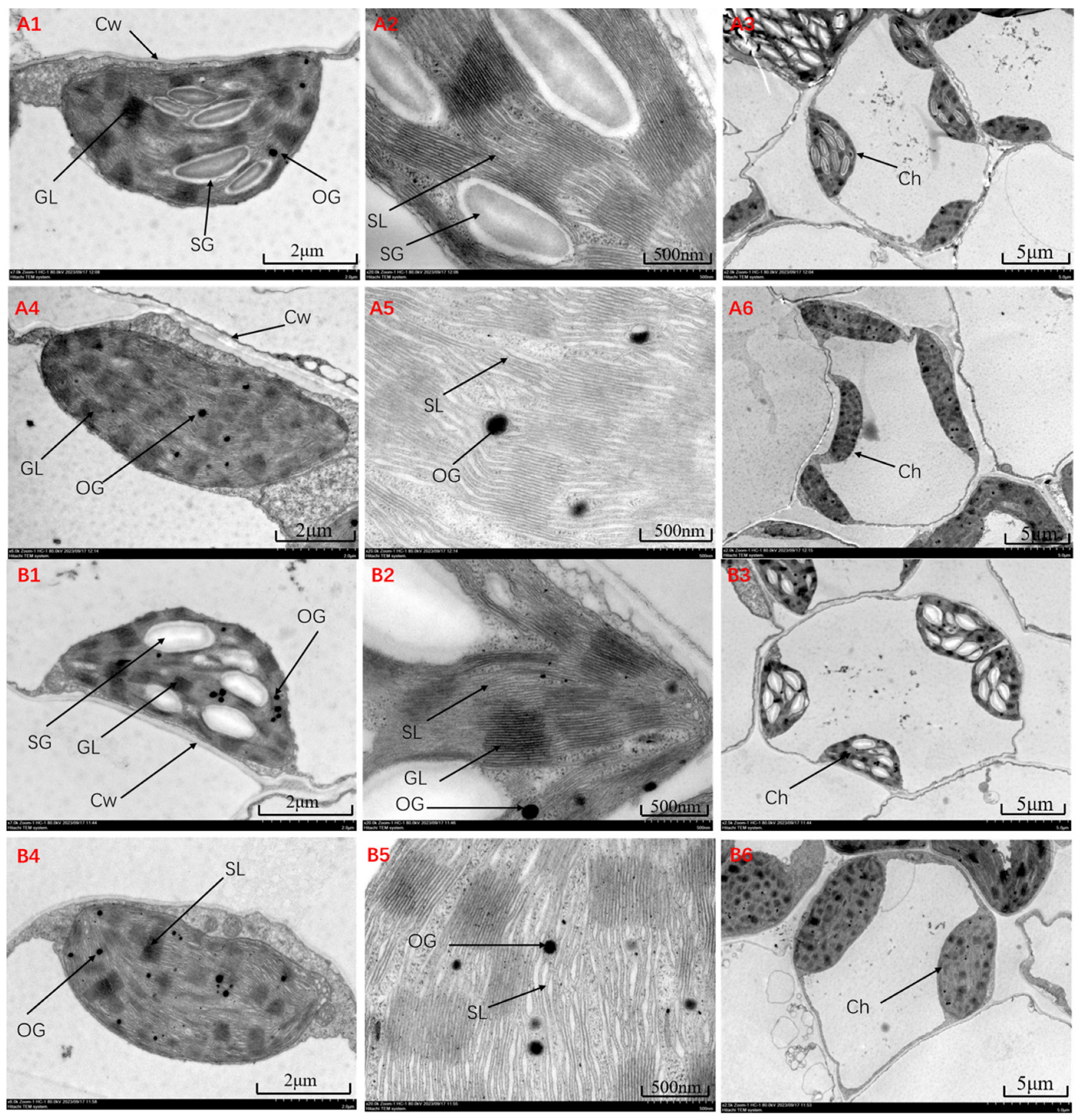
3.2. Effects of Saline-Alkali Stress on the Cellular Ultrastructure of Foxtail Millet Leaves
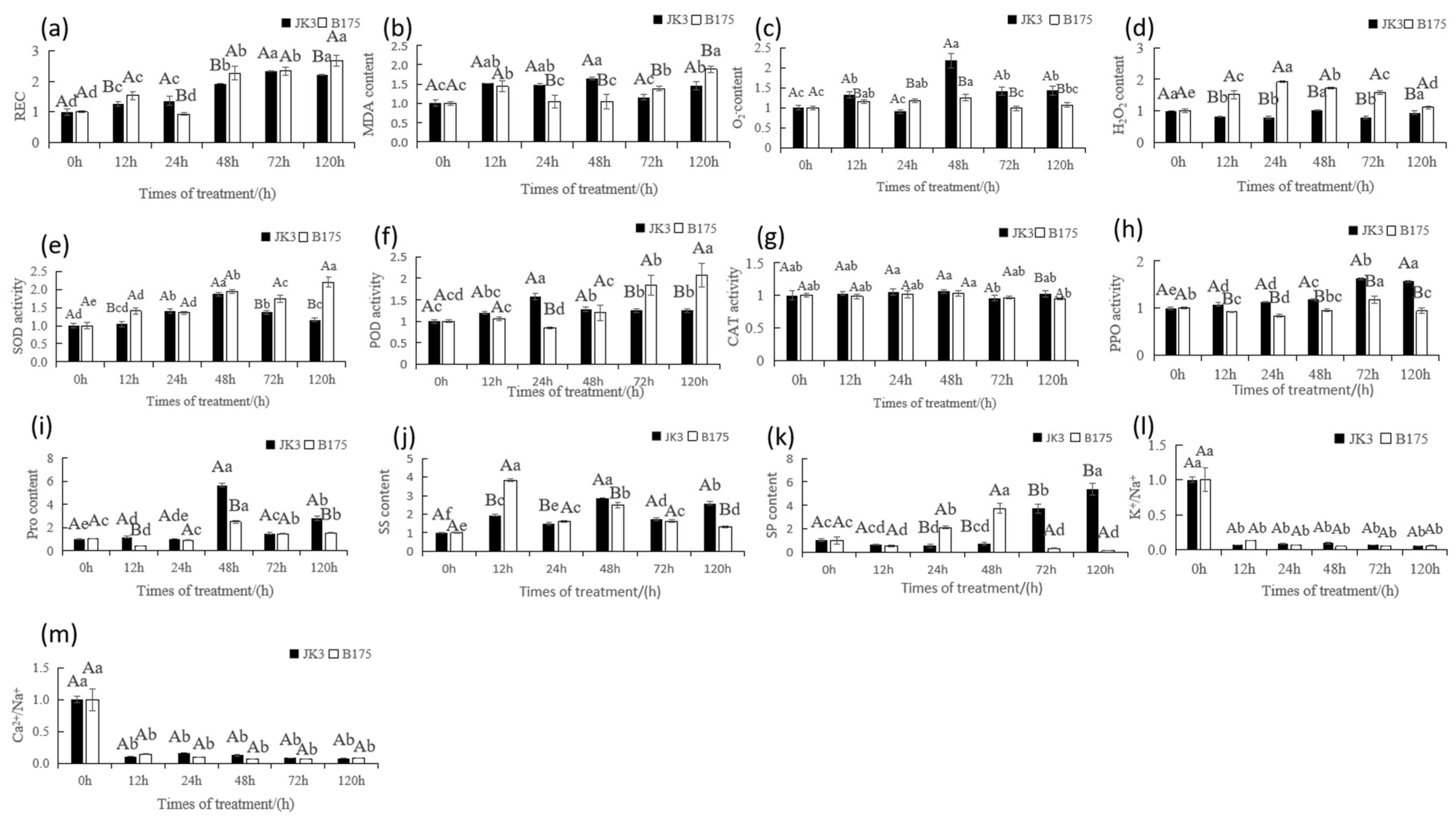
3.3. Effects of Saline-Alkali Stress on the Physiological and Biochemical Characteristics of Foxtail Millet Leaves
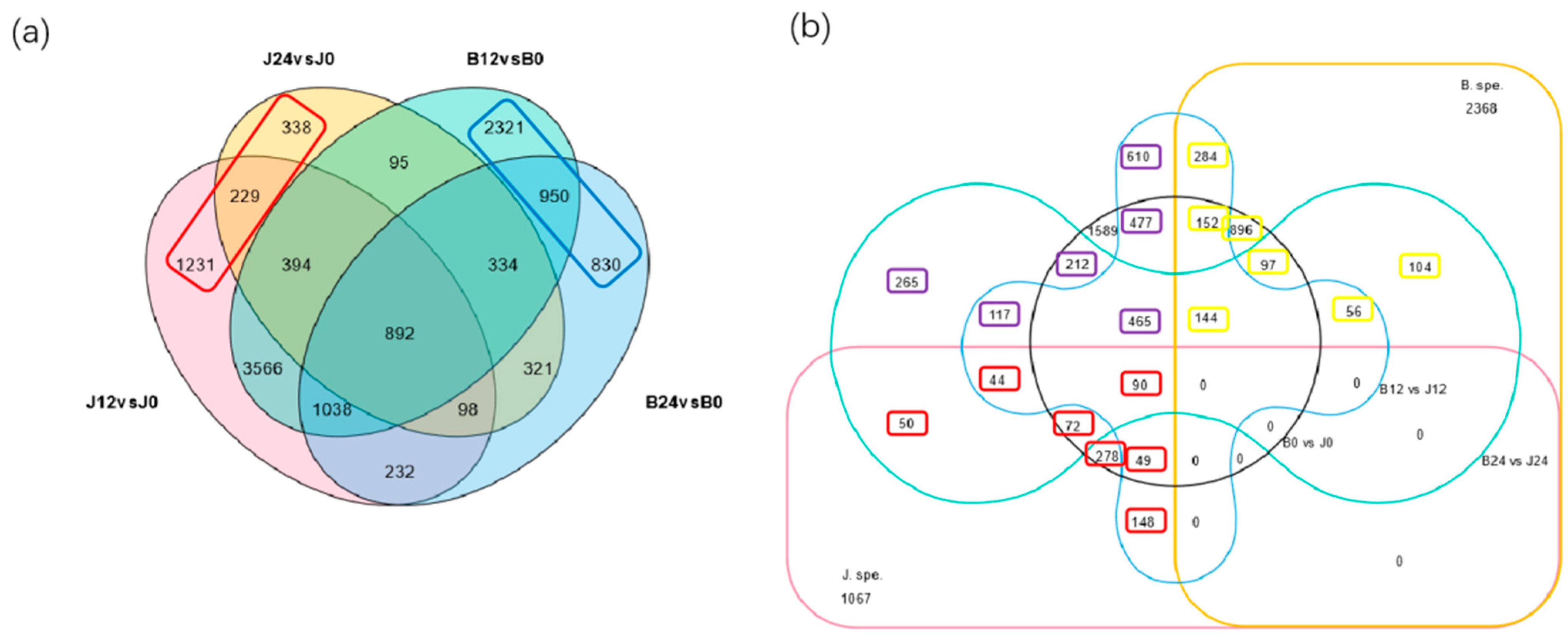
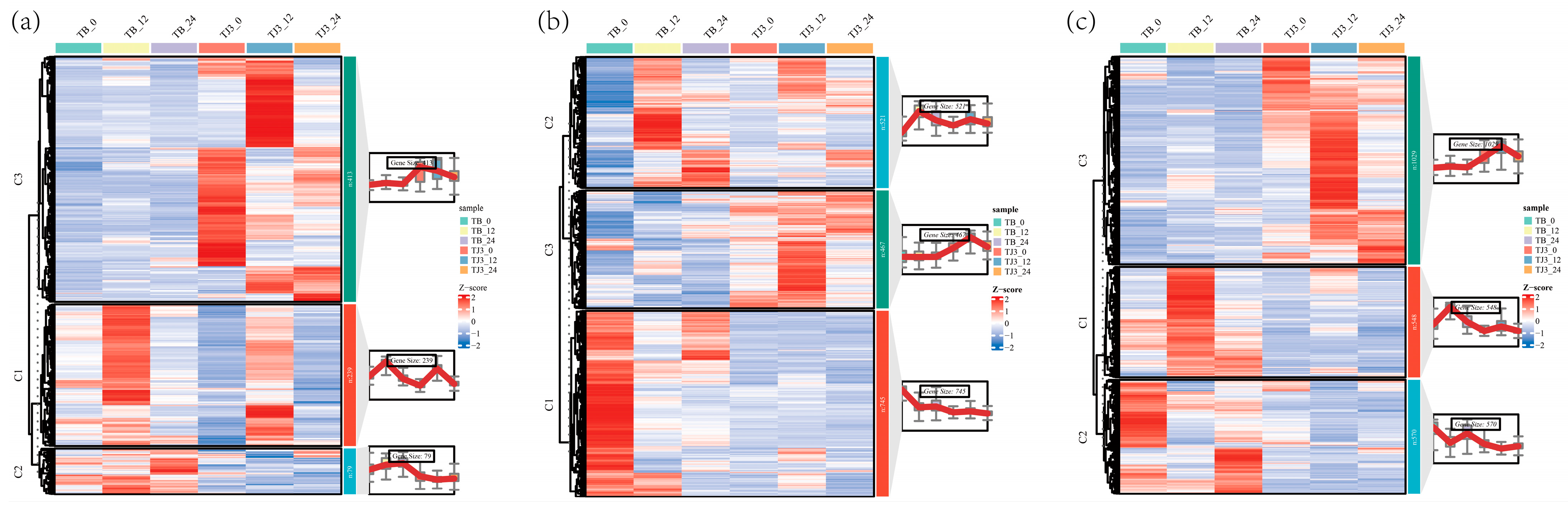
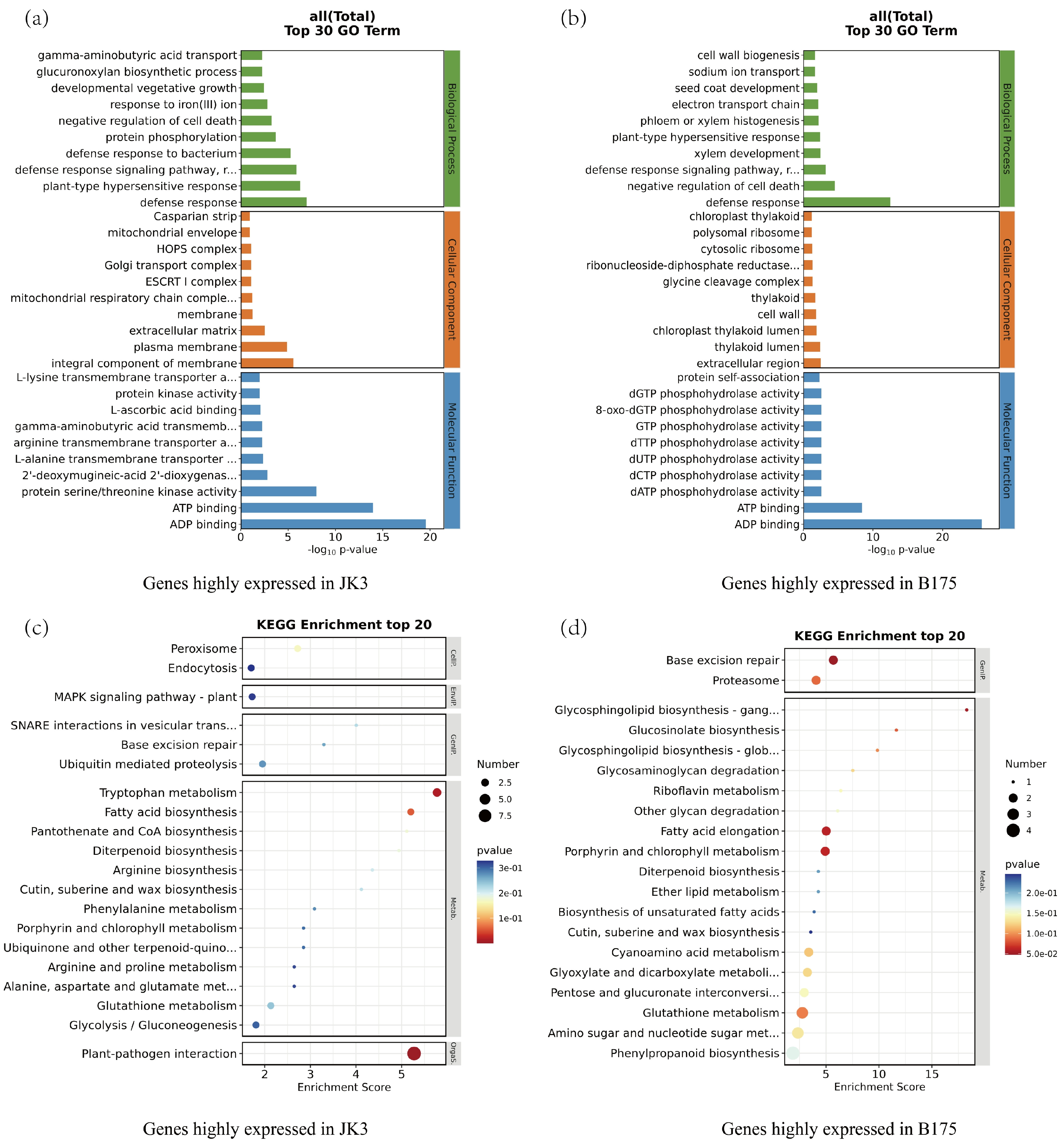
3.4. Transcriptomic Analysis of Foxtail Millet Response to Saline-Alkali Stress
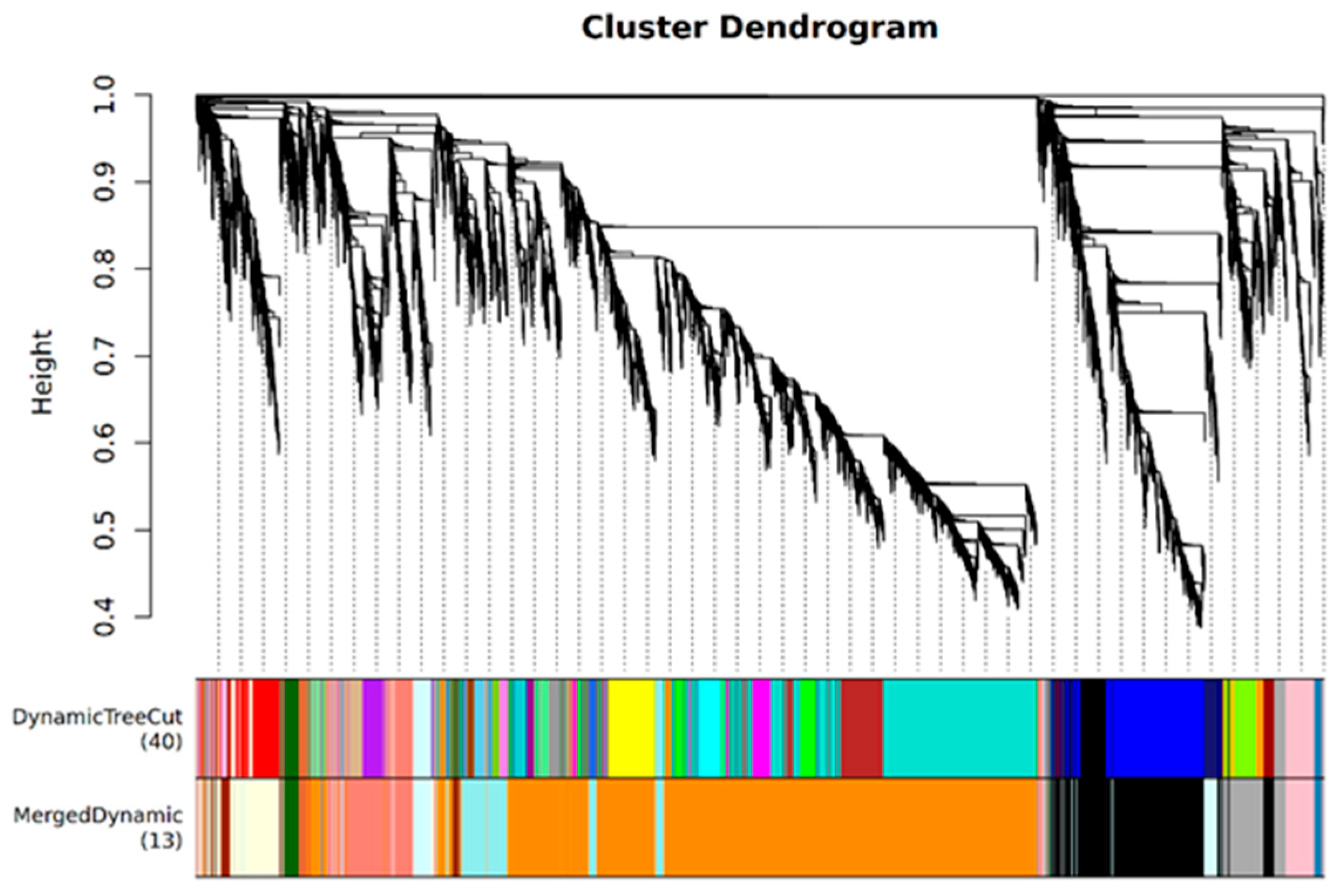
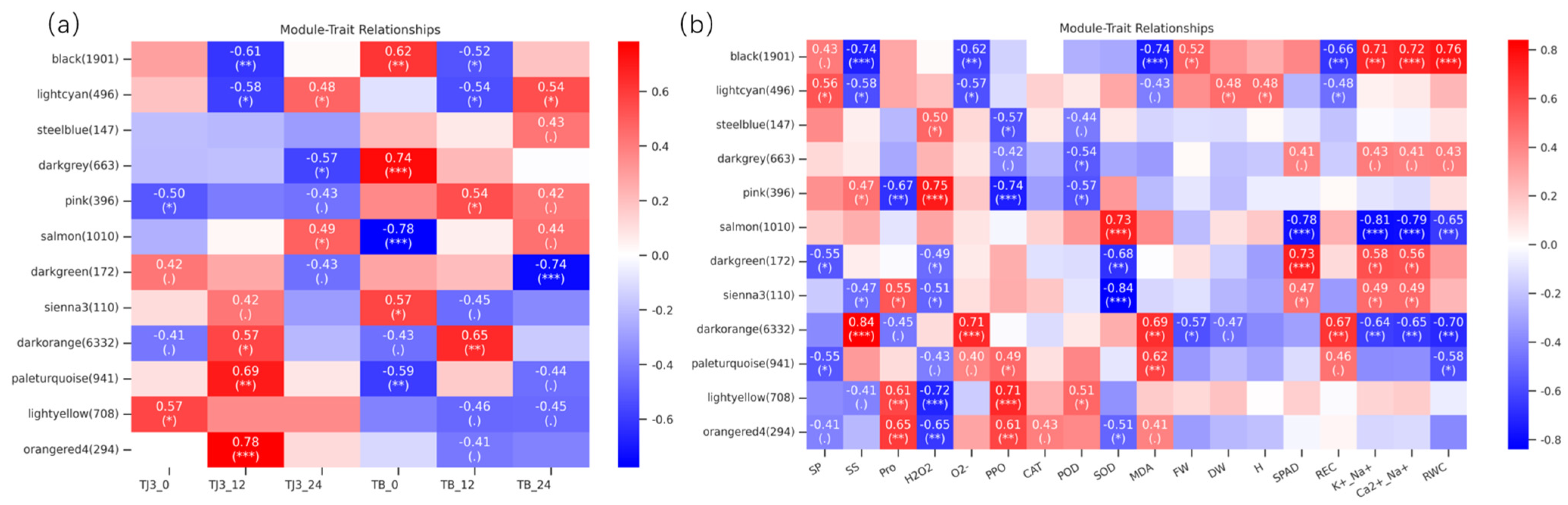
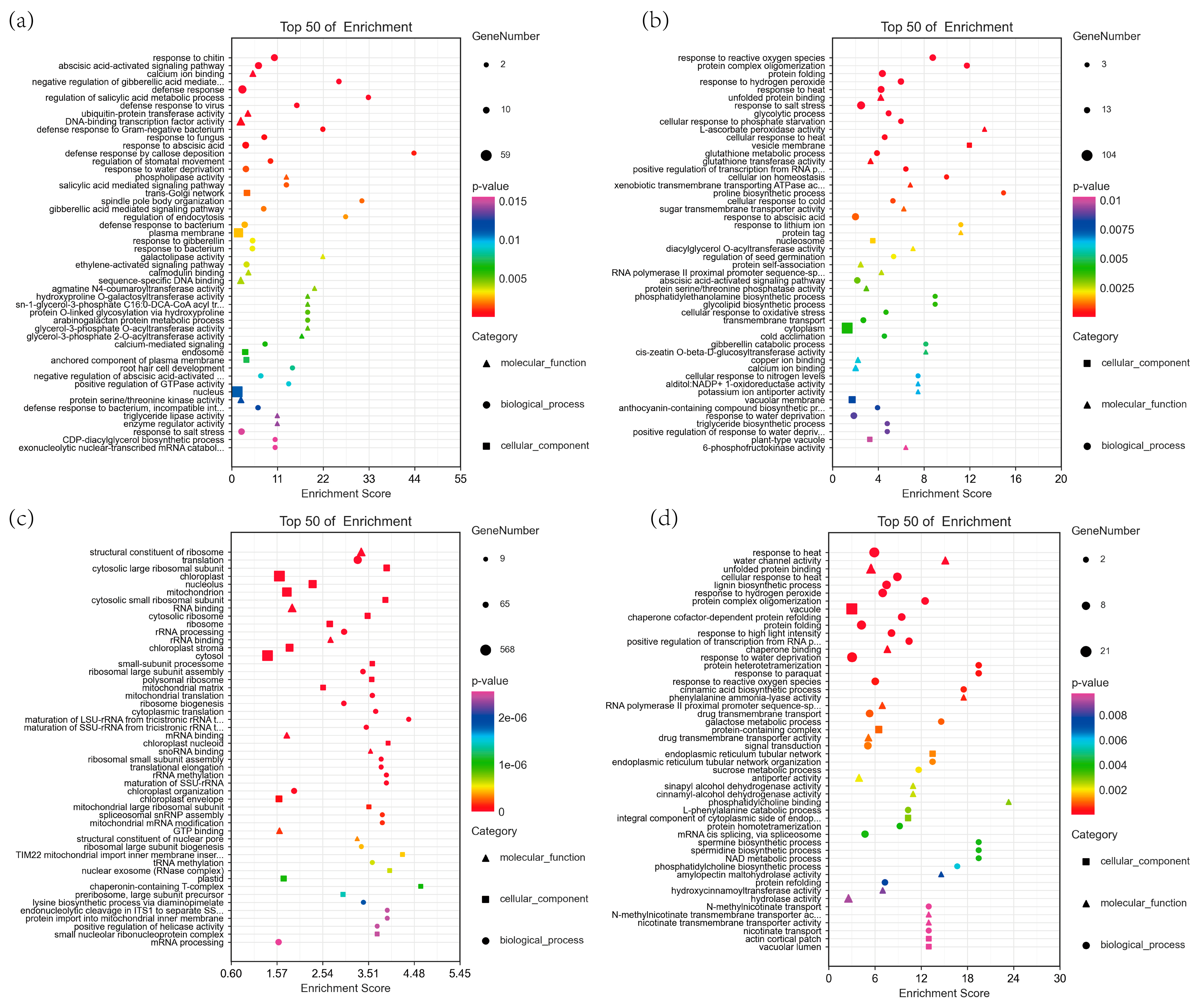
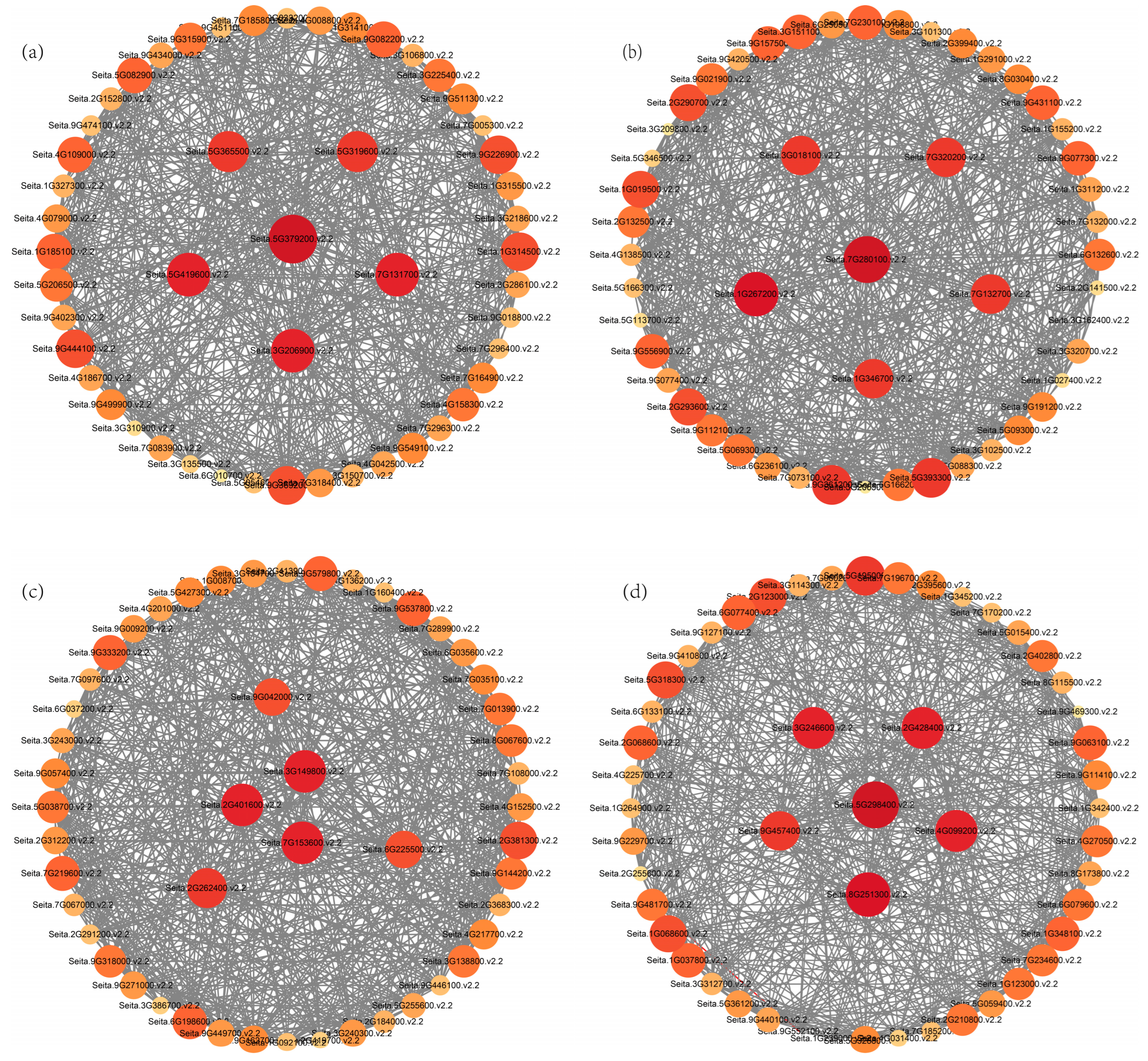
3.5. WGCNA of Foxtail Millet Response to Saline-Alkali Stress
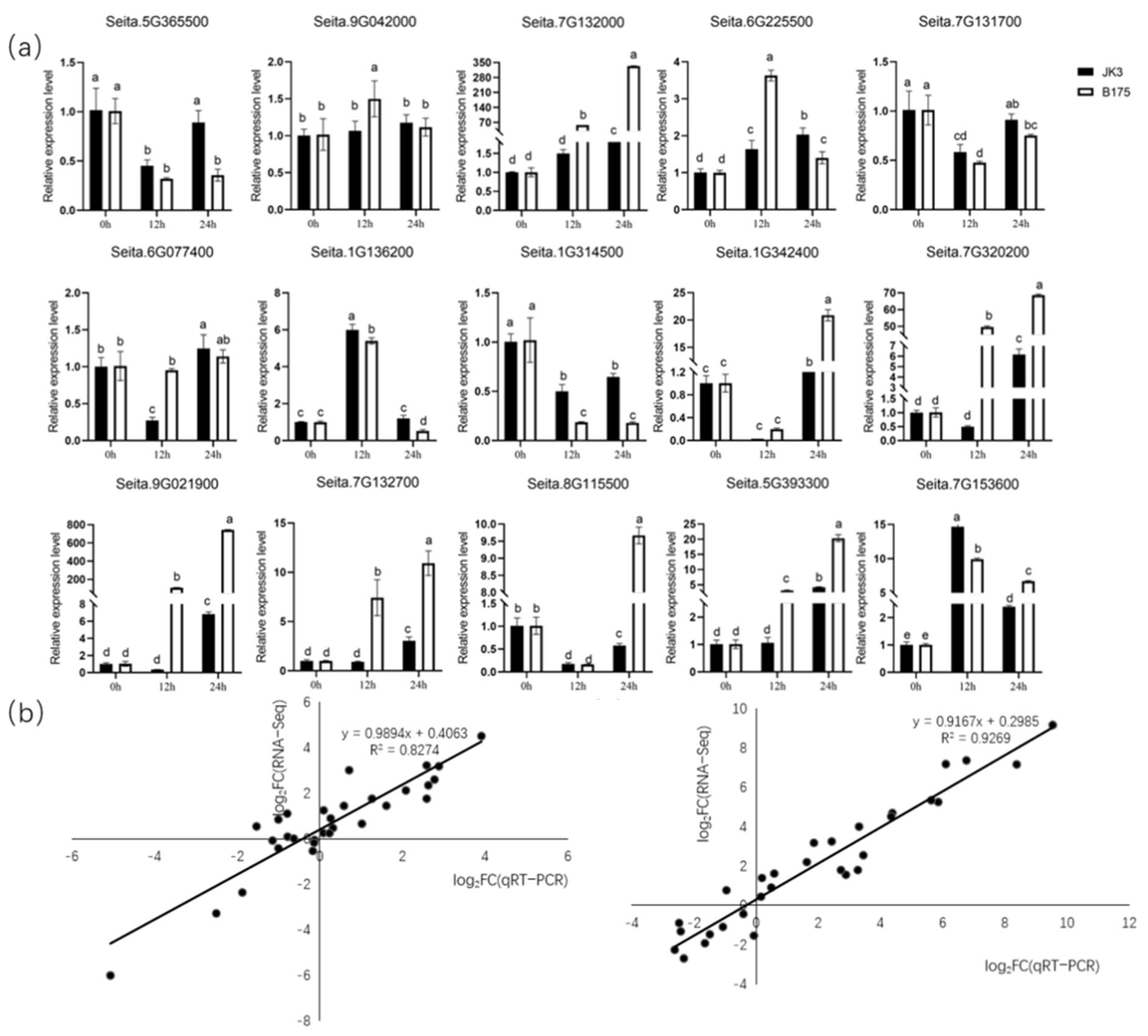
3.6. qRT-PCR Analysis
4. Discussion
4.1. Effect of Saline-Alkali Stress on Foxtail Millet Morphological Characteristics
4.2. Effect of Saline-Alkali Stress on the Ultrastructure of Foxtail Millet Leaf Cells
4.3. Physiological Mechanisms of Saline-Alkali Tolerance in Foxtail Millet
4.4. Impact of Saline-Alkali Stress on the Transcriptome of Foxtail Millet
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, L.; Li, B.; Meng, Y.; Ma, X.; Lai, Y.; Si, E.; Ren, P.; Yang, K.; Shang, X.; et al. Comparative Proteomic Analysis of Cultured Suspension Cells of the Halophyte Halogeton glomeratus by iTRAQ Provides Insights into Response Mechanisms to Salt Stress. Front. Plant Sci. 2016, 7, 110. [Google Scholar] [CrossRef]
- Leaman, D.J. The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW)—Managing Systems at Risk. Econ. Bot. 2011, 66, 418–419. [Google Scholar] [CrossRef]
- Yun, X.X.; Chen, Y.S. Dynamics of International Saline Land Development and Its Implications for China. Land Nat. Resour. Res. 2020, 1, 84–87. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Weng, Y.J. Identification and evaluation of salt tolerance in introduced spring wheat germplasm. Acta Agron. Sin. 2005, 31, 58–64. [Google Scholar] [CrossRef]
- Diao, X.M. New Advances in Research on Stress Resistance and Cultivation Techniques of Cereal and Grain Crops. Chin. Agric. Sci. 2019, 52, 3943–3949. [Google Scholar] [CrossRef]
- Diao, X.M. Basic Research Enhances the Scientific Research Innovation Level of Traditional Crops such as foxtail millet and Job’s Tears. Chin. Agric. Sci. 2016, 49, 3261–3263. [Google Scholar] [CrossRef]
- Kai, Y.; Zhao, X.Y.; Li, Z.Y.; Qiu, J.M.; Cao, Y.B. Research progress on the molecular mechanisms of plants responding to saline-alkali stress. Biotechnol. Bull. 2024, 40, 122–138. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, L.; Zhang, T. Transcriptomic Analysis of Two Foxtail Millet Varieties under Drought Stress. Mol. Plant Breed. 2025, 1–19. [Google Scholar]
- Wu, C.; Zhang, M.; Liang, Y.; Zhang, L.; Diao, X. Salt stress responses in foxtail millet: Physiological and molecular regulation. Crop J. 2023, 11, 1011–1021. [Google Scholar] [CrossRef]
- Yang, Y.L. Revealing the Response Mechanism of Foxtail Millet to Saline-Alkali Stress Based on Integrated Multi-Omics Analysis. Doctoral Dissertation, Shanxi Agricultural University, Jinzhong, China, 2023. [Google Scholar]
- Jayaraman, A.; Puranik, S.; Rai, N.K.; Vidapu, S.; Sahu, P.P.; Lata, C. cDNA-AFLP analysis reveals differential gene expression in response to salt stress in foxtail millet (Setaria italica L.). Mol. Biotechnol. 2008, 40, 241–251. [Google Scholar] [CrossRef]
- Valença, D.D.C.; De Moura, S.M.; Travassos-Lins, J.; Alves-Ferreira, M.; Medici, L.O.; Ortiz-Silva, B.; Macrae, A.; Reinert, F. Physiological and molecular responses of Setaria viridis to osmotic stress. Plant Physiol. Biochem. 2020, 155, 114–125. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Dai, S.; Ding, H.; Wang, Q.; Li, X.; Ding, G.; Wang, P.; Guan, Y.; Liu, W. Integrative analyses of transcriptomics and metabolomics upon seed germination of foxtail millet in response to salinity. Sci. Rep. 2020, 10, 13660. [Google Scholar] [CrossRef]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Blumwald, E. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr. Opin. Plant Biol. 2014, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef]
- Zepeda-Jazo, I.; Shabala, S.; Chen, Z.; Pottosin, I.I. Na−-K+ transport in roots under salt stress. Plant Signal. Behav. 2008, 3, 401–403. [Google Scholar] [CrossRef]
- Chen, X.L.; Cai, Q.Y.; Gao, H.B. The effect of applying biochar on water movement in coastal saline-alkali soil. J. Irrig. Drain. 2023, 42 (Suppl. S1), 33–38. [Google Scholar]
- Xu, Y.; Tao, S.; Zhu, Y.; Zhang, Q.; Li, P.; Wang, H.; Zhang, Y.; Bakirov, A.; Cao, H.; Qin, M.; et al. Identification of Alkaline Salt Tolerance Genes in Brassica napus L. by Transcriptome Analysis. Genes 2022, 13, 1493. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still going strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Ghnaya, T.; Messedi, D.; Hessini, K.; Labidi, N.; Savoure, A.; Abdelly, C. Effect of sodium chloride on the response of the halophyte species Sesuvium portulacastrum grown in mannitol-induced water stress. Plant Res. 2007, 120, 291–299. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, G.X.; Zheng, F.C. Research progress on plant salt stress response. Mol. Plant Breed. 2018, 16, 3006–3015. [Google Scholar] [CrossRef]
- Yang, C.W.; Shi, D.C.; Wang, D.L. Comparative effects of saline-alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Wang, G.; Yu, L.H.; Zhao, H.J.; Liu, Y.; Geng, G. Effects of NaCl stress at different periods on the growth and photosynthesis of sugar beet. Crop J. 2021, 4, 99–104. [Google Scholar] [CrossRef]
- Jiang, J.H.; Guan, R.X.; Guo, Y.; Chang, R.Z.; Qiu, L.J. A simple method for identifying salt tolerance in soybean seedlings. Acta Agron. Sin. 2013, 39, 1248–1256. [Google Scholar] [CrossRef]
- Tian, R.N.; Xin, J.P. Research Progress on European Holly. For. Sci. Technol. Dev. 2014, 28, 12–17. [Google Scholar] [CrossRef]
- Shao, B.X.; Wang, H.M.; Zhao, Y.L.; Chen, W.; Guo, Z.J.; Gong, H.Y.; Sang, X.H.; Ye, W.W.; Wang, J.J. Analysis of the Association between Salt Tolerance Traits and SSR Molecular Markers in Upland Cotton. J. Cotton Res. 2015, 27, 118–125. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, X.X.; Liu, J.X. Effects of Salt Stress on Ion Content and Photosynthesis in Salix SH31 Clonal Lines. J. For. Sci. Res. 2015, 28, 565–569. [Google Scholar] [CrossRef]
- Nefissi Ouertani, R.; Arasappan, D.; Ruhlman, T.A.; Ben Chikha, M.; Abid, G.; Mejri, S.; Ghorbel, A.; Jansen, R.K. Effects of Salt Stress on Transcriptional and Physiological Responses in Barley Leaves with Contrasting Salt Tolerance. Int. J. Mol. Sci. 2022, 23, 5006. [Google Scholar] [CrossRef]
- Cui, Q.Q.; Dong, Y.H.; Li, M.; Zhang, W.D.; Liu, B.B.; Ai, X.Z.; Bi, H.G.; Li, Q.M. Effect of water-nitrogen coupling on photosynthesis and ultrastructure of cucumber leaves under CO2 enrichment. Ying Yong Sheng Tai Xue Bao 2017, 28, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Zhu, Y.L.; Zhang, J.T.; Zhai, X.Q. Effects of Salt Stress on Chlorophyll Fluorescence and Leaf Anatomical Structure in Robinia Pseudoacacia Seedlings. Chin. Agric. Bull. 2018, 34, 29–35. [Google Scholar]
- Tao, S.X.; Zhang, Y.; Li, P.; Yan, J.Q.; Xu, A.X.; Huang, Z. Effects of NaHCO3 stress on the growth of cabbage-type rapeseed seedlings and prediction of salt tolerance candidate genes. Northwest Agric. J. 2019, 28, 1978–1986. [Google Scholar] [CrossRef]
- Zou, C.L. Physiological Mechanisms of Sugar Beet Adaptation to Alkaline Salt Stress and Its Transcriptome Analysis. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, September 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.L.; Zeng, F.H.; Hu, M.L.; Luo, X.Y. Changes in the ultrastructure of leaf cells of two cool-season turf grasses under high temperature and drought. Northwest J. Bot. 2003, 23, 304–308. [Google Scholar]
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Xie, B.T.; Wang, Q.M.; Zhang, H.Y.; Li, A.X.; Hou, F.Y.; Dong, S.X.; Wang, B.Q.; Zhang, L.M. Research progress on salt and alkali tolerance of sweet potato. North China Agric. J. 2013, 28, 219–226. [Google Scholar]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Xinquan-Zhang Linkai-Huang Li, Z.; Nie, W.X. Analysis of Antioxidant Enzyme Activity and Antioxidant Genes Expression During Germination of Two Different Genotypes of Lolium multiflorum Under Salt Tolerance. Protein Pept. Lett. 2016, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Chu, M. Screening of Alkali-Tolerant Sorghum Varieties and Effects of Alkali Stress on Sorghum Seedlings. Master’s Thesis, Heilongjiang Bayi Agricultural University, Daqing, China, February 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, X.; Wang, Q.N.; Liu, J.; Zhang, X.Z.; Hu, G.F. Physiological characteristics and isoenzyme studies of three species of salix under salt stress. Chin. J. Grassl. 2019, 41, 43–52. [Google Scholar]
- Hong, Y.T. Salt Tolerance of Capsicum Annuum and Its Physiological and Biochemical Responses to Salt Stress. Master’s Thesis, Northwest A&F University, Xianyang, China, 2019. [Google Scholar]
- Bao, L.G. Identification of Salt Tolerance in Sorghum Germplasm Resources and Full-Length Transcriptome Analysis Under Salt Stress. Master’s Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
- Li, N. Response Characteristics of Different Genotypes of Chinese Cabbage to Salt-Alkali Stress. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2023. [Google Scholar]
- Li, N.; Liu, H.; Sun, J.; Zheng, H.; Wang, J.; Yang, L.; Zhao, H.; Zou, D. Transcriptome analysis of two contrasting rice cultivars during alkaline stress. Sci. Rep. 2018, 8, 9586. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Tariq, R.; Rizwan, M.; Wang, X.; Zhang, X.; Huang, L. Genome-wide expression and physiological profiling of pearl millet genotype reveal the biological pathways and various gene clusters underlying salt rsistance. Front. Plant Sci. 2022, 13, 849618. [Google Scholar] [CrossRef]
- Kumar, K.; Raina, S.K.; Sultan, S.M. Arabidopsis MAPK signaling pathways and their cross talks in abiotic stress response. J. Plant Biochem. Biotechnol. 2020, 29, 700–714. [Google Scholar] [CrossRef]
- Moustafa, K.; AbuQamar, S.; Jarrar, M.; Al-Rajab, A.J.; Trémouillaux-Guiller, J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014, 33, 1217–1225. [Google Scholar] [CrossRef]
- Zhu, D.; Chang, Y.; Pei, T. MAPK-like protein 1 positively regulates maize seedling drought sensitivity by suppressing ABA biosynthesis. Plant J. 2020, 102, 747–760. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of membrane lipids enhances the tolerance of saccharomyces cerevisiae to freezing and salt stress. Appl. Environ. Microbiol. 2007, 73, 110. [Google Scholar] [CrossRef]
- Mikami, K.; Murata, N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res. 2003, 42, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, H.; Yang, M.; Xu, W.; Zhao, W.; Qiu, R.; Kang, N.; Cui, G. Unveiling Salt Tolerance Mechanisms and Hub Genes in Alfalfa (Medicago sativa L.) Through Transcriptomic and WGCNA Analysis. Plants 2024, 13, 3141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xie, H.; Wei, X.; Dossa, K.; Yu, Y.; Hui, S.; Tang, G.; Zeng, X.; Yu, Y.; Hu, P.; et al. WGCNA Analysis of Salt-Responsive Core Transcriptome Identifies Novel Hub Genes in Rice. Genes 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.L.; Sun, P.Y.; Wang, J.R.; Sun, X.Q.; Zheng, C.Z.; Fan, T.; Chen, Q.F.; Li, H.Y. Comparative physiological, transcriptomic, and WGCNA analyses reveal the key genes and regulatory pathways associated with drought tolerance in Tartary buckwheat. Front. Plant Sci. 2022, 13, 985088. [Google Scholar] [CrossRef]
- Lu, C.; Pu, Y.; Liu, Y.; Li, Y.; Qu, J.; Huang, H.; Dai, S. Comparative transcriptomics and weighted gene co-expression correlation network analysis (WGCNA) reveal potential regulation mechanism of carotenoid accumulation in Chrysanthemum × morifolium. Plant Physiol Biochem. 2019, 142, 415–428. [Google Scholar] [CrossRef]
- Espinoza, C.; Liang, Y.; Stacey, G. Chitin receptor CERK1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant J. 2017, 89, 984–995. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, M.F.; Chen, H.; Qu, L.Q.; Chen, F.; Shen, S.H. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: Proteomic evidence. Biochim Biophys Acta. 2010, 1804, 929–940. [Google Scholar] [CrossRef]
- Fu, L.; Liu, J.Z.; Tao, B.X. A review of the adaptation mechanisms of salt-tolerant plants to saline soil environments. Jiangsu Agric. Sci. 2021, 49, 32–39. [Google Scholar] [CrossRef]
- Zhang, X.Y. Study on the Mechanism of Apple Rootstock Response to Saline-Alkali Stress and the Function of MdWRKY75d. Ph.D. Thesis, Shihezi University, Shihezi, China, 2023. [Google Scholar]
- Si, A.J.; Chen, H.; Yu, Y.; Kong, X.H.; Liu, L.; Wang, X.W. Application of WRKY transcription factors in breeding for abiotic stress tolerance in plants. Jiangsu Agric. Sci. 2019, 47, 5. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Seita.5G365500.v2.2 | ACCATCTGCGGCTGCCTCAA | CAGTTGACGCTGGTGCTGATCT |
| Seita.7G131700.v2.2 | GTCGCCTACGTGGTCTTCTCCT | GCGTTGATGCGGTCAGTCTTGA |
| Seita.1G314500.v2.2 | AACGCCGCCGTCAGATACCA | AAGGTCCACTGAGCCGTCACTC |
| Seita.7G320200.v2.2 | TCGCTCTTGTCGCTCTGGTCTC | CTTCTGCCGTCGTGTTGTTGGT |
| Seita.7G153600.v2.2 | TGCATGGCCGGACTGGTTCT | CTCCTCCTGGTCTGCGAGCATT |
| Seita.9G042000.v2.2 | GCGAGAGCCTGCCGATCTGTAT | AGTTCTGCCAACGCTACTTGCC |
| Seita.6G225500.v2.2 | ATCCGTACCCTCCTCCCTCTCA | GGTGCTGCTGGTCGTTGAAGT |
| Seita.6G077400.v2.2 | GCAAGAGCGTCGGCAAGAACA | TTCACCACGGCCACCTTATCCT |
| Seita.1G342400.v2.2 | ACGTGCGGTCTACGGGTGAT | TCCTGATGAAGTTGTCGGCGAA |
| Seita.8G115500.v2.2 | AGTTGTGCGGGTGGGAAGTGT | CGTCGTACTCCTCCAGCAGGTT |
| Seita.7G132000.v2.2 | TCGCAGCCCATCGCATAAGC | TGCTGGTTGCCGCCGTAGAA |
| Seita.1G136200.v2.2 | GCTTTGCGTTCGGTCCTTGAGA | GCAGGTGCATCATCAGCAGGTT |
| Seita.9G021900.v2.2 | ACGTTGCGGTTGTACCTCACTT | AGCTCCACCTCTGGCTTGTTGT |
| Seita.5G393300.v2.2 | TGTGCTCGACTCTGGTGATGGT | TCGGCGGTTGTGGTGAAGGA |
| Seita.7G132700.v2.2 | ACCTCCCAATTCCCAACATGCC | GCCACCTCTGCCGTCTTCTCTA |
| SiEF-1a | TGACTGTGCTGTCCTCATCA | GTTGCAGCAGCAAATCATCT |
| Module | Pathway ID | Pathway | Number of Genes | p-Value |
|---|---|---|---|---|
| orangered4 | ko04626 | Plant–pathogen interaction | 12 | p < 0.001 |
| ko04016 | MAPK signaling pathway—plant | 9 | p < 0.001 | |
| ko00561 | Glycerolipid metabolism | 3 | 0.036 | |
| salmon | ko00480 | Glutathione metabolism | 20 | p < 0.001 |
| ko04141 | Protein processing in endoplasmic reticulum | 25 | p < 0.001 | |
| ko00010 | Glycolysis/Gluconeogenesis | 17 | p < 0.001 | |
| ko00561 | Glycerolipid metabolism | 11 | 0.001 | |
| ko00440 | Phosphonate and phosphinate metabolism | 3 | 0.002 | |
| ko00260 | Glycine, serine and threonine metabolism | 9 | 0.004 | |
| ko00053 | Ascorbate and aldarate metabolism | 7 | 0.006 | |
| ko00620 | Pyruvate metabolism | 9 | 0.007 | |
| ko00564 | Glycerophospholipid metabolism | 11 | 0.009 | |
| ko00052 | Galactose metabolism | 7 | 0.010 | |
| ko04016 | MAPK signaling pathway-plant | 13 | 0.012 | |
| ko00051 | Fructose and mannose metabolism | 7 | 0.0112 | |
| ko00270 | Cysteine and methionine metabolism | 10 | 0.015 | |
| ko00710 | Carbon fixation in photosynthetic organisms | 7 | 0.034 | |
| ko00906 | Carotenoid biosynthesis | 4 | 0.044 | |
| darkorange | ko03010 | Ribosome | 263 | p < 0.001 |
| ko03008 | Ribosome biogenesis in eukaryotes | 59 | p < 0.001 | |
| ko03013 | RNA transport | 86 | p < 0.001 | |
| ko00290 | Valine, leucine, and isoleucine biosynthesis | 12 | p < 0.001 | |
| ko00280 | Valine, leucine, and isoleucine degradation | 28 | p < 0.001 | |
| ko00030 | Pentose phosphate pathway | 30 | p < 0.001 | |
| ko00230 | Purine metabolism | 46 | p < 0.001 | |
| ko00240 | Pyrimidine metabolism | 33 | 0.001 | |
| ko00300 | Lysine biosynthesis | 12 | 0.001 | |
| ko00340 | Histidine metabolism | 11 | 0.002 | |
| ko00220 | Arginine biosynthesis | 18 | 0.002 | |
| ko00640 | Propanoate metabolism | 19 | 0.003 | |
| ko00261 | Monobactam biosynthesis | 9 | 0.005 | |
| lightcyan | ko00330 | Arginine and proline metabolism | 7 | p < 0.001 |
| ko04141 | Protein processing in endoplasmic reticulum | 13 | 0.001 | |
| ko00940 | Phenylpropanoid biosynthesis | 14 | 0.004 | |
| ko00440 | Phosphonate and phosphinate metabolism | 2 | 0.013 | |
| ko00052 | Galactose metabolism | 5 | 0.015 | |
| ko00500 | Starch and sucrose metabolism | 9 | 0.020 | |
| ko00360 | Phenylalanine metabolism | 4 | 0.022 | |
| ko00941 | Flavonoid biosynthesis | 4 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Y.; Yang, Y.; Xu, Y.; Fan, X.; Guo, Z.; Han, Y.; Lin, X. Physiological Mechanisms and Core Genes in Response to Saline-Alkali Stress in Foxtail Millet (Setaria italica L.). Biomolecules 2025, 15, 859. https://doi.org/10.3390/biom15060859
Wang H, Li Y, Yang Y, Xu Y, Fan X, Guo Z, Han Y, Lin X. Physiological Mechanisms and Core Genes in Response to Saline-Alkali Stress in Foxtail Millet (Setaria italica L.). Biomolecules. 2025; 15(6):859. https://doi.org/10.3390/biom15060859
Chicago/Turabian StyleWang, Huimin, Yun Li, Yanan Yang, Yanrui Xu, Xiaoying Fan, Zhenqing Guo, Yucui Han, and Xiaohu Lin. 2025. "Physiological Mechanisms and Core Genes in Response to Saline-Alkali Stress in Foxtail Millet (Setaria italica L.)" Biomolecules 15, no. 6: 859. https://doi.org/10.3390/biom15060859
APA StyleWang, H., Li, Y., Yang, Y., Xu, Y., Fan, X., Guo, Z., Han, Y., & Lin, X. (2025). Physiological Mechanisms and Core Genes in Response to Saline-Alkali Stress in Foxtail Millet (Setaria italica L.). Biomolecules, 15(6), 859. https://doi.org/10.3390/biom15060859





