Current Modalities in Soft-Tissue Reconstruction and Vascularized Adipose Engineering
Abstract
1. Introduction
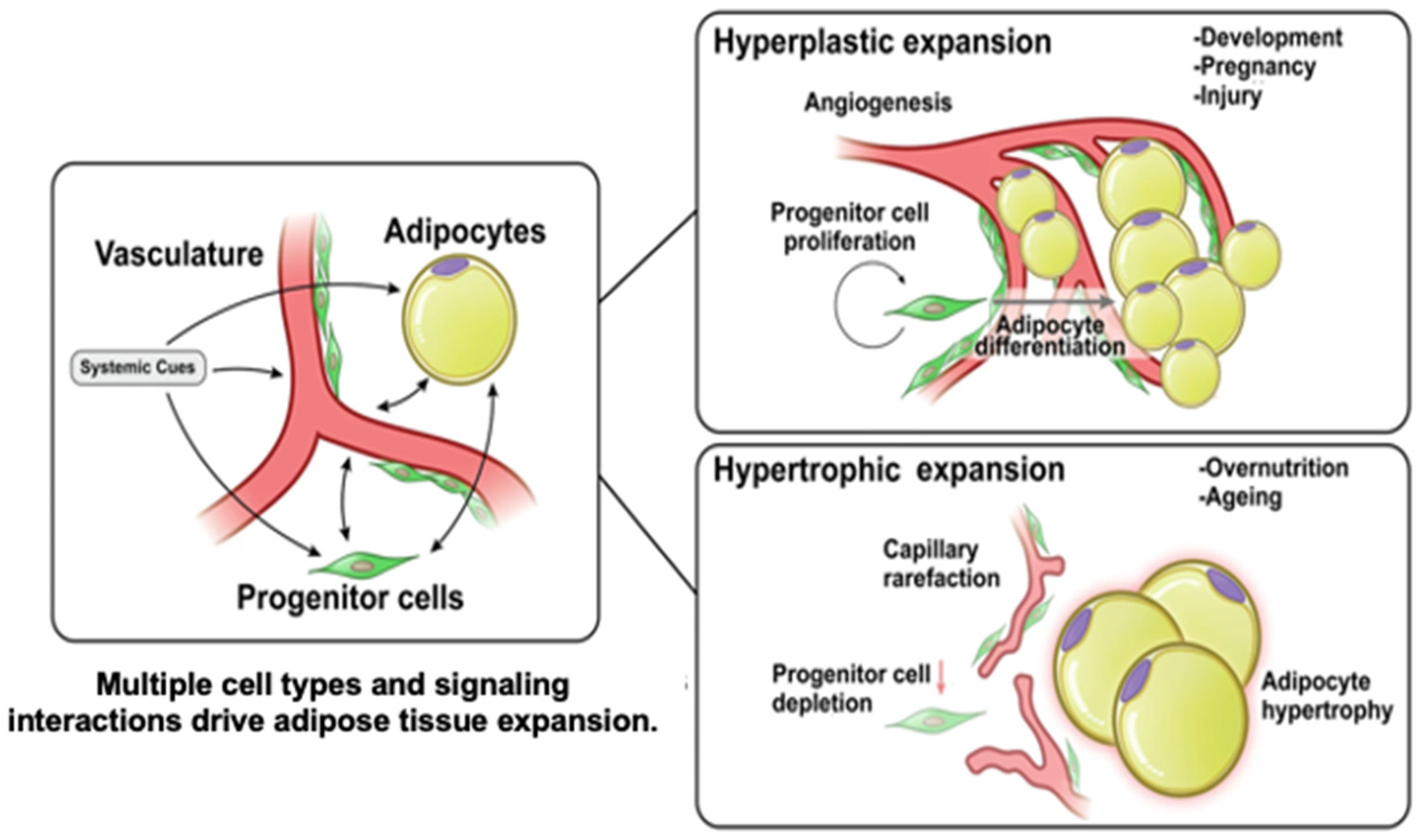
1.1. Adipose Development
1.2. Adipose Angiogenesis
2. Soft-Tissue Reconstruction
2.1. Fat-Graft Principles
2.2. Fat Grafts in Clinical Care

2.3. Graft and Recipient-Site Preparation
2.4. Adipose-Flap Principles
3. Adipose Engineering
3.1. Stem Cell Applications in Adipose Engineering
3.2. Scaffolds
3.2.1. Biopolymer-Based Scaffolds
3.2.2. Synthetic Scaffolds
3.2.3. Decellularized ECM
3.2.4. Adipose Collagen Fragments
3.3. Growth Factors/Biologics
3.3.1. Extracellular Vesicles
3.3.2. Platelet-Rich Plasma
3.4. Approaches to Engineering Vascularized Adipose Tissue
3.4.1. Top–Down Approach
3.4.2. Bottom–Up Approach
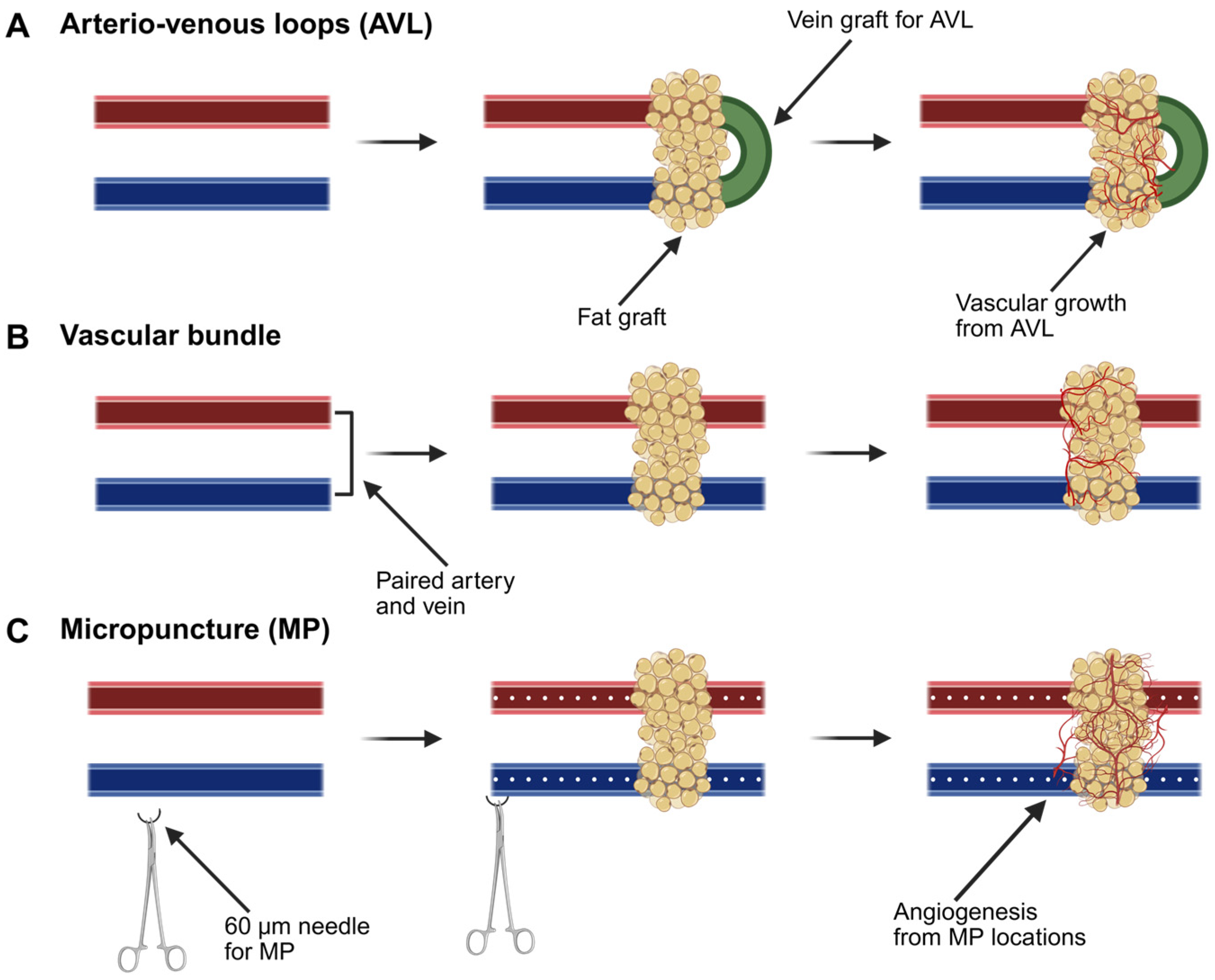
3.5. Initiating Perfusion
3.5.1. Arteriovenous Loops
3.5.2. Vascular Bundles
3.5.3. Micropuncture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The developmental origins of adipose tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Arts, I.C.W.; Peeters, R.L.M.; de Kok, T.M.; Ertaylan, G. Adipose tissue in health and disease through the lens of its building blocks. Sci. Rep. 2020, 10, 10433. [Google Scholar] [CrossRef] [PubMed]
- Teresa Minjung, O.; Chan, K.; Brennan, T.; Roden, D.; Shamouelian, D.; Chung, H.Y.; Waner, M. Autologous Fat Grafting Restores Soft-tissue Contour Deformities after Vascular Anomaly Surgery. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2196. [Google Scholar] [CrossRef]
- Abu-Ghname, A.; Perdanasari, A.T.; Reece, E.M. Principles and Applications of Fat Grafting in Plastic Surgery. Semin. Plast. Surg. 2019, 33, 147–154. [Google Scholar] [CrossRef]
- Mota de Sa, P.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Siersbaek, R.; Mandrup, S. Transcriptional networks controlling adipocyte differentiation. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 247–255. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes. Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ge, K. Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci. 2014, 4, 29. [Google Scholar] [CrossRef]
- Imai, T.; Takakuwa, R.; Marchand, S.; Dentz, E.; Bornert, J.M.; Messaddeq, N.; Wendling, O.; Mark, M.; Desvergne, B.; Wahli, W.; et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. USA 2004, 101, 4543–4547. [Google Scholar] [CrossRef]
- Linhart, H.G.; Ishimura-Oka, K.; DeMayo, F.; Kibe, T.; Repka, D.; Poindexter, B.; Bick, R.J.; Darlington, G.J. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 12532–12537. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Tamori, Y.; Masugi, J.; Nishino, N.; Kasuga, M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 2002, 51, 2045–2055. [Google Scholar] [CrossRef]
- Wu, Z.; Bucher, N.L.; Farmer, S.R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell. Biol. 1996, 16, 4128–4136. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes. Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes. Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919; quiz 920. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, J.E.; Jin, J.; Lim, J.S.; Oh, N.; Kim, K.; Chang, S.I.; Shibuya, M.; Kim, H.; Koh, G.Y. The spatiotemporal development of adipose tissue. Development 2011, 138, 5027–5037. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 2022, 25, 439–453. [Google Scholar] [CrossRef]
- Strong, A.L.; Adidharma, W.; Brown, O.H.; Cederna, P.S. Fat Grafting Subjectively Improves Facial Skin Elasticity and Hand Function of Scleroderma Patients. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3373. [Google Scholar] [CrossRef]
- Pu, L.L. Mechanisms of Fat Graft Survival. Ann. Plast. Surg. 2016, 77 (Suppl. S1), S84–S86. [Google Scholar] [CrossRef]
- Evans, B.G.A.; Gronet, E.M.; Saint-Cyr, M.H. How Fat Grafting Works. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2705. [Google Scholar] [CrossRef]
- Gan, F.; Liu, L.; Zhou, Q.; Huang, W.; Huang, X.; Zhao, X. Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis. PLoS ONE 2022, 17, e0261498. [Google Scholar] [CrossRef]
- Shauly, O.; Gould, D.J.; Ghavami, A. Fat Grafting: Basic Science, Techniques, and Patient Management. Plast. Reconstr. Surg. Glob. Open 2022, 10, e3987. [Google Scholar] [CrossRef]
- Valente, D.S.; Ely, P.B.; Kieling, L.; Konzen, A.T.; Steffen, L.P.; Lazzaretti, G.S.; Zanella, R.K. Breast fat grafting and cancer: A systematic review of the science behind enhancements and concerns. Transl. Breast Cancer Res. 2024, 5, 14. [Google Scholar] [CrossRef]
- Delaporte, T.; Delay, E.; Toussoun, G.; Delbaere, M.; Sinna, R. Breast volume reconstruction by lipomodeling technique: About 15 consecutive cases. Ann. Chir. Plast. Esthet. 2009, 54, 303–316. [Google Scholar] [CrossRef]
- Khouri, R.K., Jr.; Khouri, R.E.; Lujan-Hernandez, J.R.; Khouri, K.R.; Lancerotto, L.; Orgill, D.P. Diffusion and perfusion: The keys to fat grafting. Plast. Reconstr. Surg. Glob. Open 2014, 2, e220. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Davis, M.J.; Winocour, S.J. The Science of Fat Grafting. Semin. Plast. Surg. 2020, 34, 5–10. [Google Scholar] [CrossRef]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef]
- Bagheri, S.C.; Bohluli, B.; Consky, E.K. Current Techniques in Fat Grafting. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2018, 26, 7–13. [Google Scholar] [CrossRef]
- Fuentes-Felix, C. BRAVA: Results did not meet expectations. Aesthet. Surg. J. 2003, 23, 42. [Google Scholar] [CrossRef]
- Oranges, C.M.; Striebel, J.; Tremp, M.; Madduri, S.; Kalbermatten, D.F.; Schaefer, D.J. The Impact of Recipient Site External Expansion in Fat Grafting Surgical Outcomes. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1649. [Google Scholar] [CrossRef]
- Topcu, A.; Aydin, O.E.; Unlu, M.; Barutcu, A.; Atabey, A. Increasing the viability of fat grafts by vascular endothelial growth factor. Arch. Facial Plast. Surg. 2012, 14, 270–276. [Google Scholar] [CrossRef]
- Gassman, A.A.; Lewis, M.S.; Bradley, J.P.; Lee, J.C. Remote Ischemic Preconditioning Improves the Viability of Donor Lipoaspirate during Murine Fat Transfer. Plast. Reconstr. Surg. 2015, 136, 495–502. [Google Scholar] [CrossRef]
- Samdal, F.; Skolleborg, K.C.; Berthelsen, B. The effect of preoperative needle abrasion of the recipient site on survival of autologous free fat grafts in rats. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1992, 26, 33–36. [Google Scholar] [CrossRef]
- Leong, D.T.; Hutmacher, D.W.; Chew, F.T.; Lim, T.C. Viability and adipogenic potential of human adipose tissue processed cell population obtained from pump-assisted and syringe-assisted liposuction. J. Dermatol. Sci. 2005, 37, 169–176. [Google Scholar] [CrossRef]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.A.; Valentino, J.; Hoffman, H.T. Long-term result of vocal cord augmentation with autogenous fat. Ann. Otol. Rhinol. Laryngol. 1995, 104, 871–874. [Google Scholar] [CrossRef]
- Nguyen, A.; Pasyk, K.A.; Bouvier, T.N.; Hassett, C.A.; Argenta, L.C. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast. Reconstr. Surg. 1990, 85, 378–386; discussion 387–379. [Google Scholar] [CrossRef]
- Delay, E.; Garson, S.; Tousson, G.; Sinna, R. Fat injection to the breast: Technique, results, and indications based on 880 procedures over 10 years. Aesthet. Surg. J. 2009, 29, 360–376. [Google Scholar] [CrossRef]
- Schipper, J.A.M.; Tuin, A.J.; Loonen, T.G.J.; Dijkstra, P.U.; Spijkervet, F.K.L.; Schepers, R.H.; Jansma, J. Volume and patient satisfaction, 5 years of follow up after facial fat grafting. J. Plast. Reconstr. Aesthet. Surg. 2025, 102, 231–237. [Google Scholar] [CrossRef]
- Mehrara, B.J.; Santoro, T.D.; Arcilla, E.; Watson, J.P.; Shaw, W.W.; Da Lio, A.L. Complications after microvascular breast reconstruction: Experience with 1195 flaps. Plast. Reconstr. Surg. 2006, 118, 1100–1109. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, D.H.; Perng, C.K. Vascular Complications and Free Flap Salvage in Head and Neck Reconstructive Surgery: Analysis of 150 Cases of Reexploration. Ann. Plast. Surg. 2017, 78, S83–S88. [Google Scholar] [CrossRef]
- Pan, X.L.; Chen, G.X.; Shao, H.W.; Han, C.M.; Zhang, L.P.; Zhi, L.Z. Effect of heparin on prevention of flap loss in microsurgical free flap transfer: A meta-analysis. PLoS ONE 2014, 9, e95111. [Google Scholar] [CrossRef]
- Senchenkov, A.; Lemaine, V.; Tran, N.V. Management of perioperative microvascular thrombotic complications—The use of multiagent anticoagulation algorithm in 395 consecutive free flaps. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 1293–1303. [Google Scholar] [CrossRef]
- Chang, E.I.; Chang, E.I.; Soto-Miranda, M.A.; Zhang, H.; Nosrati, N.; Robb, G.L.; Chang, D.W. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast. Reconstr. Surg. 2013, 132, 1383–1391. [Google Scholar] [CrossRef]
- Atisha, D.M.; Tessiatore, K.M.; Rushing, C.N.; Dayicioglu, D.; Pusic, A.; Hwang, S. A National Snapshot of Patient-Reported Outcomes Comparing Types of Abdominal Flaps for Breast Reconstruction. Plast. Reconstr. Surg. 2019, 143, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Las, D.E.; de Jong, T.; Zuidam, J.M.; Verweij, N.M.; Hovius, S.E.; Mureau, M.A. Identification of independent risk factors for flap failure: A retrospective analysis of 1530 free flaps for breast, head and neck and extremity reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 894–906. [Google Scholar] [CrossRef]
- Largo, R.D.; Selber, J.C.; Garvey, P.B.; Chang, E.I.; Hanasono, M.M.; Yu, P.; Butler, C.E.; Baumann, D.P. Outcome Analysis of Free Flap Salvage in Outpatients Presenting with Microvascular Compromise. Plast. Reconstr. Surg. 2018, 141, 20e–27e. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Peirsman, A.; Nguyen, H.T.; Van Waeyenberge, M.; Ceballos, C.; Bolivar, J.; Kawakita, S.; Vanlauwe, F.; Tirpakova, Z.; Van Dorpe, S.; Van Damme, L.; et al. Vascularized adipose tissue engineering: Moving towards soft tissue reconstruction. Biofabrication 2023, 15, 32003. [Google Scholar] [CrossRef]
- Bianco, P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wu, M.; Zou, R.; Mao, S.; Cong, P.; Hou, M.; Jin, H.; Zhao, Y.; Bao, Y. Adipose-derived mesenchymal stem cell-loaded beta-chitin nanofiber hydrogel promote wound healing in rats. J. Mater. Sci. Mater. Med. 2022, 33, 12. [Google Scholar] [CrossRef]
- Pacelli, S.; Chakravarti, A.R.; Modaresi, S.; Subham, S.; Burkey, K.; Kurlbaum, C.; Fang, M.; Neal, C.A.; Mellott, A.J.; Chakraborty, A.; et al. Investigation of human adipose-derived stem-cell behavior using a cell-instructive polydopamine-coated gelatin-alginate hydrogel. J. Biomed. Mater. Res. A 2021, 109, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Karbasi, S.; Morshed, M.; Zarkesh Esfahani, H.; Golozar, M.; Vaezifar, S. Cell Attachment and Proliferation of Human Adipose-Derived Stem Cells on PLGA/Chitosan Electrospun Nano-Biocomposite. Cell J. 2015, 17, 429–437. [Google Scholar] [CrossRef]
- Yang, J.Z.; Qiu, L.H.; Xiong, S.H.; Dang, J.L.; Rong, X.K.; Hou, M.M.; Wang, K.; Yu, Z.; Yi, C.G. Decellularized adipose matrix provides an inductive microenvironment for stem cells in tissue regeneration. World J. Stem Cells 2020, 12, 585–603. [Google Scholar] [CrossRef]
- Hong, K.Y.; Bae, H.; Park, I.; Park, D.Y.; Kim, K.H.; Kubota, Y.; Cho, E.S.; Kim, H.; Adams, R.H.; Yoo, O.J.; et al. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 2015, 142, 2623–2632. [Google Scholar] [CrossRef]
- Tsekouras, A.; Mantas, D.; Tsilimigras, D.I.; Moris, D.; Kontos, M.; Zografos, G.C. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. In Vivo 2017, 31, 1229–1234. [Google Scholar] [CrossRef]
- Dani, V.; Yao, X.; Dani, C. Transplantation of fat tissues and iPSC-derived energy expenditure adipocytes to counteract obesity-driven metabolic disorders: Current strategies and future perspectives. Rev. Endocr. Metab. Disord. 2022, 23, 103–110. [Google Scholar] [CrossRef]
- Patsch, C.; Challet-Meylan, L.; Thoma, E.C.; Urich, E.; Heckel, T.; O’Sullivan, J.F.; Grainger, S.J.; Kapp, F.G.; Sun, L.; Christensen, K.; et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015, 17, 994–1003. [Google Scholar] [CrossRef]
- Kong, A.M.; Yap, K.K.; Lim, S.Y.; Marre, D.; Pebay, A.; Gerrand, Y.W.; Lees, J.G.; Palmer, J.A.; Morrison, W.A.; Mitchell, G.M. Bio-engineering a tissue flap utilizing a porous scaffold incorporating a human induced pluripotent stem cell-derived endothelial cell capillary network connected to a vascular pedicle. Acta Biomater. 2019, 94, 281–294. [Google Scholar] [CrossRef]
- Koduru, S.V.; Leberfinger, A.N.; Pasic, D.; Forghani, A.; Lince, S.; Hayes, D.J.; Ozbolat, I.T.; Ravnic, D.J. Cellular Based Strategies for Microvascular Engineering. Stem Cell Rev. Rep. 2019, 15, 218–240. [Google Scholar] [CrossRef]
- Koivunotko, E.; Snirvi, J.; Merivaara, A.; Harjumaki, R.; Rautiainen, S.; Kelloniemi, M.; Kuismanen, K.; Miettinen, S.; Yliperttula, M.; Koivuniemi, R. Angiogenic Potential of Human Adipose-Derived Mesenchymal Stromal Cells in Nanofibrillated Cellulose Hydrogel. Biomedicines 2022, 10, 2584. [Google Scholar] [CrossRef]
- Sarkanen, J.R.; Vuorenpaa, H.; Huttala, O.; Mannerstrom, B.; Kuokkanen, H.; Miettinen, S.; Heinonen, T.; Ylikomi, T. Adipose stromal cell tubule network model provides a versatile tool for vascular research and tissue engineering. Cells Tissues Organs 2012, 196, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Johnson, J.A.; Dunne, L.W.; Chen, Y.; Iyyanki, T.; Wu, Y.; Chang, E.I.; Branch-Brooks, C.D.; Robb, G.L.; Butler, C.E. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016, 35, 166–184. [Google Scholar] [CrossRef] [PubMed]
- Kocherova, I.; Bryja, A.; Mozdziak, P.; Angelova Volponi, A.; Dyszkiewicz-Konwinska, M.; Piotrowska-Kempisty, H.; Antosik, P.; Bukowska, D.; Bruska, M.; Izycki, D.; et al. Human Umbilical Vein Endothelial Cells (HUVECs) Co-Culture with Osteogenic Cells: From Molecular Communication to Engineering Prevascularised Bone Grafts. J. Clin. Med. 2019, 8, 1602. [Google Scholar] [CrossRef] [PubMed]
- Prasad Chennazhy, K.; Krishnan, L.K. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 2005, 26, 5658–5667. [Google Scholar] [CrossRef]
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef]
- Freiman, A.; Shandalov, Y.; Rozenfeld, D.; Shor, E.; Segal, S.; Ben-David, D.; Meretzki, S.; Egozi, D.; Levenberg, S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res. Ther. 2016, 7, 5. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vuckovic, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Kimura, Y.; Inamoto, T.; Tabata, Y. Adipose tissue formation in collagen scaffolds with different biodegradabilities. J. Biomater. Sci. Polym. Ed. 2010, 21, 463–476. [Google Scholar] [CrossRef]
- Kimura, Y.; Ozeki, M.; Inamoto, T.; Tabata, Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials 2003, 24, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, T.; Song, K.; Jiang, B.; Ma, X.; Cui, Z. Collagen-chitosan polymer as a scaffold for the proliferation of human adipose tissue-derived stem cells. J. Mater. Sci. Mater. Med. 2009, 20, 799–808. [Google Scholar] [CrossRef]
- Albrecht, F.B.; Schmidt, F.F.; Volz, A.C.; Kluger, P.J. Bioprinting of 3D Adipose Tissue Models Using a GelMA-Bioink with Human Mature Adipocytes or Human Adipose-Derived Stem Cells. Gels 2022, 8, 611. [Google Scholar] [CrossRef]
- Cheng, M.H.; Chang, C.W.; Wang, J.; Bupphathong, S.; Huang, W.; Lin, C.H. 3D-Bioprinted GelMA Scaffold with ASCs and HUVECs for Engineering Vascularized Adipose Tissue. ACS Appl. Bio Mater. 2024, 7, 406–415. [Google Scholar] [CrossRef]
- Gilchrist, A.E.; Serrano, J.F.; Ngo, M.T.; Hrnjak, Z.; Kim, S.; Harley, B.A.C. Encapsulation of murine hematopoietic stem and progenitor cells in a thiol-crosslinked maleimide-functionalized gelatin hydrogel. Acta Biomater. 2021, 131, 138–148. [Google Scholar] [CrossRef]
- Chang, K.H.; Liao, H.T.; Chen, J.P. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: In vitro and in vivo studies. Acta Biomater. 2013, 9, 9012–9026. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Wittmann, K.; Dietl, S.; Ludwig, N.; Berberich, O.; Hoefner, C.; Storck, K.; Blunk, T.; Bauer-Kreisel, P. Engineering vascularized adipose tissue using the stromal-vascular fraction and fibrin hydrogels. Tissue Eng. Part. A 2015, 21, 1343–1353. [Google Scholar] [CrossRef]
- Holnthoner, W.; Hohenegger, K.; Husa, A.M.; Muehleder, S.; Meinl, A.; Peterbauer-Scherb, A.; Redl, H. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J. Tissue Eng. Regen. Med. 2015, 9, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Griffith, M.; Hincke, M. Characterization and inhibition of fibrin hydrogel-degrading enzymes during development of tissue engineering scaffolds. Tissue Eng. 2007, 13, 1469–1477. [Google Scholar] [CrossRef]
- Louis, F.; Sowa, Y.; Irie, S.; Higuchi, Y.; Kitano, S.; Mazda, O.; Matsusaki, M. Injectable Prevascularized Mature Adipose Tissues (iPAT) to Achieve Long-Term Survival in Soft Tissue Regeneration. Adv. Healthc. Mater. 2022, 11, e2201440. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kang, S.W.; Cha, B.H.; Park, H.; Park, K.S.; Lee, K.Y.; Lee, S.H. The effect of conjugating RGD into 3D alginate hydrogels on adipogenic differentiation of human adipose-derived stromal cells. Macromol. Biosci. 2011, 11, 673–679. [Google Scholar] [CrossRef]
- Yoo, B.; Kim, S.; Shin, B.H.; Lee, M.H.; Choy, Y.B.; Lee, K.; Heo, C.Y.; Koh, W.G. Preparation of alginate hydrogel with human-derived adipose tissue to improve fat graft survival and adipogenesis. J. Ind. Eng. Chem. 2021, 95, 148–155. [Google Scholar] [CrossRef]
- Kim, W.S.; Mooney, D.J.; Arany, P.R.; Lee, K.; Huebsch, N.; Kim, J. Adipose tissue engineering using injectable, oxidized alginate hydrogels. Tissue Eng. Part. A 2012, 18, 737–743. [Google Scholar] [CrossRef]
- Brandl, F.P.; Seitz, A.K.; Tessmar, J.K.; Blunk, T.; Gopferich, A.M. Enzymatically degradable poly(ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials 2010, 31, 3957–3966. [Google Scholar] [CrossRef]
- Salvatore, L.; Natali, M.L.; Brunetti, C.; Sannino, A.; Gallo, N. An Update on the Clinical Efficacy and Safety of Collagen Injectables for Aesthetic and Regenerative Medicine Applications. Polymers 2023, 15, 1020. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Tytgat, L.; Ryx, M.; Blondeel, P.; Stillaert, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Van Vlierberghe, S. Soft tissue fillers for adipose tissue regeneration: From hydrogel development toward clinical applications. Acta Biomater. 2017, 63, 37–49. [Google Scholar] [CrossRef]
- Vashi, A.V.; Keramidaris, E.; Abberton, K.M.; Morrison, W.A.; Wilson, J.L.; O’Connor, A.J.; Cooper-White, J.J.; Thompson, E.W. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials 2008, 29, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Weiser, B.; Prantl, L.; Schubert, T.E.; Zellner, J.; Fischbach-Teschl, C.; Spruss, T.; Seitz, A.K.; Tessmar, J.; Goepferich, A.; Blunk, T. In vivo development and long-term survival of engineered adipose tissue depend on in vitro precultivation strategy. Tissue Eng. Part. A 2008, 14, 275–284. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Yue, Y.; Sun, J.; Cui, L. Reconstruction of epidural fat with engineered adipose tissue from adipose derived stem cells and PLGA in the rabbit dorsal laminectomy model. Biomaterials 2012, 33, 6965–6973. [Google Scholar] [CrossRef]
- Patrick, C.W., Jr.; Chauvin, P.B.; Hobley, J.; Reece, G.P. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999, 5, 139–151. [Google Scholar] [CrossRef]
- Fukushima, K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater. Sci. 2016, 4, 9–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef]
- Jain, S.; Yassin, M.A.; Fuoco, T.; Liu, H.; Mohamed-Ahmed, S.; Mustafa, K.; Finne-Wistrand, A. Engineering 3D degradable, pliable scaffolds toward adipose tissue regeneration; optimized printability, simulations and surface modification. J. Tissue Eng. 2020, 11, 2041731420954316. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Cheng, M.H.; Engel, H.; Kao, S.W.; Larson, J.C.; Gupta, S.; Brey, E.M. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.S.; Chung, J.J.; Kim, S.H.; Park, J.W.; Lee, K.; Jung, Y. Enhanced Regeneration of Vascularized Adipose Tissue with Dual 3D-Printed Elastic Polymer/dECM Hydrogel Complex. Int. J. Mol. Sci. 2021, 22, 2886. [Google Scholar] [CrossRef]
- Banyard, D.A.; Borad, V.; Amezcua, E.; Wirth, G.A.; Evans, G.R.; Widgerow, A.D. Preparation, Characterization, and Clinical Implications of Human Decellularized Adipose Tissue Extracellular Matrix (hDAM): A Comprehensive Review. Aesthet. Surg. J. 2016, 36, 349–357. [Google Scholar] [CrossRef]
- Wang, L.; Johnson, J.A.; Zhang, Q.; Beahm, E.K. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 2013, 9, 8921–8931. [Google Scholar] [CrossRef] [PubMed]
- Flynn, L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 2010, 31, 4715–4724. [Google Scholar] [CrossRef]
- Han, T.T.; Toutounji, S.; Amsden, B.G.; Flynn, L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 2015, 72, 125–137. [Google Scholar] [CrossRef]
- Cheung, H.K.; Han, T.T.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, B.S.; Kim, J.Y.; Kim, J.D.; Choi, Y.C.; Yang, H.J.; Park, K.; Lee, H.Y.; Cho, Y.W. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J. Biomed. Mater. Res. A 2011, 97, 292–299. [Google Scholar] [CrossRef]
- Pati, F.; Ha, D.H.; Jang, J.; Han, H.H.; Rhie, J.W.; Cho, D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef]
- Flynn, L.; Semple, J.L.; Woodhouse, K.A. Decellularized placental matrices for adipose tissue engineering. J. Biomed. Mater. Res. A 2006, 79, 359–369. [Google Scholar] [CrossRef]
- Xu, M.; He, Y.; Li, Y.; Liu, K.; Zhang, Y.; Su, T.; Yao, Y.; Jin, X.; Zhang, X.; Lu, F. Combined Use of Autologous Sustained-Release Scaffold of Adipokines and Acellular Adipose Matrix to Construct Vascularized Adipose Tissue. Plast. Reconstr. Surg. 2024, 153, 348e–360e. [Google Scholar] [CrossRef]
- Lee, E.Y.; Xia, Y.; Kim, W.S.; Kim, M.H.; Kim, T.H.; Kim, K.J.; Park, B.S.; Sung, J.H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair. Regen. 2009, 17, 540–547. [Google Scholar] [CrossRef]
- Irvin, J.; Danchik, C.; Rall, J.; Babcock, A.; Pine, M.; Barnaby, D.; Pathakamuri, J.; Kuebler, D. Bioactivity and composition of a preserved connective tissue matrix derived from human placental tissue. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2731–2740. [Google Scholar] [CrossRef]
- Magana, A.; Giovanni, R.; Essien, E.; Epel, B.; Kotecha, M.; Liu, S.; Mathew, M.T.; Hagarty, S.E.; Bijukumar, D. Amniotic growth factors enhanced human pre-adipocyte cell viability and differentiation under hypoxia. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 15, 236–242. [Google Scholar] [CrossRef]
- Khanna, A.; Oropeza, B.P.; Huang, N.F. Engineering Spatiotemporal Control in Vascularized Tissues. Bioengineering 2022, 9, 555. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Liu, Y. VEGF heparinized-decellularized adipose tissue scaffolds enhance tissue engineering vascularization in vitro. RSC Adv. 2018, 8, 33614–33624. [Google Scholar] [CrossRef]
- Murohara, T.; Shintani, S.; Kondo, K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr. Pharm. Des. 2009, 15, 2784–2790. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2023, 18, 100522. [Google Scholar] [CrossRef]
- Mou, S.; Zhou, M.; Li, Y.; Wang, J.; Yuan, Q.; Xiao, P.; Sun, J.; Wang, Z. Extracellular Vesicles from Human Adipose-Derived Stem Cells for the Improvement of Angiogenesis and Fat-Grafting Application. Plast. Reconstr. Surg. 2019, 144, 869–880. [Google Scholar] [CrossRef]
- Nie, F.; Ding, P.; Zhang, C.; Zhao, Z.; Bi, H. Extracellular vesicles derived from lipoaspirate fluid promote fat graft survival. Adipocyte 2021, 10, 293–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T. Adipose-derived stem cells exosome and its potential applications in autologous fat grafting. J. Plast. Reconstr. Aesthet. Surg. 2023, 76, 219–229. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, H.; Qian, M.; Ji, G.; Wei, Y.; He, J.; Tian, H.; Zhao, Q. MSC-derived sEV-loaded hyaluronan hydrogel promotes scarless skin healing by immunomodulation in a large skin wound model. Biomed. Mater. 2022, 17, 034104. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.S.; Vasconez, H.C.; Morrison, S.; Mogni, B.; Linton, S.; Hockensmith, L.; Kabir, T.; Zielins, E.; Najor, A.; Bakri, K.; et al. Fat Graft Enrichment Strategies: A Systematic Review. Plast. Reconstr. Surg. 2020, 145, 827–841. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Chen, W. Influence of repeatedly injecting platelet-rich plasma on survival and quality of fat grafts in nude mice. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2013, 27, 454–459. [Google Scholar]
- Sasaki, G.H. The Safety and Efficacy of Cell-Assisted Fat Grafting to Traditional Fat Grafting in the Anterior Mid-Face: An Indirect Assessment by 3D Imaging. Aesthetic Plast. Surg. 2015, 39, 833–846. [Google Scholar] [CrossRef]
- Gentile, P.; Di Pasquali, C.; Bocchini, I.; Floris, M.; Eleonora, T.; Fiaschetti, V.; Floris, R.; Cervelli, V. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg. Innov. 2013, 20, 370–376. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomedicine 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Shimizu, T. Cell sheet-based tissue engineering for fabricating 3-dimensional heart tissues. Circ. J. 2014, 78, 2594–2603. [Google Scholar] [CrossRef]
- Villalona, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T.; et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. Part. B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef]
- Brett, E.; Chung, N.; Leavitt, W.T.; Momeni, A.; Longaker, M.T.; Wan, D.C. A Review of Cell-Based Strategies for Soft Tissue Reconstruction. Tissue Eng. Part. B Rev. 2017, 23, 336–346. [Google Scholar] [CrossRef]
- Zhang, Q.; Hubenak, J.; Iyyanki, T.; Alred, E.; Turza, K.C.; Davis, G.; Chang, E.I.; Branch-Brooks, C.D.; Beahm, E.K.; Butler, C.E. Engineering vascularized soft tissue flaps in an animal model using human adipose-derived stem cells and VEGF+PLGA/PEG microspheres on a collagen-chitosan scaffold with a flow-through vascular pedicle. Biomaterials 2015, 73, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Spheroids as vascularization units: From angiogenesis research to tissue engineering applications. Biotechnol. Adv. 2017, 35, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.B.; Urrata, V.; Trapani, M.; Moschella, F.; Cordova, A.; Toia, F. Systematic review on spheroids from adipose-derived stem cells: Spontaneous or artefact state? J. Cell. Physiol. 2022, 237, 4397–4411. [Google Scholar] [CrossRef]
- Banerjee, D.; Singh, Y.P.; Datta, P.; Ozbolat, V.; O’Donnell, A.; Yeo, M.; Ozbolat, I.T. Strategies for 3D bioprinting of spheroids: A comprehensive review. Biomaterials 2022, 291, 121881. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Xia, X.; Han, M.; Li, F.; Li, C.; Li, Y.; Gao, D. Organoid technology for tissue engineering. J. Mol. Cell Biol. 2020, 12, 569–579. [Google Scholar] [CrossRef]
- Mandl, M.; Viertler, H.P.; Hatzmann, F.M.; Brucker, C.; Grossmann, S.; Waldegger, P.; Rauchenwald, T.; Mattesich, M.; Zwierzina, M.; Pierer, G.; et al. An organoid model derived from human adipose stem/progenitor cells to study adipose tissue physiology. Adipocyte 2022, 11, 164–174. [Google Scholar] [CrossRef]
- Strobel, H.A.; Gerton, T.; Hoying, J.B. Vascularized adipocyte organoid model using isolated human microvessel fragments. Biofabrication 2021, 13, 035022. [Google Scholar] [CrossRef]
- Schmidt, V.J.; Hilgert, J.G.; Covi, J.M.; Leibig, N.; Wietbrock, J.O.; Arkudas, A.; Polykandriotis, E.; de Wit, C.; Horch, R.E.; Kneser, U. Flow increase is decisive to initiate angiogenesis in veins exposed to altered hemodynamics. PLoS ONE 2015, 10, e0117407. [Google Scholar] [CrossRef]
- Leibig, N.; Wietbrock, J.O.; Bigdeli, A.K.; Horch, R.E.; Kremer, T.; Kneser, U.; Schmidt, V.J. Flow-Induced Axial Vascularization: The Arteriovenous Loop in Angiogenesis and Tissue Engineering. Plast. Reconstr. Surg. 2016, 138, 825–835. [Google Scholar] [CrossRef]
- Fischer, K.S.; Henn, D.; Zhao, E.T.; Sivaraj, D.; Litmanovich, B.; Hahn, W.W.; Hostler, A.C.; Mojadidi, S.M.; Gonzalez, J.; Knochel, A.B.; et al. Elevated Shear Stress Modulates Heterogenous Cellular Subpopulations to Induce Vascular Remodeling. Tissue Eng. Part. A 2024, 30, 752–765. [Google Scholar] [CrossRef]
- Schmidt, V.J.; Hilgert, J.G.; Covi, J.M.; Weis, C.; Wietbrock, J.O.; de Wit, C.; Horch, R.E.; Kneser, U. High flow conditions increase connexin43 expression in a rat arteriovenous and angioinductive loop model. PLoS ONE 2013, 8, e78782. [Google Scholar] [CrossRef]
- Meyer, A.; Horch, R.E.; Schoengart, E.; Beier, J.P.; Taeger, C.D.; Arkudas, A.; Lang, W. Results of combined vascular reconstruction by means of AV loops and free flap transfer in patients with soft tissue defects. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 545–553. [Google Scholar] [CrossRef]
- Debels, H.; Palmer, J.; Han, X.L.; Poon, C.; Abberton, K.; Morrison, W. In vivo tissue engineering of an adipose tissue flap using fat grafts and Adipogel. J. Tissue Eng. Regen. Med. 2020, 14, 633–644. [Google Scholar] [CrossRef]
- Henn, D.; Chen, K.; Fischer, K.; Rauh, A.; Barrera, J.A.; Kim, Y.J.; Martin, R.A.; Hannig, M.; Niedoba, P.; Reddy, S.K.; et al. Tissue Engineering of Axially Vascularized Soft-Tissue Flaps with a Poly-(varepsilon-Caprolactone) Nanofiber-Hydrogel Composite. Adv. Wound Care 2020, 9, 365–377. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Gerrand, Y.W.; Wray, L.S.; Tan, B.; Joukhdar, H.; Kaplan, D.L.; Morrison, W.A.; Mitchell, G.M. Vascular Pedicle and Microchannels: Simple Methods Toward Effective In Vivo Vascularization of 3D Scaffolds. Adv. Healthc. Mater. 2019, 8, e1901106. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sung, K.C.; Fumimoto, M.; Tsutsumi, A.; Kondo, S.; Hinohara, Y.; Morrison, W.A. Prefabricated engineered skin flap using an arteriovenous vascular bundle as a vascular carrier in rabbits. Plast. Reconstr. Surg. 2006, 117, 1860–1875. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tamai, M.; Taguchi, N.; Niyazi, A.; Ueno, M.; Nagasao, T. Spontaneously generated large adipose flaps in vivo tissue engineering chambers. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1889–1896. [Google Scholar] [CrossRef]
- Lu, Z.; Yuan, Y.; Gao, J.; Lu, F. Adipose tissue extract promotes adipose tissue regeneration in an adipose tissue engineering chamber model. Cell Tissue Res. 2016, 364, 289–298. [Google Scholar] [CrossRef]
- Horchler, S.N.; Hancock, P.C.; Sun, M.; Liu, A.T.; Massand, S.; El-Mallah, J.C.; Goldenberg, D.; Waldron, O.; Landmesser, M.E.; Agrawal, S.; et al. Vascular persistence following precision micropuncture. Microcirculation 2024, 31, e12835. [Google Scholar] [CrossRef]
- Hancock, P.C.; Koduru, S.V.; Sun, M.; Ravnic, D.J. Induction of scaffold angiogenesis by recipient vasculature precision micropuncture. Microvasc. Res. 2021, 134, 104121. [Google Scholar] [CrossRef]



| Cell Type | Source | Differentiation Potential | Qualities Relevant to Adipose Engineering |
|---|---|---|---|
| Adipose-derived stem cells (ASCs) | Adipose tissue, stromal vascular fraction (SVF) | Differentiate into adipocytes, endothelial cells, pericyte-like cells |
|
| Adult somatic cells (e.g., skin fibroblasts) | Potential to differentiate into various cell lines, including endothelial cells |
| |
| Human umbilical vein endothelial cells (HUVECs) | Umbilical cord blood vessels | Endothelial cells |
|
| Endothelial progenitor cells (EPCs) | Circulating blood, bone marrow, umbilical cord blood, adipose tissue | Endothelial cells |
|
| Human adipose-derived microvascular endothelial cells (hAMECs) | Adipose tissue, SVF | Endothelial cells |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mallah, J.C.; Wen, C.; Waldron, O.; Jikaria, N.R.; Asgardoon, M.H.; Schlidt, K.; Goldenberg, D.; Horchler, S.; Landmesser, M.E.; Park, J.H.; et al. Current Modalities in Soft-Tissue Reconstruction and Vascularized Adipose Engineering. Biomolecules 2025, 15, 780. https://doi.org/10.3390/biom15060780
El-Mallah JC, Wen C, Waldron O, Jikaria NR, Asgardoon MH, Schlidt K, Goldenberg D, Horchler S, Landmesser ME, Park JH, et al. Current Modalities in Soft-Tissue Reconstruction and Vascularized Adipose Engineering. Biomolecules. 2025; 15(6):780. https://doi.org/10.3390/biom15060780
Chicago/Turabian StyleEl-Mallah, Jessica C., Connie Wen, Olivia Waldron, Neekita R. Jikaria, Mohammad Hossein Asgardoon, Kevin Schlidt, Dana Goldenberg, Summer Horchler, Mary E. Landmesser, Ji Ho Park, and et al. 2025. "Current Modalities in Soft-Tissue Reconstruction and Vascularized Adipose Engineering" Biomolecules 15, no. 6: 780. https://doi.org/10.3390/biom15060780
APA StyleEl-Mallah, J. C., Wen, C., Waldron, O., Jikaria, N. R., Asgardoon, M. H., Schlidt, K., Goldenberg, D., Horchler, S., Landmesser, M. E., Park, J. H., Hasegawa, U., Wang, Y., & Ravnic, D. J. (2025). Current Modalities in Soft-Tissue Reconstruction and Vascularized Adipose Engineering. Biomolecules, 15(6), 780. https://doi.org/10.3390/biom15060780







