Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions
Abstract
1. Introduction
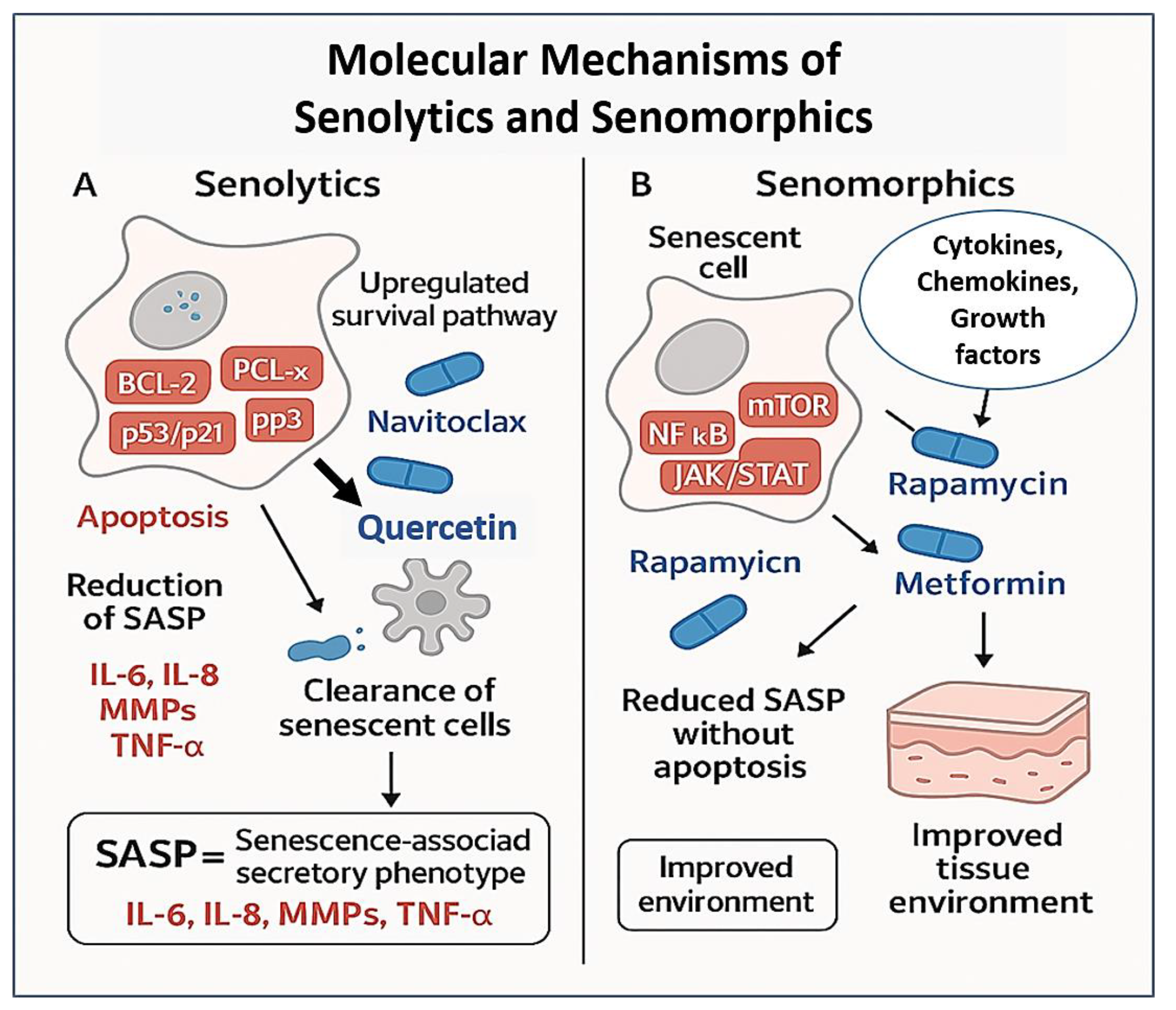
2. Senolytics: Eliminating Senescent Cells
| Senolytic Class | Molecular Targets | Represent Agents | Mode of Action | Strengths | Limitations/Challenges | Ref. |
|---|---|---|---|---|---|---|
| Tyrosine Kinase Inhibitors | Src family kinases, Eph receptors | Dasatinib | Inhibits pro-survival tyrosine kinases upregulated in certain SnC types | Effective in senescent preadipocytes and progenitors | Cell-type specificity; potential for systemic toxicity | [33] |
| Flavonoid Polyphenols | PI3K/AKT, NF-κB, ROS pathways | Quercetin, Fisetin | Induces apoptosis via oxidative stress and suppression of anti-apoptotic signaling | Low toxicity; orally bioavailable; broad applicability | Variable potency; poor bioavailability in vivo | [34] |
| BCL-2 Family Inhibitors | BCL-2, BCL-xL, BCL-w | Navitoclax (ABT-263), ABT-737 | Blocks anti-apoptotic proteins, sensitizing SnCs to apoptosis | Potent and broad-acting across senescent phenotypes | Thrombocytopenia due to BCL-xL inhibition in platelets | [35] |
| FOXO4-p53 Disruptors | FOXO4-p53 complex | FOXO4-DRI peptide | Disrupts nuclear retention of p53, restoring apoptotic signaling | Selective SnC clearance; rejuvenates aged tissues | Peptide delivery limitations; currently preclinical | [36] |
| HSP90 Inhibitors | Heat shock protein 90 | 17-DMAG, Geldanamycin derivatives | Destabilizes chaperone-dependent survival proteins in SnCs | Targets multiple stress response pathways | General cytotoxicity; lacks SnC specificity | [37] |
| CDK/p53 Pathway Modulators | MDM2-p53, CDK4/6 | UBX0101, Nutlin-3 | Modulates cell cycle regulators and apoptotic checkpoints | Targeted for local intra-articular applications | Short half-life; mixed clinical trial results | [38] |
| Natural Senolytics (Plant-Derived) | ROS generation, NF-κB, SASP factors | Piperlongumine, Curcumin analogues | Promotes redox imbalance and downregulates SASP-related pathways | Low-cost, multi-targeted; dietary sources | Low potency; unclear pharmacokinetics and dosing strategies | [39] |
3. Senomorphics: Modulating the SASP
| Agent/Class | Primary Targets | Mechanism of Action | Current Status | Key Notes | Ref. |
|---|---|---|---|---|---|
| Rapamycin (Sirolimus) | mTORC1 | Inhibits mTOR-mediated translation of SASP factors (e.g., IL-1α) | Multiple trials in aging (e.g., NCT02432287) | Shown to improve immune function, reduce inflammation | [71] |
| Ruxolitinib/Baricitinib | JAK1/2 | Blocks JAK-STAT signaling, suppressing IL-6/IL-8 mediated SASP amplification | Phase 2 trials for frailty, inflammation | Also used for myelofibrosis and rheumatoid arthritis | [72] |
| p38 MAPK Inhibitors | p38 MAPK | Reduces SASP via inhibition of upstream inflammatory signaling | Preclinical/early clinical | Reduces IL-6, TNF-α production in senescent cells | [73] |
| BET Inhibitors | BRD4, chromatin modifiers | Represses transcription of SASP-related genes by reducing enhancer/promoter activity | Preclinical | Emerging class with epigenetic modulation potential | [74] |
| NF-κB Inhibitors | NF-κB pathway (IKK complex) | Blocks transcription of pro-inflammatory cytokines central to the SASP | Mostly preclinical | Non-specific immunosuppression is a challenge | [75] |
| Glucocorticoids (e.g., Dexamethasone) | Glucocorticoid receptor/NF-κB | Represses SASP cytokine expression, suppresses general inflammation | Clinically approved; repurposing debated | Broad-spectrum effects; not ideal for long-term use | [76] |
| Metformin | AMPK/NF-κB/mTOR | Indirectly suppresses SASP by activating AMPK, inhibiting mTOR, and dampening NF-κB | Widely used; TAME trial (NCT04245771) ongoing | Mild SASP modulation; favorable safety profile | [77] |
| Resveratrol | SIRT1/NF-κB | Activates SIRT1, inhibits NF-κB signaling and oxidative stress | Nutraceutical; limited clinical trials | Low potency; bioavailability limitations | [78] |
| HDAC Inhibitors | Histone deacetylases | Alters chromatin accessibility of inflammatory gene promoters | Preclinical/repurposing from oncology | Potential for selective SASP modulation | [79] |
4. Challenges and Limitations
| Challenge | Description | Implications | Possible Solutions |
|---|---|---|---|
| Heterogeneity of Senescent Cells | Senescent cell features vary by tissue, trigger, and aging context | Limits development of one-size-fits-all senotherapeutics; variable drug response | Use tissue-specific profiling (e.g., single-cell omics); design context-dependent or combinatorial therapies |
| Lack of Specific Biomarkers | No robust, non-invasive markers to quantify senescent cell burden in vivo | Difficult to identify target patients, track efficacy, or determine optimal dosing | Develop circulating biomarkers, imaging tracers, and senescence-specific transcriptomic signatures |
| Safety Concerns of Senolytics | Off-target effects on non-senescent cells (e.g., platelets, immune cells) | Heightened toxicity risk, especially in frail elderly patients | Engineer targeted delivery systems (e.g., nanoparticles, prodrugs, ADCs); explore intermittent “hit-and-run” dosing |
| Limitations of Senomorphics | Broad-acting effects, immunomodulation, lack of clearance | Potential SASP rebound; unclear long-term benefits | Develop pathway-selective senomorphics; combine with senolytics or regenerative therapies |
| Context-Dependent Role of Senescence | Senescence contributes positively to tissue repair and tumor suppression in some settings | Risk of unintended tissue damage or impaired regeneration if senescent cells are eliminated indiscriminately | Adopt adaptive modulation strategies; tailor timing and duration of intervention to specific physiological contexts |
| Regulatory and Ethical Uncertainty | Aging is not a recognized medical indication; endpoints and trial designs lack standardization | Slows approval and integration into mainstream care; public skepticism | Define aging-related surrogate endpoints; develop ethical guidelines for preventive gerotherapeutics |
5. Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, R.P.; Hu, S.A.; Du, X.H.; Wang, Y.W.; Fang, K.; Zhu, Y.B.; Lou, N.B.; Yuan, C.H.; Yang, J. Revolutionizing Senescence Detection: Advancements from Traditional Methods to Cutting-Edge Techniques. Aging Dis. 2025, 16, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, N.Y.; Zhang, H.; Liang, G.Y.; Lin, Y.; Qian, Z.H.; Yang, X.; Yang, J.K.; Fu, Y.G.; Zhang, C.T.; et al. Systemic aging and aging-related diseases. FASEB J. 2025, 39, e70430. [Google Scholar] [CrossRef] [PubMed]
- Tamatta, R.; Pai, V.R.; Jaiswal, C.; Singh, I.; Singh, A.K. Neuroinflammaging and the Immune Landscape: The Role of Autophagy and Senescence in Aging Brain. Biogerontology 2025, 26, 52. [Google Scholar] [CrossRef]
- García-Domínguez, M. Pathological and Infla mmatory Consequences of Aging. Biomolecules 2025, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Fukumoto, T.; Noma, K. Therapeutic strategies targeting cellular senescence for cancer and other diseases. J. Biochem. 2024, 175, 525–537. [Google Scholar] [CrossRef]
- Balducci, L.; Falandry, C.; Monfardini, S. Senotherapy, cancer, and aging. J. Geriatr. Oncol. 2024, 15, 101671. [Google Scholar] [CrossRef]
- Wang, B.S.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef]
- Noh, S.G.; Kim, H.W.; Kim, S.; Chung, K.W.; Jung, Y.S.; Yoon, J.H.; Yu, B.P.; Lee, J.; Chung, H.Y. Senoinflammation as the underlying mechanism of aging and its modulation by calorie restriction. Ageing Res. Rev. 2024, 101, 102503. [Google Scholar] [CrossRef]
- Imawari, Y.; Nakanishi, M. Senescence and senolysis in cancer: The latest findings. Cancer Sci. 2024, 115, 2107–2116. [Google Scholar] [CrossRef]
- Wang, S.; Huo, T.; Lu, M.; Zhao, Y.; Zhang, J.; He, W.; Chen, H. Recent Advances in Aging and Immunosenescence: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 499. [Google Scholar] [CrossRef]
- Williams, Z.J.; Chow, L.; Dow, S.; Pezzanite, L.M. The potential for senotherapy as a novel approach to extend life quality in veterinary medicine. Front. Vet. Sci. 2024, 11, 1369153. [Google Scholar] [CrossRef] [PubMed]
- Samiminemati, A.; Aprile, D.; Siniscalco, D.; Di Bernardo, G. Methods to Investigate the Secretome of Senescent Cells. Method Protocol. 2024, 7, 52. [Google Scholar] [CrossRef]
- Konstantinou, E.; Longange, E.; Kaya, G. Mechanisms of Senescence and Anti-Senescence Strategies in the Skin. Biology 2024, 13, 647. [Google Scholar] [CrossRef]
- Kondratyeva, L.G.; Matveeva, D.K.; Ezdakova, M.I.; Utkina, M.V.; Ratushnyy, A.Y. The influence of the geroprotective cytokine on the transcriptome of young and senescent mesenchymal stem cells. BMC Res. Notes 2025, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Wyles, S.P.; Yu, G.T.; Gold, M.; Behfar, A. Topical Platelet Exosomes Reduce Senescence Signaling in Human Skin: An Exploratory Prospective Trial. Dermatol. Surg. 2024, 50, S160–S165. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Matsumoto, M.; Yamanaka, M.; Irikura, R.; Nakajima, K.; Tada, K.; Nakayama, Y.; Konishi, M.; Itoh, N.; Funayama, R.; et al. BACH1 inhibits senescence, obesity, and short lifespan by ferroptotic FGF21 secretion. Cell Rep. 2024, 43, 114403. [Google Scholar] [CrossRef]
- Poblocka, M.; Bassey, A.L.; Smith, V.M.; Falcicchio, M.; Manso, A.S.; Althubiti, M.; Sheng, X.; Kyle, A.; Barber, R.; Frigerio, M.; et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 2021, 11, 20358. [Google Scholar] [CrossRef]
- Zumerle, S.; Sarill, M.; Saponaro, M.; Colucci, M.; Contu, L.; Lazzarini, E.; Sartori, R.; Pezzini, C.; Rinaldi, A.; Scanu, A.; et al. Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice. Nat. Aging 2024, 4, 1231–1248. [Google Scholar] [CrossRef]
- Dorronsoro, A.; Santiago, F.E.; Grassi, D.; Zhang, T.; Lai, R.C.; McGowan, S.J.; Angelini, L.; Lavasani, M.; Corbo, L.; Lu, A.; et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell 2021, 20, e13337. [Google Scholar] [CrossRef]
- Sun, W.T.; Gao, Y.; Wu, Y.B.; Wu, W.; Wang, C.F.; Chen, J.X.; Luan, C.J.; Hua, M.; Liu, W.L.; Gong, W.J.; et al. Targeted apoptosis of senescent cells by valproic acid alleviates therapy-induced cellular senescence and lung aging. Phytomedicine 2024, 135, 156131. [Google Scholar] [CrossRef]
- Mansfield, L.; Ramponi, V.; Gupta, K.; Stevenson, T.; Mathew, A.B.; Barinda, A.J.; Herbstein, F.; Morsli, S. Emerging insights in senescence: Pathways from preclinical models to therapeutic innovations. NPJ Aging 2024, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, Z.; Zhang, H.; Zhang, N.; Liu, Z.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Regulation of cellular senescence in tumor progression and therapeutic targeting: Mechanisms and pathways. Mol. Cancer 2025, 24, 106. [Google Scholar] [CrossRef]
- Li, C.; Yuan, Y.; Jia, Y.; Zhou, Q.; Wang, Q.; Jiang, X. Cellular senescence: From homeostasis to pathological implications and therapeutic strategies. Front. Immunol. 2025, 16, 1534263. [Google Scholar] [CrossRef]
- Melo dos Santos, L.S.; Trombetta-Lima, M.; Eggen, B.J.L.; Demaria, M. Cellular senescence in brain aging and neurodegeneration. Ageing Res. Rev. 2024, 93, 102141. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Buonfiglio, F.; Li, J.Y.; Pfeiffer, N.; Gericke, A. Mechanisms Underlying Vascular Inflammaging: Current Insights and Potential Treatment Approaches. Aging Dis. 2025. ahead of print. [Google Scholar]
- Stojanovic, S.D.; Thum, T.; Bauersachs, J. Anti-senescence therapies: A new concept to address cardiovascular disease. Cardiovasc. Res. 2025, 121, 730–747. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, H.X.; Wu, H.L. Cellular Senescence in Health, Disease, and Lens Aging. Pharmaceuticals 2025, 18, 244. [Google Scholar] [CrossRef] [PubMed]
- Burdusel, D.; Doeppner, T.R.; Surugiu, R.; Hermann, D.M.; Olaru, D.G.; Popa-Wagner, A. The Intersection of Epigenetics and Senolytics in Mechanisms of Aging and Therapeutic Approaches. Biomolecules 2025, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Al-Hashimi, A.; Benedetto, M.; Ruchaya, P.J. From bench to bedside: The critical need for standardized senescence detection. Arch. Cardiovasc. Dis. 2025, 118, 205–211. [Google Scholar] [CrossRef]
- Baier, M.P.; Ranjit, R.; Owen, D.B.; Wilson, J.L.; Stiles, M.A.; Masingale, A.M.; Thomas, Z.; Bredegaard, A.; Sherry, D.M.; Logan, S. Cellular Senescence Is a Central Driver of Cognitive Disparities in Aging. Aging Cell 2025. ahead of print. [Google Scholar] [CrossRef]
- Gadecka, A.; Nowak, N.; Bulanda, E.; Janiszewska, D.; Dudkowska, M.; Sikora, E.; Bielak-Zmijewska, A. The senolytic cocktail, dasatinib and quercetin, impacts the chromatin structure of both young and senescent vascular smooth muscle cells. Geroscience 2025. ahead of print. [Google Scholar] [CrossRef]
- Tang, J.R.; Li, J.Y.; Hou, Z.Y.; He, R.; Li, B.Z.; Gong, J.J.; Xie, Y.H.; Meng, W.R.; Liu, Y.K.; Ouchi, T.; et al. Dasatinib and Quercetin Mitigate Age-Related Alveolar Bone Inflammaging and Neutrophil Infiltration. Oral. Dis. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, D.P.; Maldonado, V.V.; Ardana, I.K.K.; Porter, R.M.; Samsonraj, R.M. Assessing the Effects of Dasatinib on Mesenchymal Stem/Stromal Cells. Cell Mol. Bioeng. 2024, 17, 609–618. [Google Scholar] [CrossRef]
- Maurer, S.; Kirsch, V.; Ruths, L.; Brenner, R.E.; Riegger, J. Senolytic therapy combining Dasatinib and Quercetin restores the chondrogenic phenotype of human osteoarthritic chondrocytes by the release of pro-anabolic mediators. Aging Cell 2025, 24, e14361. [Google Scholar] [CrossRef]
- Greenberg, E.F.; Voorbach, M.J.; Smith, A.; Reuter, D.R.; Zhuang, Y.C.; Wang, J.Q.; Wooten, D.W.; Asque, E.; Hu, M.; Hoft, C.; et al. Navitoclax safety, tolerability, and effect on biomarkers of senescence and neurodegeneration in aged nonhuman primates. Heliyon 2024, 10, e36483. [Google Scholar] [CrossRef]
- Born, E.; Lipskaia, L.; Breau, M.; Houssaini, A.; Beaulieu, D.; Marcos, E.; Pierre, R.; Do Cruzeiro, M.; Lefevre, M.; Derumeaux, G.; et al. Eliminating Senescent Cells Can Promote Pulmonary Hypertension Development and Progression. Circulation 2023, 147, 650–666. [Google Scholar] [CrossRef]
- Hassan, J.W.; Bhatwadekar, A.D. Senolytics in the treatment of diabetic retinopathy. Front. Pharmacol. 2022, 13, 896907. [Google Scholar] [CrossRef]
- Chin, A.F.; Han, J.; Clement, C.C.; Choi, Y.; Zhang, H.; Browne, M.; Jeon, O.H.; Elisseeff, J.H. Senolytic treatment reduces oxidative protein stress in an aging male murine model of post-traumatic osteoarthritis. Aging Cell 2023, 22, 13979. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Neganova, M. Antioxidant Senotherapy by Natural Compounds: A Beneficial Partner in Cancer Treatment. Antioxidants 2025, 14, 199. [Google Scholar] [CrossRef]
- Guo, Y.L.; Fenwick, P.; Viola, P.; Al-Sahaf, M.; Barnes, P.J.; Donnelly, L.E. Navitoclax inhibits senescence in human alveolar type 2 cells from COPD patients. Eur. Respir. J. 2024, 64, PA897. [Google Scholar] [CrossRef]
- Agha-Mir-Salim, D.; Bhayadia, R.; Heckl, D.; Klusmann, J.H. Evaluation of Navitoclax (ABT-263) as a promising Therapy for Pediatric Acute Myeloid Leukemia. Klin. Padiatr. 2024, 236, 200. [Google Scholar] [CrossRef]
- Kolodkin-Gal, D.; Roitman, L.; Ovadya, Y.; Azazmeh, N.; Assouline, B.; Schlesinger, Y.; Kalifa, R.; Horwitz, S.; Khalatnik, Y.; Hochner-Ger, A.; et al. Senolytic elimination of Cox2-expressing senescent cells inhibits the growth of premalignant pancreatic lesions. Gut 2022, 71, 345–355. [Google Scholar] [CrossRef]
- Rad, A.N.; Grillari, J. Current senolytics: Mode of action, efficacy and limitations, and their future. Mech. Ageing Dev. 2024, 217, 111888. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Q.H.; Wang, R.; Liu, Y.; Cheng, Z.S. FOXO4-D-Retro-Inverso targets extracellular matrix production in fibroblasts and ameliorates bleomycin-induced pulmonary fibrosis in mice. Arch. Pharmacol. 2023, 396, 2393–2403. [Google Scholar] [CrossRef]
- Xu, X.F.; Lou, Z.L.; Li, J.S.; Liang, F.X.; Yu, Y.F.; Wu, M. Inhibition of Hsp90 Alleviates Necroptosis and Inflammation in Lung Epithelial Cells During Pulmonary Ischemia-Reperfusion Injury. Clin. Exp. Pharmacol. Physiol. 2025, 52, e70037. [Google Scholar] [CrossRef]
- Qin, M.T.; Ren, J.; Chen, X.D.; Zhou, W.; Zhang, S.Y.; Zhang, W.L.; Shi, M.X.; Zhang, M.Z.; Liu, H.S.; Ma, Y.F.; et al. CD19-targeted HSP90 inhibitor nanoparticle combined with TKIs reduces tumor burden and enhances T-cell immunity in murine B-cell malignancies. Theranostics 2025, 15, 3589–3609. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, J.X.; Yuan, Q.Y.; Wang, X.Y.; Zeng, W.D.; Mi, Y.L.; Zhang, C.Q. Flavonoid Fisetin Alleviates Ovarian Aging of Laying Chickens by Enhancing Antioxidant Capacity and Glucose Metabolic Homeostasis. Antioxidants 2024, 13, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Zhang, X.Y.; Liao, Q.Y.; Rui, X.L.; Wang, R. Study on the Mechanism of Raspberry (Rubi fructus) in Treating Type 2 Diabetes Based on UPLC-Q-Exactive Orbitrap MS, Network Pharmacology, and Experimental Validation. Phytochem. Anal. 2025, 36, 744–758. [Google Scholar] [CrossRef]
- Das, G.; Kameswaran, S.; Ramesh, B.; Bangeppagari, M.; Nath, R.; Das Talukdar, A.; Shin, H.S.; Patra, J.K. Anti-Aging Effect of Traditional Plant-Based Food: An Overview. Foods 2024, 13, 3785. [Google Scholar] [CrossRef]
- Lamichhane, G.; Liu, J.; Lee, S.J.; Lee, D.Y.; Zhang, G.L.; Kim, Y. Curcumin Mitigates the High-Fat High-Sugar Diet-Induced Impairment of Spatial Memory, Hepatic Metabolism, and the Alteration of the Gut Microbiome in Alzheimer’s Disease-Induced (3xTg-AD) Mice. Nutrients 2024, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Riessland, M.; Orr, M.E. Translating the Biology of Aging into New Therapeutics for Alzheimer’s Disease: Senolytics. J. Prev. Alzheimer’s Dis. 2023, 10, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Poisa-Beiro, L.; Landry, J.J.M.; Yan, B.; Kardorff, M.; Eckstein, V.; Villacorta, L.; Krammer, P.H.; Zaugg, J.; Gavin, A.-C.; Benes, V.; et al. A Senescent Cluster in Aged Human Hematopoietic Stem Cell Compartment as Target for Senotherapy. Int. J. Mol. Sci. 2025, 26, 787. [Google Scholar] [CrossRef] [PubMed]
- Sarad, K.; Jankowska, U.; Skupien-Rabian, B.; Babler, A.; Kramann, R.; Dulak, J.; Jaźwa-Kusior, A. Senescence of endothelial cells promotes phenotypic changes in adventitial fibroblasts: Possible implications for vascular aging. Mol. Cell Biochem. 2024, 480, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Dong, Y.; Cruickshank-Taylor, A.B.; Gnawali, G.; Bi, F.C.; Wang, W. Senolytic Prodrugs: A Promising Approach to Enhancing Senescence-Targeting Intervention. Chembiochem 2024, 25, e202400355. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vicente, P.; López-Martínez, C.; Rioseras, B.; Albaiceta, G.M. Activation of senescence in critically ill patients: Mechanisms, consequences and therapeutic opportunities. Ann. Intensive Care 2024, 14, 2. [Google Scholar] [CrossRef]
- Imb, M.; Véghelyi, Z.; Maurer, M.; Kühnel, H. Exploring Senolytic and Senomorphic Properties of Medicinal Plants for Anti-Aging Therapies. Int. J. Mol. Sci. 2024, 25, 10419. [Google Scholar] [CrossRef]
- Wan, T.; Fielder, E.; Alimohammadiha, G.; Ishaq, A.; Low, E.; Weigand, B.M.; Kelly, G.; Parker, C.; Griffin, B.; Jurk, D.; et al. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. eLife 2022, 11, 75492. [Google Scholar] [CrossRef]
- Mikawa, T.; Yoshida, K.; Kondoh, H. Senotherapy preserves resilience in aging. Geriatr. Gerontol. Int. 2024, 24, 845–849. [Google Scholar] [CrossRef]
- McHugh, D.; Durán, I.; Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat. Rev. Drug Discov. 2025, 24, 57–71. [Google Scholar] [CrossRef]
- Ya, J.; Bayraktutan, U. Senolytics and Senomorphics Targeting p38MAPK/NF-κB Pathway Protect Endothelial Cells from Oxidative Stress-Mediated Premature Senescence. Cells 2024, 13, 1292. [Google Scholar] [CrossRef]
- Kita, A.; Yamamoto, S.; Saito, Y.; Chikenji, T.S. Cellular senescence and wound healing in aged and diabetic skin. Front. Physiol. 2024, 15, 1344116. [Google Scholar] [CrossRef]
- Gallage, S.; Irvine, E.E.; Avila, J.E.B.; Reen, V.; Pedroni, S.M.A.; Duran, I.; Ranvir, V.; Khadayate, S.; Pombo, J.; Brookes, S.; et al. Ribosomal S6 kinase 1 regulates inflammaging via the senescence secretome. Nat. Aging 2024, 4, 1544–1561. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Salech, F.; Lim, A.; Vogrin, S.; Duque, G. The effect of rapamycin and its analogues on age-related musculoskeletal diseases: A systematic review. Aging Clin. Exp. Res. 2022, 34, 2317–2333. [Google Scholar] [CrossRef]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience 2021, 43, 1135–1158. [Google Scholar] [CrossRef]
- Politano, D.; Tonduti, D.; Battini, R.; Fazzi, E.; Orcesi, S. Exploring emerging JAK inhibitors in the treatment of Aicardi-Goutières syndrome. Expert. Opin. Emerg. Drugs 2025, 30, 21–39. [Google Scholar] [CrossRef]
- Hu, Z.H.; Lu, L.; Feng, J.D.; Song, H.B.; Zhang, S.Y.; Yang, L.; Liu, Y.H.; Wang, T. Low-Dose Baricitinib Plus Narrow-Band Ultraviolet B for the Treatment of Progressive Non-Segmental Vitiligo: A Prospective, Controlled, Open-Label Study. Pigment. Cell Melanoma Res. 2025, 38, 13209. [Google Scholar] [CrossRef]
- Utama, A.; Wijesinghe, R.; Thng, S. Janus kinase inhibitors and the changing landscape of vitiligo management: A scoping review. Int. J. Dermatol. 2024, 63, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ruminski, P.; Singh, M.; Staser, K.; Ashami, K.; Ritchey, J.; Lim, S.; Dipersio, J.F.; Choi, J. Novel JAK Inhibitors to Reduce Graft-Versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation in a Preclinical Mouse Model. Molecules 2024, 29, 1801. [Google Scholar] [CrossRef] [PubMed]
- Solignac, M.; Cabrera, N.; Fouillet-Desjonqueres, M.; Duquesne, A.; Laurent, A.; Foray, A.P.; Viel, S.; Zekre, F.; Belot, A. JAK inhibitors in refractory juvenile rheumatic diseases: Efficacy, tolerance and type-I interferon profiling, a single center retrospective study. J. Autoimmun. 2024, 147, 103248. [Google Scholar] [CrossRef]
- Mansilla-Polo, M.; Morgado-Carrasco, D. Biologics Versus JAK Inhibitors. Part I: Cancer Risk. A Narrative Review. Dermatology Ther. 2024, 14, 1389–1442. [Google Scholar] [CrossRef]
- Chrienova, Z.; Rysanek, D.; Oleksak, P.; Stary, D.; Bajda, M.; Reinis, M.; Mikyskova, R.; Novotny, O.; Andrys, R.; Skarka, A.; et al. Discovery of small molecule mechanistic target of rapamycin inhibitors as anti-aging and anti-cancer therapeutics. Front. Aging Neurosci. 2022, 14, 1048260. [Google Scholar] [CrossRef] [PubMed]
- Frede, N.; Lorenzetti, R.; Hüppe, J.M.; Janowska, I.; Troilo, A.; Schleyer, M.T.; Venhoff, A.C.; Voll, R.E.; Thiel, J.; Venhoff, N.; et al. JAK inhibitors differentially modulate B cell activation, maturation and function: A comparative analysis of five JAK inhibitors in an B cell differentiation model and in patients with rheumatoid arthritis. Front. Immunol. 2023, 14, 1087986. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.W.; Zhao, L.J.; Xu, Q.L.; Zhao, J.H. The journey of p38 MAP kinase inhibitors: From bench to bedside in treating inflammatory diseases. Eur. J. Med. Chem. 2024, 280, 116950. [Google Scholar] [CrossRef]
- Leal, A.S.; Liby, K.T. The BRD4 Inhibitor I-BET-762 Reduces HO-1 Expression in Macrophages and the Pancreas of Mice. Int. J. Mol. Sci. 2024, 25, 9985. [Google Scholar] [CrossRef]
- Cheng, J.J.; Wu, L.; Chen, X.W.; Li, S.; Sun, R.J.; Huang, Y.W.; Wang, P.; Ouyang, J.W.; Pei, P.P.; Yang, H.C.; et al. Polo-like kinase 2 promotes microglial activation via regulation of the HSP90a/IKKR a /IKK R pathway. Cell Rep. 2024, 43, 114827. [Google Scholar] [CrossRef]
- Thrikawala, S.U.; Anderson, M.H.; Rosowski, E.E. Glucocorticoids Suppress NF-kB-Mediated Neutrophil Control of Hyphal Growth. J. Immunol. 2024, 213, 2400021. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.H.; Yang, H.Q.; Hu, Z.Y.; Jin, X.; Chen, Z.C.; Jiang, W.; Sun, F.H. The Research Progress of Metformin Regulation of Metabolic Reprogramming in Malignant Tumors. Pharm. Res. 2024, 41, 2143–2159. [Google Scholar] [CrossRef]
- Tshivhase, A.M.; Matsha, T.; Raghubeer, S. Resveratrol attenuates high glucose-induced inflammation and improves glucose metabolism in HepG2 cells. Sci. Rep. 2024, 14, 1106. [Google Scholar] [CrossRef]
- Makgoba, T.B.; Kapp, E.; Egieyeh, S.; Joubert, J. HDAC3 inhibitors: A patent review of their broad-spectrum applications as therapeutic agents. Expert Opin. Ther. Pat. 2024, 34, 273–295. [Google Scholar] [CrossRef]
- Han, Z.Q.; Wang, L.J.; Xu, S.Y.; Zhang, H.R.; Cheng, J.; Pan, S.F. Microvesicle-Shuttled microRNA-130b Activates the Hepatic Inflammation by Inhibiting Glucocorticoid-Receptor-Mediated Immunosuppression in High-Fat Diet-Induced Obese Mice. Vet. Sci. 2024, 11, 565. [Google Scholar] [CrossRef]
- Meng, Q.Q.; Bai, M.; Guo, M.L.; Li, Z.X.; Liu, W.W.; Fan, X.J.; Sun, R.; Yang, X.R.; Yuan, D.F.; Shi, Y.L.; et al. Inhibition of Serum- and Glucocorticoid-Regulated Protein Kinase-1 Aggravates Imiquimod-Induced Psoriatic Dermatitis and Enhances Proinflammatory Cytokine Expression through the NF-kB Pathway. J. Investig. Dermatol. 2023, 143, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Mostafa, M.M.; Kooi, C.; Sasse, S.K.; Michi, A.N.; Shah, S.V.; Leigh, R.; Gerber, A.N.; Newton, R. Interplay between nuclear factor-KB, p38 MAPK, and glucocorticoid receptor signaling synergistically induces functional TLR2 in lung epithelial cells. J. Biol. Chem. 2022, 298, 101747. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alqahtani, T.; Venkatesan, K.; Sivadasan, D.; Ahmed, R.; Sirag, N.; Elfadil, H.; Abdullah Mohamed, H.; TA, H.; Elsayed Ahmed, R.; et al. SASP Modulation for Cellular Rejuvenation and Tissue Homeostasis: Therapeutic Strategies and Molecular Insights. Cells 2025, 14, 608. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Xiao, D.Y.; Veviorskiy, A.; Xin, Y.; Lok, S.W.Y.; Pulous, F.E.; Zhang, P.R.; Zhu, Y.F.; Ma, Y.M.; Hu, X.; et al. AI-Driven Robotics Laboratory Identifies Pharmacological TNIK Inhibition as a Potent Senomorphic Agent. Aging Dis. 2026. ahead of print. [Google Scholar] [CrossRef]
- Malaquin, N.; Rodier, F. In Cellular Senescence and Aging; Dynamic and scalable assessment of the senescence-associated secretory phenotype (SASP). Academic Press, Elsevier: London, UK, 2024; pp. 181–195. [Google Scholar] [CrossRef]
- Giroud, J.; Bouriez, I.; Paulus, H.; Pourtier, A.; Debacq-Chainiaux, F.; Pluquet, O. Exploring the Communication of the SASP: Dynamic, Interactive, and Adaptive Effects on the Microenvironment. Int. J. Mol. Sci. 2023, 24, 10788. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Giannoula, Y.; Kroemer, G.; Pietrocola, F. Cellular senescence and the host immune system in aging and age-related disorders. Biomed. J. 2023, 46, 100581. [Google Scholar] [CrossRef]
- Wang, L.; Hong, W.; Zhu, H.; He, Q.; Yang, B.; Wang, J.; Weng, Q. Macrophage senescence in health and diseases. Acta Pharm. Sin. B 2024, 14, 1508–1524. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Q.; Kirkland, J.L. Targeting senescent cells for a healthier longevity: The roadmap for an era of global aging. Life Med. 2022, 1, 103–119. [Google Scholar] [CrossRef]
- Xia, W.; Chen, H.; Yang, H.; Zhu, L.; Xie, C.; Hou, M. Depletion of SASP senescent cardiomyocytes with senolytic drugs confers therapeutic effects in doxorubicin-related cardiotoxicity. FEBS J. 2024, 291, 4029–4042. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Revskij, D.; Woitas, A.; Koelle, B.; Umstätter, C.; Zechner, D.; Khan, F.M.; Fuellen, G.; Jaster, R. Effects of triggers of senescence and senolysis in murine pancreatic cancer cells. Hepatobiliary Pancreat. Dis. 2024, 23, 628–637. [Google Scholar] [CrossRef]
- McCorkle, J.R.; Ahn, R.; Cao, C.D.; Hill, K.S.; Dietrich, C.S.; Kolesar, J.M. Antineoplastic Drug Synergy of Artesunate with Navitoclax in Models of High-Grade Serous Ovarian Cancer. Cancers 2024, 16, 1321. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.; Tamilanban, T.; Kumar, P.S.; Guru, A.; Muthupandian, S.; Kathiravan, M.K.; Arockiaraj, J. Role and mechanistic actions of protein kinase inhibitors as an effective drug target for cancer and COVID. Arch. Microbiol. 2023, 205, 238. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Ring, N.A.R.; Valdivieso, K.; Grillari, J.; Redl, H.; Ogrodnik, M. The role of senescence in cellular plasticity: Lessons from regeneration and development and implications for age-related diseases. Dev. Cell 2022, 57, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.E.; Zhou, Z. Senescent cells as a target for anti-aging interventions: From senolytics to immune therapies. J. Transl. Intern. Med. 2025, 13, 33–47. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Bi, J.; Zeng, J.; Liu, X.; Mo, C.; Yao, M.; Zhang, J.; Yuan, P.; Jia, B.; Xu, S. Drug delivery for age-related bone diseases: From therapeutic targets to common and emerging therapeutic strategies. Saudi Pharm. J. 2024, 32, 102209. [Google Scholar] [CrossRef]
- Wilar, G.; Suhandi, C.; Wathoni, N.; Fukunaga, K.; Kawahata, I. Nanoparticle-Based Drug Delivery Systems Enhance Treatment of Cognitive Defects. Int. J. Nanomed. 2024, 19, 11357–11378. [Google Scholar] [CrossRef]
- Lin, M.; Guo, J.; Gu, Z.; Tang, W.; Tao, H.; You, S.; Jia, D.; Sun, Y.; Jia, P. Machine learning and multi-omics integration: Advancing cardiovascular translational research and clinical practice. J. Transl. Med. 2025, 23, 388. [Google Scholar] [CrossRef]
- Silva, N.; Rajado, A.T.; Esteves, F.; Brito, D.; Apolónio, J.; Roberto, V.P.; Binnie, A.; Araújo, I.; Nóbrega, C.; Bragança, J.; et al. Measuring healthy ageing: Current and future tools. Biogerontology 2023, 24, 845–866. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saliev, T.; Singh, P.B. Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions. Biomolecules 2025, 15, 860. https://doi.org/10.3390/biom15060860
Saliev T, Singh PB. Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions. Biomolecules. 2025; 15(6):860. https://doi.org/10.3390/biom15060860
Chicago/Turabian StyleSaliev, Timur, and Prim B. Singh. 2025. "Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions" Biomolecules 15, no. 6: 860. https://doi.org/10.3390/biom15060860
APA StyleSaliev, T., & Singh, P. B. (2025). Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions. Biomolecules, 15(6), 860. https://doi.org/10.3390/biom15060860






