Recovery of Lysosomal Acidification and Autophagy Flux by Attapulgite Nanorods: Therapeutic Potential for Lysosomal Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Preparations of ATT
2.3. Preparations of FITC-ATT
2.4. Construction of mRFP-GFP-LC3/HeLa Cells
2.5. Cell Culture
2.6. Flow Cytometric Analysis of the Internalized FITC-Labeled ATT
2.7. Observation of ATT by Biological TEM (Bio-TEM)
2.8. Western Blotting
2.9. Cell Viability
2.10. Palmitate: BSA Preparation
2.11. Nile Red Staining
2.12. Construction and Treatment of the NAFLD Model
2.13. Oral Glucose-Tolerance Test (OGTT)
2.14. Detection of Serum Biochemical Indexes
2.15. Hematoxylin–Eosin Staining
2.16. Liver Oil Red O Staining
2.17. Cell Lysosomal Acid Detection
2.18. Statistical Analysis
3. Results
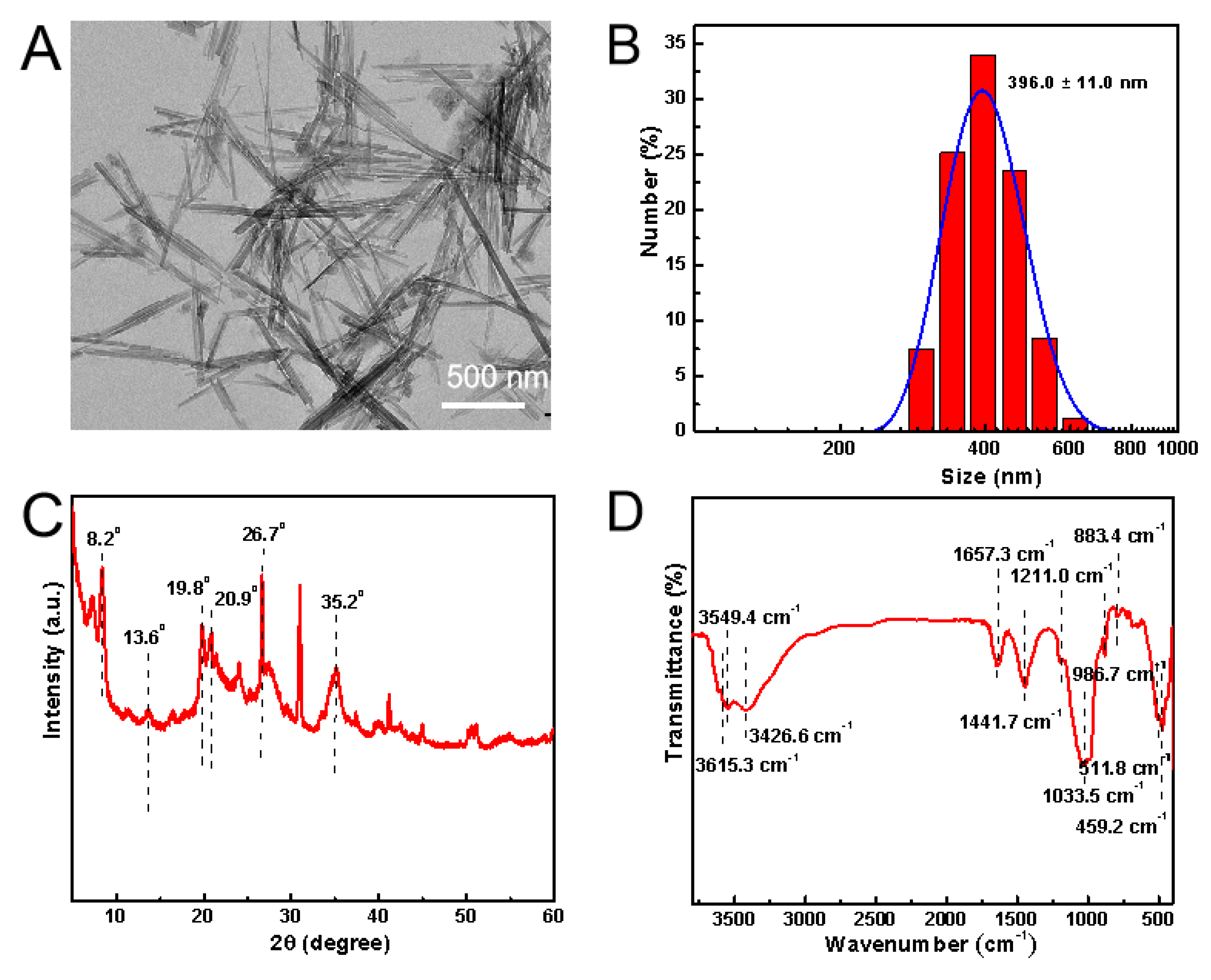
3.1. Characterizations of ATT
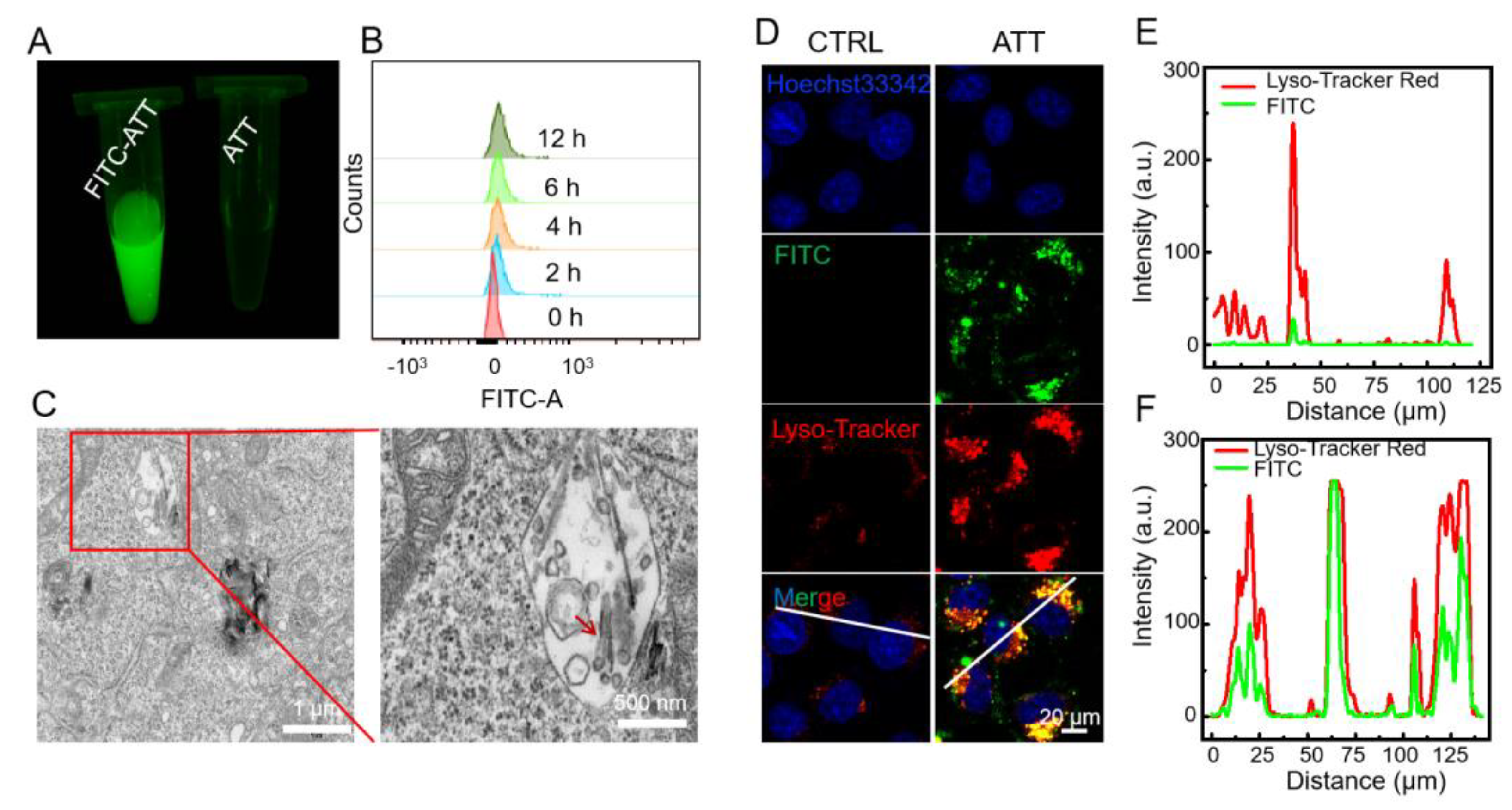
3.2. Cellular Uptake and Intracellular Localization of ATT
3.3. Restoration of Lysosomal Acidity and CTSD Maturation by ATT
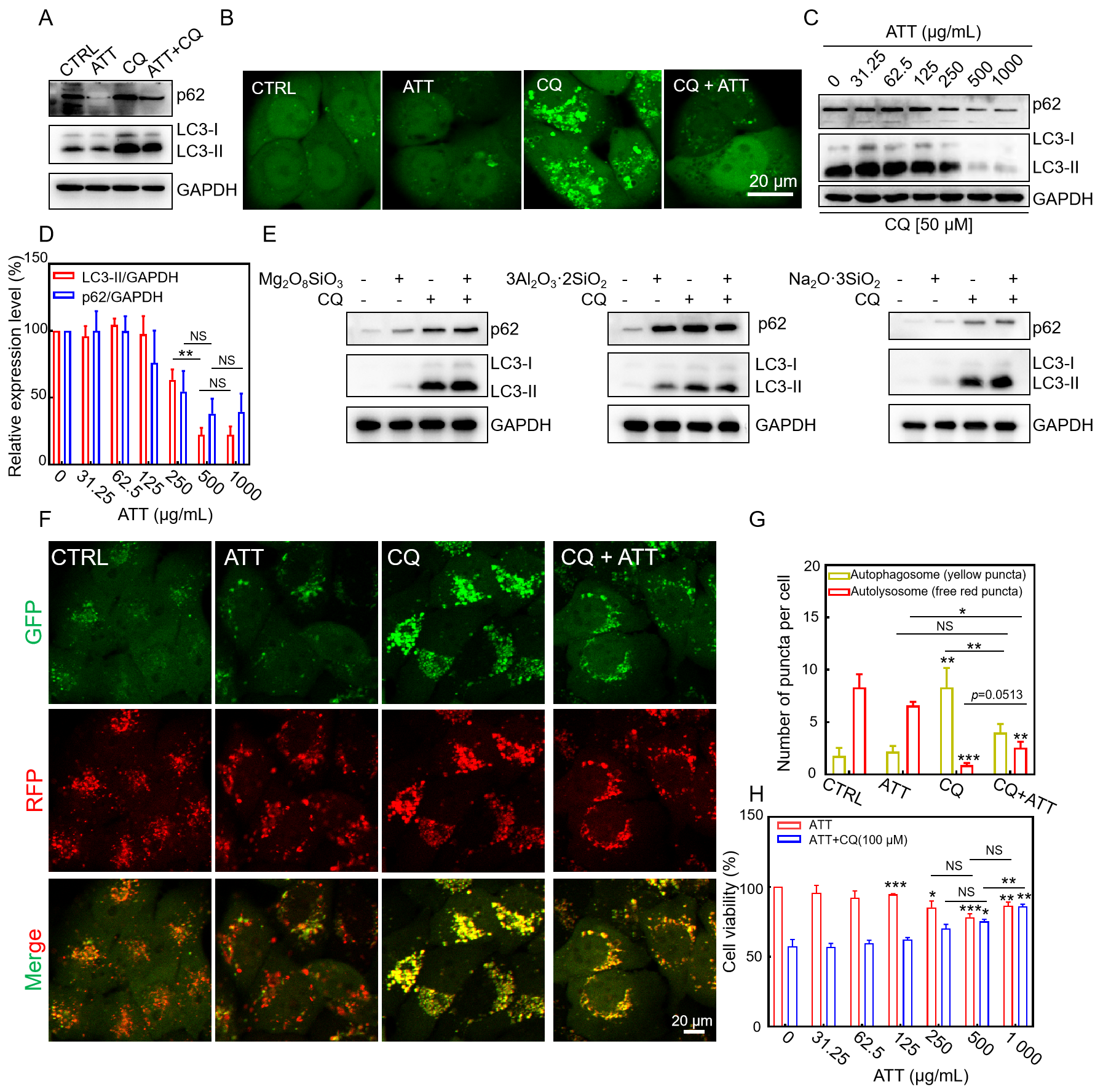
3.4. Restoring CQ-Blocked Autophagic Flux and Reducing CQ-Induced Cytotoxicity by ATT
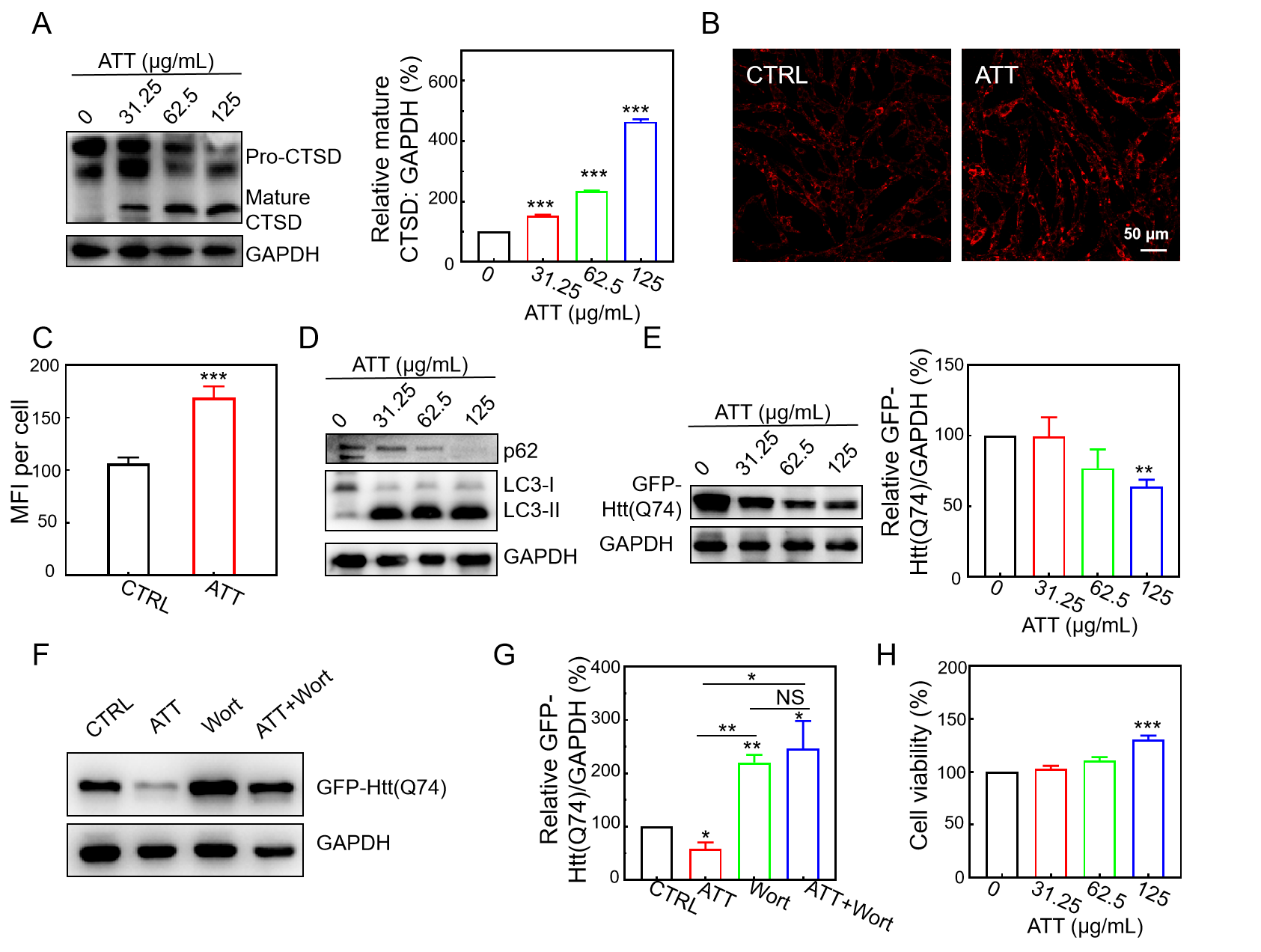
3.5. Increasing Lysosomal Acidity and Accelerating the Clearance of Mutant Huntingtin by ATT
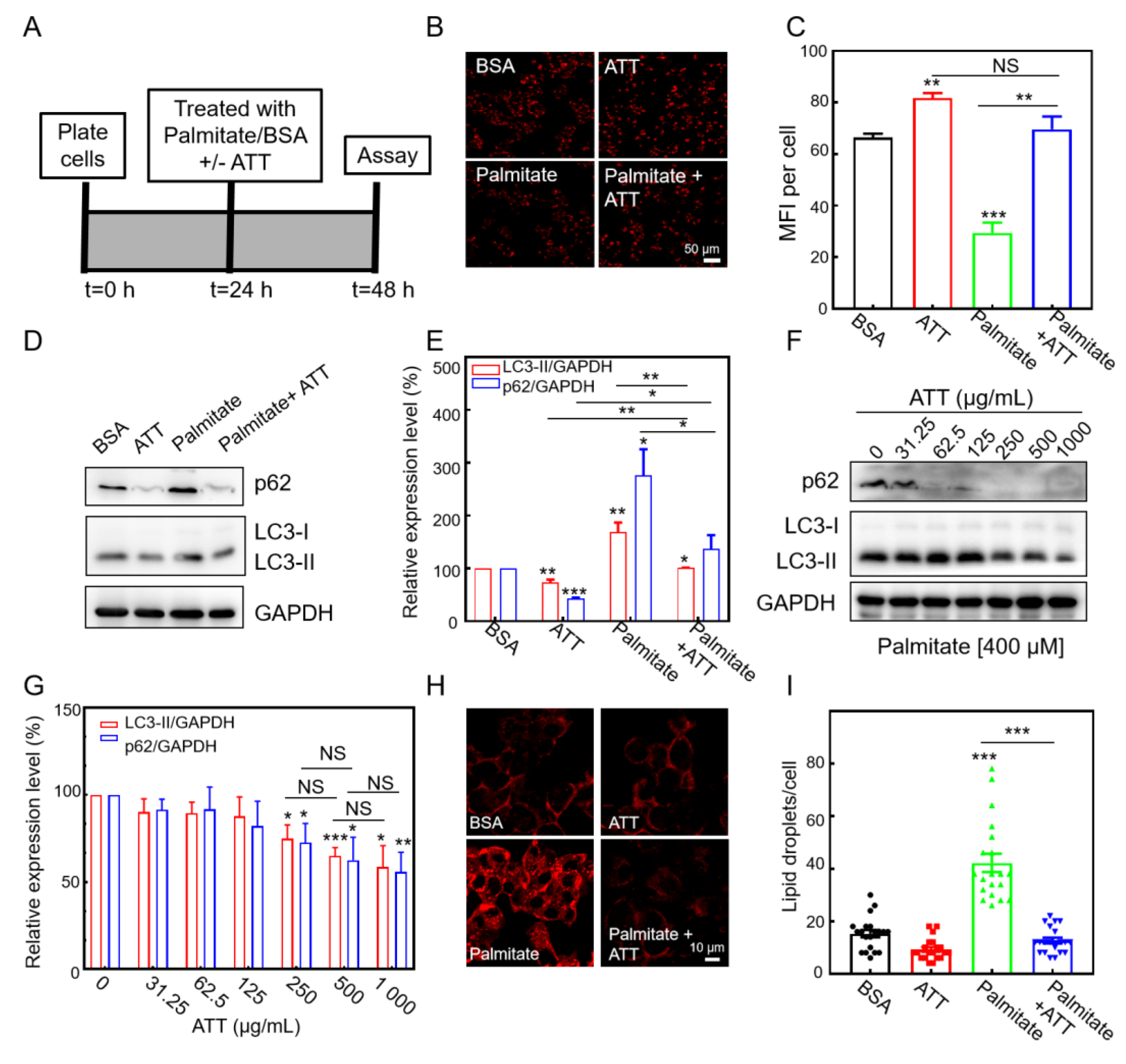
3.6. ATT Reacidifies Lysosomes and Restores Autophagic Flux Under Lipotoxicity
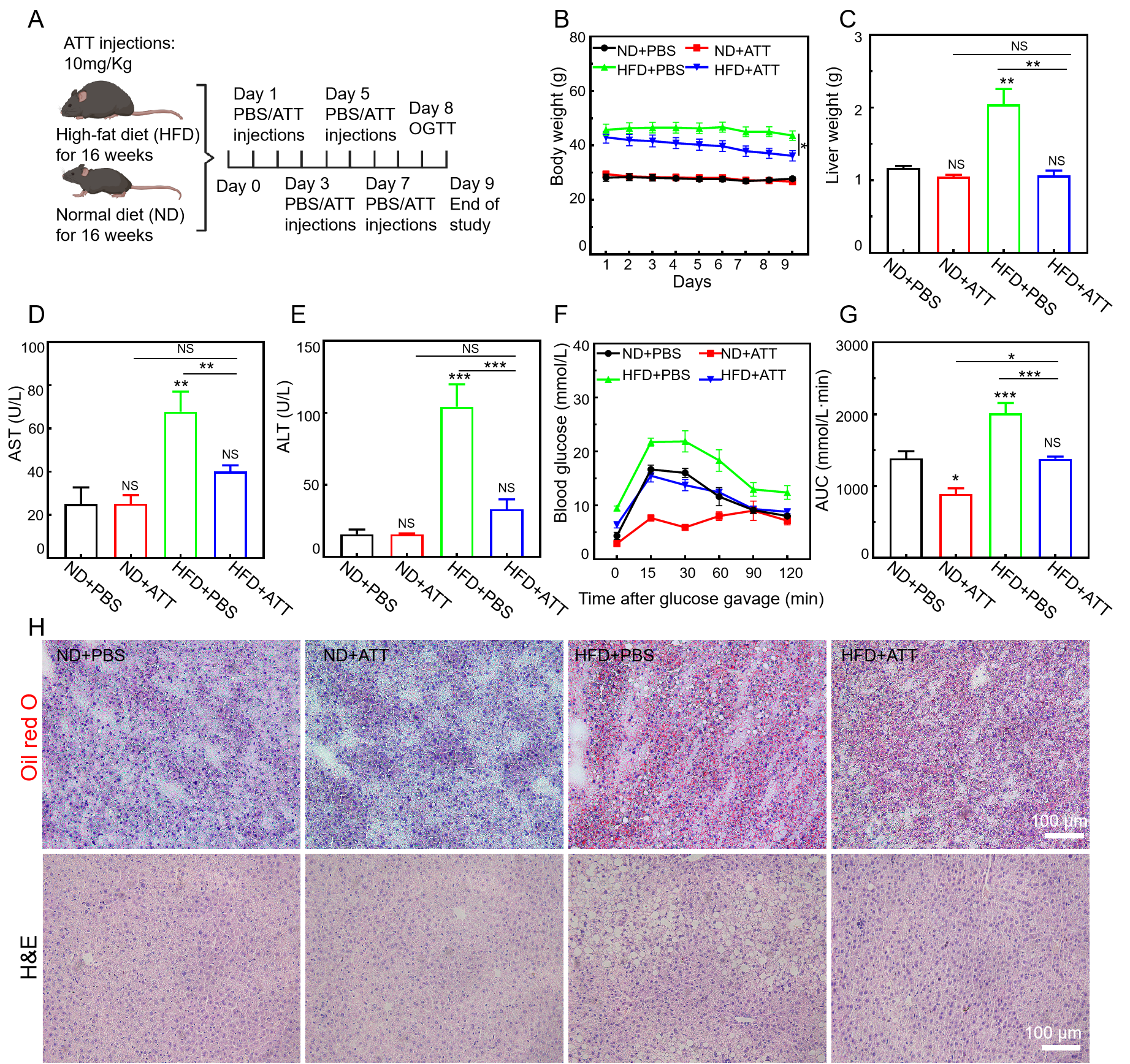
3.7. ATT Alleviates the Pathological Condition in High-Fat-Diet-Induced NAFLD Mice
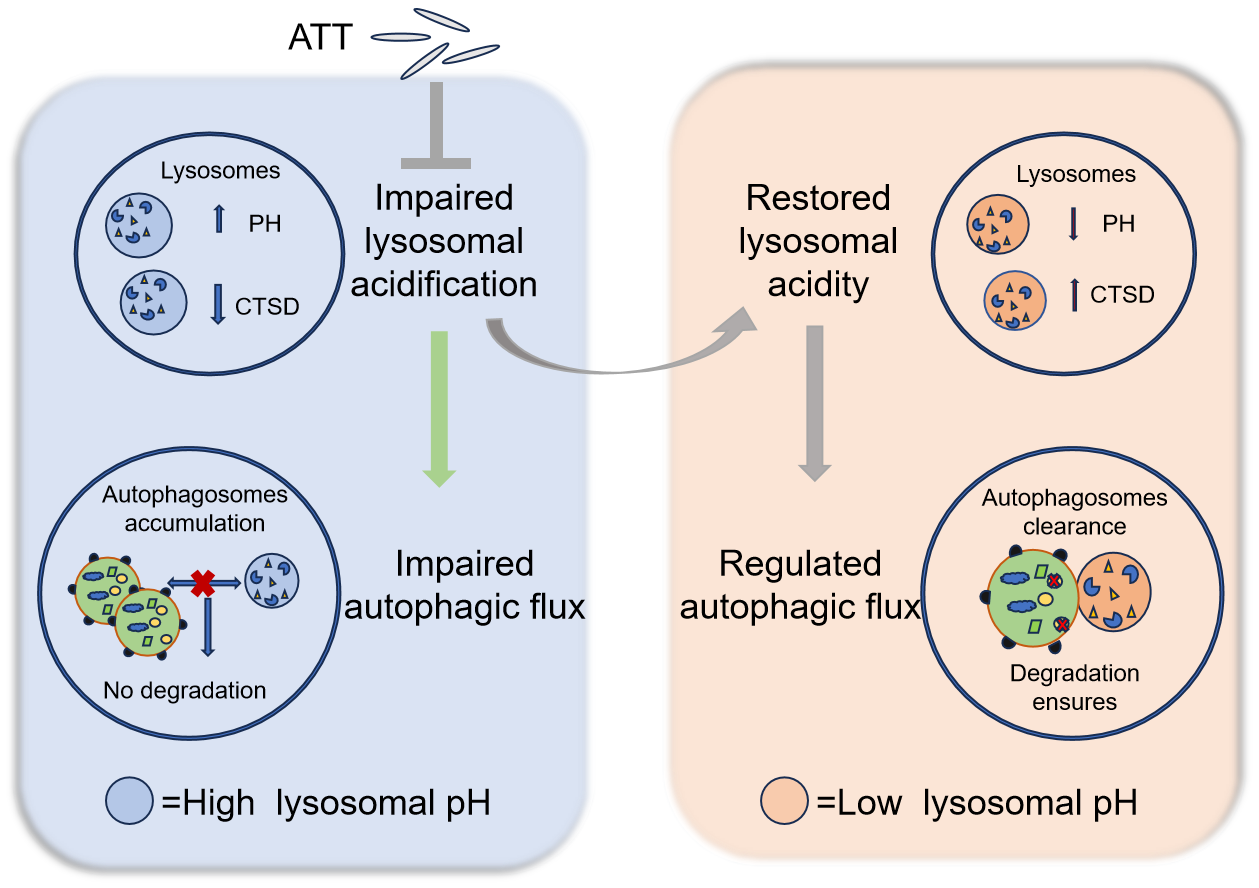
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2019, 21, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Alhowyan, A.A.; Harisa, G.I. From Molecular Therapies to Lysosomal Transplantation and Targeted Drug Strategies: Present Applications, Limitations, and Future Prospects of Lysosomal Medications. Biomolecules 2025, 15, 327. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Liu, J.; Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Ther. 2022, 29, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Perera, R.M. Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology. Nat. Rev. Mol. Cell Biol. 2024, 25, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Gros, F.; Muller, S. The role of lysosomes in metabolic and autoimmune diseases. Nat. Rev. Nephrol. 2023, 19, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A.; Rubinsztein, D.C. Mechanisms of autophagy–lysosome dysfunction in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2024, 25, 926–946. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, P.; Lu, T.; Wang, Y.; Wei, Y.; Wei, X. Role of lysosomes in physiological activities, diseases, and therapy. J. Hematol Oncol. 2021, 14, 79. [Google Scholar]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and Incidence of Huntington’s Disease: An Updated Systematic Review and Meta-Analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef]
- Martinez-Vicente, M.; Talloczy, Z.; Wong, E.; Tang, G.; Koga, H.; Kaushik, S.; de Vries, R.; Arias, E.; Harris, S.; Sulzer, D.; et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010, 13, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Alshehabi, Y.; Martin, D.D.O. Protective Proteolysis in Huntington’s Disease: Unraveling the Role of Post-Translational Myristoylation of Huntingtin in Autophagy. J. Huntington's Dis. 2024, 13, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.J.; Veeranna; Rosa, C.M.; Kumar, A.; Mohan, P.S.; Stavrides, P.; Marchionini, D.M.; Yang, D.-S.; Nixon, R.A. Pathobiology of the autophagy-lysosomal pathway in the Huntington’s disease brain. bioRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Valionyte, E.; Yang, Y.; Roberts, S.L.; Kelly, J.; Lu, B.; Luo, S. Lowering Mutant Huntingtin Levels and Toxicity: Autophagy-Endolysosome Pathways in Huntington’s Disease. J. Mol. Biol. 2020, 432, 2673–2691. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, Q.; Fu, J. Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy 2023, 20, 221–241. [Google Scholar] [CrossRef]
- Grander, C.; Grabherr, F.; Tilg, H. Non-alcoholic fatty liver disease: Pathophysiological concepts and treatment options. Cardiovasc. Res. 2023, 119, 1787–1798. [Google Scholar] [CrossRef]
- Teng, M.L.P.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.H.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Zeng, J.; Acin-Perez, R.; Assali, E.A.; Martin, A.; Brownstein, A.J.; Petcherski, A.; Fernández-del-Rio, L.; Xiao, R.; Lo, C.H.; Shum, M.; et al. Restoration of lysosomal acidification rescues autophagy and metabolic dysfunction in non-alcoholic fatty liver disease. Nat. Commun. 2023, 14, 2573. [Google Scholar] [CrossRef]
- Dima, A.; Jurcut, C.; Arnaud, L. Hydroxychloroquine in systemic and autoimmune diseases: Where are we now? Jt. Bone Spine 2021, 88, 105143. [Google Scholar] [CrossRef]
- Mondal, K.; Porter, H.; Cole, J.; Pandya, H.K.; Basu, S.K.; Khanam, S.; Chiu, C.-Y.; Shah, V.; Stephenson, D.J.; Chalfant, C.E.; et al. Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome–Lysosomal Pathway and Sphingolipid Metabolism in the Retina. Mol. Neurobiol. 2022, 59, 3873–3887. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Seydi, E.; Hassani, M.K.; Naderpour, S.; Arjmand, A.; Pourahmad, J. Cardiotoxicity of chloroquine and hydroxychloroquine through mitochondrial pathway. BMC Pharmacol. Toxicol. 2023, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.H.; Hall, M.J.; Burgoyne, T.; Fale, P.; Storm, T.; Escrevente, C.; Antas, P.; Seabra, M.C.; Futter, C.E. Impaired Lysosome Reformation in Chloroquine-Treated Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Visual Sci. 2023, 64, 10. [Google Scholar] [CrossRef]
- Chen, X.; Yan, G.; Chen, M.; Yang, P.; Xu, B. Alkylated chitosan-attapulgite composite sponge for rapid hemostasis. Biomater. Adv. 2023, 153, 213569. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Shen, M.; Tomás, H.; Zhou, B.; Shi, X. Antitumor Efficacy of Doxorubicin-Loaded Electrospun Attapulgite–Poly(lactic-co-glycolic acid) Composite Nanofibers. J. Funct. Biomater. 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Deng, D.; Gao, J.; Cai, C. Tubelike Gold Sphere–Attapulgite Nanocomposites with a High Photothermal Conversion Ability in the Near-Infrared Region for Enhanced Cancer Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 10243–10252. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, Y.; Wen, C.; Zhou, Y. Dietary supplementation with a silicate clay mineral (palygorskite) alleviates inflammatory responses and intestinal barrier damage in broiler chickens challenged with Escherichia coli. Poult. Sci. 2024, 103, 104017. [Google Scholar] [CrossRef]
- Yang, Q.-L.; Yang, L.; Qu, X.-Y.; Xiao, D.-F. Effects of dietary supplementation by modified palygorskite and essential oil/palygorskite complex on growth performance and intestinal flora composition of broilers with diarrhea. Poult. Sci. 2024, 103, 104379. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezabakhsh, A.; Rezaie, J.; Nouri, M.; Rahbarghazi, R. Exosomal cargos modulate autophagy in recipient cells via different signaling pathways. Cell Biosci. 2020, 10, 92. [Google Scholar] [CrossRef]
- Lee, D.; Hong, J.H. Nanoparticle-Mediated Therapeutic Application for Modulation of Lysosomal Ion Channels and Functions. Pharmaceutics 2020, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Shi, A.; Yin, H.; Wang, Y.; Liu, Q.; Chen, W.; Wu, J. Nanomaterials Respond to Lysosomal Function for Tumor Treatment. Cells 2022, 11, 3348. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-F.; Zhang, L.; Nethi, S.K.; Barui, A.K.; Lin, J.; Zhou, W.; Shen, Y.; Man, N.; Zhang, Y.-J.; Xu, J.; et al. Accelerating the clearance of mutant huntingtin protein aggregates through autophagy induction by europium hydroxide nanorods. Biomaterials 2014, 35, 899–907. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, P.; Liu, L.; Ding, T.; Yang, Y.; Jin, P.; Zhang, L.; Zhao, Z.; Wang, M.; Hu, B.; et al. AIE-enabled transfection-free identification and isolation of viable cell subpopulations differing in the level of autophagy. Autophagy 2023, 19, 3062–3078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Misra, S.K.; Moitra, P.; Zhang, X.; Jeong, S.-J.; Stitham, J.; Rodriguez-Velez, A.; Park, A.; Yeh, Y.-S.; Gillanders, W.E.; et al. Use of acidic nanoparticles to rescue macrophage lysosomal dysfunction in atherosclerosis. Autophagy 2022, 19, 886–903. [Google Scholar] [CrossRef]
- Morgan, M.J.; Fitzwalter, B.E.; Owens, C.R.; Powers, R.K.; Sottnik, J.L.; Gamez, G.; Costello, J.C.; Theodorescu, D.; Thorburn, A. Metastatic cells are preferentially vulnerable to lysosomal inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, E8479–E8488. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Wei, P.-F.; Jin, P.-P.; Barui, A.K.; Hu, Y.; Zhang, L.; Zhang, J.-Q.; Shi, S.-S.; Zhang, H.-R.; Lin, J.; Zhou, W.; et al. Differential ERK activation during autophagy induced by europium hydroxide nanorods and trehalose: Maximum clearance of huntingtin aggregates through combined treatment. Biomaterials 2015, 73, 160–174. [Google Scholar] [CrossRef]
- Di, Y.-Q.; Han, X.-L.; Kang, X.-L.; Wang, D.; Chen, C.-H.; Wang, J.-X.; Zhao, X.-F. Autophagy triggers CTSD (cathepsin D) maturation and localization inside cells to promote apoptosis. Autophagy 2020, 17, 1170–1192. [Google Scholar] [CrossRef]
- Sala, G.L.; Bellocci, M.; Callegari, F.; Rossini, G.P. Azaspiracid-1 Inhibits the Maturation of Cathepsin D in Mammalian Cells. Chem. Res. Toxicol. 2013, 26, 444–455. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T. How to Interpret LC3 Immunoblotting. Autophagy 2014, 3, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rehman, S.K.; Zhang, W.; Wen, A.; Yao, L.; Zhang, J. Autophagy is a therapeutic target in anticancer drug resistance. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 220–229. [Google Scholar] [CrossRef]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Enhancement of immunotoxin activity using chemical and biological reagents. Br. J. Cancer 1997, 75, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, Y.; Chen, Y.; Song, X.; Zheng, X.; Li, J.; He, J.; Chen, X.; Huang, C.; Wang, W.; et al. A Specific Mini-Intrabody Mediates Lysosome Degradation of Mutant Huntingtin. Adv. Sci. 2023, 10, 2301120. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Kaur, G.; Seth, P.; Jana, N.R.; Jana, N.R. Autophagy-Induced Nanoparticle-Based Clearing of Toxic Huntingtin Protein Aggregates from Neuron Cells. ACS Appl. Nano Mater. 2024, 7, 3468–3478. [Google Scholar] [CrossRef]
- Trudeau, K.M.; Colby, A.H.; Zeng, J.; Las, G.; Feng, J.H.; Grinstaff, M.W.; Shirihai, O.S. Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J. Cell Biol. 2016, 214, 25–34. [Google Scholar] [CrossRef]
- Flessa, C.-M.; Nasiri-Ansari, N.; Kyrou, I.; Leca, B.M.; Lianou, M.; Chatzigeorgiou, A.; Kaltsas, G.; Kassi, E.; Randeva, H.S. Genetic and Diet-Induced Animal Models for Non-Alcoholic Fatty Liver Disease (NAFLD) Research. Int. J. Mol. Sci. 2022, 23, 15791. [Google Scholar] [CrossRef]
- Recena Aydos, L.; Aparecida do Amaral, L.; Serafim de Souza, R.; Jacobowski, A.C.; Freitas dos Santos, E.; Rodrigues Macedo, M.L. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 2019, 11, 3067. [Google Scholar] [CrossRef]
- Hui, A.; Yan, R.; Wang, W.; Wang, Q.; Zhou, Y.; Wang, A. Incorporation of quaternary ammonium chitooligosaccharides on ZnO/palygorskite nanocomposites for enhancing antibacterial activities. Carbohydr. Polym. 2020, 247, 116685. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Colilla, M.; Vallet-Regí, M. Zwitterionic ceramics for biomedical applications. Acta Biomater. 2016, 40, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zhao, C.; Zhai, Q.; Liu, W.; Ke, X.; Liu, X. The highly efficient simultaneous removal of Pb2+ and methylene blue induced by the release of endogenous active sites of montmorillonite. Water Sci. Technol. 2022, 86, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chen, M.; Zhang, Y.; Wu, L. Hydrothermal synthesis and formation mechanism of controllable magnesium silicate nanotubes derived from coal fly ash. Nanotechnology 2023, 34, 365701. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, M.; Daniel, J.; Genin, E.; Soria, F.N.; Blanchard-Desce, M.; Bezard, E.; Dehay, B. Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 2016, 12, 472–483. [Google Scholar] [CrossRef]
- Santin, Y.; Formoso, K.; Haidar, F.; Fuentes, M.D.P.O.; Bourgailh, F.; Hifdi, N.; Hnia, K.; Doghri, Y.; Resta, J.; Champigny, C.; et al. Inhalation of acidic nanoparticles prevents doxorubicin cardiotoxicity through improvement of lysosomal function. Theranostics 2023, 13, 5435–5451. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, B.-M.; Li, X.; Yu, Y.-C.; Deng, D.-K.; Sun, L.-J.; Qu, H.-L.; Wu, R.-X.; Xu, X.-Y.; Sun, H.-H.; et al. Gold nanoparticles targeting the autophagy–lysosome system to combat the inflammation-compromised osteogenic potential of periodontal ligament stem cells: From mechanism to therapy. Biomaterials 2022, 288, 121743. [Google Scholar] [CrossRef]
- Song, W.; Soo Lee, S.; Savini, M.; Popp, L.; Colvin, V.L.; Segatori, L. Ceria Nanoparticles Stabilized by Organic Surface Coatings Activate the Lysosome-Autophagy System and Enhance Autophagic Clearance. ACS Nano 2014, 8, 10328–10342. [Google Scholar] [CrossRef]
- Kang, I.; Yoo, J.M.; Kim, D.; Kim, J.; Cho, M.K.; Lee, S.-E.; Kim, D.J.; Lee, B.-C.; Lee, J.Y.; Kim, J.-J.; et al. Graphene Quantum Dots Alleviate Impaired Functions in Niemann-Pick Disease Type C in Vivo. Nano Lett. 2021, 21, 2339–2346. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Jin, P.; Huang, Y.; Dai, Q.; Zhu, Q.; Wei, P.; Yang, Z.; Zhang, L.; Liu, H.; et al. Graphene oxide improves postoperative cognitive dysfunction by maximally alleviating amyloid beta burden in mice. Theranostics 2020, 10, 11908–11920. [Google Scholar] [CrossRef]
- Pei, X.; Liu, D.; Li, J.; Li, L.; Ding, X.; Zhang, W.; Li, Z.; Xu, G.; Li, C.; Li, D. TFEB coordinates autophagy and pyroptosis as hepatotoxicity responses to ZnO nanoparticles. Sci. Total Environ. 2023, 865, 161242. [Google Scholar] [CrossRef]
- Della Porta, A.; Bornstein, K.; Coye, A.; Montrief, T.; Long, B.; Parris, M.A. Acute chloroquine and hydroxychloroquine toxicity: A review for emergency clinicians. Am. J. Emerg. Med. 2020, 38, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Jordaan, P.; Dumotier, B.; Traebert, M.; Miller, P.E.; Ghetti, A.; Urban, L.; Abi-Gerges, N. Cardiotoxic Potential of Hydroxychloroquine, Chloroquine and Azithromycin in Adult Human Primary Cardiomyocytes. Toxicol. Sci. 2021, 180, 356–368. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Fan, X.; Huang, X.; Li, Z.; Jing, Z.; Zhang, G.; Xu, Y.; Zhang, N.; Wei, P. Recovery of Lysosomal Acidification and Autophagy Flux by Attapulgite Nanorods: Therapeutic Potential for Lysosomal Disorders. Biomolecules 2025, 15, 728. https://doi.org/10.3390/biom15050728
Hao Y, Fan X, Huang X, Li Z, Jing Z, Zhang G, Xu Y, Zhang N, Wei P. Recovery of Lysosomal Acidification and Autophagy Flux by Attapulgite Nanorods: Therapeutic Potential for Lysosomal Disorders. Biomolecules. 2025; 15(5):728. https://doi.org/10.3390/biom15050728
Chicago/Turabian StyleHao, Yuanjing, Xinru Fan, Xiaodan Huang, Zhaoying Li, Zhiyuan Jing, Guilong Zhang, Yuxue Xu, Na Zhang, and Pengfei Wei. 2025. "Recovery of Lysosomal Acidification and Autophagy Flux by Attapulgite Nanorods: Therapeutic Potential for Lysosomal Disorders" Biomolecules 15, no. 5: 728. https://doi.org/10.3390/biom15050728
APA StyleHao, Y., Fan, X., Huang, X., Li, Z., Jing, Z., Zhang, G., Xu, Y., Zhang, N., & Wei, P. (2025). Recovery of Lysosomal Acidification and Autophagy Flux by Attapulgite Nanorods: Therapeutic Potential for Lysosomal Disorders. Biomolecules, 15(5), 728. https://doi.org/10.3390/biom15050728




