Recent Advances in Multiple Strategies for the Biosynthesis of Sesquiterpenols
Abstract
1. Introduction
2. Sesquiterpenols and Their Bioactivities
2.1. Acyclic Sesquiterpenols
2.2. Monocyclic Sesquiterpenols
2.3. Bicyclic Sesquiterpenols
2.4. Polycyclic Sesquiterpenols
3. Sesquiterpenol Biosynthesis
3.1. Biosynthetic Pathway
3.2. Sesquiterpenol Synthases
4. Common Strategies for Optimizing Sesquiterpenol Biosynthesis
4.1. Enzyme Engineering
4.1.1. Identification and Functional Verification of Key Enzymes
4.1.2. Post-Modification of Enzymes
4.1.3. Enzyme Colocalization
4.1.4. Improving Enzymes Catalytic Activity
4.2. The Strategies for the Regulation of the Synthesis Pathway
4.2.1. The Engineering of Host Metabolism
4.2.2. The Enhancement of the Central Carbon Flux
4.3. Subcellular Organelle Engineering
4.4. Toxicity Tolerance of Terpenoids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, S.; Sun, J.; Zhang, C.; Li, D.; Lu, W. Yarrowia lipolytica construction for heterologous synthesis of α-santalene and fermentation optimization. Appl. Microbiol. Biotechnol. 2019, 103, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.P.; Figueiredo, C.P.; Calixto, J.B. Themed Section: Mediators and Receptors in the Resolution of Inflammation Research Paper. Br. J. Pharmacol. 2009, 158, 933–1172. [Google Scholar]
- Chan, W.-K.; Tan, L.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.; Wang, L.; Ma, Y.; Fan, T.-P.; Deng, H.; Cai, Y. Constructing High-Yielding Serratia marcescens for (−)-α-Bisabolol Production Based on the Exogenous Haloarchaeal MVA Pathway and Endogenous Molecular Chaperones. J. Agric. Food Chem. 2024, 73, 747–755. [Google Scholar] [CrossRef]
- Lei, M.; Qiu, Z.; Guan, L.; Xiang, Z.; Zhao, G.-R. Metabolic Engineering for Efficient Production of Z,Z-Farnesol in E. coli. Microorganisms 2023, 11, 1583. [Google Scholar] [CrossRef]
- Luo, Y.; Li, B.-Z.; Liu, D.; Zhang, L.; Chen, Y.; Jia, B.; Zeng, B.-X.; Zhao, H.; Yuan, Y.-J. Engineered biosynthesis of natural products in heterologous hosts. Chem. Soc. Rev. 2015, 44, 5265–5290. [Google Scholar] [CrossRef] [PubMed]
- Chemler, J.A.; Koffas, M.A. Metabolic engineering for plant natural product biosynthesis in microbes. Curr. Opin. Biotechnol. 2008, 19, 597–605. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, G.; Sun, Z. From chemical synthesis to biosynthesis: Trends toward total synthesis of natural products. Synth. Biol. J. 2021, 2, 674–696. [Google Scholar]
- Lima, I.S.D.; Ferreira, M.O.G.; Barros, E.M.L.; Rizzo, M.D.S.; Santos, J.D.A.; Ribeiro, A.B.; Anteveli Osajima Furtini, J.; C. Silva-Filho, E.; Estevinho, L.M. Antibacterial and Healing Effect of Chicha Gum Hydrogel (Sterculia striata) with Nerolidol. Int. J. Mol. Sci. 2023, 24, 2210. [Google Scholar] [CrossRef]
- Sousa, G.; Alves, M.I.; Neves, M.; Tecelão, C.; Ferreira-Dias, S. Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods 2022, 11, 439. [Google Scholar] [CrossRef]
- De Moura, D.F.; Rocha, T.A.; De Melo Barros, D.; Da Silva, M.M.; Dos Santos Santana, M.; Neta, B.M.; Cavalcanti, I.M.F.; Martins, R.D.; Da Silva, M.V. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol. Arch. Microbiol. 2021, 203, 4303–4311. [Google Scholar] [CrossRef]
- Arunachalam, S.; Nagoor Meeran, M.F.; Azimullah, S.; Sharma, C.; Goyal, S.N.; Ojha, S. Nerolidol Attenuates Oxidative Stress, Inflammation, and Apoptosis by Modulating Nrf2/MAPK Signaling Pathways in Doxorubicin-Induced Acute Cardiotoxicity in Rats. Antioxidants 2021, 10, 984. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Oliveira, G.; De Carvalho, R.; De Sousa, D.; Freitas, R.; Pinto, P.; Moraes, J. Antischistosomal Activity of the Terpene Nerolidol. Molecules 2014, 19, 3793–3803. [Google Scholar] [CrossRef]
- Kadhum, W.R.; See, G.L.; Alhijjaj, M.; Kadhim, M.M.; Arce, F., Jr.; Al-Janabi, A.S.; Al-Rashidi, R.R.; Khadom, A.A. Evaluation of the Skin Permeation-Enhancing Abilities of Newly Developed Water-Soluble Self-Assembled Liquid Crystal Formulations Based on Hexosomes. Crystals 2022, 12, 1238. [Google Scholar] [CrossRef]
- Fonsêca, D.V.; Salgado, P.R.R.; De Carvalho, F.L.; Salvadori, M.G.S.S.; Penha, A.R.S.; Leite, F.C.; Borges, C.J.S.; Piuvezam, M.R.; Pordeus, L.C.D.M.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABA ergic system and proinflammatory cytokines. Fundamemntal Clin. Pharma 2016, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Glumac, M.; Čikeš Čulić, V.; Marinović-Terzić, I.; Radan, M. Mechanism of cis-Nerolidol-Induced Bladder Carcinoma Cell Death. Cancers 2023, 15, 981. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef]
- Polke, M.; Jacobsen, I.D. Quorum sensing by farnesol revisited. Curr. Genet. 2017, 63, 791–797. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on elemol. Food Chem. Toxicol. 2008, 46, S147–S148. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.S.; Ferreira, O.O.; Lobato, L.G.N.; De Santana Botelho, A.; Mali, S.N.; Kumar, R.; De Jesus Pereira Franco, C.; Rosa, U.A.; Correia, Z.A.; Da Silva, M.P.; et al. Exploring phytochemistry, antioxidant capacity, and biological potential of essential oils obtained from Euphorbiaceae species. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Andersson, M.; Bergendorff, O.; Shan, R.; Zygmunt, P.; Sterner, O. Minor Components with Smooth Muscle Relaxing Properties from Scented Myrrh (Commiphora guidotti). Planta Med. 1997, 63, 251–254. [Google Scholar] [CrossRef]
- Claeson, P.; Zygmunt, P.; Högestätt, E.D. Calcium Antagonistic Properties of the Sesquiterpene T-Cadinol: A Comparison with Nimodipine in the Isolated Rat Aorta. Pharmacol. Toxicol. 1991, 69, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Claeson, P.; Andersson, R.; Samuelsson, G. T-cadinol: A pharmacologically active constituent of scented myrrh: Introductory pharmacological characterization and high field 1H- and 13C-NMR data. Planta Medica 1991, 57, 352–356. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, S.; Fu, X.; Lai, C.; Guo, D. De novo biosynthesis of τ-cadinol in engineered Escherichia coli. Bioresour. Bioprocess. 2022, 9, 29. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Su, Z.-R.; Yang, W.; Zhong, M.; Xian, Y.-F.; Lin, Z.-X. Patchouli alcohol attenuates the cognitive deficits in a transgenic mouse model of Alzheimer’s disease via modulating neuropathology and gut microbiota through suppressing C/EBPβ/AEP pathway. J. Neuroinflamm. 2023, 20, 19. [Google Scholar] [CrossRef]
- Bommareddy, A.; Rule, B.; VanWert, A.L.; Santha, S.; Dwivedi, C. α-Santalol, a derivative of sandalwood oil, induces apoptosis in human prostate cancer cells by causing caspase-3 activation. Phytomedicine 2012, 19, 804–811. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Benkhaira, N.; Mssillou, I.; Touhtouh, J.; Aanniz, T.; Chamkhi, I.; El Omari, N.; Khalid, A.; Abdalla, A.N.; Aboulagras, S.; et al. Natural sources and pharmacological properties of santalenes and santalols. Ind. Crops Prod. 2024, 214, 118567. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of α-santalol and sandalwood oil. Phytomedicine 2013, 20, 409–416. [Google Scholar] [CrossRef]
- Paulpandi, M.; Kannan, S.; Thangam, R.; Kaveri, K.; Gunasekaran, P.; Rejeeth, C. In vitro anti-viral effect of β-santalol against influenza viral replication. Phytomedicine 2012, 19, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Asgharzade, S.; Ahmadzadeh, A.M.; Pourbagher-Shahri, A.M.; Forouzanfar, F. Protective effects of cedrol against transient global cerebral ischemia/reperfusion injury in rat. BMC Complement. Med. Ther. 2025, 25, 83. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Pourbagher-Shahri, A.M.; Ghazavi, H. Evaluation of Antiarthritic and Antinociceptive Effects of Cedrol in a Rat Model of Arthritis. Oxidative Med. Cell. Longev. 2022, 2022, 4943965. [Google Scholar] [CrossRef]
- Özek, G.; Schepetkin, I.A.; Yermagambetova, M.; Özek, T.; Kirpotina, L.N.; Almerekova, S.S.; Abugalieva, S.I.; Khlebnikov, A.I.; Quinn, M.T. Innate Immunomodulatory Activity of Cedrol, a Component of Essential Oils Isolated from Juniperus Species. Molecules 2021, 26, 7644. [Google Scholar] [CrossRef]
- Nie Tianqing; Meng Xiangwei; Ying Yuchen; Zhang Xingxian Research progress on anti-tumor activity of curcumol and its derivatives. Chin. Tradit. Herbal. Drugs 2020, 51, 5613–5621.
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Jiménez, I.A.; Bazzocchi, I.L.; Valladares, B.; Akkari, H.; Lorenzo-Morales, J.; Piñero, J.E. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 2018, 117, 2855–2867. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Pharmacological and biological effects of alpha-bisabolol: An updated review of the molecular mechanisms. Life Sci. 2022, 304, 120728. [Google Scholar] [CrossRef]
- Han, G.H. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell Factories 2016, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Bai, J.; Yang, W.; Liu, Y.; Sun, B. Identification of the Key Odorants in Fresh Amomum tsaoko Fruit. Food Sci. 2020, 41, 173–178. [Google Scholar]
- Cai, Z.-M.; Peng, J.-Q.; Chen, Y.; Tao, L.; Zhang, Y.-Y.; Fu, L.-Y.; Long, Q.-D.; Shen, X.-C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- Khalil, M.; Khizar, M.; Alshaya, D.S.; Hameed, A.; Muhammad, N.; Binyameen, M.; Azeem, M.; Hussain, M.; Abbas, Q.; Attia, K.A.; et al. Insecticidal and Repellent Activity of Essential Oils from Seven Different Plant Species against Tribolium castaneum (Coleoptera: Tenebrionidae). Insects 2024, 15, 755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Q.; Shen, J.; Li, Y.; Zhang, H.; Zhang, X.; Yang, S.; Jiang, Z.; Wang, M.; Li, J.; et al. Metabolic engineering of glycolysis in Escherichia coli for efficient production of patchoulol and τ-cadinol. Bioresour. Technol. 2024, 391, 130004. [Google Scholar] [CrossRef]
- Dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol—A Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)—Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, M.; Li, Z.-H.; Tao, X.; Wei, D.-Z.; Wang, F.-Q. Significantly Enhanced Production of Patchoulol in Metabolically Engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2019, 67, 8590–8598. [Google Scholar] [CrossRef]
- Verešová, A.; Terentjeva, M.; Ban, Z.; Li, L.; Vukic, M.; Vukovic, N.; Kluz, M.I.; Ben Sad, R.; Ben Hsouna, A.; Bianchi, A.; et al. Enhancing Antimicrobial Efficacy of Sandalwood Essential Oil Against Salmonella enterica for Food Preservation. Foods 2024, 13, 3919. [Google Scholar] [CrossRef]
- Luo, F.; Ling, Y.; Li, D.S.; Tang, T.; Liu, Y.-C.; Liu, Y.; Li, S.-H. Characterization of a sesquiterpene cyclase from the glandular trichomes of Leucosceptrum canum for sole production of cedrol in Escherichia coli and Nicotiana benthamiana. Phytochemistry 2019, 162, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.L.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Production of curcuminoids from tyrosine by a metabolically engineered Escherichia coli using caffeic acid as an intermediate. Biotechnol. J. 2015, 10, 599–609. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, K. Production of Terpenoids by Synthetic Biology Approaches. Front. Bioeng. Biotechnol. 2020, 8, 347. [Google Scholar] [CrossRef]
- Navale, G.R.; Dharne, M.S.; Shinde, S.S. Metabolic engineering and synthetic biology for isoprenoid production in Escherichia coli and Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 457–475. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Behrendorff, J.; Douw, A.; Vickers, C.E. The methylerythritol phosphate pathway as an oxidative stress sense and response system. Nat. Commun. 2024, 15, 5303. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Lindley, N.D. Metabolic Engineering Strategies for Sustainable Terpenoid Flavor and Fragrance Synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.-L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Vattekkatte, A.; Garms, S.; Brandt, W.; Boland, W. Enhanced structural diversity in terpenoid biosynthesis: Enzymes, substrates and cofactors. Org. Biomol. Chem. 2018, 16, 348–362. [Google Scholar] [CrossRef]
- Segura, M.J.R.; Jackson, B.E.; Matsuda, S.P.T. Mutagenesis approaches to deduce structure–function relationships in terpene synthases. Nat. Prod. Rep. 2003, 20, 304–317. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Zhang, W.; Liu, W. Metabolic engineering of Saccharomyces cerevisiae to improve farnesol production. Acta Microbiol. Sin. 2021, 61, 1257–1268. [Google Scholar]
- Cheah, L.C.; Liu, L.; Stark, T.; Plan, M.R.; Peng, B.; Lu, Z.; Schenk, G.; Sainsbury, F.; Vickers, C.E. Metabolic flux enhancement from the translational fusion of terpene synthases is linked to terpene synthase accumulation. Metab. Eng. 2023, 77, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, H.; Li, Y.; Yao, H.; Luo, H. The new advance of terpene synthase research in the plant. Plant Physiol. J. 2017, 53, 1139–1149. [Google Scholar]
- Aguilar, F.; Ekramzadeh, K.; Scheper, T.; Beutel, S. Whole-Cell Production of Patchouli Oil Sesquiterpenes in Escherichia coli: Metabolic Engineering and Fermentation Optimization in Solid–Liquid Phase Partitioning Cultivation. ACS Omega 2020, 5, 32436–32446. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X.; Li, F.; Zuo, S.; Li, M.; Zhao, J.; Han, X.; Wen, M. Optimized biosynthesis of santalenes and santalols in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 8795–8804. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Møller, K.; Nielsen, K.F.; Schalk, M.; Clark, A.; Nielsen, J. Production of plant sesquiterpenes in Saccharomyces cerevisiae: Effect of ERG9 repression on sesquiterpene biosynthesis. Biotech. Bioeng. 2008, 99, 666–677. [Google Scholar] [CrossRef]
- Ohto, C.; Muramatsu, M.; Obata, S.; Sakuradani, E.; Shimizu, S. Overexpression of the gene encoding HMG-CoA reductase in Saccharomyces cerevisiae for production of prenyl alcohols. Appl. Microbiol. Biotechnol. 2009, 82, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yoon, S.; Shah, A.A.; Chung, Y.; Kim, J.; Choi, E.; Keasling, J.D.; Kim, S. Farnesol production from Escherichia coli by harnessing the exogenous mevalonate pathway. Biotechnol. Bioeng. 2010, 107, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Peng, B.; Ebert, B.E.; Dumsday, G.; Vickers, C.E. Auxin-mediated protein depletion for metabolic engineering in terpene-producing yeast. Nat. Commun. 2021, 12, 1051. [Google Scholar] [CrossRef]
- Peng, B.; Bandari, N.C.; Lu, Z.; Howard, C.B.; Scott, C.; Trau, M.; Dumsday, G.; Vickers, C.E. Engineering eukaryote-like regulatory circuits to expand artificial control mechanisms for metabolic engineering in Saccharomyces cerevisiae. Commun. Biol. 2022, 5, 135. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.; Zhang, Y.; Liang, D.; Caiyin, Q.; Qiao, J. Characterization of trans-Nerolidol Synthase from Celastrus angulatus Maxim and Production of trans-Nerolidol in Engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 2236–2244. [Google Scholar] [CrossRef]
- Liu, F.; Liu, S.C.; Qi, Y.K.; Liu, Z.; Chen, J.; Wei, L.J.; Hua, Q. Enhancing Trans.-Nerolidol Productivity in Yarrowia lipolytica by Improving Precursor Supply and Optimizing Nerolidol Synthase Activity. J. Agric. Food Chem. 2022, 70, 15157–15165. [Google Scholar] [CrossRef]
- Bai, R.; Xie, J.; Zhang, Y.; Sun, L.; Zhang, Z.; Wang, L.; Hu, J. Combination with isopentenyl diphosphate isomerase gene affects expression of two linalool/nerolidol synthases isoforms from Lingzhi. Gene 2025, 951, 149394. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Plan, M.R.; Chrysanthopoulos, P.; Hodson, M.P.; Nielsen, L.K.; Vickers, C.E. A squalene synthase protein degradation method for improved sesquiterpene production in Saccharomyces cerevisiae. Metab. Eng. 2017, 39, 209–219. [Google Scholar] [CrossRef]
- Gao, L.; Hou, R.; Cai, P.; Yao, L.; Wu, X.; Li, Y.; Zhang, L.; Zhou, Y.J. Engineering Yeast Peroxisomes for α-Bisabolene Production from Sole Methanol with the Aid of Proteomic Analysis. JACS Au 2024, 4, 2474–2483. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Mai, J.; Wang, J.; Wei, Y.; Ledesma-Amaro, R.; Ji, X.-J. Engineering Yarrowia lipolytica for sustainable production of the chamomile sesquiterpene (−)-α-bisabolol. Green. Chem. 2021, 23, 780–787. [Google Scholar] [CrossRef]
- Li, Y.W.; Yang, C.L.; Shen, Q.; Peng, Q.-Q.; Guo, Q.; Nie, Z.-K.; Sun, X.-M.; Shi, T.-Q.; Ji, X.-J.; Huang, H. YALIcloneNHEJ: An Efficient Modular Cloning Toolkit for NHEJ Integration of Multigene Pathway and Terpenoid Production in Yarrowia lipolytica. Front. Bioeng. Biotechnol. 2022, 9, 816980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Y.; Wang, Y.; Lu, Z.; Miao, L.; Wang, S.; Li, Z.; Sun, X.; Han, Y.; He, S.; et al. Biosynthesis of α-bisabolene from low-cost renewable feedstocks by peroxisome engineering and systems metabolic engineering of the yeast Yarrowia lipolytica. Green. Chem. 2023, 25, 8145–8159. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, L.; Gao, S.; Li, N.; Yu, S.; Zhou, J. Engineering Saccharomyces cerevisiae for enhanced (–)-α-bisabolol production. Synth. Syst. Biotechnol. 2023, 8, 187–195. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Han, L.; Wang, Q.; Liu, H.; Cheng, P.; Li, R.; Guo, X.; Zhou, Z. Enhancement of Patchoulol Production in Escherichia coli via Multiple Engineering Strategies. J. Agric. Food Chem. 2021, 69, 7572–7580. [Google Scholar] [CrossRef]
- Mitsui, R.; Nishikawa, R.; Yamada, R.; Matsumoto, T.; Ogino, H. Construction of yeast producing patchoulol by global metabolic engineering strategy. Biotech. Bioeng. 2020, 117, 1348–1356. [Google Scholar] [CrossRef]
- Tao, Q.; Du, G.; Chen, J.; Zhang, J.; Peng, Z. Metabolic Engineering for Efficient Synthesis of Patchoulol in Saccharomyces cerevisiae. Fermentation 2024, 10, 211. [Google Scholar] [CrossRef]
- Liu, M.; Lin, Y.C.; Guo, J.J.; Du, M.M.; Tao, X.; Gao, B.; Zhao, M.; Ma, Y.; Wang, F.Q.; Wei, D.Z. High-Level Production of Sesquiterpene Patchoulol in Saccharomyces cerevisiae. ACS Synth. Biol. 2021, 10, 158–172. [Google Scholar] [CrossRef]
- Tao, X.Y.; Lin, Y.C.; Wang, F.Q.; Liu, Q.H.; Ma, Y.S.; Liu, M.; Wei, D.Z. Production of sesquiterpene patchoulol in mitochondrion-engineered Saccharomyces cerevisiae. Biotechnol. Lett. 2022, 44, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Lin, Y.; Ruan, S.; Xiao, R.; Zhang, X.; Liang, S. Dual cytoplasmic-mitochondrial engineering for improved patchoulol production in Komagataella phaffii. Biochem. Eng. J. 2024, 206, 109286. [Google Scholar] [CrossRef]
- Luo, G.; Lin, Y.; Chen, S.; Xiao, R.; Zhang, J.; Li, C.; Sinskey, A.J.; Ye, L.; Liang, S. Overproduction of Patchoulol in Metabolically Engineered Komagataella phaffii. J. Agric. Food Chem. 2023, 71, 2049–2058. [Google Scholar] [CrossRef]
- Peng, Q.-Q.; Guo, Q.; Chen, C.; Song, P.; Wang, Y.-T.; Ji, X.-J.; Ye, C.; Shi, T.-Q. High-Level Production of Patchoulol in Yarrowia lipolytica via Systematic Engineering Strategies. J. Agric. Food Chem. 2023, 71, 4638–4645. [Google Scholar] [CrossRef]
- Zha, W.; An, T.; Li, T.; Zhu, J.; Gao, K.; Sun, Z.; Xu, W.; Lin, P.; Zi, J. Reconstruction of the Biosynthetic Pathway of Santalols under Control of the GAL Regulatory System in Yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef]
- Green, S.A.; Chen, X.; Nieuwenhuizen, N.J.; Matich, A.J.; Wang, M.Y.; Bunn, B.J.; Yauk, Y.-K.; Atkinson, R.G. Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J. Exp. Bot. 2012, 63, 1951–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lei, P. The Functional Identification of Two Alternative Splicing Transcripts of CsNES. J. Tea Sci. 2021, 41, 753–760. [Google Scholar]
- Booth, J.K.; Yuen, M.M.S.; Jancsik, S.; Madilao, L.L.; Page, J.E.; Bohlmann, J. Terpene Synthases and Terpene Variation in Cannabis sativa. Plant Physiol. 2020, 184, 130–147. [Google Scholar] [CrossRef]
- Guimarães, P.M.; Brasileiro, A.C.; Morgante, C.V.; Martins, A.C.; Pappas, G.; Silva, O.B.; Togawa, R.; Leal-Bertioli, S.C.; Araujo, A.C.; Moretzsohn, M.C.; et al. Global transcriptome analysis of two wild relatives of peanut under drought and fungi infection. BMC Genom. 2012, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Wang, H.; Yao, P.; Sun, J.; Guo, C.; Jin, Y.; Yang, L.; Chen, Y.; Shi, F.; Yu, L.; et al. Biosynthesis of α-Bisabolol by Farnesyl Diphosphate Synthase and α-Bisabolol Synthase and Their Related Transcription Factors in Matricaria recutita L. Int. J. Mol. Sci. 2023, 24, 1730. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Kim, S.U.; Ro, D.K. Molecular cloning and characterization of (+)-epi-α-bisabolol synthase, catalyzing the first step in the biosynthesis of the natural sweetener, hernandulcin, in Lippia dulcis. Arch. Biochem. Biophys. 2012, 527, 37–44. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Wang, L.; Zhang, Y.; Hua, W.; Li, D.; Lv, H.; Zhang, X. Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genom. 2011, 12, 451. [Google Scholar] [CrossRef]
- Ro, D.K.; Ehlting, J.; Keeling, C.I.; Lin, R.; Mattheus, N.; Bohlmann, J. Microarray expression profiling and functional characterization of AtTPS genes: Duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g13300 encode root-specific and wound-inducible (Z)-γ-bisabolene synthases. Arch. Biochem. Biophys. 2006, 448, 104–116. [Google Scholar] [CrossRef]
- Crouch, J.A.; Dawe, A.; Aerts, A.; Barry, K.; Churchill, A.C.L.; Grimwood, J.; Hillman, B.I.; Milgroom, M.G.; Pangilinan, J.; Smith, M.; et al. Genome Sequence of the Chestnut Blight Fungus Cryphonectria parasitica EP155: A Fundamental Resource for an Archetypical Invasive Plant Pathogen. Phytopathology® 2020, 110, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.; Rao, S.; Bomzan, D.P.; Kumar, S.R.; Shanmugam, P.V.; Olsson, S.B.; Nagegowda, D.A. Functional characterization of a defense-responsive bulnesol/elemol synthase from potato. Physiol. Plant. 2021, 171, 7–21. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, M.; Li, M.; Zhao, J.; Han, X. [Progress in biosynthesis of santalene and santalol]. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2018, 34, 862–875. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Wang, Y.; Sun, H.; Wang, S.; Wang, D.; Duan, Y.; Niu, J.; Wang, Z. Integrative analysis of transcriptome and metabolome reveals the sesquiterpenoids and polyacetylenes biosynthesis regulation in Atractylodes lancea (Thunb.) DC. Int. J. Biol. Macromol. 2023, 253, 127044. [Google Scholar] [CrossRef]
- Ren, F.; Mao, H.; Liang, J.; Liu, J.; Shu, K.; Wang, Q. Functional characterization of ZmTPS7 reveals a maize τ-cadinol synthase involved in stress response. Planta 2016, 244, 1065–1074. [Google Scholar] [CrossRef]
- Jullien, F.; Moja, S.; Bony, A.; Legrand, S.; Petit, C.; Benabdelkader, T.; Poirot, K.; Fiorucci, S.; Guitton, Y.; Nicolè, F.; et al. Isolation and functional characterization of a τ-cadinol synthase, a new sesquiterpene synthase from Lavandula angustifolia. Plant Mol. Biol. 2014, 84, 227–241. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, L.; Wu, M.; Xia, G.; Lin, P.; Zi, J. Characterization of a Sesquiterpene Synthase Catalyzing Formation of Cedrol and Two Diastereoisomers of Tricho-Acorenol from Euphorbia fischeriana. J. Nat. Prod. 2021, 84, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.S.P.; Vikström, D.; Houben, D.; Van Den Berg Van Saparoea, H.B.; De Gier, J.-W.; Luirink, J. Application of an E. coli signal sequence as a versatile inclusion body tag. Microb. Cell Fact. 2017, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Song, J.M.; Seo, S.H.; Wang, C.; Lee, S.; Lee, H.; Kim, S.; Choi, E. Ty1-fused protein-body formation for spatial organization of metabolic pathways in Saccharomyces cerevisiae. Biotech. Bioeng. 2018, 115, 694–704. [Google Scholar] [CrossRef]
- Nguyen, T.K.M.; Ki, M.R.; Son, R.G.; Pack, S.P. The NT11, a novel fusion tag for enhancing protein expression in Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 2205–2216. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G. [Progress in co-immobilization of multiple enzymes]. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2015, 31, 469–480. [Google Scholar]
- Wang, X.; Pereira, J.H.; Tsutakawa, S.; Fang, X.; Adams, P.D.; Mukhopadhyay, A.; Lee, T.S. Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450. Metab. Eng. 2021, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wang Chen; Zhao Meng; Ding Mingzhu; Wang Ying; Yao Mingdong; Xiao Wenhai Application of biological scaffold system on synthetic biology. Chem. Ind. Eng. Prog. 2020, 39, 4557–4567.
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlén, M.; Nielsen, J.; Hudson, E.P. Affibody Scaffolds Improve Sesquiterpene Production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.L.; Johns, S.T.; Walters, R.; Miller, D.J.; Van Der Kamp, M.W.; Allemann, R.K. Active Site Loop Engineering Abolishes Water Capture in Hydroxylating Sesquiterpene Synthases. ACS Catal. 2023, 13, 14199–14204. [Google Scholar] [CrossRef]
- Cheng, J.; Pu, Z.; Chen, J.; Chen, D.; Li, B.; Wen, Z.; Jin, Y.; Yao, Y.; Shao, K.; Gu, X.; et al. Development of a green Komagataella phaffii cell factory for sustainable production of plant-derived sesquiterpene (–)-α-bisabolol. Synth. Syst. Biotechnol. 2025, 10, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J. Systems biology of lipid metabolism: From yeast to human. FEBS Lett. 2009, 583, 3905–3913. [Google Scholar] [CrossRef]
- Donald, K.A.; Hampton, R.Y.; Fritz, I.B. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1997, 63, 3341–3344. [Google Scholar] [CrossRef]
- Yamada, R.; Wakita, K.; Ogino, H. Global Metabolic Engineering of Glycolytic Pathway via Multicopy Integration in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 659–666. [Google Scholar] [CrossRef]
- Muramatsu, M.; Ohto, C.; Obata, S.; Sakuradani, E.; Shimizu, S. Accumulation of prenyl alcohols by terpenoid biosynthesis inhibitors in various microorganisms. Appl. Microbiol. Biotechnol. 2008, 80, 589. [Google Scholar] [CrossRef]
- Chatzivasileiou, A.O.; Ward, V.; Edgar, S.M.; Stephanopoulos, G. Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zu, Y.; Huang, S.; Stephanopoulos, G. Engineering a universal and efficient platform for terpenoid synthesis in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2207680120. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Qian, S.; Tan, Z.; Cheong, S.; Gonzalez, R. The isoprenoid alcohol pathway, a synthetic route for isoprenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 12810–12815. [Google Scholar] [CrossRef]
- Ma, Y.R.; Wang, K.F.; Wang, W.J.; Ding, Y.; Shi, T.Q.; Huang, H.; Ji, X.J. Advances in the metabolic engineering of Yarrowia lipolytica for the production of terpenoids. Bioresour. Technol. 2019, 281, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Scalcinati, G.; Knuf, C.; Partow, S.; Chen, Y.; Maury, J.; Schalk, M.; Daviet, L.; Nielsen, J.; Siewers, V. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab. Eng. 2012, 14, 91–103. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Machine learning applications in systems metabolic engineering. Curr. Opin. Biotechnol. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Sha, Y.; Ge, M.; Lu, M.; Xu, Z.; Zhai, R.; Jin, M. Advances in metabolic engineering for enhanced acetyl-CoA availability in yeast. Crit. Rev. Biotechnol. 2024, 1–19. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Jian, X.X.; Lv, Y.B.; Nian, K.Q.; Gao, Q.; Chen, J.; Wei, L.-J.; Hua, Q. Enhanced squalene biosynthesis in Yarrowia lipolytica based on metabolically engineered acetyl-CoA metabolism. J. Biotechnol. 2018, 281, 106–114. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Yang, Y.; Wang, S.; Wang, Q.; Wang, X.; Yan, Z.; Cheng, J.; Liu, C.; Yang, X.; et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design. Nat. Commun. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Shen, X.; Wang, J.; Li, C.; Yuan, Q.; Yan, Y. Dynamic gene expression engineering as a tool in pathway engineering. Current Opin. Biotechnol. 2019, 59, 122–129. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, S.; Bi, H.; Wang, K.; Fang, Y.; Wang, M.; Tan, T. Development of a specific biosensor for sesquiterpene based on SELEX and directed evolution platforms. Talanta 2025, 283, 127186. [Google Scholar] [CrossRef] [PubMed]
- Subash Chandra Bose, K.; Shah, M.I.; Krishna, J.; Sankaranarayanan, M. Genome-scale metabolic model analysis of Pichia pastoris for enhancing the production of S-adenosyl-l-methionine. Bioprocess. Biosyst. Eng. 2023, 46, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yu, W.; Chen, Y.; Yang, S.; Zhao, Z.K.; Nielsen, J.; Luan, H.; Zhou, Y.J. Engineering yeast for high-level production of diterpenoid sclareol. Metab. Eng. 2023, 75, 19–28. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Kory, N. Principles and functions of metabolic compartmentalization. Nat. Metab. 2022, 4, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Ayer, A.; Sanwald, J.; Pillay, B.A.; Meyer, A.J.; Perrone, G.G.; Dawes, I.W. Distinct Redox Regulation in Sub-Cellular Compartments in Response to Various Stress Conditions in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e65240. [Google Scholar] [CrossRef]
- Hirose, T.; Ninomiya, K.; Nakagawa, S.; Yamazaki, T. A guide to membraneless organelles and their various roles in gene regulation. Nat. Rev. Mol. Cell Biol. 2023, 24, 288–304. [Google Scholar] [CrossRef]
- Xu, K.; Qin, L.; Bai, W.; Wang, X.; Li, F.; Ren, S.; Gao, X.; Chen, B.; Tong, Y.; Li, J.; et al. Multilevel Defense System (MDS) Relieves Multiple Stresses for Economically Boosting Ethanol Production of Industrial Saccharomyces cerevisiae. ACS Energy Lett. 2020, 5, 572–582. [Google Scholar] [CrossRef]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Andrade, N.J.; Ismail, F.M.D.; Barbosa, M.C.A. Exposure to Anacardiaceae Volatile Oils and Their Constituents Induces Lipid Peroxidation within Food-Borne Bacteria Cells. Molecules 2012, 17, 9728–9740. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Yu, K.; Zang, Y.; Qu, Z.; Wei, C.; Yuan, W. Expression of the human antiapoptotic protein Bcl-2 increases nerolidol production in engineered yeast. Process Biochem. 2022, 119, 90–95. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, X.; Huang, J.; Qi, F. [Reinforcement of Rhodobacter sphaeroides cofactor NADPH to increase the production of farnesol]. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2020, 36, 90–99. [Google Scholar] [CrossRef]
- Wang, J.F.; Xiong, Z.Q.; Li, S.Y.; Wang, Y. Enhancing isoprenoid production through systematically assembling and modulating efflux pumps in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 8057–8067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytologist 2020, 226, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Sattayawat, P.; Yunus, I.S.; Jones, P.R. Bioderivatization as a concept for renewable production of chemicals that are toxic or poorly soluble in the liquid phase. Proc. Natl. Acad. Sci. USA 2020, 117, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
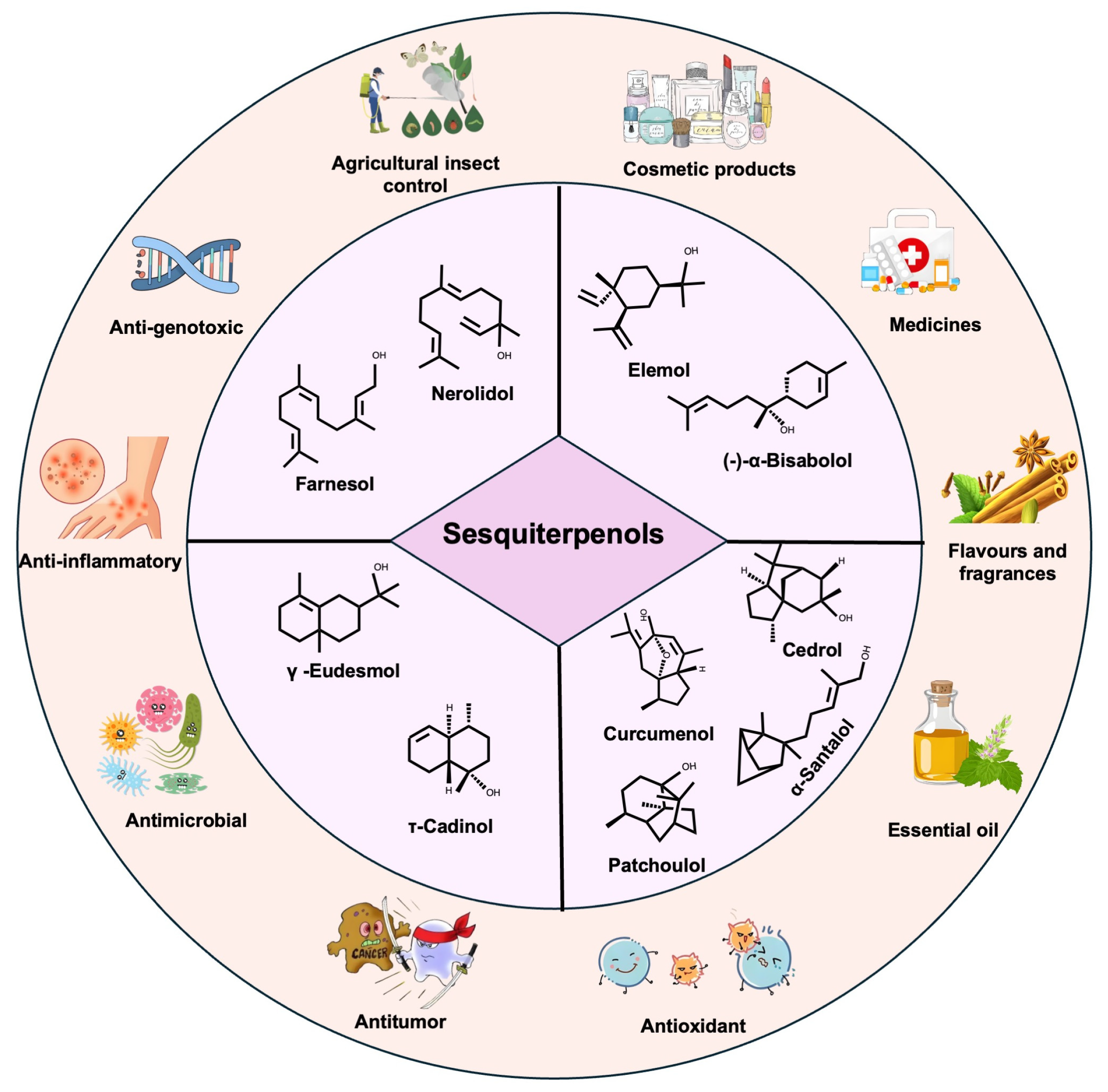
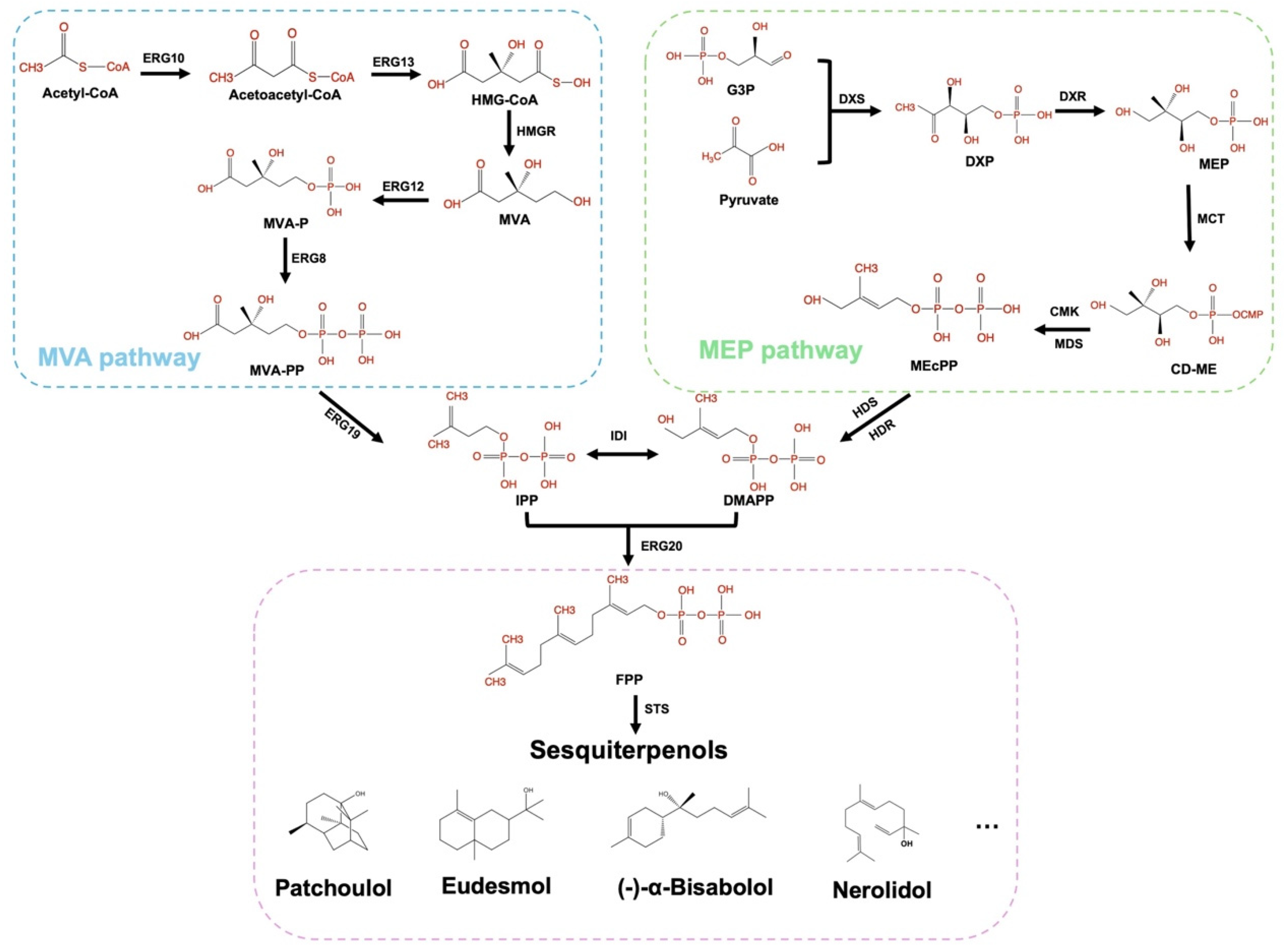

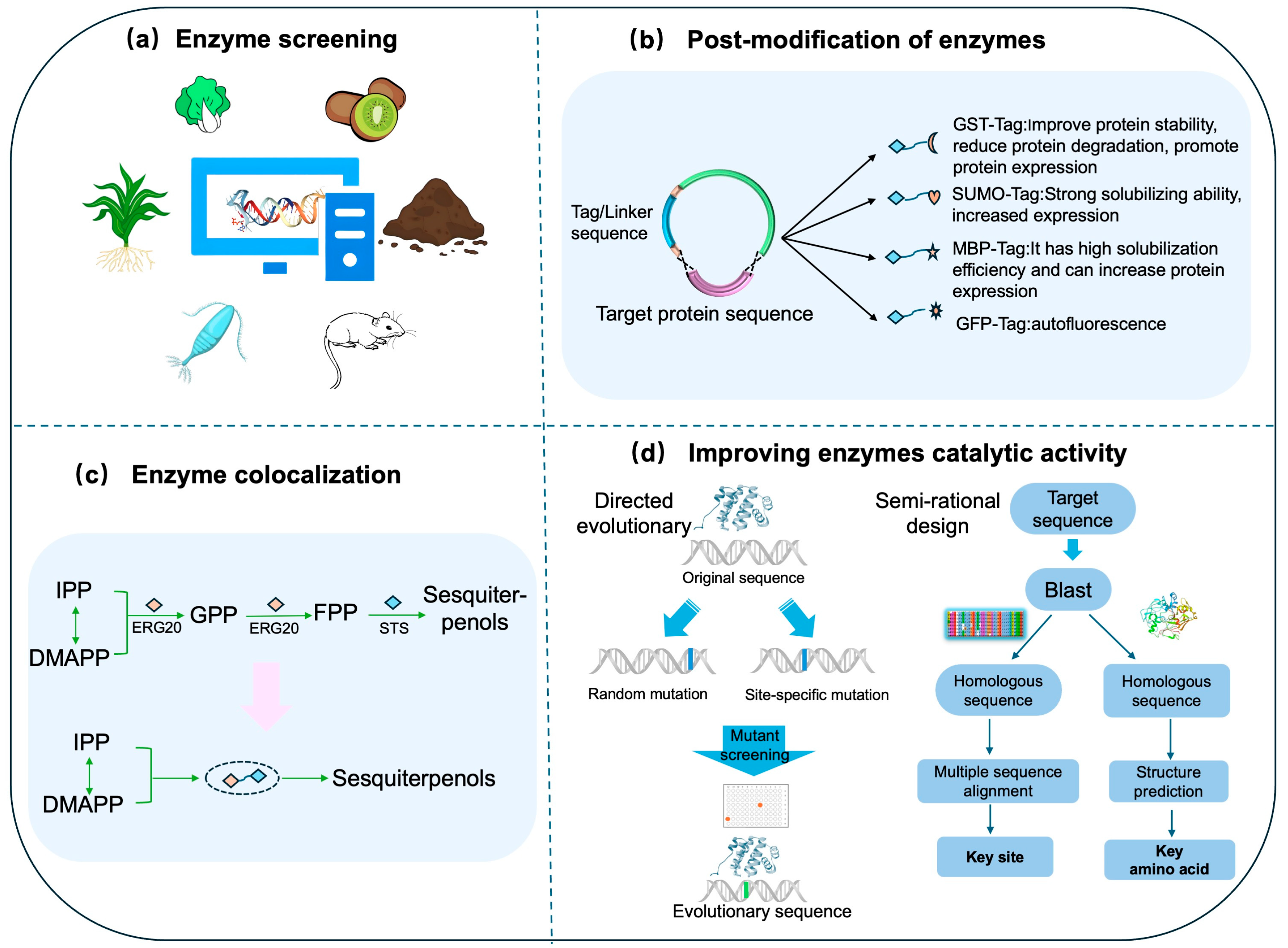

| Classification | Sesquiterpenol | Functions and Applications | References |
|---|---|---|---|
| acyclic | nerolidol | antimicrobial, anti-biofilm, antioxidant, antiparasitic, transdermal permeation-enhancing, antinociceptive, and anticancer properties | [11,12,13,14,15,16,17] |
| farnesol | essential oils, intercellular signaling, quorum sensing modulation, apoptosis regulation | [19] | |
| monocyclic | (-)-α-bisabolol | ulcer healing, gallstone dissolution, analgesia, anti-inflammatory, bacteriostasis, anti-genotoxic, melanin inhibition | [9,20] |
| elemol | fragrance formulation, personal care products, laundry detergents, household commodities | [21] | |
| bicyclic | eudesmol | fragrance fixative, antimicrobial, insecticidal properties | [22] |
| τ-cadinol | pest-repellent, nutritional substrate, membrane stabilizer, energy metabolism modulator, smooth muscle relaxation, antidiarrheal effects, calcium channel antagonism, phyto germination regulation, acaricidal action, insect repellency | [23,24,25,26] | |
| polycyclic | patchoulol | woody aroma, cosmetics and household products, anti-anginal activity, arrhythmia suppression, antihypertensive effects | [27] |
| α-santalol | antitumor, antimicrobial, antibacterial prophylaxis, virucidal activity, antioxidant properties | [28,29,30,31] | |
| cedrol | oriental fragrance formulations | [32,33,34] | |
| curcumol | anticancer, antimicrobial, antifungal, antiviral, anti-inflammatory | [35] |
| Classification | Sesquiterpenol | Host | STRATEGIES | Scales | Titers (mg/L) | Reference |
|---|---|---|---|---|---|---|
| Acyclic | Z,Z- farnesol | Escherichia coli | tested five Z, Z-farnesyl diphosphate (Z, Z-FPP) synthases; screened thirteen phosphatases; site-directed mutagenesis of cis-prenyltransferase | flask | 572.13 | [6] |
| farnesol | Saccharomyces cerevisiae | overexpressed tHMGR1 and ERG20; replacement of the natural ERG9 promoter with the hxt1 promoter; overexpressed of endogenous phosphatase PAH1 | flask | 393.13 | [55] | |
| farnesol | Saccharomyces cerevisiae | replaced the natural ERG9 promoter with the tunable MET3 promoter | flask | 20.2 | [60] | |
| farnesol | Saccharomyces cerevisiae | overexpressed tHMGR1 | bioreactor | 145.7 | [61] | |
| farnesol | Escherichia coli | overexpressed ispA; introduced the MVA pathway | flask | 135.5 | [62] | |
| nerolidol | Saccharomyces cerevisiae | fused of nerolidol synthase to farnesyl diphosphate synthase | flask | 4200 | [56] | |
| nerolidol | Saccharomyces cerevisiae | depleted hexokinase-2 to lifts glucose repression; depleted acetyl-CoA carboxylase (Acc1p) | flask | 3500 | [63] | |
| nerolidol | Saccharomyces cerevisiae | engineered a eukaryote-like tetracycline-mediated circuit to minimize metabolic burden during strain development and maintenance | flask | 2540 | [64] | |
| trans- nerolidol | Saccharomyces cerevisiae | overexpressed the mevalonate pathway; knockout of GAL80 gene; replaced the natural ERG9 promoter with the tunable HXT1 promoter | bioreactor | 7010 | [65] | |
| nerolidol | Yarrowia lipolytica | used homology modeling and docking studies to design the FaNES1G498Q mutant of nerolidol synthase; enhanced the expression of an endogenous carnitine acetyltransferase (CAT2); introduced the nerolidol biosynthesis pathway into the peroxisomal | bioreactor | 11,100 | [66] | |
| nerolidol | Escherichia coli | overexpressed nerolidol synthase; fusion nerolidol synthase and ispA | flask | 0.016 | [67] | |
| nerolidol | Saccharomyces cerevisiae | overexpressed the MVA pathway synthase; engineered CLN2PEST sequence-dependent degradation of ER-associated protein with the Erg9p signal peptide | flask | 150 | [68] | |
| Monocyclic | α-bisabolol | Pichia pastoris | optimized the mevalonate pathway; peroxisomal compartmentalization; overexpressed the limiting enzyme EfmvaE | flask | 1100 | [69] |
| α-bisabolol | Yarrowia lipolytica | overexpressed tHMG1 and ERG20; truncated the native promoter of the squalene synthase gene SQS1; increased the copy number of the bisabolenol synthase gene MrBBS;enhanced the β-oxidation pathway | flask | 364.23 | [70] | |
| α-bisabolol | Yarrowia lipolytica | used YALI clone NHEJ system to overexpress the mevalonate pathway; | bioreactor | 4400 | [71] | |
| α-bisabolol | Yarrowia lipolytica | introduced the α-bisabolene biosynthesis pathway into the peroxisomal; efflux pump-mediated product export; optimized the gene copy number of rate-limiting enzymes; balanced the distribution of the common precursor acetyl-CoA between natural lipids | bioreactor | 15,500 | [72] | |
| α-bisabolol | Saccharomyces cerevisiae | used the low-transcription-level HXT1 promoter to replace the initial promoter of ERG9; fusion expression of ERG20 and MrBBS; knockout of DPP1; overexpressed ERG20 and ADH2; overexpressed global activator of MVA pathway Upc2G888A; knockout of the ROX1 and Ypl062w | bioreactor | 7020 | [73] | |
| α-bisabolol | Serratia marcescens | introduce heterology MVA pathway; knockout of PhoA; co-expression DnaK/J | bioreactor | 30,200 | [5] | |
| Bicyclic | τ-cadinol | Escherichia coli | overexpressed of IDI; two-phase fermentation | flask | 133.5 | [26] |
| τ-cadinol | Escherichia coli | knock out the cAMP synthesis pathway; constructed a glycolysis-control device mediated by pyruvate sensing; dynamic regulated the glycolysis | bioreactor | 15,200 | [26] | |
| Polycyclic | patchoulol | Escherichia coli | overexpressed the exogenous mevalonate pathway; overexpressed a patchoulol synthase; optimized the pH and temperature of culture broth | bioreactor | 40.2 | [58] |
| patchoulol | Escherichia coli | semi-rational mutant Patchoulol synthase (PTS); fused of PTS with FPP synthase; deleted the competitive routes for acetate, lactate, ethanol, and succinate synthesis; enhanced the expression of efflux transporters | bioreactor | 970.1 | [74] | |
| patchoulol | Escherichia coli | knocked out the cAMP synthesis pathway to alleviate glucose repression; constructed a glycolysis-control device mediated by pyruvate sensing; dynamic regulated the glycolysis | bioreactor | 1675.1 | [42] | |
| patchoulol | Saccharomyces cerevisiae | optimized the expression of eight genes in the MVA pathway: integrated multiple copies of the patchoulol synthase gene (LibPTS) | flask | 42.1 | [75] | |
| patchoulol | Saccharomyces cerevisiae | overexpressed the mevalonate pathway; integrated multiple copies of the patchoulol synthase gene; knocked out the farnesol synthase genes DPP1 and LPP1; replaced the natural ERG9 promoter with the tunable ERG1 promoter; enhanced the supply of acetyl-CoA by overexpressing ADH2 and knocking out the transcription factors YPL062W, YNR063W, and YJL064W | flask | 195.96 | [76] | |
| patchoulol | Saccharomyces cerevisiae | overexpressed tHMGR1; fusion of FPP synthase ERG20 and PTS; replaced the natural ERG9 promoter with the tunable HXT1 promoter; enhanced the supply of acetyl-CoA by knocking out the transcription factors YPL062W, YNR063W, and YJL064W; overexpressed the cytoplasmic catalase CTT1 and the peroxisomal catalase CTA1 to reduce the levels of reactive oxygen species (ROS);constructing the PTS mutant PTST404S | flask | 141.5 | [77] | |
| patchoulol | Saccharomyces cerevisiae | fusion of farnesyl pyrophosphate (FPP) synthase and patchoulol synthase to increase the utilization of FPP precursors; enhanced the expression of rate-limiting genes in the mevalonate pathway; squalene synthase was weakened by the glucose-inducible promoter of HXT1 to reduce the metabolic flux from FPP to ergosterol; inhibited the biosynthesis of farnesol, thereby reducing the consumption of FPP | flask | 466.8 | [44] | |
| patchoulol | Saccharomyces cerevisiae | fusion of farnesyl pyrophosphate (FPP) synthase and patchoulol synthase; introducing the patchoulol biosynthesis pathway into the mitochondria of Saccharomyces cerevisiae | flask | 19.24 | [78] | |
| patchoulol | Pichia pastoris | dual cytoplasmic–mitochondrial engineering; introducing the patchoulol biosynthesis pathway into the mitochondria | flask | 109 | [79] | |
| patchoulol | Pichia pastoris | fusion of farnesyl pyrophosphate (FPP) synthase and patchoulol synthase; increased precursor supply; added auxiliary carbon source | flask | 149.64 | [80] | |
| patchoulol | Yarrowia lipolytica | downregulated squalene synthesis based on Cu2+-repressible promoter; expanded the mevalonate precursor pool; fusion of farnesyl pyrophosphate (FPP) synthase and patchoulol synthase | flask | 235 | [81] | |
| santalol | Saccharomyces cerevisiae | downregulated ERG9 gene; truncated the N-terminal 46 amino acid of ATR1 | flask | 68.8 | [59] | |
| santalol | Saccharomyces cerevisiae | replaced the natural ERG9 promoter with the tunable HXT1 promoter; overexpressed santalol synthesis pathway; overexpressed GAL4 (a transcriptional activator of GAL promotors) and PGM2 (a yeast phosphoglucomutase) | bioreactor | 1300 | [82] |
| Crucial Genes | Sources | Involved in Biosynthesis of Which Sesquiterpenols | Validation Strategies | Reference |
|---|---|---|---|---|
| farnesyl diphosphate synthase; α-bisabolol synthase | Matricaria recutita L. | α-bisabolol | purification from tissue; in vitro biochemical assays; transcriptomics; | [87] |
| α-bisabolol synthase | Lippia dulcis | α-bisabolol | transcriptomics; biochemical assays; heterologous expression in Saccharomyces cerevisiae | [88] |
| nerolidol synthase | Actinidia chinensis | nerolidol | purification from tissue; transcriptomics; in vitro biochemical assays; | [83] |
| nerolidol synthase | Celastrus angulatus | nerolidol | purification from tissue; transcriptomics; in vitro biochemical assays; heterologous expression in Saccharomyces cerevisiae | [65] |
| nerolidol synthase | Camellia sinensis | nerolidol | transcriptomics; purification from tissue; | [84] |
| α-bisabolol synthase | Sesamum indicum L. | α-bisabolol | transcriptomics; | [89] |
| α-bisabolol synthase | Arabidopsis thaliana | α-bisabolol | genomics; | [90] |
| nerolidol synthase | Cannabis sativa | nerolidol | genomics; | [85] |
| nerolidol synthase | peanut | nerolidol | global transcriptome analysis | [86] |
| farnesol synthase | Cryphonectria parasitica | farnesol | genomics; | [91] |
| elemol synthase | potato | elemol | purification from tissue; transcriptomics; | [92] |
| cytochrome P450 monooxygenase | Santalum album | α-santalol | transcriptomics; | [93] |
| eudesmol synthase | Atractylodes lancea | eudesmol | transcriptomics; genomics | [94] |
| τ-cadinol synthase | Zea mays | τ-cadinol | purification from tissue; in vitro biochemical assays; | [95] |
| τ-cadinol synthase | Lavandula angustifolia | τ-cadinol | transcriptomics | [96] |
| cedrol synthase | Euphorbia fischeriana | cedrol | transcriptomics; in vitro biochemical assays; | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Chen, R.; Qiao, J.; Li, W.; Zhu, H. Recent Advances in Multiple Strategies for the Biosynthesis of Sesquiterpenols. Biomolecules 2025, 15, 664. https://doi.org/10.3390/biom15050664
Li M, Chen R, Qiao J, Li W, Zhu H. Recent Advances in Multiple Strategies for the Biosynthesis of Sesquiterpenols. Biomolecules. 2025; 15(5):664. https://doi.org/10.3390/biom15050664
Chicago/Turabian StyleLi, Mengyuan, Ruiqi Chen, Jianjun Qiao, Weiguo Li, and Hongji Zhu. 2025. "Recent Advances in Multiple Strategies for the Biosynthesis of Sesquiterpenols" Biomolecules 15, no. 5: 664. https://doi.org/10.3390/biom15050664
APA StyleLi, M., Chen, R., Qiao, J., Li, W., & Zhu, H. (2025). Recent Advances in Multiple Strategies for the Biosynthesis of Sesquiterpenols. Biomolecules, 15(5), 664. https://doi.org/10.3390/biom15050664






