The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research
Abstract
1. Introduction
Literature Search
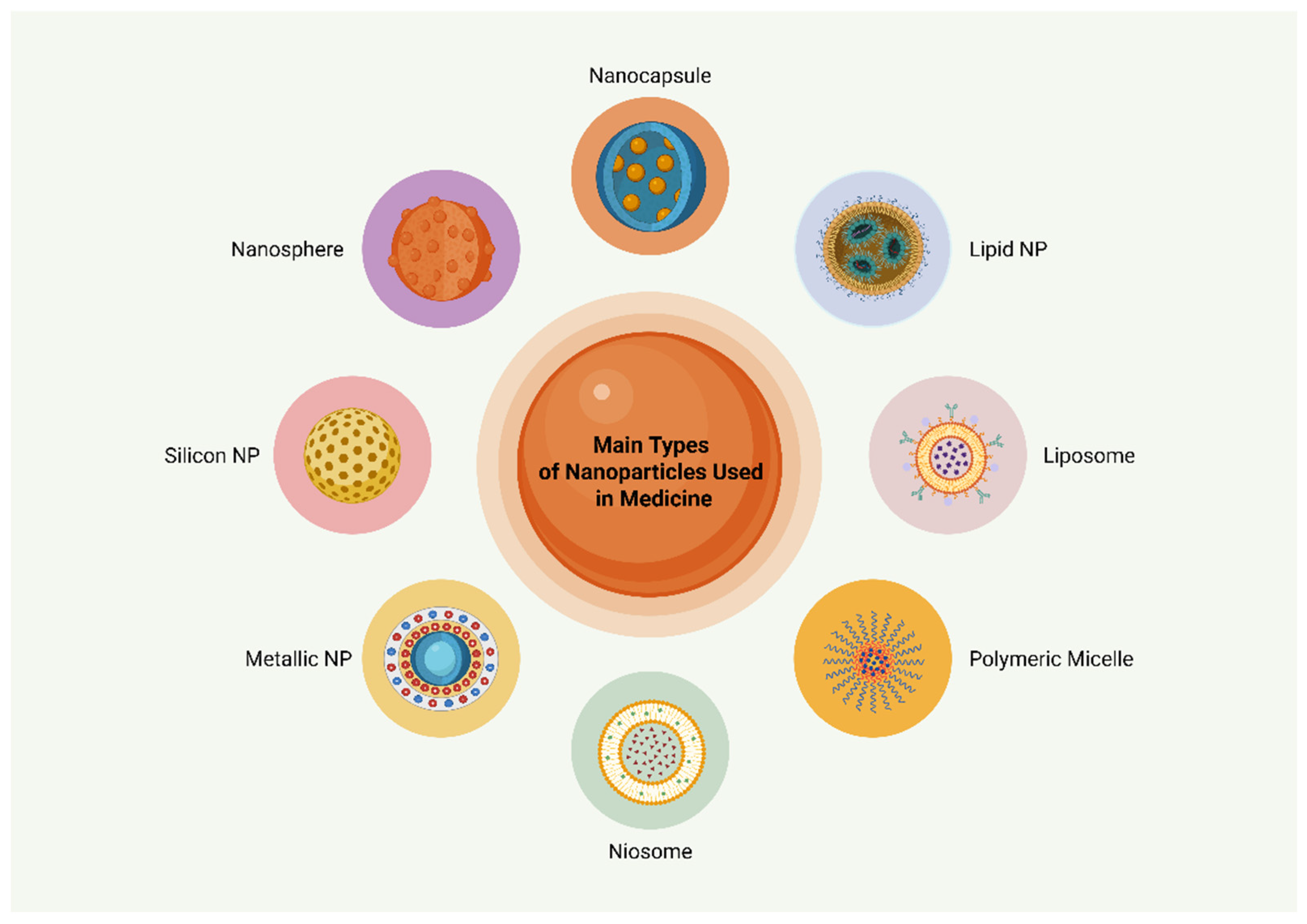
2. Nanotechnology in Medicine
3. Antidiabetic Therapies and Nanotechnology
3.1. Insulin Therapy
3.2. Other Antidiabetic Agents
4. Phytomedicines with Antidiabetic Properties and Nanotechnology
5. Gene Therapy and Nanomedicine in Diabetes Mellitus
6. Nanomedicine for Transplantation of Pancreatic Cells for Diabetes Mellitus Management
7. Nanotechnology and Diabetes Treatment: Local Applications for Diabetic Wound Healing
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO Facts. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 February 2025).
- WHO Facts. Urgent Action is Needed As Global Diabetes Cases Increase Four-Fold over Past Decades. Available online: https://www.who.int/news/item/13-11-2024-urgent-action-needed-as-global-diabetes-cases-increase-four-fold-over-past-decades (accessed on 2 February 2025).
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef]
- Manral, K.; Singh, A.; Singh, Y. Nanotechnology as a potential treatment for diabetes and its complications: A review. Diabetes Metab. Syndr. 2024, 18, 103159. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthet. Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-Physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S.S. Materials for Oral Delivery of Proteins and Peptides. Nat. Rev. Mater. 2019, 5, 127–148. [Google Scholar] [CrossRef]
- Andreadi, A.; Lodeserto, P.; Todaro, F.; Meloni, M.; Romano, M.; Minasi, A.; Bellia, A.; Lauro, D. Nanomedicine in the Treatment of Diabetes. Int. J. Mol. Sci. 2024, 25, 7028. [Google Scholar] [CrossRef]
- Lee, Y.; Kamada, N.; Moon, J.J. Oral Nanomedicine for Modulating Immunity, Intestinal Barrier Functions, and Gut Microbiome. Adv. Drug Deliv. Rev. 2021, 179, 114021. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Turasan, H. Latest developments in the applications of microfluidization to modify the structure of macromolecules leading to improved physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 2021, 62, 4481–4503. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Guo, Y.; Lara Ochoa, S.; Fathordoobady, F.; Singh, A. Optimal ultrasonication process time remains constant for a specific nanoemulsion size reduction system. Sci. Rep. 2021, 11, 9241. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Lemmerman, L.R.; Das, D.; Higuita-Castro, N.; Mirmira, R.G.; Gallego-Perez, D. Nanomedicine-based strategies for diabetes: Diagnostics, monitoring, and treatment. Trends Endocrinol. Metab. 2020, 31, 448–458. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control Release 2022, 345, 512–536. [Google Scholar] [CrossRef]
- Yadav, K. Nanotechnology in Diabetes Management: Revolutionizing Treatment and Diagnostics. J. Mol. Liq. 2024, 414, 126117. [Google Scholar] [CrossRef]
- Purohit, D.; Jalwal, P.; Manchanda, D.; Saini, S.; Verma, R.; Kaushik, D.; Mittal, V.; Kumar, M.; Bhattacharya, T.; Rahman, H.; et al. Nanocapsules: An Emerging Drug Delivery System. Recent Pat. Nanotechnol. 2023, 17, 190–207. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Sarkhel, S.; Shuvo, S.M.; Ansari, M.A.; Mondal, S.; Kapat, P.; Ghosh, A.; Sarkar, T.; Biswas, R.; Atanase, L.I.; Carauleanu, A. Nanotechnology-Based Approaches for the Management of Diabetes Mellitus: An Innovative Solution to Long-Lasting Challenges in Antidiabetic Drug Delivery. Pharmaceutics 2024, 16, 1572. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef]
- Pang, H.; Huang, X.; Xu, Z.P.; Chen, C.; Han, F.Y. Progress in Oral Insulin Delivery by PLGA Nanoparticles for the Management of Diabetes. Drug Discov. Today 2023, 28, 103393. [Google Scholar] [CrossRef]
- Bahman, F.; Greish, K.; Taurin, S. Nanotechnology in Insulin Delivery for Management of Diabetes. Pharm. Nanotechnol. 2019, 7, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Mu, T. Materials and Structure of Polysaccharide-Based Delivery Carriers for Oral Insulin: A Review. Carbohydr. Polym. 2024, 323, 121364. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Lockwood, G.P.; Heffernan, S.J.; Daymond, J.; Ngu, M.; Narayanan, R.K.; Westwood, L.J.; Mohanty, B.; Esser, L.; Williams, C.C.; et al. Oral Nanotherapeutic Formulation of Insulin with Reduced Episodes of Hypoglycaemia. Nat. Nanotechnol. 2024, 19, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Barfar, A.; Alizadeh, H.; Masoomzadeh, S.; Javadzadeh, Y. Oral Insulin Delivery: A Review on Recent Advancements and Novel Strategies. Curr. Drug Deliv. 2024, 21, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Sumaila, M.; Marimuthu, T.; Kumar, P.; Choonara, Y.E. Lipopolysaccharide Nanosystems for the Enhancement of Oral Bioavailability. AAPS PharmSciTech 2021, 22, 242. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Xue, M.; Wang, M.; Guo, R.; Wang, B.; Zhang, H. Net-Neutral Nanoparticles-Extruded Microcapsules for Oral Delivery of Insulin. ACS Appl. Mater. Interfaces 2023, 15, 33491–33503. [Google Scholar] [CrossRef]
- Chellathurai, M.S.; Yong, C.L.; Sofian, Z.M.; Sahudin, S.; Hasim, N.B.M.; Mahmood, S. Self-assembled chitosan-insulin oral nanoparticles—A critical perspective review. Int. J. Biol. Macromol. 2023, 243, 125125. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Guo, Y.; Baldelli, A.; Singh, A. Mercaptonicotinic acid activated thiolated chitosan (MNA-TG-chitosan) to enable peptide oral delivery by opening cell tight junctions and enhancing transepithelial transport. Sci. Rep. 2023, 13, 17343. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Ren, S.; Pan, J.; Wang, Y.; Shen, Y.; Zeng, Z.; Cui, H.; Zhao, X. Versatile Oral Insulin Delivery Nanosystems: From Materials to Nanostructures. Int. J. Mol. Sci. 2022, 23, 3362. [Google Scholar] [CrossRef]
- Anastasiou, I.A.; Kounatidis, D.; Vallianou, N.G.; Skourtis, A.; Dimitriou, K.; Tzivaki, I.; Tsioulos, G.; Rigatou, A.; Karampela, I.; Dalamaga, M. Beneath the Surface: The Emerging Role of Ultra-Processed Foods in Obesity-Related Cancer. Curr. Oncol. Rep. 2025, 27, 390–414. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and Chitosan—A review. J. Control Release. 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mohanraj, V.; Parkin, J. Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Int. J. Pept. Res. Ther. 2003, 10, 621Y629. [Google Scholar] [CrossRef]
- Shen, S.; Tang, H.; Zhang, X.; Ren, Z.; Wang, D.; Gao, H.; Qian, Y.; Jiang, X.; Yang, W. Targeting mesoporous silica-encapsulated gold nanorods for chemo–465 photothermal therapy with near-infrared radiation. Biomaterials 2013, 4, 3158. [Google Scholar] [CrossRef]
- Fathy, M.M.; Hassan, A.A.; Elsayed, A.A.; Fahmy, H.M. Controlled release of silica-coated insulin-loaded chitosan nanoparticles as a promising oral administration system. BMC Pharmacol. Toxicol. 2023, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, A.B.; Silvestre, A.L.P.; Sposito, L.; de Souza, M.P.C.; Sábio, R.M.; Araújo, V.H.S.; Cury, B.S.F.; Chorilli, M. The role of polysaccharides from natural resources to design oral insulin micro- and nanoparticles intended for the treatment of Diabetes mellitus: A review. Carbohydr. Polym. 2021, 256, 117504. [Google Scholar] [CrossRef]
- Kurniawati, D.; Kurniati, N.F.; Ratnaningsih, E.; Hertadi, R. Study on the development of nanoparticles based on levan for oral insulin delivery. Biomed. Mater. 2025, 20, 025028. [Google Scholar] [CrossRef] [PubMed]
- Seyam, S.; Choukaife, H.; Al Rahal, O.; Alfatama, M. Colonic targeting insulin-loaded trimethyl chitosan nanoparticles coated pectin for oral delivery: In vitro and In vivo studies. Int. J. Biol. Macromol. 2024, 281 Pt 4, 136549. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, Y.; Wu, L.; Zhu, X.; Zhang, Z.; Huang, Y. Novel solid lipid nanoparticle with endosomal escape function for oral delivery of insulin. ACS Appl. Mater. Interfaces 2018, 10, 9315–9324. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid lipid nanoparticles (SLNs): An advanced drug delivery system targeting the brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, M.; Ni, X.; Wang, J.; Rong, H.; Su, Y.; Yu, S.; Mohammad, I.S.; Leung, S.S.Y.; Hu, H. Virus-mimicking mesoporous silica nanoparticles with an electrically neutral and hydrophilic surface to improve the oral absorption of insulin by breaking through dual barriers of the mucus layer and the intestinal epithelium. ACS Appl. Mater. Interfaces 2021, 13, 18077–18088. [Google Scholar] [CrossRef]
- Rao, R.; Liu, X.; Li, Y.; Tan, X.; Zhou, H.; Bai, X.; Yang, X.; Liu, W. Bioinspired zwitterionic polyphosphoester modified porous silicon nanoparticles for efficient oral insulin delivery. Biomater. Sci. 2021, 9, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Natesan, V.; Kim, S.J. The trend of organic based nanoparticles in the treatment of diabetes and its perspectives. Biomol. Ther. 2023, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Alyoussef Alkrad, J.; Neubert, R.H.H. Dermal and transdermal peptide delivery using enhancer molecules and colloidal carrier systems. Part V: Transdermal administration of insulin. Int. J. Pharm. 2022, 616, 121511. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Ibrahima, M.M. BÜCHI nano spray dryer B-90: A promising technology for the production of metformin hydrochloride-loaded alginate-gelatin nanoparticles. Drug Dev. Ind. Pharm. 2019, 45, 1907–1914. [Google Scholar] [CrossRef]
- Cesur, S.; Cam, M.E.; Sayın, F.S.; Su, S.; Harker, A.; Edirisinghe, M.; Gunduz, O. Metformin-Loaded Polymer-Based Microbubbles/Nanoparticles Generated for the Treatment of Type 2 Diabetes Mellitus. Langmuir 2022, 38, 5040–5051. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Nnamani, D.O.; Duhu, J.C.; Nmesirionye, B.U.; Momoh, M.A.; Akpa, P.A.; Attama, A.A. Potential enhancement of metformin hydrochloride in solidified reverse micellar solution-based PEGylated lipid nanoparticles targeting therapeutic efficacy in diabetes treatment. Heliyon 2022, 8, e09099. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Mohamed, N.A.; Macasa, S.S.; Basha, H.K.; Adan, A.M.; Crovella, S.; Ding, H.; Triggle, C.R.; Marei, I.; Abou-Saleh, H. Metformin-loaded nanoparticles reduce hyperglycemia-associated oxidative stress and induce eNOS phosphorylation in vascular endothelial cells. Sci. Rep. 2024, 14, 30870. [Google Scholar] [CrossRef]
- Sun, S.; Hou, X.; Li, K.; Huang, C.; Rong, Y.; Bi, J.; Li, X.; Wu, D. Curcumin and Metformin Infinite Coordination Polymer Nanoparticles for Combined Therapy of Diabetic Mice via Intraperitoneal Injections. J. Funct. Biomater. 2024, 15, 388. [Google Scholar] [CrossRef]
- Ilyas, U.; Asif, M.; Wang, M.; Altaf, R.; Zafar, H.; Faran Ashraf Baig, M.M.; Paiva-Santos, A.C.; Abbas, M. Nanostructured Lipid Carrier-Based Delivery of Pioglitazone for Treatment of Type 2 Diabetes. Front. Pharmacol. 2022, 13, 934156. [Google Scholar] [CrossRef]
- Lupascu, F.G.; Sava, A.; Tătărușanu, S.M.; Iacob, A.T.; Dascălu, A.; Profire, B.Ș.; Vasincu, I.M.; Apotrosoaei, M.; Gîscă, T.C.; Turin-Moleavin, I.A.; et al. New Chitosan-Based Co-Delivery Nanosystem for Diabetes Mellitus Therapy. Polymers 2024, 16, 1825. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Rebelos, E.; Kouveletsou, M.; Kontrafouri, P.; Eleftheriadou, I.; Diakoumopoulou, E.; Karampela, I.; Tentolouris, N.; Dalamaga, M. The Many Facets of PPAR-γ Agonism in Obesity and Associated Comorbidities: Benefits, Risks, Challenges, and Future Directions. Curr. Obes. Rep. 2025, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Demirturk, E.; Ugur Kaplan, A.B.; Cetin, M.; Dönmez Kutlu, M.; Köse, S.; Akıllıoğlu, K. Preparation of nanoparticle and nanoemulsion formulations containing repaglinide and determination of pharmacokinetic parameters in rats. Eur. J. Pharm. Sci. 2024, 200, 106844. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Antoniadis, A.P.; Fragakis, N. Diabetes-Driven Atherosclerosis: Updated Mechanistic Insights and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 2196. [Google Scholar] [CrossRef]
- You, L.; Wang, Q.; Ma, Y.; Li, Y.; Ye, H.; Xu, L.; Lei, M. Precise dapagliflozin delivery by cardiac homing peptide functionalized mesoporous silica nanocarriers for heart failure repair after myocardial infarction. Front. Chem. 2022, 10, 1013910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, C.K.; Wu, T.T.; Ho, C.Y.; Yeh, T.C.; Sun, G.C.; Tseng, C.J.; Cheng, P.W. Attenuation of epithelial-mesenchymal transition via SGLT2 inhibition and diabetic cataract suppression by dapagliflozin nanoparticles treatment. Life Sci. 2023, 330, 122005. [Google Scholar] [CrossRef]
- Al-Tantawy, S.M.; Eraky, S.M.; Eissa, L.A. Promising renoprotective effect of gold nanoparticles and dapagliflozin in diabetic nephropathy via targeting miR-192 and miR-21. J. Biochem. Mol. Toxicol. 2023, 37, e23430. [Google Scholar] [CrossRef]
- Kweon, S.; Park, S.J.; Lee, H.K.; Kang, S.H.; Chang, K.Y.; Choi, J.U.; Park, J.; Shim, J.H.; Park, J.W.; Byun, Y. Coordinated ASBT and EGFR Mechanisms for Optimized Liraglutide Nanoformulation Absorption in the GI Tract. Int. J. Nanomed. 2024, 19, 2973–2992. [Google Scholar] [CrossRef]
- Subedi, L.; Bamjan, A.D.; Phuyal, S.; Shim, J.H.; Cho, S.S.; Seo, J.B.; Chang, K.Y.; Byun, Y.; Kweon, S.; Park, J.W. An oral liraglutide nanomicelle formulation conferring reduced insulin-resistance and long-term hypoglycemic and lipid metabolic benefits. J. Control Release 2025, 378, 637–655. [Google Scholar] [CrossRef]
- Li, Y.; Tian, H.; Zeng, H.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. Chitosan based surface modulation of core-shell nanoparticles for oral delivery of exenatide via balancing mucus penetration and cellular uptake. Int. J. Pharm. 2025, 672, 125319. [Google Scholar] [CrossRef]
- Pinto, S.; Hosseini, M.; Buckley, S.T.; Yin, W.; Garousi, J.; Gräslund, T.; van Ijzendoorn, S.; Santos, H.A.; Sarmento, B. Nanoparticles targeting the intestinal Fc receptor enhance intestinal cellular trafficking of semaglutide. J. Control Release 2024, 366, 621–636. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S. Development of Phytochemical Delivery Systems by NanoSuspension and Nano-Emulsion Techniques. Int. J. Mol. Sci. 2023, 24, 9824. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to NanoPharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A Review on Chemical and Physical Modifications of Phytosterols and Their Influence on Bioavailability and Safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Al-Joufi, F.; Anwar, M.; Attia, M.F.; El-Bana, M.A. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf. B. Biointerfaces 2019, 177, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Momenkiaei, F.; Raofie, F. Preparation of Curcuma longa L. Extract Nanoparticles Using Supercritical Solution Expansion. J. Pharm. Sci. 2019, 108, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; ArabSadeghabadi, Z.; Ziamajidi, N.; Abbasalipourkabir, R.; RezaeiFarimani, A. Oral administration of resveratrol-loaded solid lipid nanoparticle improves insulin resistance through targeting expression of SNARE proteins in adipose and muscle tissue in rats with type 2 diabetes. Nanoscale Res. Lett. 2019, 14, 227. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycaemic effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef]
- Kazazis, C.E.; Evangelopoulos, A.A.; Kollas, A.; Vallianou, N.G. The therapeutic potential of milk thistle in diabetes. Rev. Diabet. Stud. 2014, 11, 167–174. [Google Scholar] [CrossRef]
- Singh, M.K.; Pooja, D.; Ravuri, H.G.; Gunukula, A.; Kulhari, H.; Sistla, R. Fabrication of Surfactant-Stabilized Nanosuspension of Naringenin to Surpass Its Poor Physicochemical Properties and Low Oral Bioavailability. Phytomedicine 2018, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zucca, G.; Vigani, B.; Valentino, C.; Ruggeri, M.; Marchesi, N.; Pascale, A.; Giovilli, G.; Malavasi, L.; Sandri, G.; Rossi, S. Chondroitin Sulphate-Chitosan Based Nanogels Loaded with Naringenin-β-Cyclodextrin Complex as Potential Tool for the Treatment of Diabetic Retinopathy: A Formulation Study. Int. J. Nanomed. 2025, 20, 907–932. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, T.; Zeng, H.; Lin, L.; Xie, H.; Lin, R.; Huang, M. Naringenin Inhibits Ferroptosis in Renal Tubular Epithelial Cells of Diabetic Nephropathy Through SIRT1/FOXO3a Signaling Pathway. Drug Dev. Res. 2025, 86, e70044. [Google Scholar] [CrossRef]
- Basaldúa-Maciel, V.; Guzmán-Flores, J.M.; Reyes-Chaparro, A.; Martínez-Esquivias, F. Therapeutic Potential of Quercetin in Type 2 Diabetes Based on a Network Pharmacology Study. Curr. Top. Med. Chem. 2025. [Google Scholar] [CrossRef]
- Singh, S.; Kushwah, V.; Agrawal, A.K.; Jain, S. Insulin- and quercetin-loaded liquid crystalline nanoparticles: Implications on oral bioavailability, antidiabetic and antioxidant efficacy. Nanomedicine 2018, 13, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Y.J.; Chen, C.; Wang, X.J.; Li, W. Targeting pyroptosis: A novel strategy of ginseng for the treatment of diabetes and its chronic complications. Phytomedicine 2025, 138, 156430. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Zhao, H.; You, P.; You, H.; Wu, H.; Shou, X.; Cheng, G. Rosmarinic acid ameliorated cardiac dysfunction and mitochondrial injury in diabetic cardiomyopathy mice via activation of the SIRT1/PGC-1α pathway. Biochem. Biophys. Res. Commun. 2021, 546, 29–34. [Google Scholar] [CrossRef]
- Wani, T.U.; Raza, S.N.; Khan, N.A. Rosmarinic acid loaded chitosan nanoparticles for wound healing in rats. Int. J. Pharm. Sci. Res. 2019, 10, 1126–1135. [Google Scholar]
- Alrubaye, A.; Motovali-Bashi, M.; Miroliaei, M. Rosmarinic acid inhibits DNA glycation and modulates the expression of Akt1 and Akt3 partially in the hippocampus of diabetic rats. Sci. Rep. 2021, 11, 20605. [Google Scholar] [CrossRef]
- Vieira, L.C.; Moreira, C.P.S.; Castro, B.F.M.; Cotta, O.A.L.; Silva, L.M.; Fulgêncio, G.O.; Silva-Cunha, A.; Fialho, S.L. Rosmarinic Acid Intravitreal Implants: A New Therapeutic Approach for Ocular Neovascularization. Planta Med. 2020, 86, 1286–1297. [Google Scholar] [CrossRef]
- Chatterjee, G.; Saha, A.K.; Khurshid, S.; Saha, A. A Comprehensive Review of the Antioxidant, Antimicrobial, and Therapeutic Efficacies of Black Cumin (Nigella sativa L.) Seed Oil and Its Thymoquinone. J. Med. Food 2025, 28, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Hussain, F.; Naeem, M.; Khan, A.; Al-Harrasi, A. Nanotechnology Approach for Exploring the Enhanced Bioactivities and Biochemical Characterization of Freshly Prepared Nigella sativa L. Nanosuspensions and Their Phytochemical Profile. Front. Bioeng. Biotechnol. 2022, 10, 888177. [Google Scholar] [CrossRef]
- Anuradha, U.; Bhavana, V.; Chary, P.S.; Rajana, N.; Parida, K.K.; Kalia, N.P.; Khatri, D.K.; Mehra, N.K. Thymoquinone loaded nanoemulgel in streptozotocin induced diabetic wound. Nanomedicine 2024, 19, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Hofni, A.; Ali, F.E.M.; Ibrahim, A.R.N.; Aboubaker, E.M. Renoprotective Effect of Thymoquinone against Streptozotocin-Induced Diabetic Nephropathy: Role of NOX2 and Nrf2 Signals. Curr. Mol. Pharmacol. 2023, 16, 905–914. [Google Scholar] [CrossRef]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef]
- Ma, R.; He, Y.; Fang, Q.; Xie, G.; Qi, M. Ferulic acid ameliorates renal injury via improving autophagy to inhibit inflammation in diabetic nephropathy mice. Biomed. Pharmacother. 2022, 153, 113424. [Google Scholar] [CrossRef]
- Wehbe, N.; Bechelany, M.; Badran, A.; Al-Sawalmih, A.; Mesmar, J.E.; Baydoun, E. A Phytochemical Analysis and the Pharmacological Implications of the Seagrass Halodule uninervis: An Overview. Pharmaceuticals 2024, 17, 993. [Google Scholar] [CrossRef] [PubMed]
- Al-Mijalli, S.H.; Mrabti, H.N.; Ouassou, H.; Flouchi, R.; Abdallah, E.M.; Sheikh, R.A.; Alshahrani, M.M.; Awadh, A.A.A.; Harhar, H.; Omari, N.E. Chemical Composition, Antioxidant, Anti-Diabetic, Anti-Acetylcholinesterase, Anti-Inflammatory, and Antimicrobial Properties of Arbutus unedo L. and Laurus nobilis L. Essential Oils. Life 2022, 12, 1876. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2016, 12, 279–294. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Yap, W.S.; Suhaimi, N.A.B.A.; Gupta, G.; Dua, K. Current Therapies and Targets for Type 2 Diabetes Mellitus. Panminerva Med. 2018, 60, 117–131. [Google Scholar] [CrossRef]
- Oh, S.; Lee, M.; Ko, K.S.; Choi, S.; Kim, S.W. GLP-1 Gene Delivery for the Treatment of Type 2 Diabetes. Mol. Ther. 2003, 7, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.H.; Ho, J.S.S.; Chen, S.; Tam, Y.Y.C.; Cullis, P.R.; Kieffer, T.J. Lipid Nanoparticle Delivery of Glucagon Receptor siRNA Improves Glucose Homeostasis in Mouse Models of Diabetes. Mol. Metab. 2017, 6, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, J.G.; Reza-López, S.A.; González-Rodríguez, E.; Siqueiros-Cendón, T.S.; Escareño Contreras, A.; Rascón-Cruz, Q.; Leal-Berumen, I. Genetic Variants of SLC22A1 rs628031 and rs622342 and Glycemic Control in T2DM Patients from Northern Mexico. Genes 2025, 16, 139. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S. Recent Progress in Genetic and Epigenetic Research on Type 2 Diabetes. Exp. Mol. Med. 2016, 48, e220. [Google Scholar] [CrossRef]
- Ko, K.S.; Lee, M.; Koh, J.J.; Kim, S.W. Combined Administration of Plasmids Encoding IL-4 and IL-10 Prevents the Development of Autoimmune Diabetes in Nonobese Diabetic Mice. Mol. Ther. 2001, 4, 313–316. [Google Scholar] [CrossRef]
- Koh, J.J.; Ko, K.S.; Lee, M.; Han, S.; Park, J.S.; Kim, S.W. Degradable Polymeric Carrier for the Delivery of IL-10 Plasmid DNA to prevent Autoimmune Insulitis of NOD Mice. Gene Ther. 2000, 7, 2099–2104. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Sajjadi-Jazi, S.M. Diabetic Stem Cell Therapy and Nanomedicine: Advancements in Treating Diabetes. J. Diabetes Metab. Disord. 2023, 22, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Xu, C.F.; Li, H.J.; Cao, Z.T.; Liu, J.; Wang, J.L.; Du, X.J.; Yang, X.Z.; Gu, Z.; Wang, J. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano 2018, 12, 994–1005. [Google Scholar] [CrossRef]
- Bai, X.; Pei, Q.; Pu, C.; Chen, Y.; He, S.; Wang, B. Multifunctional islet transplantation hydrogel encapsulating A20 high-expressing islets. Drug Des. Devel. Ther. 2020, 14, 4021–4027. [Google Scholar] [CrossRef]
- Chen, S.; Du, K.; Zou, C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res. Ther. 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Stabler, C.L.; Giraldo, J.A.; Berman, D.M.; Gattás-Asfura, K.M.; Willman, M.A.; Rabassa, A.; Geary, J.; Diaz, W.; Kenyon, N.M.; Kenyon, N.S. Transplantation of PEGylated islets enhances therapeutic efficacy in a diabetic nonhuman primate model. Am. J. Transplant. 2020, 20, 689–700. [Google Scholar] [CrossRef]
- Warshauer, J.T.; Bluestone, J.A.; Anderson, M.S. New frontiers in the treatment of type 1 diabetes. Cell Metab. 2020, 31, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.Y.; Ryu, J.Y.; Lee, H.A.R.; Hong, S.H.; Park, H.S.; Hong, K.S.; Park, S.G.; Kim, H.P.; Yoon, T.J. Lecithin nano-liposomal particle as a CRISPR/Cas9 complex delivery system for treating type 2 diabetes. J. Nanobiotechnol. 2019, 17, 19. [Google Scholar] [CrossRef]
- Othman, S.I.; Alturki, A.M.; Abu-Taweel, G.M.; Altoom, N.G.; Allam, A.A.; Abdelmonem, R. Chitosan for biomedical applications, promising antidiabetic drug delivery system, and new diabetes mellitus treatment based on stem cell. Int. J. Biol. Macromol. 2021, 190, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Burganova, G.; Bridges, C.; Thorn, P.; Landsman, L. The role of vascular cells in pancreatic beta-cell function. Front. Endocrinol. 2021, 12, 667170. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Xia, D.; Guo, Y.; Xu, R.; Li, N. Emerging strategies for nitric oxide production and their topical application as nano dressings to promote diabetic wound healing. J. Nanobiotechnol. 2025, 23, 53. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
- Haque, S.T.; Saha, S.K.; Haque, M.E.; Biswas, N. Nanotechnology-Based Therapeutic Applications: In Vitro and in Vivo Clinical Studies for Diabetic Wound Healing. Biomater. Sci. 2021, 9, 7705–7747. [Google Scholar] [CrossRef]
- Qi, X.; Huan, Y.; Si, H.; Zou, J.; Mu, Z. Study of the Effect of Epidermal Growth Factor Nanoparticles in the Treatment of Diabetic Rat Ulcer Skin and Regeneration. J. Nanosci. Nanotechnol. 2021, 21, 3028–3034. [Google Scholar] [CrossRef]
- Lopes Rocha Correa, V.; Martins, J.A.; De Souza, T.R.; De Castro Nunes Rincon, G.; Miguel, M.P.; De Menezes, L.B.; Amaral, A.C. Melatonin Loaded Lecithin-Chitosan Nanoparticles Improved the Wound Healing in Diabetic Rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Borah, S.J.; Bhawna Kumar, S.; Gupta, A.; Kumari, V.; Kumar, R.; Dubey, K.K.; Kumar, V. Emerging trends in nano-based antidiabetic therapeutics: A path to effective diabetes management. Mater. Adv. 2023, 4, 3091–3113. [Google Scholar] [CrossRef]
- Siwach, R.; Pandey, P.; Chawla, V.; Dureja, H. Role of nanotechnology in diabetic management. Recent Pat. Nanotechnol. 2019, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, S.; Ji, W.; Yao, H.; Lin, L.; Cui, H.; Santos, H.A.; Pan, G. Emerging theranostic nanomaterials in diabetes and its complications. Adv. Sci. 2022, 9, 2102466. [Google Scholar] [CrossRef]
- Yan, Y.; Cai, H.; Yang, M. The application of nanotechnology for the diagnosis and treatment of endocrine disorders: A review of current trends, toxicology and future perspective. Int. J. Nanomed. 2024, 19, 9921–9942. [Google Scholar] [CrossRef] [PubMed]
- Saber, S.; Abdelhady, R.; Elhemely, M.A.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Negm, S.; et al. Nanoscale Systems for Local Activation of Hypoxia-Inducible Factor-1 Alpha: A New Approach in Diabetic Wound Management. Int. J. Nanomed. 2024, 19, 13735–13762. [Google Scholar] [CrossRef]
- Parveen, R.; Ali, F.; Singh, S.D. Innovative Nanocomposites for Drug Delivery: A Novel Approach for Diabetic Foot Ulcer. Curr. Drug Deliv. 2024. [Google Scholar] [CrossRef]
- Shan, X.; Cai, Y.; Zhu, B.; Zhou, L.; Sun, X.; Xu, X.; Yin, Q.; Wang, D.; Li, Y. Rational strategies for improving the efficiency of design and discovery of nanomedicines. Nat. Commun. 2024, 15, 9990. [Google Scholar] [CrossRef]

| Material | Carrier Compound | Method Used | Size (nm) |
|---|---|---|---|
| Chitosan | Chitosan, alginate | Electrostatic bonds and chemical interactions | 104 |
| Chitosan | Chitosan | Self-assembly | 277 |
| Chitosan | Carboxymethyl chitosan | Chemical cross linking interactions | 190 |
| Chitosan | Chitosan, γ-PGA | Electrostatic bonds | 250 |
| Alginate, dextran sulfate | Emulsification/gelation | 300 | |
| HPMCP | Emulsification/solvent diffusion | 200 | |
| Proanthocyanidines, glucans | Recrystallization | 100–300 | |
| PLA | PLA/PEG | Nanoprecipitation | 63 |
| PLGA | PLGA 20 kDa/50 kDa | Double emulsion | 157/247 |
| MOFs | Fe-based MOF | Physical absorption | 100 |
| Zr6-based MOF | Physical absorption | (-) | |
| DOTAP, EPC | BSA | Thin-film hydration | 195 |
| EP, CH, DOTAP | Chitosan | Thin-film hydration | 439 |
| DODA-501, NIPAAm, AAC | Free radical polymerization | 94–200 | |
| Soybean lecithin | Peptide | Double emulsion | 161.6 |
| Soy lecithin | Propylene glycol | Emulsification/solvent evaporation | 203.6 |
| Hyaluronic Acid, HPMCP | Penetratin peptide | FNC | 103 |
| Mesoporous silica NPs | KLPVM peptide | Physical absorption | 263.5 |
| Hydroxyapatite, PEG | Gallic acid | Homogeneous Precipitation/esterification/amidation | 150 |
| Mesoporous silica NPs | APBA | Aqueous polymerization/physical absorption | 202.8 |
| Pros of NPs | Cons of NPs |
|---|---|
| Improved absorption | Unknown safety in the long term |
| More controllable release allowing for plausible better compliance | Lack of clinical trials |
| Resistance to various pH values as well as to enzymatic degradation throughout the GIT | High costs |
| Improved entry into the targeted cells | Research is in its very early stages |
| PHYs. | Antidiabetic Properties/Action |
|---|---|
| Curcumin | ↓ FPG; ↓ IR Also used in diabetic wounds in a nanoformula hydrogel as it has healing properties due to its inhibition of MMP-9 [67,68]. |
| Resveratrol | ↓ FPG; ↓ IR It is undergoing evaluation on the treatment of DR due to its inhibition of VEGF-1,ICAM-1, MCP-1 and ERK1/2 [69,70]. |
| Berberine | ↓ FPG; ↓ IR [71]. |
| Silymarin | ↓ FPG; ↓ IR [72]. |
| Naringenin | Under investigation for improvement in early DR due to its antioxidant properties. Amelioration in DKD due to inhibition of ferroptosis via the SIRT1/FOXO3a pathway [73,74,75]. |
| Quercetin | It may be useful in DR, DKD and DN due to its antioxidant, anti-fibrotic, anti-inflammatory potential and by affecting pyroptosis. As a hydrogel, it is postulated to improve wound healing due to its antioxidant properties [76,77]. |
| Rosmarinic Acid | It has been suggested to ameliorate cardiac dysfunction (cardiomyopathy) in DM due to its antioxidant properties. Instillation on the eyes has been proposed to improve DR due to its antioxidant capacity. Also, it is undergoing evaluation as a gel for diabetic wounds. In addition, it has been suggested to interfere with the deposition of β-amyloid in the brain [78,79,80,81]. |
| Thymoquinone (from Nigella sativa) | It is suggested to possess nephroprotective potential via the Nrf2/NOX2 pathway. It has been suggested to be useful in diabetic wounds due to its antioxidant, anti-inflammatory and antimicrobial properties as well as its angiogenesis amelioration [82,83,84,85,86]. |
| Ferulic Acid | It has been implicated in ameliorating DKD by means of improving autophagy. It has been suggested as a nanogel to be involved in healing diabetic wounds due to its antioxidant and antimicrobial potential [87,88,89]. |
| Seagrass Halodule uninervis | Very recently, it has been suggested to exhibit antioxidant and anti-inflammatory properties [90]. |
| Arbutus unedo | It has been proposed to exert antidiabetic, antioxidant, anti-inflammatory as well as antimicrobial potential [91]. |
| Epigallocatechin-3 gallate | This polyphenolic compound of tea has been suggested to inhibit angiogenesis in the eye by targeting integrins; as such, it may be further exploited in DR [92]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallianou, N.G.; Dalamaga, M.; Pavlou, A.; Rebelos, E.; Karamanolis, N.N.; Papachristoforou, E.; Mavrothalassitis, E.; Eleftheriadou, I.; Tentolouris, N.; Kounatidis, D. The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research. Biomolecules 2025, 15, 653. https://doi.org/10.3390/biom15050653
Vallianou NG, Dalamaga M, Pavlou A, Rebelos E, Karamanolis NN, Papachristoforou E, Mavrothalassitis E, Eleftheriadou I, Tentolouris N, Kounatidis D. The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research. Biomolecules. 2025; 15(5):653. https://doi.org/10.3390/biom15050653
Chicago/Turabian StyleVallianou, Natalia G., Maria Dalamaga, Argyro Pavlou, Eleni Rebelos, Nikolaos Nektarios Karamanolis, Eleftheria Papachristoforou, Evangelos Mavrothalassitis, Ioanna Eleftheriadou, Nikolaos Tentolouris, and Dimitris Kounatidis. 2025. "The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research" Biomolecules 15, no. 5: 653. https://doi.org/10.3390/biom15050653
APA StyleVallianou, N. G., Dalamaga, M., Pavlou, A., Rebelos, E., Karamanolis, N. N., Papachristoforou, E., Mavrothalassitis, E., Eleftheriadou, I., Tentolouris, N., & Kounatidis, D. (2025). The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research. Biomolecules, 15(5), 653. https://doi.org/10.3390/biom15050653








